Inactivation Kinetics of Listeria monocytogenes Applying Mild Temperatures and Fractionated Mexican Oregano Essential Oil (Poliomintha longiflora Gray) in a Modified Simulated Meat Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture and Growth Conditions
2.2. Essential Oil of P. longiflora
2.3. Preparation of Oil Working Solutions
2.4. Antimicrobial Activity: Minimum Bactericidal Concentration and Sublethal Concentrations
2.5. Thermal Inactivation Treatments
2.6. Estimation of the L. monocytogenes Inactivation Parameters
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| °C | Degree Celsius |
| μL | Microliter |
| Af | Accuracy factor |
| AIC | Akaike Information Criterion |
| Bf | Bias factor |
| BHI | Brain Heart Infusion |
| CFU/mL | Colony-Forming Units per milliliter |
| EO | Essential Oils |
| FIV | Fractionated Oregano Essential Oil |
| g/L | Grams per liter |
| GRAS | Generally Recognized As Safe |
| h | Hours |
| HPP | High-Pressure Processing |
| MBC | Minimum Bactericidal Concentration |
| min | Minutes |
| mL | Milliliter |
| OD | Optical Density |
| OEO | Oregano Essential Oil |
| PEO | Pure Oregano Essential Oil |
| RMSE | Root Mean Square Error |
| RTE | Ready-To-Eat |
| s | Seconds |
| SER | Standard Error of Regression |
| TSA | Trypticase Soy Agar |
| v/v | Volume/volume |
References
- Food and Drug Administration. Hazard Analysis and Risk-Based Preventive Controls for Human Food: Draft Guidance for Industry. 2024. Available online: https://www.regulations.gov/document/FDA-2016-D-2343-0092 (accessed on 16 April 2025).
- Food and Drug Administration. Bad Bug Book-Foodborne Pathogenic Microorganisms and Natural Toxins-Second Edition 2 Bad Bug Book Handbook of Foodborne Pathogenic Microorganisms and Natural Toxins; Food and Drug Administration: Washington DC, USA, 2022; pp. 99–102. [Google Scholar]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; Nørrung, B.; et al. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, 5134. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; Nauta, M.; et al. Persistence of microbiological hazards in food and feed production and processing environments. EFSA J. 2024, 22, e8521. [Google Scholar] [CrossRef]
- CDC. Listeria Outbreak Linked to Leafy Greens—February. 2023. Available online: https://www.cdc.gov/listeria/outbreaks/monocytogenes-02-23/index.html (accessed on 9 March 2025).
- CDC. Listeria Outbreak Linked to Ice Cream—August. 2023. Available online: https://www.cdc.gov/listeria/outbreaks/ice-cream-08-23/index.html (accessed on 9 March 2025).
- CDC. Listeria Outbreak Linked to Queso Fresco and Cotija Cheese—February. 2024. Available online: https://www.cdc.gov/listeria/outbreaks/cheese-02-24/index.html (accessed on 9 March 2025).
- CDC. Listeria Outbreak Linked to Ready-To-Eat Meat and Poultry Products. Available online: https://www.cdc.gov/listeria/outbreaks/meat-and-poultry-products-11-24/index.html (accessed on 9 March 2025).
- CDC. More Illnesses and Deaths in Listeria Outbreak Linked to Deli Meats Is Reminder to Avoid Recalled Products. 2024. Available online: https://www.cdc.gov/media/releases/2024/s0828-listeria-outbreak-deli-meats.html (accessed on 9 March 2025).
- CDC. Outbreak Investigation of Listeria monocytogenes: Frozen Supplemental Shakes (February 2025). Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-listeria-monocytogenes-frozen-supplemental-shakes-february-2025 (accessed on 9 March 2025).
- Guel-García, P.; De León, F.J.G.; Aguilera-Arreola, G.; Mandujano, A.; Mireles-Martínez, M.; Oliva-Hernández, A.; Cruz-Hernández, M.A.; Vasquez-Villanueva, J.; Rivera, G.; Bocanegra-García, V.; et al. Prevalence and Antimicrobial Resistance of Listeria monocytogenes in Different Raw Food from Reynosa, Tamaulipas, Mexico. Foods 2024, 13, 1656. [Google Scholar] [CrossRef]
- Matle, I.; Mbatha, K.R.; Madoroba, E. A review of Listeria monocytogenes from meat and meat products: Epidemiology, virulence factors, antimicrobial resistance, and diagnosis. Onderstepoort J. Veter-Res. 2020, 87, 20. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, S.A.; El-Mekkawy, R.M.; El-Hack, M.E.A.; El-Ghareeb, W.R.; Suliman, G.M.; Alowaimer, A.N.; Swelum, A.A. Inactivation of Listeria monocytogenes in ready-to-eat smoked turkey meat by combination with packaging atmosphere, oregano essential oil and cold temperature. AMB Express 2019, 9, 54. [Google Scholar] [CrossRef]
- Labidi, S.; Jánosity, A.; Yakdhane, A.; Yakdhane, E.; Surányi, B.; Mohácsi-Farkas, C.; Kiskó, G. Effects of pH, sodium chloride, and temperature on the growth of Listeria monocytogenes biofilms. Acta Aliment. 2023, 52, 270–280. [Google Scholar] [CrossRef]
- Hong, H.; Yang, S.M.; Kim, E.; Kim, H.J.; Park, S.H. Comprehensive metagenomic analysis of stress-resistant and -sensitive Listeria monocytogenes. Appl. Microbiol. Biotechnol. 2023, 107, 6047–6056. [Google Scholar] [CrossRef]
- Magalhães, R.; Ferreira, V.; Brandão, T.; Palencia, R.C.; Almeida, G.; Teixeira, P. Persistent and non-persistent strains of Listeria monocytogenes: A focus on growth kinetics under different temperature, salt, and pH conditions and their sensitivity to sanitizers. Food Microbiol. 2016, 57, 103–108. [Google Scholar] [CrossRef]
- Yue, S.; Liu, Y.; Wang, X.; Xu, D.; Qiu, J.; Liu, Q.; Dong, Q. Modeling the effects of the preculture temperature on the lag phase of Listeria monocytogenes at 25 °C. J. Food Prot. 2019, 82, 2100–2107. [Google Scholar] [CrossRef]
- Wei, J.; Ismael, M.; Huang, M.; Han, T.; Zhong, Q. The Bactericidal Effects of Combined Sterilization Methods on Listeria monocytogenes and the Application in Prepared Salads. Elsevier, 2025. Preprint Article. Available online: https://ssrn.com/abstract=5102418 (accessed on 22 March 2025).
