Featured Application
The results presented in this article prove that it is possible to replace the non-ecological pressure–thermal treatment of banana peels with microwave or ultrasound treatment combined with enzymatic hydrolysis without a significant loss of efficiency of the cultured yeast.
Abstract
This study evaluates the feasibility of using banana peels as a substrate for cultivating fodder yeast biomass. Banana peels (BPs), representing approximately 38% of the total fruit weight, are rich in cellulose and hemicellulose, thus presenting a significant opportunity for valorisation. The study investigates the effects of microwave and ultrasound treatments on the hydrolysis efficiency of banana peels and the subsequent cultivation of yeast. Two yeast strains, Scheffersomyces stipitis and Meyerozyma guilliermondii, were cultivated in hydrolysates prepared using various methods, including acid–thermal, enzymatic, microwave, and ultrasound treatments. The results demonstrate that enzymatic hydrolysis following microwave or ultrasound pretreatment significantly enhances sugar release, supporting higher biomass yields. The maximum biomass concentration achieved was 7.68 g DM/L, with crude protein content reaching up to 45.46% DM. These results indicate that banana peels can be effectively utilised for single-cell protein production, providing a sustainable alternative for animal feed. The study underscores the potential of integrating microwave and ultrasound technologies in bioprocessing to enhance the efficiency and environmental sustainability of yeast cultivation.
1. Introduction
Bananas are tropical plants [1] and the fourth most important food crop in the world after rice, wheat and corn. They are an important source of nutrition for many people around the world because they are available in large quantities all year round [2,3]. This fruit is a rich source of starch, fibre, vitamins A, B, C and E, and minerals (potassium, phosphorus). A quantity of 100 g of peeled banana contains as much as 360 mg of potassium, which has a positive effect on the functioning of the heart muscle [4]. In addition, bananas are characterised by a high content of natural, bioactive nutrients—carotenoids and phenolic compounds. They have antioxidant, anti-inflammatory, antibacterial and anti-cancer effects [5]. The peel surrounding the edible part is the main waste when eating bananas. Banana peels (BPs) make up about 38% of the total banana weight. With around 140 million tonnes of bananas produced worldwide annually, BPs are a potential environmental pollutant [6,7].
Banana peels contain valuable compounds that can be used primarily in the food industry [8]. They are rich in bioactive compounds such as tannins, flavonoids, alkaloids, glycosides, terpenoids and anthocyanins. In addition, they are rich in dietary fibre, protein, amino acids, polyunsaturated fatty acids and potassium. The compounds mentioned in the abovementioned waste can improve the nutritional quality of certain food products [6,9]. Banana peels can be used as feed additives due to their positive composition. Thanks to their tannin content, they have a positive effect on cows’ milk yield and protein content [10]. BPs can be used to make silage to extend the feed suitability. The BP silage production process is similar to other ‘traditional’ plant-based raw materials [10]. Peels can also be used as fertiliser for plants. Due to their high potassium content, amino acids, and growth stimulants, they contribute to increasing the germination rate of seeds. Another important aspect is that they deter aphids, pests that contribute to the destruction of cultivated plants [11].
Based on predictions, the global population’s protein requirement exceeds 200 million tonnes for human nutrition [12,13]. Animals consume at least a comparable amount of protein annually, and these trends indicate continued growth [13,14]. Agriculture, facing challenges from a changing climate, may struggle to meet these needs fully [15,16,17,18]. Consequently, there is growing interest in alternative protein sources, such as microbial protein. Single-cell protein includes both whole microbial cells and proteins that are extracted from their biomass [19]. It is believed that the term SCP (Single Cell Protein) was first used by scientists at the Massachusetts Institute of Technology around 1966. Initially, the term referred only to microbial biomass containing proteins, but now it refers to both biomass and protein isolates from microbial cells (bacteria, algae, yeasts and moulds) [19]. Common substrates that can be used to produce different types of single-cell protein are whey, sugar cane bagasse, orange waste, rice husks and beet pulp. This makes it possible to reduce the impact of organic waste on the environment and convert it into animal feed [20]. The possibility of producing SCP carries many advantages, making it a much better solution than plant or animal protein. The main benefit is the significantly short generation time of microorganisms and the high protein content in the cells. It is also possible to modify the proteins’ amino acid profile by adjusting the substrate’s composition, the cultivation conditions, or the cultivated strain’s genetic modification. It is also worth noting that the production of this protein can be carried out in processes isolated from the environment, which are not influenced by climatic factors [21].
The use of yeast in animal nutrition offers many benefits. It is a rich source of B vitamins, enzymes and highly digestible proteins. Yeast is becoming increasingly important as a carrier of bioelements. Yeast also benefits the rumen flora and stimulates the growth, development and condition of animals [22,23].
Scheffersomyces stipitis and Meyerozyma guilliermondii are frequently used yeast species, both for scientific purposes and for the production of feed biomass. They reproduce efficiently by budding at 25–37 °C and can ferment various carbohydrates such as glucose, galactose, maltose and xylose [24,25,26,27,28,29,30,31,32,33]. Meyerozyma guilliermondii, used in our experiments, is known as one of the yeast strains accepted for obtaining fodder SCP [34], while the other strain—Scheffersomyces stipitis—can bioconvert lignocellulose, ferment xylose and also ferment the sugars glucose, mannose, galactose, cellobiose, mannan and xylan oligomers. This makes it a powerful organism that can simultaneously hydrolyse, saccharify and ferment the carbon sources in cheap agri-food waste [27,35,36,37,38].
Acid–thermal treatment is one of the oldest methods of at least partial hydrolysis of lignocellulosic materials. Due to the use of acids, it is considered non-ecological, while the neutralisation of the solution after the process causes salinisation and hinders subsequent bioprocesses. Therefore, various alternative methods of two-stage hydrolysis have been tested for many years. In the first stage, a pre-treatment is used to loosen the matrix of the lignocellulosic raw material. The subsequent hydrolysis is then carried out using enzymes that release simple sugars from the raw material’s polysaccharides.
Numerous studies have investigated the effects of microwaves and ultrasound on the hydrolysis of lignocellulosic materials and the extraction of biologically active compounds; however, reports on banana peels are scarce [39,40,41,42,43,44,45]. Banana peels are a waste product generated in households and through the industrial processing of bananas. Therefore, it is reasonable to seek methods for valorising this waste.
The composition of mono- and polysaccharides makes banana peels a promising raw material for effective yeast cultivation following efficient hydrolysis. Therefore, we found it interesting to investigate the impact of microwave and ultrasound treatment on the hydrolysis yield of this raw material and the cultivation of yeast SCP.
2. Materials and Methods
2.1. Banana Peels—Characteristics of the Raw Material
The raw material for the research was obtained from fresh bananas purchased at one of the chain grocery stores in Lodz, Poland. These bananas were purchased as ripe yellow fruit with no signs of spoilage. They belonged to the Cavendish variety, a world-renowned cultivar adapted to long-distance transport. The fruits were harvested unripe at the place of cultivation and stored under controlled conditions during transport, lasting up to four weeks. Before being sent to the shop, the bananas were subjected to accelerated ripening under the action of ethylene, which caused them to ripen and turn yellow. Banana peels were ground using a laboratory homogeniser (Ultra Turrax T25, Janke and Kunkel IKA, Staufen, Germany) for 3 min at 5–10 °C. Before use, the samples were stored frozen at −20 °C.
