Quality of Maize Silage After Using Meat Bone Meal as a Phosphorus Fertilizer in a Field Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Silage Preparation
2.3. Chemical Analysis
2.4. Calculations and Statistical Analysis
3. Results
3.1. Chemical Composition of Maize Silage
3.2. Macronutrient Content of Maize Silage
3.3. Fermentation Parameters and Feed Value of Maize Silage
4. Discussion
4.1. Chemical Composition of Maize Silage
4.2. Macronutrient Content of Maize Silage
4.3. Fermentation Parameters and Feed Value of Maize Silage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | acetic acid |
| ADF | acid detergent fiber |
| ADL | acid detergent lignin |
| BA | butyric acid |
| C | carbon |
| Ca | calcium |
| CA | crude ash |
| CF | crude fiber |
| CP | crude protein |
| DM | dry matter |
| EE | extract ether |
| FM | fresh matter |
| K | potassium |
| LA | lactic acid |
| LA:AA | ratio of lactic acid to acetic acid |
| MBM | meat and bone meal |
| Mg | magnesium |
| N | nitrogen |
| n/a | not applicable |
| NDF | neutral detergent fiber |
| NFC | non-fiber carbohydrate |
| N-NH3 | ammonia nitrogen |
| OM | organic matter |
| P | phosphorus |
| PA | propionic acid |
| PDIE | protein digested in the small intestine when energy is limiting |
| PDIN | protein digested in the small intestine when nitrogen is limiting |
| TN | total nitrogen |
| UFL | feed unit for milk production |
| UFV | feed unit for meat production |
| VFAs | volatile fatty acids |
References
- Tharangani, R.M.H.; Yakun, C.; Zhao, L.S.; Ma, L.; Liu, H.L.; Su, S.L.; Shan, L.; Yang, Z.N.; Kononoff, P.J.; Weiss, W.P.; et al. Corn silage quality index: An index combining milk yield, silage nutritional and fermentation parameters. Anim. Feed Sci. 2021, 273, 114817. [Google Scholar] [CrossRef]
- Wang, S. Silage Preparation, Processing and Efficient Utilization. Agriculture 2025, 15, 128. [Google Scholar] [CrossRef]
- Cooke, K.M.; Bernard, J.K.; West, J.W. Performance of dairy cows fed annual ryegrass silage and corn silage with steam-flaked or ground corn. J. Dairy. Sci. 2008, 91, 2417–2422. [Google Scholar] [CrossRef]
- Dewhurst, R.J. Milk production from silage: Comparison of grass, legume and maize silages and their mixtures. Agric. Food Sci. 2013, 22, 57–69. [Google Scholar] [CrossRef]
- Khan, N.A.; Yu, P.; Ali, M.; Cone, J.W.; Hendriks, W.H. Nutritive value of maize silage in relation to dairy cow performance and milk quality. J. Sci. Food Agri. 2015, 95, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Ferraretto, L.F.; Vanderwerff, L.M.; Salvati, G.G.S.; Dias Júnior, G.S.; Shaver, R.D. Corn Shredlage: Equipment, storage and animal perspectives. In Proceedings of the 17th International Silage Conference, Piracicaba, Brazil, 1–3 July 2015; ESALQ: Piracicaba, Brazil, 2015; pp. 150–157. [Google Scholar]
- Neumann, M.; Baldissera, E.; Alessi Ienke, L.; Martins de Souza, A.; Piemontez de Oliveira, P.E.; Harry Bumbieris Junior, V. Nutritional Value Evaluation of Corn Silage from Different Mesoregions of Southern Brazil. Agriculture 2024, 14, 1055. [Google Scholar] [CrossRef]
- Cox, W.J.; Charney, D.J.R. Row spacing, plant density, and nitrogen effects on corn silage. Agron. J. 2005, 93, 597–602. [Google Scholar] [CrossRef]
- Kim, K.; Clay, D.E.; Carlson, C.G.; Clay, S.A.; Trooien, T. Do synergistic relationships between nitrogen and water influence the ability of corn to use nitrogen derived from fertilizer and soil? Agron. J. 2008, 100, 551–556. [Google Scholar] [CrossRef]
- Kodaolu, B.; Mohammed, I.; Gillespie, A.W.; Audette, Y.; Longstaffe, J.G. Phosphorus availability and corn (Zea mays L.) response to application of P-based commercial organic fertilizers to a calcareous soil. Soil. Sci. Soc. Am. J. 2023, 87, 1386–1397. [Google Scholar] [CrossRef]