- Ghabraie, M.; Vu, K.D.; Huq, T.; Khan, A.; Lacroix, M. Antilisterial effects of antibacterial formulations containing essential oils, nisin, nitrite and organic acid salts in a sausage model. J. Food Sci. Technol. 2016, 53, 2625–2633. [Google Scholar] [CrossRef]
- Mani-López, E.; García, H.; López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Puvača, N.; Milenković, J.; Coghill, T.G.; Bursić, V.; Petrović, A.; Tanasković, S.; Pelić, M.; Pelić, D.L.; Miljković, T. Antimicrobial activity of selected essential oils against selected pathogenic bacteria: In vitro study. Antibiotics 2021, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- de Souza Pedrosa, G.T.; Pimentel, T.C.; Gavahian, M.; de Medeiros, L.L.; Pagán, R.; Magnani, M. The combined effect of essential oils and emerging technologies on food safety and quality. LWT 2021, 147, 111593. [Google Scholar] [CrossRef]
- Vidaković Knežević, S.; Knežević, S.; Vranešević, J.; Kravić, S.; Lakićević, B.; Kocić-Tanackov, S.; Karabasil, N. Effects of Selected Essential Oils on Listeria monocytogenes in Biofilms and in a Model Food System. Foods 2023, 12, 1930. [Google Scholar] [CrossRef]
- Tejada-Muñoz, S.; Cortez, D.; Rascón, J.; Chavez, S.G.; Caetano, A.C.; Díaz-Manchay, R.J.; Sandoval-Bances, J.; Huyhua-Gutierrez, S.; Gonzales, L.; Chenet, S.M.; et al. Antimicrobial Activity of Origanum vulgare Essential Oil against Staphylococcus aureus and Escherichia coli. Pharmaceuticals 2024, 17, 1430. [Google Scholar] [CrossRef]
- Pinto, L.; Cervellieri, S.; Netti, T.; Lippolis, V.; Baruzzi, F. Antibacterial Activity of Oregano (Origanum vulgare L.) Essential Oil Vapors against Microbial Contaminants of Food-Contact Surfaces. Antibiotics 2024, 13, 371. [Google Scholar] [CrossRef]
- Maggio, F.; Rossi, C.; Chaves-López, C.; Valbonetti, L.; Desideri, G.; Paparella, A.; Serio, A. A single exposure to a sublethal concentration of Origanum vulgare essential oil initiates response against food stressors and restoration of antibiotic susceptibility in Listeria monocytogenes. Food Control 2022, 132, 108562. [Google Scholar] [CrossRef]
- Ruiz-Hernández, K.; Sosa-Morales, M.E.; Cerón-García, A.; Gómez-Salazar, J.A. Physical, Chemical and Sensory Changes in Meat and Meat Products Induced by the Addition of Essential Oils: A Concise Review. Food Rev. Int. 2021, 39, 2027–2056. [Google Scholar] [CrossRef]
- Ortega, A.R.; Guinoiseau, E.; Poli, J.-P.; Quilichini, Y.; Serra, D.d.R.; Novelles, M.d.C.T.; Castaño, I.E.; Pérez, O.P.; Berti, L.; Lorenzi, V. The Primary Mode of Action of Lippia graveolens Essential Oil on Salmonella enterica subsp. Enterica Serovar Typhimurium. Microorganisms 2023, 11, 2943. [Google Scholar] [CrossRef]
- Ortega-Nieblas, M.; Robles-Burgueño, M.; Acedo-Félix, E.; González-León, A.; Morales-Trejo, A.; Vázquez-Moreno, L. Chemical Composition and Antimicrobial Activity of Oregano (Lippia palmeri S. Wats) Essential Oil. Rev Fitotec Nex. 2011, 34, 11–17. [Google Scholar] [CrossRef]
- Zapién-Chavarría, K.A.; Plascencia-Terrazas, A.; Venegas-Ortega, M.G.; Varillas-Torres, M.; Rivera-Chavira, B.E.; Adame-Gallegos, J.R.; González-Rangel, M.O.; Nevárez-Moorillón, G.V. Susceptibility of multidrug-resistant and biofilm-forming uropathogens to Mexican oregano essential oil. Antibiotics 2019, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Levario-Gómez, A.; Ávila-Sosa, R.; Gutiérrez-Méndez, N.; López-Malo, A.; Nevárez-Moorillón, G.V. Modeling the Combined Effect of pH, Protein Content, and Mexican Oregano Essential Oil Against Food Spoilage Molds. Front. Sustain. Food Syst. 2020, 4, 34. [Google Scholar] [CrossRef]
- Mora-Zúñiga, A.E.; Treviño-Garza, M.Z.; Guerra, C.A.A.; Rodríguez, S.A.G.; Castillo, S.; Martínez-Rojas, E.; Rodríguez-Rodríguez, J.; Báez-González, J.G. Comparison of Chemical Composition, Physicochemical Parameters, and Antioxidant and Antibacterial Activity of the Essential Oil of Cultivated and Wild Mexican Oregano Poliomintha longiflora Gray. Plants 2022, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- García, E.S.; Torres-Alvarez, C.; Sosa, E.G.M.; Pimentel-González, M.; Treviño, L.V.; Guerra, C.A.A.; Castillo, S.; Rodríguez, J.R. Essential Oil of Fractionated Oregano as Motility Inhibitor of Bacteria Associated with Urinary Tract Infections. Antibiotics 2024, 13, 665. [Google Scholar] [CrossRef]