2.2. Yeast Strains and Inoculum Preparation
Our research used pure yeast cultures: Scheffersomyces stipitis LOCK 0049 (from the Lodz Pure Culture Collection LOCK 105) and Meyerozyma guilliermondii ATCC 6260. The commonly used YPD medium was used to prepare the inoculum. Yeast cells, Scheffersomyces stipitis LOCK 0049 or Meyerozyma guilliermondii ATCC 6260, were transferred into 1 L round-bottomed flasks containing 150 mL of YPD medium, which had been sterilised in an autoclave at 121 °C for 21 min. The initial pH of the medium, measured using a pH meter (HandyLab 100, SI Analytics, Mainz, Germany), was adjusted to 5.2 ± 0.1 with 25% H2SO4. Subsequently, shaken cultivations were conducted for 48 h at 32 ± 1 °C using a Reciprocal Shaker (Eberbach E5900, Belleville, NJ, USA). Subsequently, the yeast cells were centrifuged (Laboratory Centrifuge MPW380R, Warsaw, Poland) at a relative centrifugal force (RCF) of 5000× g for 10 min. The cells were washed thrice with sterile deionised water and resuspended in a 0.9% NaCl solution. These suspensions served as the inoculum source for specific cultivations in banana peel hydrolysates. The cell concentration in the inoculum was determined spectrophotometrically (Rayleigh Analytical Instrument, Beijing, China) at 540 nm, with results expressed in grams of dry matter (DM) per litre, calculated from a prepared standard curve.
2.3. Preparation of Banana Peel Hydrolysates
Banana peel hydrolysates were prepared in four variants:
- (a)
- A quantity of 800 g of ground banana peel (approximately 11.75% dry weight) was weighed into a plastic container, into which 1000 mL of a 2.5% H2SO4 solution was then added. The mixture was stirred and autoclaved at 121 °C for 1 h. Afterwards, the sample was cooled to room temperature and neutralised with 30% NaOH to achieve a pH of 5.3–5.5. This hydrolysis method was used as a control and was abbreviated as PT.
- (b)
- A quantity of 800 g of ground banana peel (approximately 11.75% dry weight) was weighed into a plastic container, into which 1000 mL of a 2.5% H2SO4 solution was then measured. The mixture was stirred and autoclaved at 121 °C for 1 h. Afterwards, the sample was cooled to room temperature and neutralised with 30% NaOH to achieve a pH between 5.3 and 5.5. Then, enzymatic hydrolysis of banana peels was carried out using a mixture of commercially available preparations, Viscozyme and UltrafloMax (Novozymes A/S, Bagsværd, Denmark), which exhibit cellulase, pectinase, xylanase and invertase activities. Both enzyme preparations were dosed at 0.05 mL per gram (DM) of banana peels. The enzymatic treatment was carried out at 50 °C for 6 h. The hydrolysate obtained in this way was labelled with the abbreviation PTE.
- (c)
- A quantity of 800 g of ground banana peel (approximately 11.75% dry weight) was weighed into a plastic container, into which 1000 mL of a 2.5% H2SO4 solution was then measured. After mixing, the sample was microwaved using an LG MH 6842B microwave oven (LG Electronics, Warsaw, Poland) with a peak power output of 900 W. The sample was treated for 3 min at 600 W microwave power. Afterwards, the sample was cooled and neutralised with 30% NaOH to achieve a pH value between 5.3 and 5.5. Then, the enzymatic hydrolysis of the banana peels was carried out as described above. The hydrolysate obtained in this way was labelled with the abbreviation ME.
- (d)
- A quantity of 800 g of ground banana peel (approximately 11.75% dry weight) was weighed into a plastic container, into which 1000 mL of a 2.5% H2SO4 solution was then added. After mixing, the sample was ultrasonicated using a UP400S Ultrasonic Generator (Hielscher Ultrasonics GmbH, Teltow, Germany). The sonication parameters were as follows: operational frequency, 24 kHz; output power, 300 W/mL; and pulse mode factor, 50% per second. The sonotrode (H3) utilised had a maximum immersion depth of 90 mm, a tip diameter of 3 mm, and a peak amplitude of 210 µm. Ultrasound treatment was administered for 20 min. Afterwards, the sample was cooled and neutralised with 30% NaOH to achieve a pH value between 5.3 and 5.5. Then, the enzymatic hydrolysis of the banana peels was carried out as described above. The hydrolysate obtained after ultrasonication was labelled with the abbreviation UE.
2.4. Preparation of Culture Media and Yeast Cultivation
The obtained hydrolysates were centrifuged at 8000× g for 10 min using a Laboratory Centrifuge MPW380R (Warsaw, Poland) to remove solid residues. The clear supernatant was transferred into 150 mL to 1 L round-bottom glass flasks. 3 g/L of diammonium hydrogen phosphate (NH4)2HPO4 was added to the hydrolysate to supply the necessary nitrogen and phosphorus for yeasts. The initial pH was then adjusted to 5.2 ± 0.1. Cultivation was initiated with the addition of pre-prepared seeding suspensions of either Scheffersomyces stipitis or Meyerozyma guilliermondii (dose: 1 g DM/L). The flasks were then sealed with cotton wool plugs. Cultivation was then performed at 32 ± 1 °C for 48 h on a reciprocal laboratory shaker set to 140 oscillations per minute (Eberbach E5900, Belleville, NJ, USA).
2.5. Sample Designations After Cultivations
PT + S; PT + G—cultivations of Scheffersomyces stipitis (+S) or Meyerozyma guilliermondii (+G) in PT (control) hydrolysate.
PTE + S; PTE + G—cultivations of Scheffersomyces stipitis (+S) or Meyerozyma guilliermondii (+G) in PTE hydrolysate.
ME + S; ME + G—cultivations of Scheffersomyces stipitis (+S) or Meyerozyma guilliermondii (+G) in ME hydrolysate.
UE + S; UE + G—cultivations of Scheffersomyces stipitis (+S) or Meyerozyma guilliermondii (+G) in UE hydrolysate.
2.6. Determination of Biomass Content After Cultivation
A quantity of 7 mL of the culture suspension was taken and centrifuged (Laboratory Centrifuge MPW380R, Warsaw, Poland) at 5000× g for 10 min to determine the biomass content in the post-culture medium. The supernatant was preserved for HPLC measurements, and the biomass, after being washed twice with distilled water, was transferred to glass weighing vessels and dried to a constant weight at 105 ± 1 °C (Suslab Bio 005 Laboratory Drier, Lodz, Poland) until a stable weight of the dried samples was achieved. The biomass content present in 7 mL of the suspension after cultivation was the basis for calculating the biomass concentration in 1 L.
2.7. Crude Protein Assay
Crude protein was assayed using the standard Kjeldahl method [46].
2.8. Dry Matter Content in the Raw Material
The dry matter content was quantified utilising a Radwag WPS-30S weighing dryer (Radwag, Radom, Poland). The drying time and temperature sequence were as follows: 50 °C for 3 min, followed by 70 °C for 3 min, and finally 120 °C until the mass change was less than 1 mg per 60 s.
2.9. Cellulose, Hemicellulose, Lignin and Pectin Assay in Banana Peels
The BPs’ concentrations of key constituents, including holocellulose (cellulose and hemicellulose) and lignin, were determined following the NREL protocol [47]. Pectins were assayed as described by Oberoi et al. [48], after they were extracted from the raw material through hot water treatment at 70 °C for 1 h. The residue, following two hot-water extractions, was subjected to 0.5% ammonium oxalate treatment and subsequently exposed to 0.05 M HCl at 80 °C for 1 h. The filtrate from these extractions was collected and precipitated using 96% ethanol. The precipitate was dried, weighed, and quantified as pectin (%).
2.10. Enzymatic Activity
The enzymatic activity for cellulose, pectin and xylan degradation by enzymes contained in the Viscozyme and UltrafloMax preparations was evaluated at 50 °C and pH 5.0. The substrates used were 0.4% carboxymethylcellulose (CMC) (Merck, Kenilworth, NJ, USA), 0.5% citrus pectin (Merck) and 0.5% birch xylan (Merck). The release of reducing sugars from these substrates was measured within 5 min using the 3′,5′-dinitrosalicylic acid (DNS; Merck) reagent [49]. Enzymatic activity was quantified in international activity units, defined as the micromoles of reducing sugars released per 1 min by the enzymes contained within 1 mL of preparation.