- Jeng, A.S.; Haraldsen, T.K.; Vagstad, N.; Grønlund, N. Meat and bone meal as nitrogen fertilizer to cereals in Norway. Agric. Food Sci. 2004, 13, 268–275. [Google Scholar] [CrossRef]
- Jeng, A.S.; Haraldsen, T.K.; Gronlund, A.; Pedersen, P.A. Meat and bone meal as nitrogen and phosphorus fertilizer to cereals and rye grass. Nutr. Cycl. Agroecosyst. 2006, 76, 183–191. [Google Scholar] [CrossRef]
- Nogalska, A.; Czapla, J.; Nogalski, Z.; Skwierawska, M.; Kaszuba, M. The effect of increasing doses of meat and bone meal (MBM) on maize (Zea mays L.) grown for grain. Agric. Food Sci. 2012, 21, 325–331. [Google Scholar] [CrossRef][Green Version]
- Brod, E.; Øgaard, A.F.; Krogstad, T.; Haraldsen, T.K.; Frossard, E.; Oberson, A. Drivers of phosphorus uptake by barley following secondary resource application. Front. Nutr. 2016, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Stępień, A.; Wojtkowiak, K.; Kolankowska, E. Use of Meat Industry Waste in the Form of Meat and Bone Meal in Fertilising Maize (Zea mays L.) for Grain. Sustainability 2021, 13, 2857. [Google Scholar] [CrossRef]
- Silvasy, T.; Ahmad, A.A.; Wang, K.H.; Radovich, T.J.K. Rate and timing of meat and bone meal applications influence growth, yield, and soil water nitrate concentrations in sweet corn production. Agronomy 2021, 11, 1945. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Nogalska, A. Meat and bone meal and the energy balance of winter oilseed rape—A case study in north-eastern Poland. Energies 2022, 15, 3853. [Google Scholar] [CrossRef]
- Chaves, C.; Canet, R.; Albiach, R.; Marin, J.; Pomares, F. Meat and bone meal: Fertilizing value and rates of nitrogen mineralization. Nutr. Carbon. Cycl. Sustain. Plant-Soil. Syst. 2005, 1, 177–180. [Google Scholar]
- Nogalska, A.; Skwierawska, M.; Nogalski, Z.; Kaszuba, M. The effect of increasing doses of meat and bone meal (MBM) applied every second year on maize grown for grain. Chil. J. Agr. Res. 2013, 73, 430–434. [Google Scholar]
- Nogalska, A.; Zalewska, M. The effect of meat and bone meal (MBM) on phosphorus concentrations in soil and crop plants. Plant Soil. Environ. 2013, 59, 575–580. [Google Scholar] [CrossRef]
- Mäkelä, P.S.; Wasonga, D.O.; Solano Hernandez, A.; Santanen, A. Seedling growth and phosphorus uptake in response to different phosphorus sources. Agronomy 2020, 10, 1089. [Google Scholar] [CrossRef]
- Nogalska, A.; Borsuk-Stanulewicz, M.; Nogalski, Z. The Effect of Meat and Bone Meal on Yield and Herbage Quality in Silage Maize. Appl. Sci. 2025, 15, 117. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Washington, DC, USA, 2016. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods of dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Kostulak–Zielińska, M.; Potkański, A. Quality of baled grass-clover silages ensiled with chemical additives. Chemical composition. Ann. Anim. Sci. 2001, 1, 153–165. [Google Scholar]
- Gąsior, R. Oznaczanie Lotnych Kwasów Tłuszczowych i Kwasu Mlekowego w Kiszonkach i w Treści Żwacza; Biuletyn Informacyjny Instytutu Zootechniki: Balice, Poland, 2002. [Google Scholar]
- Purwin, C.; Żuk-Gołaszewska, K.; Tyburski, J.; Borsuk-Stanulewicz, M.; Stefańska, B. Quality of Red Clover Forage in Different Organic Production Systems. Agriculture 2024, 14, 1159. [Google Scholar] [CrossRef]
- Khan, N.A.; Tewoldebrhan, T.A.; Zom, R.L.G.; Cone, J.W.; Hendriks, W.H. Effect of corn silage harvest maturity and concentrate type on milk fatty acid composition of dairy cows. J. Dairy Sci. 2012, 95, 1472–1483. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Research Council, National Academy of Science: Washington, DC, USA, 2001.