- Rostro-Alanis, M.d.J.; Báez-González, J.; Torres-Alvarez, C.; Parra-Saldívar, R.; Rodriguez-Rodriguez, J.; Castillo, S. Chemical composition and biological activities of oregano essential oil and its fractions obtained by vacuum distillation. Molecules 2019, 24, 1904. [Google Scholar] [CrossRef]
- Cabrera-Díaz, E.; Martínez-Chávez, L.; Gutiérrez-González, P.; Pérez-Montaño, J.A.; Rodríguez-García, M.O.; Martínez-Gonzáles, N.E. Effect of storage temperature and time on the behavior of Salmonella, Listeria monocytogenes, and background microbiota on whole fresh avocados (Persea americana var Hass). Int. J. Food Microbiol. 2022, 369, 109614. [Google Scholar] [CrossRef]
- Márquez-González, M.; Osorio, L.F.; Velásquez-Moreno, C.G.; García-Lira, A.G. Thermal Inactivation of Salmonella enterica and Listeria monocytogenes in Quesillo Manufactured from Raw Milk. Int. J. Food Sci. 2022, 2022, 2507867. [Google Scholar] [CrossRef]
- Rubio Lozano, S.M.; Martínez Bruno, F.J.; Hernández Castro, R.; Bonilla Contreras, C.; Danilo Méndez Medina, R.; Núñez Espinosa, F.J.; Echeverry, A. Detection of Listeria monocytogenes, Salmonella and Yersinia enterocolitica in beef at points of sale in Mexico. Rev. Mex. Cienc. Pecu. 2013, 4, 107–115. [Google Scholar]
- Agregán, R.; Munekata, P.E.; Zhang, W.; Zhang, J.; Pérez-Santaescolástica, C.; Lorenzo, J.M. High-pressure processing in inactivation of Salmonella spp. in food products. Trends Food Sci. Technol. 2021, 107, 31–37. [Google Scholar] [CrossRef]
- Soni, A.; Bremer, P.; Brightwell, G. A Comprehensive Review of Variability in the Thermal Resistance (D-Values) of Food-Borne Pathogens—A Challenge for Thermal Validation Trials. Foods 2022, 11, 4117. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Bezirtzoglou, E. Predictive modeling of microbial behavior in food. Foods 2019, 8, 654. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, F.; Valero, A. Application of Predictive Models in Quantitative Risk Assessment and Risk Management. In Predictive Microbiology in Foods; Harter, R.W., Ed.; Springer: New York, NY, USA; Berlin/Heidelberg, Germany; Dordrecht, The Netherlands; London, UK, 2013; pp. 87–97. [Google Scholar]
- Possas, A.; Posada-Izquierdo, G.D.; Pérez-Rodríguez, F.; Valero, A.; García-Gimeno, R.M.; Duarte, M.C. Application of predictive models to assess the influence of thyme essential oil on Salmonella enteritidis behaviour during shelf life of ready-to-eat turkey products. Int. J. Food Microbiol. 2017, 240, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Dogruyol, H.; Mol, S.; Cosansu, S. Increased thermal sensitivity of Listeria monocytogenes in sous-vide salmon by oregano essential oil and citric acid. Food Microbiol. 2020, 90, 103496. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, N.F.; Ruiz-Montero, R.; Briones, E.; Baños, E.; Rodríguez-Alarcón, L.G.S.M.; Chaves, J.A.; Abad, R.; Varela, C.; on behalf of the LISMOAN team; Lorusso, N. Listeriosis outbreak caused by contaminated stuffed pork, Andalusia, Spain, July to October 2019. Eurosurveillance 2022, 27, 2200279. [Google Scholar] [CrossRef]
- Lazou, T.P.; Chaintoutis, S.C. Comparison of disk diffusion and broth microdilution methods for antimicrobial susceptibility testing of Campylobacter isolates of meat origin. J. Microbiol. Methods 2023, 204, 106649. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Gardini, F.; Lanciotti, R. Effects of sub-lethal concentrations of thyme and oregano essential oils, carvacrol, thymol, citral and trans-2-hexenal on membrane fatty acid composition and volatile molecule profile of Listeria monocytogenes, Escherichia coli and Salmonella enteritidis. Food Chem. 2015, 182, 185–192. [Google Scholar] [CrossRef]
- Juneja, V.K.; Huang, L.; Yan, X. Thermal inactivation of foodborne pathogens and the USDA pathogen modeling program. J. Therm. Anal. Calorim. 2011, 106, 191–198. [Google Scholar] [CrossRef]