2.11. HPLC Analysis of Banana Peels’ Hydrolysates and Post-Cultivation Effluents
The concentrations of glucose (GLU), xylose (XYL), arabinose (ARA), cellobiose (CEL), glycerol (GlyOH), ethanol (EtOH), acetic acid (AcA), galacturonic acid (GalA), succinic (SucA) and formic acid (ForA) in the media were analysed by high-performance liquid chromatography (HPLC) using an Agilent 1260 Infinity system (Agilent Technologies, Santa Clara, CA, USA) and a Hi-Plex Ca column (7.7 × 300 mm, 8 μm) (Agilent Technologies, Santa Clara, CA, USA) equipped with a refractive index detector (RID) operating at 55 °C. The column temperature was maintained at 80 °C. HPLC grade water was used as the mobile phase, flowing at a 0.6 mL/min rate with a sample volume of 20 μL. Before analysis, samples were mixed with zinc sulphate solution to achieve a final concentration of 10% for protein precipitation. Sediments were removed by centrifugation at 7000 g for 10 min, and the samples were then filtered through 0.45 μm Teflon membranes before analysis.
2.12. Statistical Analysis
All experimental procedures and assays were conducted in quadruplicate. The ensuing statistical analyses, including analysis of variance (ANOVA), calculation of standard deviation (SD), and Student’s t-test at a significance level of α = 0.05, were performed using Origin 7.5 software.
3. Results and Discussion
3.1. Banana Peels Analysis
In the study’s first phase, we evaluated the basic qualitative and quantitative parameters of the banana peels used as raw material. The results are shown in Table 1.

Table 1.
Results of the analysis of the banana peels used in the experiments.
The dry matter content was 11.75%. This result is consistent with the 8.7–18.7% range reported by Emoga et al. [50]. The cellulose content was 10.09% DM, which is confirmed by the data presented by Jamil et al. [51] (7.5–18.7% DM) and Yasin et al. (6–15% DM) [52]. Kabenge et al. [53] also reported a cellulose concentration in dry matter of 9.9% DM, which is very close to our result. Pereira et al. [54] report a relatively wide range of cellulose content in banana peels of 18–60% (w/w). It should also be emphasised that the authors cited above stress that the BP chemical composition depends on the variety of fruit, their degree of ripeness at the time of harvesting, and the method of storing whole fruit and, later, the peel. Therefore, when comparing the results of banana peel composition, it is better to focus on ranges of values rather than specific values, as they may be the result of the factors mentioned above, which are usually not precisely defined in the articles.
The hemicellulose fraction determined by us was 36.89% DM (Table 1). Together with cellulose, they make up almost half of the dry matter of the peels, which we considered a promising potential for use for yeast cell conversion in the case of efficient hydrolysis. Pereira et al. [54] (17–40% DM), Kabenge et al. [53] (41.38% DM) and Jamil et al. [51] (40–41.4% DM) reported similar hemicellulose contents in banana peels to ours. The lignin content we determined was 9.62% of the dry matter of the peels, which is confirmed in the scientific publications presented so far. Jamal et al. [55] report a concentration range of 6–12% DM for lignin; other reports are Pereira et al. [54] 16–31% DM, Jamil et al. [51] 3.2–16.8% DM, Kabenge et al. [53] 8.9% DM and Yasin et al. [52] 7.3–15% DM.
The pectin content of our raw material was 8.27% DM (Table 1), which is close to the range reported by Jamal et al. [55] (10–21% DM) and the range of 10–20% DM given by Pereira et al. [54]. Oberoi et al. [48] also reported a similar pectin content in peels of 13–5.9% DM. The valuable crude protein content for feed purposes in our sample was 7.22% DM, which is confirmed by the general range of 2.2–10.6% reported in the literature [50,51,52]. Summarising the obtained contents, it should be emphasised that due to the vast number of banana varieties, the way they are grown, harvested, ripened, and stored, the composition of all parts of the plant, including banana peels, is subject to fluctuations, and individual results can be verified by referring to the ranges of values given in the literature. The high total content of cellulose and hemicellulose was promising for us, as it suggested the potential yield of biomass from yeast grown in banana peel hydrolysates.
3.2. Activity of the Enzymatic Preparations Used in Our Experiments
Since we used commercial enzyme preparations, it was deemed useful to determine the actual pectinase, cellulase, and xylanase activity values in the commercial batches of Viscozyme and UltrafloMax. The results are presented in Table 2.

Table 2.
Activity of selected enzymes in Viscozyme and UltrafloMax preparations.
The enzymatic activities we determined for Viscozyme correlate with those presented by Dygas et al. [25], who reported cellulase, xylanase and pectinase activities of 20.9, 25.9 and 312.6 U/mL. Berłowska et al. [56] also reported similar activities of the same enzymes as ours (13.9, 25.1 and 412 U/mL). Similarly, the results presented by us for the UltrafloMax preparation are confirmed in the publication by Dygas et al. [25], who reported the following values: 32.7, 64.7 and 21.2 U/mL for cellulase, xylanase and pectinase activity, respectively. For the same preparation, Berłowska et al. [56] reported similar cellulase and pectinase activities (27.8 and 24.2 U/mL). In comparison, the xylanase activity they reported (127.4 U/mL) is more than twice as high as the one presented in Table 2 (58.29 U/mL), as well as the value provided by Dygas and colleagues [25]. This difference may result from using preparations from different production batches, which, in the case of multi-enzyme industrial preparations, may differ in the activity of individual enzymes. Similarly, other transport and storage conditions may also have affected the activity of the preparations analysed.
The determined cellulase activity was used to establish the dose of both enzyme preparations at 0.05 mL/g dry weight. Based on literature reports [25,26,57] and our previous work in this field [36,37,58], we assumed that the cellulase dose should be in the range of 20–30 FPU/g cellulose. The enzyme dose we adopted (0.05 mL/g DM) corresponded to approximately 27 FPU/g of cellulose contained in banana peels.
3.3. Selected Sugars in Banana Peel Hydrolysates Before and After Yeast Cultivation
One of the most crucial research objectives was to determine the impact of ultrasound and microwave treatment on the efficiency of banana peel hydrolysis into simple sugars assimilated by yeast. Table 3 (below) summarises the results of the content of selected sugars in the hydrolysate samples before cultivation and in the culture effluents.

Table 3.
Concentration of selected sugars before yeast cultivation in banana peel hydrolysate.
Analysis of the hydrolysate samples before cultivation showed that the highest glucose content (9.27 ± 0.327 g/L) was found in the samples after enzymatic hydrolysis preceded by pressure–thermal treatment (PTE sample). A statistically similar (p > 0.05) glucose level (8.55 ± 0.81 g/L) was determined in the sample after enzymatic hydrolysis preceded by ultrasonic treatment (UE). Slightly less glucose (8.08 ± 0.439 g/L) was found in the ME sample after combined microwave and enzymatic treatment. The least amount of glucose was found in the reference hydrolysate obtained after acid–thermal hydrolysis alone. Comparing the dependence of xylose, arabinose and cellobiose concentrations on the hydrolysis method, it can be concluded that the trend observed for glucose was maintained. The lowest amounts of cellobiose, xylose and arabinose were found in the PT sample, 0.02 ± 0.001 g/L, 5.56 ± 0.298 g/L and 0.73 ± 0.071 g/L, respectively. The highest amounts of these sugars were found in the PTE hydrolysate, with 0.29 ± 0.026 g/L of cellobiose, 9.24 ± 0.703 g/L of xylose and 1.93 ± 0.154 g/L of arabinose. After taking into account other substrate loads, our results are similar to those obtained by Oberoi et al. [48] after hydrolysis of banana peels with a mixture of Celluclast 1.5 L, Novozyme-188 and Pectinase P-2611 preparations. After converting the results to the same ratio of peel dry matter to total sample weight, they determined in the hydrolysate about 0.8 g/L xylose, 1.5 g/L arabinose and 6.1 to 13.2 g/L glucose. Akita et al. [59] hydrolysed banana peels using the cellulolytic preparation Onozuka R-10 to obtain significantly lower glucose and xylose concentrations (<1 g/L), although it should be emphasised that the hydrolysis time was very short at just 3 h, not allowing for the hydrolysis of the entire pool of polysaccharides present in the raw material. Pereira et al. [60] obtained more than 25 g/L of reducing sugar after 30 h of hydrolysis with a cellulolytic preparation, Cellic CTec2, when hydrolysing banana peels with a similar substrate load (1 g DM/20 mL) as ours. Palacios et al. [61] obtained 32.5 g/L of glucose after hydrolysis (with Celluclast 1.5 L) of BP at 20% substrate load. It is worth emphasising the significant amount of sugars released after ultrasound- and microwave-assisted hydrolysis, for which the total pool of sugars in these trials was very similar to those present after traditional acid–thermal treatment with subsequent enzymatic hydrolysis.