- Kruse, S.; Herrmann, A.; Kornher, A.; Taube, F. Evaluation of genotype and environmental variation in fibre content of silage maize using a model-assisted approach. Eur. J. Agron. 2008, 28, 210–223. [Google Scholar] [CrossRef]
- Keady, T.W.J. Ensiled maize and whole crop wheat forages for beef and dairy cattle: Effects on animal performance. In Silage Production and Utilization Technology: Proceedings of the XIV International Silage Conference; Park, R.S., Stronge, M.D., Eds.; Wageningen Academic: Belfast, UK, 2005; pp. 65–82. [Google Scholar]
- Abeysekara, S.; Christensen, D.A.; Niu, Z.; Theodoridou, K.; Yu, P. Molecular structure, chemical and nutrient profiles, and metabolic characteristics of the proteins and energy in new cool-season corn varieties harvested as fresh forage for dairy cattle. J. Dairy Sci. 2013, 96, 6631–6643. [Google Scholar] [CrossRef]
- Akay, V.; Jackson, J.A., Jr. Effects of NutriDense and waxy corn hybrids on the rumen fermentation, digestibility and lactational performance of dairy cows. J. Dairy Sci. 2001, 84, 1698–1706. [Google Scholar] [CrossRef]
- Weiss, W.P.; Wyatt, D.J. Effect of oil content and kernel processing of corn silage on digestibility and milk production by dairy cows. J. Dairy Sci. 2000, 83, 351–358. [Google Scholar] [CrossRef]
- Peng, Q.; Khan, N.A.; Wang, Z.; Yu, P. Moist and dry heating-induced changes in protein molecular structure, protein subfractions, and nutrient profiles in camelina seeds. J. Dairy Sci. 2014, 97, 446–457. [Google Scholar] [CrossRef]
- Mandić, V.; Bijelić, Z.; Krnjaja, V.; Ružić, D.; Muslić, V.C.P.; Đorđević, S.; Petričević, M. Effect of different nitrogen fertilization levels on maize forage yield and quality. In Proceedings of the 11th International Symposium Modern Trends in Livestock Production, Belgrade, Serbia, 11–13 October 2017; pp. 290–303. [Google Scholar]