- Miller, F.A.; Gil, M.M.; Brandão, T.R.; Teixeira, P.; Silva, C.L. Sigmoidal thermal inactivation kinetics of Listeria innocua in broth: Influence of strain and growth phase. Food Control 2009, 20, 1151–1157. [Google Scholar] [CrossRef]
- Possas, A.; Pérez-Rodríguez, F.; Valero, A.; Rincón, F.; García-Gimeno, R.M. Mathematical approach for the Listeria monocytogenes inactivation during high hydrostatic pressure processing of a simulated meat medium. Innov. Food Sci. Emerg. Technol. 2018, 47, 271–278. [Google Scholar] [CrossRef]
- Garre, A.; Georgalis, L.; Lindqvist, R.; Fernández, P.S. Development and Validation of Microbial Inactivation Models Using Bioinactivation4. In Basic Protocols in Predictive Microbiology Softwares. Methods and Protocols in Food Science; Pérez-Rodríguez, F., Valero, A., Bolivar, A., Eds.; Humana: New York, NY, USA, 2025. [Google Scholar] [CrossRef]
- Garre, A.; González-Tejedor, G.A.; Aznar, A.; Fernández, P.S.; Egea, J.A. Mathematical modelling of the stress resistance induced in Listeria monocytogenes during dynamic, mild heat treatments. Food Microbiol. 2019, 84, 103238. [Google Scholar] [CrossRef]
- Albert, I.; Mafart, P. A modified Weibull model for bacterial inactivation. Int. J. Food Microbiol. 2005, 100, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, W.D. The Logarithmic Nature of Thermal Death Time Curves. J. Infect. Dis. 1921, 29, 528–536. [Google Scholar] [CrossRef]
- Huertas, J.-P.; Ros-Chumillas, M.; Garre, A.; Fernández, P.S.; Aznar, A.; Iguaz, A.; Esnoz, A.; Palop, A. Impact of Heating Rates on Alicyclobacillus acidoterrestris Heat Resistance under Non-Isothermal Treatments and Use of Mathematical Modelling to Optimize Orange Juice Processing. Foods 2021, 10, 1496. [Google Scholar] [CrossRef] [PubMed]
- Buzrul, S. The Weibull Model for Microbial Inactivation. Food Eng. Rev. 2022, 14, 45–61. [Google Scholar] [CrossRef]
- Valenzuela-Melendres, M.; Peña-Ramos, E.; Juneja, V.K.; Camou, J.P.; Cumplido-Barbeitia, G. Effect of grapefruit seed extract on thermal inactivation of Listeria monocytogenes during sous-vide processing of two marinated Mexican meat entrées. J. Food Prot. 2016, 79, 1174–1180. [Google Scholar] [CrossRef]
- Zakrzewski, A.; Gajewska, J.; Chajęcka-Wierzchowska, W.; Zadernowska, A. Effect of sous-vide processing of fish on the virulence and antibiotic resistance of Listeria monocytogenes. NFS J. 2023, 31, 155–161. [Google Scholar] [CrossRef]
- Salazar, J.K.; Fay, M.L.; Fleischman, G.; Khouja, B.A.; Stewart, D.S.; Ingram, D.T. Inactivation kinetics of Listeria monocytogenes and Salmonella enterica on specialty mushroom garnishes based on ramen soup broth temperature. Front. Microbiol. 2024, 15, 1485398. [Google Scholar] [CrossRef]
- Kutner, M.H.; Nachtsheim, C.; Neter, J.; Li, W. Applied Linear Statistical Models, 5th ed.; McGraw-Hill Irwin: New York, NY, USA, 2005; pp. 15–23. [Google Scholar]
- Portet, S. A primer on model selection using the Akaike Information Criterion. Infect. Dis. Model. 2020, 5, 111–128. [Google Scholar] [CrossRef]
- Tarlak, F. The Use of Predictive Microbiology for the Prediction of the Shelf Life of Food Products. Foods 2023, 12, 4461. [Google Scholar] [CrossRef]
- Pouillot, R.; Kiermeier, A.; Guillier, L.; Cadavez, V.; Sanaa, M. Updated Parameters for Listeria monocytogenes Dose–Response Model Considering Pathogen Virulence and Age and Sex of Consumer. Foods 2024, 13, 751. [Google Scholar] [CrossRef]
- Milkievicz, T.; Badia, V.; Souza, V.B.; Longhi, D.A.; Galvão, A.C.; Robazza, W.d.S. Development of a general model to describe Salmonella spp. growth in chicken meat subjected to different temperature profiles. Food Control 2020, 112, 107151. [Google Scholar] [CrossRef]