The time and power of microwave treatment are obvious parameters affecting the intensity of the process. Due to the preliminary nature of our research, after reviewing the literature [39,41,43,44], we decided to use a time of 3 min and an intensity of 600 W. In addition to the specific effect of microwaves on the structure of the raw material, this also allowed the sample to be heated to a temperature of 85–95 °C, which, in our assumption, improved the extraction of the raw material components. The parameters we set translated into the results we presented. It would be interesting to develop this research to determine the optimal microwave dose for treating banana peels before enzymatic hydrolysis.
After reviewing the literature [39,40,42,45], we decided to adopt only one variant of BP ultrasonic treatment for this initial research. The parameters we applied translated into the results we obtained. In our opinion, determining the optimal dose of ultrasound for treating banana peels before enzymatic hydrolysis will be one of the directions of our further research.
The possibility of replacing non-ecological pressure–thermal treatment, which additionally requires pressure equipment, using microwaves or ultrasound, is promising for assessing the potential of banana peels as a raw material for producing microbial protein.
Table 4 displays the sugar concentrations in the effluent following yeast cultivation.

Table 4.
Concentration of selected sugars after yeast cultivation in banana peel hydrolysate.
The results (Table 4) show that the yeast culture effluent, compared to the hydrolysates before the culture, contained only residual concentrations of cellobiose and glucose (the concentration of which was, in many samples, below the detection limit). The concentrations of xylose and arabinose were also reduced by several dozen percent to below 0.63 g/L for xylose and below 0.28 g/L for arabinose. The results suggest that the yeast strains used were characterised by their ability to assimilate glucose, cellobiose, xylose and arabinose, which is confirmed by the data presented in the literature [38,62,63,64]. The reduction in cellobiose concentration may also result from its enzymatic breakdown into glucose by cellobiase present as a side activity, not declared on the label, in the enzyme preparations used [65].
3.4. Selected Organic Acids in Banana Peel Hydrolysates Before and After Yeast Cultivation
Organic acids are important indicators of metabolic activity, the condition of the raw material, and the hydrolysis process, so we determined the concentrations of selected organic acids in the hydrolysates before and after yeast cultivation.
Galacturonic acid reached the highest concentration among the acids determined in the hydrolysates (0.93 ± 0.081 g/L) (Table 5) for the sample subjected to the pressure–thermal-enzymatic (PTE) treatment sequence. Oberoi et al. [48] hydrolysed banana peels with a mixture of Celluclast 1.5 L, Novozyme-188 and Pectinase P-2611. After converting their results to the same ratio of dry peel mass to total sample mass, they determined the following in the hydrolysate: 0.9 to 5.1 g/L of galacturonic acid formed from the breakdown of pectin. Slightly lower concentrations of this acid were observed in hydrolysates obtained using microwaves (0.62 ± 0.025 g/L) and ultrasound (0.73 ± 0.051 g/L). It can be assumed that the decomposition of pectin during the acid and enzymatic hydrolysis contributed to the increase in the concentration of this acid in the analysed liquids. In the case of acetic acid, concentrations about 50% lower than those of galacturonic acid were observed. The concentration of acetic acid in the hydrolysates before cultivation ranged from 0.35 ± 0.001 g/L to 0.43 ± 0.0035 g/L for samples PT and UE, respectively. It can be seen that enzymatic treatment (ME, PTE and UE samples) caused a slight increase in concentration compared to the PT reference sample.

Table 5.
Concentration of selected organic acids before yeast cultivation in banana peel hydrolysate.
Succinic acid was another organic acid detected, with a maximum concentration of 0.29 ± 0.017 g/L in the case of ME hydrolysate (Table 5). Slightly lower concentrations of this acid were recorded for PTE and UE samples, while a significantly lower concentration of 0.05 ± 0.003 g/L was observed for the sample subjected only to pressure–thermal treatment. The last acid to be determined was formic acid, with a concentration of 0.03 ± 0.001 g/L in the reference PT hydrolysate. In the case of enzymatically treated hydrolysates, the concentration of this compound was approximately 25% higher.
The concentrations of the same acids initially determined in the banana peel hydrolysates were also determined in the effluent after the tested yeast strains had been cultivated (Table 6). The concentration of galacturonic acid ranged from 0.6 to 0.9 g/L. It should be emphasised that these concentrations were close to the values assayed in hydrolysates before cultivation (where the concentration range of this compound was between 0.62 and 0.93 g/L) The concentration of galacturonic acid after yeast cultivation indicates that it was not assimilated by the test strains, which is confirmed in the literature [66,67]. In the case of succinic acid, the concentration in the case of EU, ME and PTE hydrolysates remained at the same level, while in the case of cultivation in the PT hydrolysate, a slight increase in succinic acid from the initial level of 0.05 g/L to 0.14 g/L (PTG sample) and 0.13 g/L (PTS sample) was observed. In the case of formic acid, an increase in concentration was observed in all samples from 0.03–0.04 g/L to 0.1–0.36 g/L. The highest growth of this compound was observed for the cultivation of both yeast strains in ME and EU hydrolysates. In the case of acetic acid, no major trends in the change in concentration of this compound in the liquid waste after cultivation could be observed.

Table 6.
Concentration of selected organic acids after yeast cultivation in banana peel hydrolysate.
The changes in the concentrations of organic acids could result from the metabolic activity of yeast and potential contaminating microflora present during the process. Microbiological analysis of the raw material and assessment of changes in microflora during hydrolysis and cultivation were not included in the research plan. It may be assumed that the cultivation process was predominantly influenced by the selected yeast strains due to the addition of the inoculum. However, the possibility that other microorganisms were active in minor quantities during the process cannot be entirely excluded. They may have originated from the raw material, particularly in the absence of thermal treatment exceeding 100 °C, or may have been introduced through subsequent contamination. It should be noted that routine microscopic examination of the post-cultivation liquid (using an optical microscope at 480× magnification) did not reveal the presence of bacteria or yeast morphologically distinct from the intentionally applied strains.
The succinic and acetic (together with pyruvic) acids are predominantly synthesised by yeast through the tricarboxylic acid cycle, crucial to generating various intermediate carboxylic acids, including succinic acid [68]. Additionally, the glycolytic pathway, which facilitates the conversion of glucose to pyruvate, and the glyoxylate cycle, crucial for growth on two-carbon substrates such as ethanol and acetate, contribute significantly by providing precursors for biosynthesis through an anaplerotic mechanism [69]. Furthermore, the production of acetic acid under anaerobic conditions is associated with glycerol synthesis as a means of maintaining redox balance [70,71].
3.5. Biomass Concentration of Yeasts After Cultivation in Banana Peel Hydrolysates
The aim of the efficient preparation of banana peel hydrolysates was to utilise them as a medium for yeast cultivation. In Figure 1, we have presented the results of the cultivation of two test yeast strains, Scheffersomyces stipitis (S) and Meyerozyma guilliermondii (G), in hydrolysates obtained using various methods.
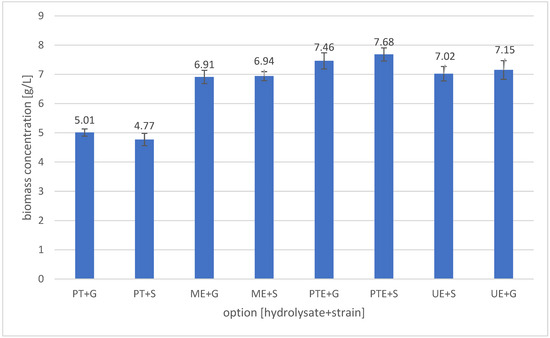
Figure 1.