- Phelps, J.; Carrasco, L.R.; Webb, E.L.; Koh, L.P.; Pascual, U. Agricultural intensification escalates future conservation costs. Proc. Natl. Acad. Sci. USA 2013, 110, 7601–7606. [Google Scholar] [CrossRef]
- Plénet, D.; Lemaire, G. Relationships between dynamics of nitrogen uptake and dry matter accumulation in maize crops. Determination of critical N concentration. Plant Soil 1999, 216, 65–82. [Google Scholar] [CrossRef]
- Nogalska, A.; Momot, M.; Sobczuk-Szul, M.; Pogorzelska-Przybyłek, P.; Nogalski, Z. The effect of milk production performance of Polish Holstein-Friesian (PHF) cows on the mineral content of milk. J. Elem. 2018, 23, 589–597. [Google Scholar] [CrossRef]
- IZPIB-INRA. Normy Żywienia Przeżuwaczy. Wartość Pokarmowa Francuskich i Krajowych Pasz dla Przeżuwaczy; IZ PIB: Cracow, Poland, 2016. (In Polish) [Google Scholar]
- Illes, A.; Bojtor, C.; Szeles, A.; Mousavi, S.M.N.; Toth, B.; Nagy, J. Analyzing the effect of intensive and low-input agrotechnical support for the physiological, phenometric, and yield parameters of different maize hybrids using multivariate statistical methods. Int. J. Agron. 2021, 2, 6682573. [Google Scholar] [CrossRef]
- Sabir, M.; Hanafi, M.M.; Malik, M.T.; Aziz, T.; Zia-ur-Rehman, M.; Ahmad, H.R.; Shahid, M. Differential effect of nitrogen forms on physiological parameters and micronutrient concentration in maize (Zea mays L.). Aust. J. Crop Sci. 2013, 7, 1836–1842. [Google Scholar]
- Huhtanen, P.; Nousiainen, J.I.; Khalili, H.; Jaakkola, S.; Heikkilä, T. Relationships between silage fermentation characteristics and milk production parameters: Analyses of literature data. Livest. Prod. Sci. 2003, 81, 57–73. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Marlow Bucks, UK, 1991. [Google Scholar]