- Dudek-Wicher, R.; Paleczny, J.; Kowalska-Krochmal, B.; Szymczyk-Ziółkowska, P.; Pachura, N.; Szumny, A.; Brożyna, M. Activity of liquid and volatile fractions of essential oils against biofilm formed by selected reference strains on polystyrene and hydroxyapatite surfaces. Pathogens 2021, 10, 515. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Li, Z.; Cai, T.; Guo, D.; Yang, B.; Zhang, C.; Shan, Z.; Wang, X.; Peng, X.; Liu, G.; et al. Inhibitory effect and mechanism of oregano essential oil on Listeria monocytogenes cells, toxins and biofilms. Microb. Pathog. 2024, 194, 106801. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, M.; Arakrak, A.; Laglaoui, A.D. Evaluation of the Antibacterial Activity of Essential Oils Against E. coli Isolated From Rabbits. Iraqi J. Agric. Sci. 2022, 53, 802–809. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Fang, T.; Wu, Y.; Xie, Y.; Sun, L.; Qin, X.; Liu, Y.; Li, H.; Dong, Q.; Wang, X. Inactivation and Subsequent Growth Kinetics of Listeria monocytogenes After Various Mild Bactericidal Treatments. Front. Microbiol. 2021, 12, 646735. [Google Scholar] [CrossRef]
- Arioli, S.; Montanari, C.; Magnani, M.; Tabanelli, G.; Patrignani, F.; Lanciotti, R.; Mora, D.; Gardini, F. Modelling of Listeria monocytogenes Scott A after a mild heat treatment in the presence of thymol and carvacrol: Effects on culturability and viability. J. Food Eng. 2019, 240, 73–82. [Google Scholar] [CrossRef]
- Shi, Y.; Tang, J.; Yue, T.; Rasco, B.; Wang, S. Pasteurizing Cold Smoked Salmon (Oncorhynchus nerka): Thermal Inactivation Kinetics of Listeria monocytogenes and Listeria innocua. J. Aquat. Food Prod. Technol. 2014, 24, 712–722. [Google Scholar] [CrossRef]
- Li, C.; Huang, L.; Hwang, C.-A. Effect of temperature and salt on thermal inactivation of Listeria monocytogenes in salmon roe. Food Control 2017, 73, 406–410. [Google Scholar] [CrossRef]
- Moura-Alves, M.; Gouveia, A.R.; de Almeida, J.M.M.; Monteiro-Silva, F.; Silva, J.A.; Saraiva, C. Behavior of Listeria monocytogenes in beef Sous vide cooking with Salvia officinalis L. essential oil, during storage at different temperatures. LWT 2020, 132, 109896. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Lu, Y.; Wang, J.; Suo, B. Synergistic effect of cinnamaldehyde on the thermal inactivation of Listeria monocytogenes in ground pork. Food Sci. Technol. Int. 2020, 26, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Kamdem, S.S.; Belletti, N.; Magnani, R.; Lanciotti, R.; Gardini, F. Effects of carvacrol, (E)-2-hexenal, and citral on the thermal death kinetics of Listeria monocytogenes. J. Food Prot. 2011, 74, 2070–2078. [Google Scholar] [CrossRef] [PubMed]
- Guevara, L.; Antolinos, V.; Palop, A.; Periago, P.M. Impact of Moderate Heat, Carvacrol, and Thymol Treatments on the Viability, Injury, and Stress Response of Listeria monocytogenes. BioMed Res. Int. 2015, 2015, 548930. [Google Scholar] [CrossRef] [PubMed]
- Essia Ngang, J.J.; Nyegue, M.A.; Ndoye, F.C.; Tchuenchieu Kamgain, A.D.; Sado Kamdem, S.L.; Lanciotti, R.; Gardini, F.; Etoa, F.-X. Characterization of Mexican coriander (Eryngium foetidum) essential oil and its inactivation of Listeria monocytogenes in vitro and during mild thermal pasteurization of pineapple juice. J. Food Prot. 2014, 77, 435–443. [Google Scholar] [CrossRef]
- Juneja, V.K.; Garcia-Dávila, J.; Lopez-Romero, J.C.; Pena-Ramos, E.A.; Camou, J.P.; Valenzuela-Melendres, M. Modeling the effects of temperature, sodium chloride, and green tea and their interactions on the thermal inactivation of Listeria monocytogenes in Turkey. J. Food Prot. 2014, 77, 1696–1702. [Google Scholar] [CrossRef]
- Butler, F.; Hunt, K.; Redmond, G.; Donofrio, F.; Barron, U.G.; Fernandes, S.; Cadavez, V.; Iannetti, L.; Centorotola, G.; Pomilio, F.; et al. Application of novel predictive microbiology techniques to shelf-life studies on Listeria monocytogenes in ready-to-eat foods (ListeriaPredict). EFSA Support. Publ. 2023, 20, 66. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Thymol; Exemption from the Requirement of a Tolerance. Federal Register 2022, 87. May 2025. Available online: https://www.govinfo.gov/content/pkg/FR-2022-09-07/pdf/2022-19294.pdf (accessed on 5 April 2025).
- Maté, J.; Periago, P.M.; Palop, A. When nanoemulsified, d-limonene reduces Listeria monocytogenes heat resistance about one hundred times. Food Control 2016, 59, 824–828. [Google Scholar] [CrossRef]
- Oficial Journal of the European Union. Commision Implementing Regulation (EU) 2024/1989. 2024. May 2025. Available online: http://data.europa.eu/eli/reg/2003/1831/oj (accessed on 5 April 2025).