Yeast biomass concentration after cultivation in banana peel hydrolysate. Sample designations: PT + S; PT + G—cultivations of Scheffersomyces stipitis (+S) or Meyerozyma guilliermondii (+G) in PT (Pressure–Thermal) hydrolysate; PTE + S; PTE + G—cultivations of Scheffersomyces stipitis (+S) or Meyerozyma guilliermondii (+G) in PTE (Pressure–Thermal + Enzymes) hydrolysate; ME + S; ME + G—cultivations of Scheffersomyces stipitis (+S) or Meyerozyma guilliermondii (+G) in ME (Microwave + Enzymes) hydrolysate; UE + S; UE + G—cultivations of Scheffersomyces stipitis (+S) or Meyerozyma guilliermondii (+G) in UE (Ultrasound + Enzymes) hydrolysate.
The lowest biomass concentration of 4.77 ± 0.21 g DM/L was observed after culturing S. stipitis yeast in the reference hydrolysate obtained only by pressure–thermal treatment of banana peels. A statistically similar (p > 0.05) biomass yield was observed after cultivating the M. guilliermondii strain in the same medium (5.01 ± 0.12 g DM/L) (Figure 1). The introduction of enzymatic treatment following pressure–thermal pretreatment resulted in a significant (p < 0.05) increase in the concentration of cultured yeast, to 7.46 ± 0.28 g DM/L for the PTE + G sample and 7.68 ± 0.22 g DM/L for the PTE + S sample. Substituting the pressure–thermal pre-treatment with microwave treatment (followed by enzymatic hydrolysis) resulted in a biomass yield of 6.91 ± 0.23 g DM/L and 6.94 ± 0.15 g DM/L for the ME + G and ME + S samples, respectively. The ultrasonic and enzymatic treatment sequence, the UE + G and UE + S samples, resulted in a final yeast yield of 7.02 ± 0.25 g DM/L and 7.15 ± 0.32 g DM/L, respectively. A statistical comparison of the source data showed that using different hydrolysis methods significantly (p < 0.05) changed the final biomass yield. In contrast, no statistically significant (p > 0.1) differences in propagation were observed between the strains used. Although it is difficult to find literature on the multiplication of biomass in experimental conditions similar to ours, we have seen several papers in which the authors used banana peels as a substrate for the multiplication of microbial biomass. Chaudhary et al. obtained 1 × 106 cells in 1 mL wort within 24 h when cultivating S. cerevisiae yeast [72]. Jiru and Melku obtained 2.4 to 8.82 g DM/L of banana peel hydrolysate when cultivating Cyberlindnera jadinii yeast for 96 h. The biomass they obtained contained up to 10% crude protein (although the authors did not state whether the result was converted to dry cell mass) [73]. Sharma and Sharma fermented BP hydrolysate with S. cerevisiae yeast, obtaining about 12 mg of protein per gram of hydrolysate after 8 days [74]. Azwar et al. [75] obtained up to 2 g DM/L after 4 days of cultivation of S. cerevisiae yeast in banana peel hydrolysate. Comparing this result to our accumulation of protein and biomass, we obtained up to about 3.5 mg of protein per gram of hydrolysate in our experiments. This difference could be due to the different methods of preparing the hydrolysates and the breeding conditions (including strain and time).
The biomass yield obtained, quantified as grams of dry yeast mass per gram of sugars present in the medium, ranged from 0.36 to 0.43 g DM/g. Comparable values have been recorded by Matos et al. [76], who reported a yield of 0.52 g/g for M. guilliermondii. For the S. stipitis strain cultivated in brewer’s spent grain hydrolysate, Duarte et al. [77] reported a Yx/s of 0.46 g/g. The sugar-to-biomass conversion results obtained in this study are economically promising and surpass those reported by Martini et al. [78], who observed the conversion ratios of 0.08–0.17 g/g for glucose and 0.13–0.19 g/g for arabinose after 60 h of cultivation of M. guilliermondii in bagasse hydrolysate. Papini et al. [64] reported a conversion ratio of 0.17 g/g for S. cerevisiae and 0.55 g/g for S. stipitis in aerobic batch cultivation with 20 g/L glucose. As sugars are also used for energy production and various metabolic by-products, yeast technologists consider the Yx/s value of 0.5 g/g very high [79].
3.6. Crude Protein Content in Yeast Biomass Grown on Banana Peel Hydrolysates
Protein is a key component of biomass for feed purposes. Yeast is characterised by high bioavailability and a rich content of essential amino acids [24,79]. Figure 2 shows the crude protein content in the dry matter of two yeast strains obtained after cultivation in banana peel hydrolysates.
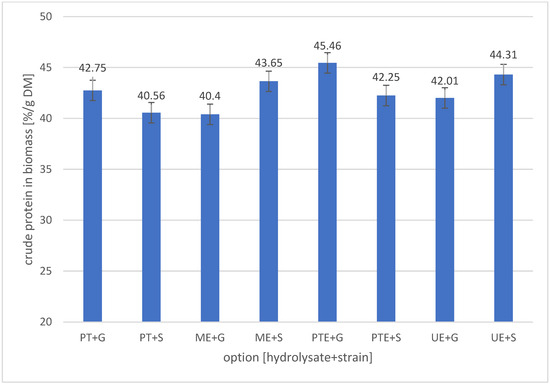
Figure 2.
Crude protein content in yeast biomass cultivated in banana peel hydrolysate. Sample designations: PT + S; PT + G—cultivations of Scheffersomyces stipitis (+S) or Meyerozyma guilliermondii (+G) in PT (Pressure–Thermal) hydrolysate; PTE + S; PTE + G—cultivations of Scheffersomyces stipitis (+S) or Meyerozyma guilliermondii (+G) in PTE (Pressure–Thermal + Enzymes) hydrolysate; ME + S; ME + G—cultivations of Scheffersomyces stipitis (+S) or Meyerozyma guilliermondii (+G) in ME (Microwave + Enzymes)hydrolysate; UE + S; UE + G—cultivations of Scheffersomyces stipitis (+S) or Meyerozyma guilliermondii (+G) in UE (Ultrasound + Enzymes) hydrolysate.
The protein content of the yeast biomass G (M. guilliermondii) grown on the reference hydrolysate PT was 42.75 ± 1.36% DM. Changing the yeast strain (S. stipitis) resulted in a protein content of 40.56 ± 0.92% DM, so it can be said that the difference in protein content between the strains was not significant (p > 0.05). Changing the treatment method by adding enzymatic hydrolysis to the pressure–thermal pretreatment resulted in an apparent increase in the protein content of the biomass of both strains to 45.46 ± 1.16% DM and 42.25 ± 0.87% DM for strains G and S, respectively. Still, statistical analysis indicates that this increase was not significant. From the point of view of assessing the effectiveness of the impact of microwave and ultrasound treatment on the hydrolysis of banana peels and the use of this raw material for the efficiency of yeast protein formation, it should be emphasised that the protein content in the dry mass of yeast cultivated after these treatments was similar (p > 0.05) to the protein content in the samples grown in the hydrolysate obtained after pressure–thermal treatment and enzymatic hydrolysis. The protein content in the dry yeast mass is the result of many factors, including the form and dose of supplemented nitrogen and the C/N ratio [24,79], and taking into account that in this respect, all the cultures carried out were similar, the final protein content was comparable. Based on the analysis of the results presented in the Figure 2, it can be concluded that implementing microwave or ultrasound treatment instead of pressure–thermal treatment did not negatively affect the protein content in yeast biomass. Jach et al., in their extensive review of yeast as a source of protein, cite a wide range of protein contents, 26–74% DM, reported in various articles, with more than 70% of the authors they cite giving a narrower range: 40–55% [24]. This range is consistent with our results. Sheth and Patel, Reed and Nagodawithana, and Patelski and colleagues also report a similar range [36,79,80].