- Gharechahi, J.; Kharazian, Z.A.; Sarikhan, S.; Jouzani, G.S.; Aghdasi, M.; Hosseini Salekdeh, G. The dynamics of the bacterial communities developed in maize silage. Microb. Biotechnol. 2017, 10, 1663–1676. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Robinson, J.R.; Ranjit, N.K.; Chen, J.H.; Golt, C.M.; Pesek, J.D. Microbial populations, fermentation endproducts, and aerobic stability of corn silage treated with ammonia or a propionic acid-based preservative. J. Dairy Sci. 2000, 83, 1479–1486. [Google Scholar] [CrossRef]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Oude Elferink, S.J.W.H.; Spoelstra, S.F. Microbiology of ensiling. In Silage Science and Technology; Buxton, D.R., Muck, R.E., H Harrison, J., Eds.; American Society of Agronomy: Madison, WI, USA, 2003; pp. 31–93. [Google Scholar]
- Mills, J.A.; Kung, L., Jr. The effect of delayed filling and application of a propionic acid-based additive on the fermentation of barley silage. J. Dairy Sci. 2002, 85, 1969–1975. [Google Scholar] [CrossRef]
- Buxton, D.R. Quality-related characteristics of forages as influenced by plant environment and agronomic factors. Anim. Feed Sci. Technol. 1996, 59, 37–49. [Google Scholar] [CrossRef]
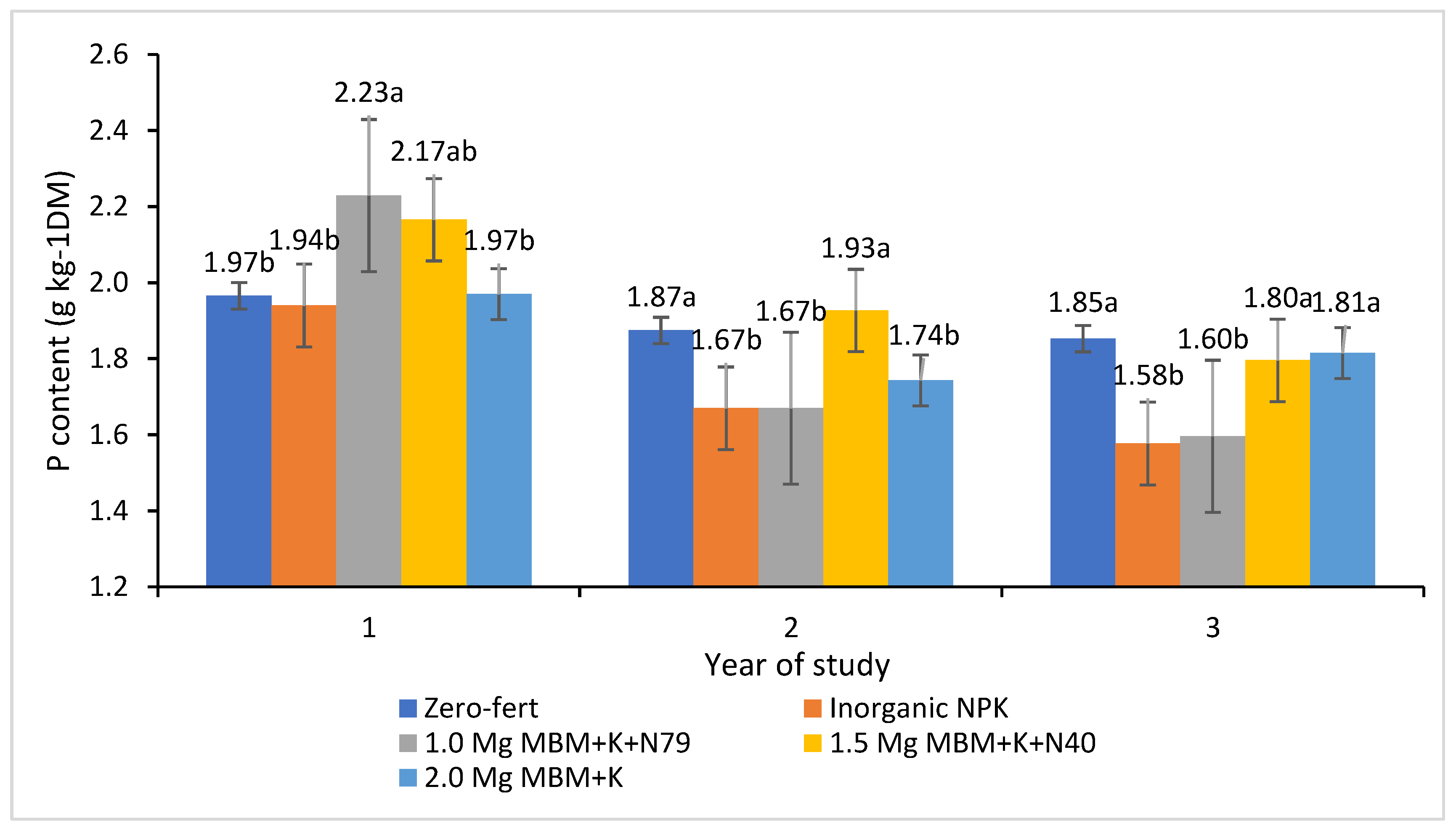
| Treatment | N | P | K |
|---|---|---|---|
| 1. Zero-fert | 0 | 0 | 0 |
| 2. Inorganic NPK 1 | 158 | 45 | 145 |
| 3. 1.0 Mg MBM + N79 2 | 158 (79 + 79) | 45 | 145 |
| 4. 1.5 Mg MBM + N40 3 | 158 (118 + 40) | 68 | 145 |
| 5. 2.0 Mg MBM 4 | 158 | 90 | 145 |
| Treatment (t) | DM 1 | CA 2 | CP 3 | EE 4 | CF 5 | NDF 6 | ADF 7 | ADL 8 | NFC 9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| g∙kg−1 FM 10 | g∙kg−1 DM | |||||||||
| Total average | 319.7 ± 7.61 | 39.2 ± 0.79 | 64.9 ± 1.89 | 26.3 ± 1.05 | 220.9 ± 5.81 | 460.7 ± 9.53 | 285.4 ± 7.17 | 19.6 ± 0.78 | 408.8 ± 5.62 | |
| 1. Zero-fert | 312.1 ± 17.99 | 39.3 ± 1.48 | 52.4 C ± 3.18 | 24.4 ± 2.28 | 224.5 ± 14.74 | 468.5 ± 25.37 | 287.0 ± 16.09 | 17.9 AB ± 1.65 | 415.4 ± 23.31 | |