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Shaw, A.; Mendonca, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L. A Review of Regulatory Standards and Advances in Essential Oils as Antimicrobials in Foods. J. Food Prot. 2023, 86, 100025. [Google Scholar] [CrossRef]
- Contreras-Soto, M.; Medrano-Félix, J.; Ibarra-Rodríguez, J.; Martínez-Urtaza, J.; Chaidez, Q.; Castro-del Campo, N. The last 50 years of Salmonella in Mexico: Sources of isolation and factors that influence its prevalence and diversity. Bio Cienc. 2019, 6, 26. [Google Scholar] [CrossRef]
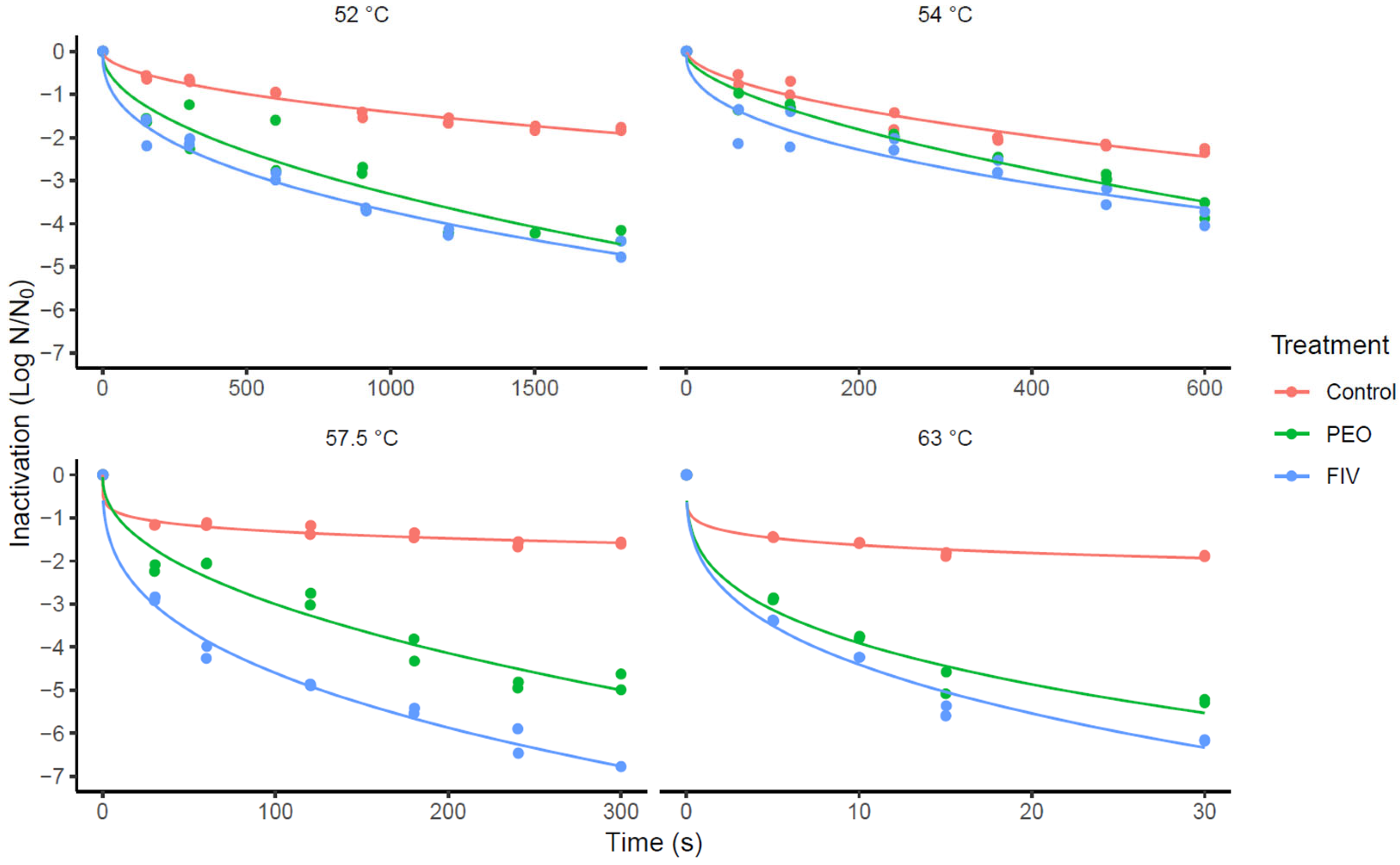
| Weibull–Mafart | Bigelow | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | T (°C) | RMSE * | Loglik | AIC | Af/Bf | RMSE | Loglik | AIC | Af/Bf |
| Control | 52 | 0.10 | 42.70 | −81.40 | 1.01/0.99 | 0.20 | 22.36 | −42.72 | 1.02/1.00 |
| 54 | 0.16 | 24.40 | −44.80 | 1.02/1.00 | 0.29 | 13.30 | −24.60 | 1.04/1.00 | |
| 57.5 | 0.08 | 47.44 | −90.88 | 1.01/1.00 | 0.37 | 11.92 | −21.84 | 1.04/1.00 | |
| 63 | 0.06 | 46.21 | −88.42 | 1.01/0.99 | 0.48 | 5.35 | −8.70 | 1.07/1.00 | |
| PEO | 52 | 0.42 | 10.36 | −16.72 | 1.07/1.00 | 0.55 | 7.57 | −13.14 | 1.09/1.00 |
| 54 | 0.18 | 22.73 | −41.46 | 1.02/1.00 | 0.30 | 14.33 | −26.66 | 1.05/1.01 | |
| 57.5 | 0.33 | 10.96 | −17.92 | 1.07/1.00 | 0.62 | 6.47 | −10.94 | 1.45/1.42 | |
| FIV | 63 | 0.34 | 11.06 | −18.12 | 1.06/1.00 | 1.00 | 2.52 | −3.04 | 1.26/0.99 |
| 52 | 0.20 | 19.14 | −34.28 | 1.06/1.03 | 0.61 | 6.22 | −10.44 | 1.12/1.02 | |
| 54 | 0.33 | 11.55 | −19.10 | 1.05/1.01 | 0.51 | 8.35 | −14.70 | 1.06/1.00 | |
| 57.5 | 0.20 | 19.50 | −35.00 | 1.10/0.98 | 1.04 | 4.14 | −6.28 | 1.30/0.94 | |
| 63 | 0.24 | 11.94 | −19.88 | 0.99/1.07 | 1.11 | 2.28 | −2.56 | 1.39/0.95 | |
| Control | PEO | FIV | ||||
|---|---|---|---|---|---|---|
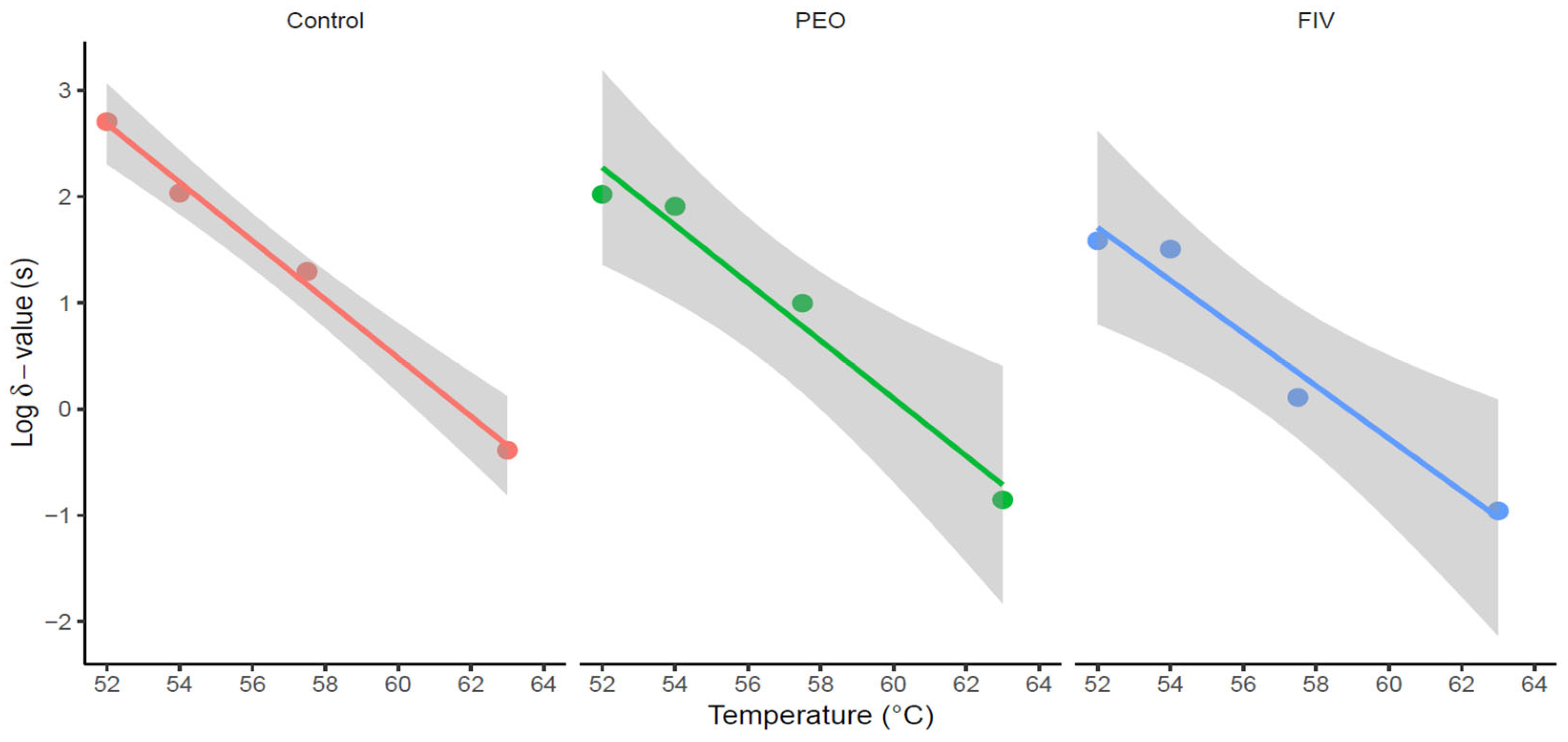
| Temp (°C) | δ-Value (min) | p | δ-Value (min) | p | δ-Value (min) | p |