4. Summary and Conclusions
The findings of our study highlight the potential of banana peels as a valuable substrate for single-cell protein production. The high content of cellulose and hemicellulose in banana peels, combined with their rich nutrient profile, makes them an excellent raw material for yeast cultivation. It was revealed that enzymatic hydrolysis following microwave or ultrasound pretreatment is more effective than traditional acid–thermal treatment. Efficient assimilation of sugars, resulting in high biomass concentrations, was achieved by the yeast strains Scheffersomyces stipitis and Meyerozyma guilliermondii, with crude protein content reaching up to 45.46% DM. Our results indicate that banana peels can be effectively utilised for single-cell protein production, providing a sustainable and environmentally friendly alternative to traditional feed sources. The integration of microwave and ultrasound technologies in bioprocessing to improve yeast cultivation’s efficiency and environmental sustainability was underscored. By replacing non-ecological pressure–thermal treatments with these innovative methods, microbial protein production from banana peels can be optimised, reducing the environmental impact and enhancing the economic viability of the process. Future research should focus on scaling up these methods and exploring their application to other types of agricultural waste, further contributing to the development of sustainable bioprocessing technologies.
Author Contributions
Conceptualisation, A.M.P. and P.D.; methodology, A.M.P. and U.D.-K.; software, J.D.; validation, U.D.-K. and J.D.; formal analysis, A.M.P.; investigation, A.M.P., M.B. and J.B.; resources, A.M.P.; data curation, U.D.-K. and K.P.-P.; writing—original draft preparation, A.M.P.; writing—review and editing, A.M.P.; visualisation, A.M.P.; supervision, J.D. and P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset is available on request from the authors. The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Emaldi, U.; Trujillo, I.; García, E. Comparison of Characteristics of Bananas (Musa sp.) from the Somaclone CIEN BTA-03 and Its Parental Clone Williams. Fruits 2004, 59, 257–263. [Google Scholar] [CrossRef]
- Menezes, E.W.; Tadini, C.C.; Tribess, T.B.; Zuleta, A.; Binaghi, J.; Pak, N.; Vera, G.; Dan, M.C.T.; Bertolini, A.C.; Cordenunsi, B.R.; et al. Chemical Composition and Nutritional Value of Unripe Banana Flour (Musa acuminata, var. Nanicão). Plant Foods Hum. Nutr. 2011, 66, 231–237. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Elayabalan, S.; Shobana, V.G.; Sivakumar, P.; Pandiyan, M. Nutritional Value of Cultivars of Banana (Musa spp.) and Its Future Prospects. J. Pharmacogn. Phytochem. 2018, 7, 2972–2977. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Irfan, S.; Nadeem, M.; Mahmood, S. A Comprehensive Review on Nutritional Value, Medicinal Uses, and Processing of Banana. Food Rev. Int. 2022, 38, 199–225. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Xu, F.; Zhou, W. Effect of LED Irradiation on the Ripening and Nutritional Quality of Postharvest Banana Fruit. J. Sci. Food Agric. 2018, 98, 5486–5493. [Google Scholar] [CrossRef] [PubMed]
- Zaini, H.B.M.; Sintang, M.D.B.; Pindi, W. The Roles of Banana Peel Powders to Alter Technological Functionality, Sensory and Nutritional Quality of Chicken Sausage. Food Sci. Nutr. 2020, 8, 5497–5507. [Google Scholar] [CrossRef]
- Bananas: Production Volume Worldwide 2023. Available online: https://www.statista.com/statistics/716037/global-banana-market-volume/ (accessed on 10 April 2025).
- Yan, L.; Fernando, W.; Brennan, M.; Brennan, C.; Jayasena, V.; Coorey, R. Effect of Extraction Method and Ripening Stage on Banana Peel Pigments. Int. J. Food Sci. Technol. 2016, 51, 1449–1456. [Google Scholar] [CrossRef]
- Carvalho, V.S.; Conti-Silva, A.C. Cereal Bars Produced with Banana Peel Flour: Evaluation of Acceptability and Sensory Profile. J. Sci. Food Agric. 2018, 98, 134–139. [Google Scholar] [CrossRef]
- Marques, O.F.C.; Sales, E.C.J.D.; Monção, F.P.; Silva, A.F.; Rigueira, J.P.S.; Pires, D.A.D.A.; Rufino, L.D.D.A.; Durães, H.F. Potential for Using Dehydrated Banana Peel as an Additive in Grass Silage. Cad. Ciênc. Agrár. 2021, 13, 1–8. [Google Scholar] [CrossRef]
- Biedunkiewicz, A. Ecophysiology of Selected Candida Species Isolated from Different Types of Utility Water under Laboratory Conditions. Appl. Ecol. Environ. Res. 2015, 13, 967–979. [Google Scholar] [CrossRef]
- Lonnie, M.; Hooker, E.; Brunstrom, J.; Corfe, B.; Green, M.; Watson, A.; Williams, E.; Stevenson, E.; Penson, S.; Johnstone, A. Protein for Life: Review of Optimal Protein Intake, Sustainable Dietary Sources and the Effect on Appetite in Ageing Adults. Nutrients 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, J.; Jose, D.; Tyler, A.; Bloxham, P.; Culling, J. The Future of Food. Can We Meet the Needs of 9bn People? Available online: https://www.research.hsbc.com/C/1/1/320/WgCK7Wv (accessed on 20 May 2024).
- Galanakis, C.M. The Future of Food. Foods 2024, 13, 506. [Google Scholar] [CrossRef]
- Robins, S.; Iley, R.; Michell, L.; Smith, S.; Gilbert, G.; McCarthy, M.; Stein, U.; Wells, P.; Lawton, J.; Landridge, A.; et al. The Future of Food: Are Food Businesses on Track to Deliver a Sustainable Protein System by 2040? Available online: https://www.forumforthefuture.org/Handlers/Download.ashx?IDMF=f2a9339c-8a62-4462-a886-f7de0e3fd729 (accessed on 13 June 2024).