| 52 | 8.470 ± 1.510 | 0.51 ± 0.05 | 1.750 ± 1.050 | 0.52 ± 0.10 | 0.640 ± 0.240 | 0.40 ± 0.04 |
| 54 | 1.800 ± 0.500 | 0.53 ± 0.07 | 1.350 ± 0.350 | 0.61 ± 0.07 | 0.540 ± 0.350 | 0.44 ± 0.09 |
| 57.5 | 0.330 ± 0.170 | 0.17 ± 0.03 | 0.170 ± 0.090 | 0.47 ± 0.07 | 0.020 ± 0.010 | 0.35 ± 0.02 |
| 63 | 0.007 ± 0.004 | 0.15 ± 0.02 | 0.002 ± 0.001 | 0.32 ± 0.05 | 0.002 ± 0.002 | 0.33 ± 0.04 |
| One-Step | Two-Step | ||||||
|---|---|---|---|---|---|---|---|
| Group | RMSE | RMSEstd | z-Value (°C) | z-Value (°C) | RSE | t Value | Pr (>|t|) |
| Control | 0.31 | 0.06 | 5.75 ± 0.28 | 3.63 ± 0.19 | 0.12 | 19.29 | 0.003 |
| PEO | 0.43 | 0.08 | 5.20 ± 0.14 | 3.69 ± 0.46 | 0.28 | 8.00 | 0.02 |
| FIV | 0.46 | 0.09 | 5.00 ± 0.13 | 4.03 ± 0.55 | 0.28 | 7.27 | 0.02 |
| Matrix | Treatment | D- or δ-Values (min) | z-Values (°C) | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D52 | D54 | D55 | D57.5 | D60 | D63 | D65 | ||||
| BHI broth supplemented with glucose and yeast extract | P. longiflora PEO 0.06%: | 1.75 | 1.35 | - | 0.17 | - | 2.00 × 10−3 | - | 5.20 | Present study |
| P. longiflora FIV 0.06%: | 0.64 | 0.54 | - | 0.02 | - | 2.00 × 10−3 | - | 5.00 | ||
| Sous-vide salmon | Origanum vulgare EO 1% | - | - | 10.03 | 4.88 | 1.81 | - | - | 5.62 | [43] |
| Sous-vide beef | Salvia officinalis EO 0.6% | - | - | 21.17 | - | - | - | - | - | [72] |
| Ground pork | Cinnamaldehyde 0.5% | - | - | 3.61 | - | 0.63 | - | 0.52 | - | [73] |
| BHI broth | Carvacrol 30 µg/mL | - | - | 8.17 | - | 0.67 | 0.17 | 0.07 | - | [74] |
| 2-Hexenal 65 µg/mL | - | - | 8.03 | - | 0.60 | 0.12 | 0.08 | - | ||
| Citral 50 µg/mL | - | - | 8.42 | - | 0.66 | 0.16 | 0.08 | - | ||
| TSBYE | Thymol | - | - | 0.25 | - | - | - | - | - | [75] |
| Carvacrol | - | - | 0.25 | - | - | - | - | - | ||
| Thymol + Carvacrol | - | - | 0.18 | - | - | - | - | - | ||
| PBS | Thymol | - | - | 1.47 | - | - | - | - | - | [69] |
| Carvacrol | - | - | 1.48 | - | - | - | - | - | ||
| Thymol + Carvacrol | - | - | 0.38 | - | - | - | - | - | ||
| Pineapple juice | Mexican coriander 15 µg/mL | - | - | 5.61 | - | 0.53 | - | 0.28 | - | [76] |
| Mexican coriander 60 µg/mL | - | - | 5.47 | - | 0.44 | - | 0.16 | |||
| Beef marinated | Grapefruit seed extract 200 ppm | - | - | 22.17 | 6.11 | 3.69 | - | - | 7.98 | [56] |
| Ground Turkey | Sodiumchloride (1%) and green tea polyphenol extract (1.5%) | - | - | 30.40 | - | 5.50 | - | 0.90 | - | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimentel-González, M.; Possas, A.; Valero, A.; Sánchez-García, E.; Rodríguez-Rodríguez, J.; Castillo, S. Inactivation Kinetics of Listeria monocytogenes Applying Mild Temperatures and Fractionated Mexican Oregano Essential Oil (Poliomintha longiflora Gray) in a Modified Simulated Meat Medium. Appl. Sci. 2025, 15, 6164. https://doi.org/10.3390/app15116164
Pimentel-González M, Possas A, Valero A, Sánchez-García E, Rodríguez-Rodríguez J, Castillo S. Inactivation Kinetics of Listeria monocytogenes Applying Mild Temperatures and Fractionated Mexican Oregano Essential Oil (Poliomintha longiflora Gray) in a Modified Simulated Meat Medium. Applied Sciences. 2025; 15(11):6164. https://doi.org/10.3390/app15116164
Chicago/Turabian StylePimentel-González, Mariana, Arícia Possas, Antonio Valero, Eduardo Sánchez-García, José Rodríguez-Rodríguez, and Sandra Castillo. 2025. "Inactivation Kinetics of Listeria monocytogenes Applying Mild Temperatures and Fractionated Mexican Oregano Essential Oil (Poliomintha longiflora Gray) in a Modified Simulated Meat Medium" Applied Sciences 15, no. 11: 6164. https://doi.org/10.3390/app15116164
APA StylePimentel-González, M., Possas, A., Valero, A., Sánchez-García, E., Rodríguez-Rodríguez, J., & Castillo, S. (2025). Inactivation Kinetics of Listeria monocytogenes Applying Mild Temperatures and Fractionated Mexican Oregano Essential Oil (Poliomintha longiflora Gray) in a Modified Simulated Meat Medium. Applied Sciences, 15(11), 6164. https://doi.org/10.3390/app15116164