- Smith, K.; Watson, A.W.; Lonnie, M.; Peeters, W.M.; Oonincx, D.; Tsoutsoura, N.; Simon-Miquel, G.; Szepe, K.; Cochetel, N.; Pearson, A.G.; et al. Meeting the Global Protein Supply Requirements of a Growing and Ageing Population. Eur. J. Nutr. 2024, 63, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Bedsaul-Fryer, J.R.; Monroy-Gomez, J.; Van Zutphen-Küffer, K.G.; Kraemer, K. Editorial: An Introduction to Traditional and Novel Alternative Proteins for Low- and Middle-Income Countries. Curr. Dev. Nutr. 2023, 8, 102014. [Google Scholar] [CrossRef]
- Sijpestijn, G.F.; Wezel, A.; Chriki, S. Can Agroecology Help in Meeting Our 2050 Protein Requirements? Livest. Sci. 2022, 256, 104822. [Google Scholar] [CrossRef]
- Lara-Parra, A.I.; Hernández-Hernández, A.A.; Jaguey-Hernández, Y.; Jiménez-Osorio, A.S.; Castañeda-Ovando, A.; Aguilar-Arteaga, K.; Añorve-Morga, J. Exploring Alternative Sources of Protein in Food: Trends in Nutrient and Functional Features. Food Res. Int. 2025, 208, 116224. [Google Scholar] [CrossRef]
- Mensah, J.K.M.; Twumasi, P. Use of Pineapple Waste for Single Cell Protein (SCP) Production and the Effect of Substrate Concentration on the Yield. J. Food Process Eng. 2017, 40, e12478. [Google Scholar] [CrossRef]
- Prosvirnikov, D.; Tuntsev, D.; Gizzatullina, L.; Kulikova, Y.; Michaud, P.; Babich, O. Protein Production from Cellulosic Waste Using Candida utilis. Environ. Technol. Innov. 2023, 32, 103445. [Google Scholar] [CrossRef]
- Ortman, K.; Pehrson, B. Selenite and Selenium Yeast as Feed Supplements for Dairy Cows. Zentralbl. Veterinarmed. A 1997, 44, 373–380. [Google Scholar] [CrossRef]
- Baker, L.M.; Kraft, J.; Karnezos, T.P.; Greenwood, S.L. Review: The Effects of Dietary Yeast and Yeast-Derived Extracts on Rumen Microbiota and Their Function. Anim. Feed Sci. Technol. 2022, 294, 115476. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Dygas, D.; Kręgiel, D.; Berłowska, J. Sugar Beet Pulp as a Biorefinery Substrate for Designing Feed. Molecules 2023, 28, 2064. [Google Scholar] [CrossRef] [PubMed]
- Dygas, D.; Liszkowska, W.; Steglińska, A.; Sulyok, M.; Kręgiel, D.; Berłowska, J. Rapeseed Meal Waste Biomass as a Single-Cell Protein Substrate for Nutritionally-Enhanced Feed Components. Processes 2023, 11, 1556. [Google Scholar] [CrossRef]
- Patelski, P.; Berłowska, J.; Balcerek, M.; Dziekońska-Kubczak, U.; Pielech-Przybylska, K.; Dygas, D.; Jędrasik, J. Conversion of Potato Industry Waste into Fodder Yeast Biomass. Processes 2020, 8, 453. [Google Scholar] [CrossRef]
- Henriques, T.; Pereira, S.; Serafim, L.; Xavier, A. Two-Stage Aeration Fermentation Strategy to Improve Bioethanol Production by Scheffersomyces stipitis. Fermentation 2018, 4, 97. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; De Vries, R.P.; Garrigues, S. Strategies for the Development of Industrial Fungal Producing Strains. J. Fungi 2023, 9, 834. [Google Scholar] [CrossRef]
- Bajić, B.; Vučurović, D.; Vasić, Đ.; Jevtić-Mučibabić, R.; Dodić, S. Biotechnological Production of Sustainable Microbial Proteins from Agro-Industrial Residues and By-Products. Foods 2022, 12, 107. [Google Scholar] [CrossRef]
- Amara, A.A.; El-Baky, N.A. Fungi as a Source of Edible Proteins and Animal Feed. J. Fungi 2023, 9, 73. [Google Scholar] [CrossRef]
- Valentino, M. Mycota of Distillery Yeast Sludge as Source of Single Cell Protein. Mycosphere 2015, 6, 241–247. [Google Scholar] [CrossRef]
- Kut, A.; Demiray, E.; Ertuğrul Karatay, S.; Dönmez, G. Second Generation Bioethanol Production from Hemicellulolytic Hydrolyzate of Apple Pomace by Pichia stipitis. Energy Sources Part Recovery Util. Environ. Eff. 2022, 44, 5574–5585. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2022/1104 of 1 July 2022 Amending Regulation (EU) No 68/2013 on the Catalogue of Feed Materials. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX%3A32022R1104 (accessed on 6 July 2024).
- Jeffries, T.W.; Van Vleet, J.R.H. Pichia stipitis Genomics, Transcriptomics, and Gene Clusters. FEMS Yeast Res. 2009, 9, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Patelski, P.; Berlowska, J.; Dziugan, P.; Pielech-Przybylska, K.; Balcerek, M.; Dziekonska, U.; Kalinowska, H. Utilisation of Sugar Beet Bagasse for the Biosynthesis of Yeast SCP. J. Food Eng. 2015, 167, 32–37. [Google Scholar] [CrossRef]
- Patelski, P.; Stanisz, M.; Antczak, A.; Balcerek, M.; Pielech-Przybylska, K.; Sapinska, E.; Dziekonska, U. Conversion of Sugar Beet Leaf Polysaccharides into Single Cell Protein. RSC Adv. 2015, 5, 20961–20965. [Google Scholar] [CrossRef]
- Papon, N.; Savini, V.; Lanoue, A.; Simkin, A.J.; Crèche, J.; Giglioli-Guivarc’h, N.; Clastre, M.; Courdavault, V.; Sibirny, A.A. Candida guilliermondii: Biotechnological Applications, Perspectives for Biological Control, Emerging Clinical Importance and Recent Advances in Genetics. Curr. Genet. 2013, 59, 73–90. [Google Scholar] [CrossRef]
- Fernandes, A.; Cruz-Lopes, L.; Esteves, B.; Evtuguin, D.V. Microwaves and Ultrasound as Emerging Techniques for Lignocellulosic Materials. Materials 2023, 16, 7351. [Google Scholar] [CrossRef] [PubMed]
- Gavrila, A.I.; Vartolomei, A.; Calinescu, I.; Vinatoru, M.; Parvulescu, O.C.; Psenovschi, G.; Chipurici, P.; Trifan, A. Ultrasound-Assisted Alkaline Pretreatment of Biomass to Enhance the Extraction Yield of Valuable Chemicals. Agronomy 2024, 14, 903. [Google Scholar] [CrossRef]
- Richel, A.; Jacquet, N. Microwave-Assisted Thermochemical and Primary Hydrolytic Conversions of Lignocellulosic Resources: A Review. Biomass Convers. Biorefinery 2015, 5, 115–124. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.A.; Chen, L.; Mustapha, A.T.; Zhou, C. Enhancement of Lignin Removal and Enzymolysis of Sugarcane Bagasse by Ultrasound-Assisted Ethanol Synergized Deep Eutectic Solvent Pretreatment. Renew. Energy 2021, 172, 304–316. [Google Scholar] [CrossRef]
- Kłosowski, G.; Mikulski, D.; Lewandowska, N. Microwave-Assisted Degradation of Biomass with the Use of Acid Catalysis. Catalysts 2020, 10, 641. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. Delignification Efficiency of Various Types of Biomass Using Microwave-Assisted Hydrotropic Pretreatment. Sci. Rep. 2022, 12, 4561. [Google Scholar] [CrossRef]
- Pradhan, D.; Tsegaye, B.; Mathew, S.S.; Jaiswal, S.; Jaiswal, A.K. Ultrasound-Assisted Pretreatment of Lignocellulosic Biomass for Bioethanol Production. In Bioethanol Fuel Production Processes. I; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-003-22653-6. [Google Scholar]
- Wrolstad, R.E.; Acree, T.E.; Decker, E.A.; Penner, M.H.; Reid, D.S.; Schwartz, S.J.; Shoemaker, C.F.; Smith, D.M.; Sporns, P. Handbook of Food Analytical Chemistry: Water, Proteins, Enzymes, Lipids, and Carbohydrates; Wrolstad, R.E., Ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.O.; Scarlata, C.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL: Golden, CO, USA, 2012.
- Oberoi, H.S.; Sandhu, S.K.; Vadlani, P.V. Statistical Optimization of Hydrolysis Process for Banana Peels Using Cellulolytic and Pectinolytic Enzymes. Food Bioprod. Process. 2012, 90, 257–265. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Emaga, T.H.; Bindelle, J.; Agneesens, R.; Buldgen, A.; Wathelet, B.; Paquot, M. Ripening Influences Banana and Plantain Peels Composition and Energy Content. Trop. Anim. Health Prod. 2011, 43, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Mohd Jamil, N.A.; Jaffar, S.S.; Saallah, S.; Misson, M.; Siddiquee, S.; Roslan, J.; Lenggoro, W. Isolation of Cellulose Nanocrystals from Banana Peel Using One-Pot Microwave and Mild Oxidative Hydrolysis System. Nanomaterials 2022, 12, 3537. [Google Scholar] [CrossRef] [PubMed]
- Yasin, M.; Gangan, S.; Panchal, S.K. Banana Peels: A Genuine Waste or a Wonderful Opportunity? Appl. Sci. 2025, 15, 3195. [Google Scholar] [CrossRef]
- Kabenge, I.; Omulo, G.; Banadda, N.; Seay, J.; Zziwa, A.; Kiggundu, N. Characterization of Banana Peels Wastes as Potential Slow Pyrolysis Feedstock. J. Sustain. Dev. 2018, 11, 14. [Google Scholar] [CrossRef]
- Pereira, M.A.F.; Cesca, K.; Poletto, P.; de Oliveira, D. New Perspectives for Banana Peel Polysaccharides and Their Conversion to Oligosaccharides. Food Res. Int. 2021, 149, 110706. [Google Scholar] [CrossRef]
- Jamal, P.K.; Saheed, O.; Alam, Z. Bio-Valorization Potential of Banana Peels (Musa sapientum): An Overview. Asian, J. Biotechnol. 2012, 4, 1–14. [Google Scholar] [CrossRef]
- Berlowska, J.; Cieciura-Włoch, W.; Kalinowska, H.; Kregiel, D.; Borowski, S.; Pawlikowska, E.; Binczarski, M.; Witonska, I. Enzymatic Conversion of Sugar Beet Pulp: A Comparison of Simultaneous Saccharification and Fermentation and Separate Hydrolysis and Fermentation for Lactic Acid Production. Food Technol. Biotechnol. 2018, 56, 188–196. [Google Scholar] [CrossRef]
- Berłowska, J.; Pielech-Przybylska, K.; Balcerek, M.; Dziekońska-Kubczak, U.; Patelski, P.; Dziugan, P.; Kręgiel, D. Simultaneous Saccharification and Fermentation of Sugar Beet Pulp for Efficient Bioethanol Production. BioMed Res. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Dziekońska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Balcerek, M.; Pielech-Przybylska, K.; Robak, K. Two-Stage Pretreatment to Improve Saccharification of Oat Straw and Jerusalem Artichoke Biomass. Energies 2019, 12, 1715. [Google Scholar] [CrossRef]
- Akita, H.; Shibata, S.; Komoriya, T.; Kamei, S.; Asamoto, H.; Matsumoto, M. Simultaneous Saccharification and Fermentation for Isobutanol Production from Banana Peel. Fermentation 2024, 10, 161. [Google Scholar] [CrossRef]
- Pereira, M.A.F.; Monteiro, C.R.M.; Pereira, G.N.; Júnior, S.E.B.; Zanella, E.; Ávila, P.F.; Stambuk, B.U.; Goldbeck, R.; de Oliveira, D.; Poletto, P. Deconstruction of Banana Peel for Carbohydrate Fractionation. Bioprocess Biosyst. Eng. 2021, 44, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S.; Ruiz, H.A.; Ramos-Gonzalez, R.; Martínez, J.; Segura, E.; Aguilar, M.; Aguilera, A.; Michelena, G.; Aguilar, C.; Ilyina, A. Comparison of Physicochemical Pretreatments of Banana Peels for Bioethanol Production. Food Sci. Biotechnol. 2017, 26, 993–1001. [Google Scholar] [CrossRef]
- NCYC 443-Meyerozyma guilliermondii|National Collection of Yeast Cultures. Available online: https://www.ncyc.co.uk/catalogue/meyerozyma-guilliermondii-443 (accessed on 11 April 2025).
- Kurtzman, C.P.; Fell, J.W. The Yeasts, A Taxonomic Study, 4th ed.; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Papini, M.; Nookaew, I.; Uhlén, M.; Nielsen, J. Scheffersomyces stipitis: A Comparative Systems Biology Study with the Crabtree Positive Yeast Saccharomyces cerevisiae. Microb. Cell Factories 2012, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Smith, J. Mixed β-Glucanase, Xylanase from Humicola Insolens; FAO: Rome, Italy, 2004. [Google Scholar]
- Martins, L.C.; Palma, M.; Angelov, A.; Nevoigt, E.; Liebl, W.; Sá-Correia, I. Complete Utilization of the Major Carbon Sources Present in Sugar Beet Pulp Hydrolysates by the Oleaginous Red Yeasts Rhodotorula toruloides and R. mucilaginosa. J. Fungi 2021, 7, 215. [Google Scholar] [CrossRef]
- Koivistoinen, O.M.; Arvas, M.; Headman, J.R.; Andberg, M.; Penttilä, M.; Jeffries, T.W.; Richard, P. Characterisation of the Gene Cluster for L-Rhamnose Catabolism in the Yeast Scheffersomyces (Pichia) stipitis. Gene 2012, 492, 177–185. [Google Scholar] [CrossRef]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory Metabolism: Glycolysis, the TCA Cycle and Mitochondrial Electron Transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef]
- Kornberg, H.L.; Madsen, N.B. The Metabolism of C2 Compounds in Micro-Organisms. 3. Synthesis of Malate from Acetate via the Glyoxylate Cycle. Biochem. J. 1958, 68, 549–557. [Google Scholar] [CrossRef]
- Eglinton, J.M.; Heinrich, A.J.; Pollnitz, A.P.; Langridge, P.; Henschke, P.A.; de Barros Lopes, M. Decreasing Acetic Acid Accumulation by a Glycerol Overproducing Strain of Saccharomyces cerevisiae by Deleting the ALD6 Aldehyde Dehydrogenase Gene. Yeast 2002, 19, 295–301. [Google Scholar] [CrossRef]
- Remize, F.; Roustan, J.L.; Sablayrolles, J.M.; Barre, P.; Dequin, S. Glycerol Overproduction by Engineered Saccharomyces cerevisiae Wine Yeast Strains Leads to Substantial Changes in By-Product Formation and to a Stimulation of Fermentation Rate in Stationary Phase. Appl. Environ. Microbiol. 1999, 65, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Shrestha, N.; Singh, L.; Khadgi, R.K.; Chaturwedi, S.B. Production of Single Cell Protein from Banana Peel, Papaya Juice and Whey and Estimation of Protein Concentration. Tribhuvan Univ. J. Microbiol. 2022, 9(1), 28–34. [Google Scholar] [CrossRef]
- Jiru, T.M.; Melku, B. Single Cell Protein Production from Torula Yeast (Cyberlindnera Sp.) Using Banana Peel Hydrolysate. J. Adv. Microbiol. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Sharma, I.; Sharma, K. Banana peel extracts for the production of single cell protein by using Saccharomyces cerevisiae. Int. J. Adv. Res. 2017, 5, 531–535. [Google Scholar] [CrossRef]
- Azwar, A.; Mukhlishien, M.; Muslim, A.; Hadissa, P.; Ningsih, U.H.; Zanil, M.F.; Ali, J.M. Production of Single Cell Protein from Banana Peel Waste in Batch Fermentation Using Saccharomyces cerevisiae. J. Bahan Alam Terbarukan 2021, 10, 104–112. [Google Scholar] [CrossRef]
- Matos, I.T.S.R.; Cassa-Barbosa, L.A.; de Galvão, R.S.M.; Nunes-Silva, C.G.; Filho, S.A. Isolation, taxonomic identification and investigation of the biotechnological potential of wild-type Meyerozyma guilliermondii associated with amazonian termites able to ferment D-xylose. Biosci. J. 2014, 30, 260–266. [Google Scholar]
- Duarte, L.C.; Lopes, F.C.S.; Neves, I.; Girio, F.M. Yeast Biomass Production in Brewery’s Spent Grains Hemicellulosic Hydrolyzate. In Applied Biochememistry and Biotechnology, Proceedings of the Twenty-Ninth Symposium on Biotechnology for Fuels and Chemicals, Denver, CO, USA, 29 April–2 May 2007; Humana Press: Totowa, NJ, USA, 2008. [Google Scholar]
- Martini, C.; Tauk-Tornisielo, S.M.; Codato, C.B.; Bastos, R.G.; Ceccato-Antonini, S.R. A Strain of Meyerozyma guilliermondii Isolated from Sugarcane Juice Is Able to Grow and Ferment Pentoses in Synthetic and Bagasse Hydrolysate Media. World J. Microbiol. Biotechnol. 2016, 32, 80. [Google Scholar] [CrossRef]
- Reed, G.; Nagodawithana, T.W. Yeast Technology; Springer: Dordrecht, The Netherlands, 1990; ISBN 978-94-011-9773-1. [Google Scholar]
- Sheth, U.; Patel, S. Production, Economics, and Marketing of Yeast Single Cell Protein. In Food Microbiology Based Entrepreneurship: Making Money from Microbes; Amaresan, N., Dharumadurai, D., Babalola, O.O., Eds.; Springer: Singapore, 2023; pp. 133–152. ISBN 978-981-19-5041-4. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).