| 2. Inorganic NPK | 328.6 ± 18.96 | 40.9 ± 1.96 | 77.2 A ± 3.03 | 28.4 ± 2.33 | 216.5 ± 9.51 | 441.1 ± 13.75 | 269.5 ± 11.35 | 16.9 B ± 1.80 | 412.4 ± 17.63 | |
| 3. 1.0 Mg MBM + N79 | 323.2 ± 18.21 | 35.5 ± 1.41 | 69.4 B ± 3.59 | 26.4 ± 2.61 | 223.8 ± 14.18 | 457.2 ± 22.34 | 283.9 ± 17.28 | 22.6 A ± 1.56 | 411.5 ± 21.54 | |
| 4. 1.5 Mg MBM + N40 | 324.6 ± 15.45 | 38.1 ± 1.92 | 64.7 B ± 3.51 | 26.7 ± 2.31 | 212.2 ± 13.85 | 464.9 ± 22.93 | 286.0 ± 16.21 | 20.1 AB ± 1.34 | 405.6 ± 18.63 | |
| 5. 2.0 Mg MBM | 310.2 ± 16.61 | 42.1 ± 1.73 | 60.9 B ± 4.55 | 25.7 ± 2.47 | 228.0 ± 13.86 | 472.1 ± 22.89 | 300.9 ± 19.65 | 20.8 AB ± 2.11 | 399.2 ± 16.32 | |
| Annual mean (y) | 2014 | 312.5 b ± 5.21 | 40.5 ± 1.62 | 79.3 a ± 2.06 | 36.0 a ± 0.67 | 169.4 c ± 4.31 | 373.2 c ± 7.71 | 221.8 c ± 6.12 | 21.7 ab ± 1.36 | 471.1 A ± 6.87 |
| 2019 | 262.1 c ± 7.53 | 39.5 ± 0.96 | 61.7 b ± 2.38 | 23.5 b ± 0.99 | 259.9 a ± 7.22 | 524.0 a ± 9.68 | 335.5 a ± 7.87 | 22.4 a ± 1.01 | 351.4 C ± 5.41 | |
| 2020 | 384.7 a ± 7.47 | 37.6 ± 1.47 | 53.9 c ± 2.42 | 19.5 c ± 1.03 | 233.6 b ± 4.04 | 485.1 b ± 6.93 | 299.1 b ± 4.99 | 14.8 b ± 1.02 | 404.0 B ± 4.58 | |
| Interaction (t × y) | ns 11 | ns | ns | ns | ns | ns | ns | ns | ns | |
| Treatment (t) | P | K | Ca | Mg | |
|---|---|---|---|---|---|
| Total average | 1.85 ± 0.05 | 12.2 ± 0.68 | 1.18 ± 0.09 | 0.97 ± 0.011 | |
| 1. Zero-fert | 1.90 ± 0.07 | 11.2 ± 0.89 | 0.86 B ± 0.14 | 0.90 b ± 0.12 | |
| 2. Inorganic NPK | 1.73 ± 0.05 | 12.2 ± 0.86 | 1.44 A ± 0.19 | 1.00 a ± 0.15 | |
| 3. 1.0 Mg MBM + N79 | 1.83 ± 0.09 | 12.0 ± 1.21 | 1.02 B ± 0.17 | 0.91 b ± 0.12 | |
| 4. 1.5 Mg MBM + N40 | 1.96 ± 0.06 | 12.4 ± 1.24 | 1.07 B ± 0.16 | 0.94 ab ± 0.13 | |
| 5. 2.0 Mg MBM | 1.84 ± 0.07 | 13.5 ± 1.19 | 1.49 A ± 0.14 | 0.95 ab ± 0.13 | |
| Annual mean (y) | 2014 | 2.05 a ± 0.04 | 8.4 C ± 0.35 | 1.05 ± 0.19 | 1.15 A ± 0.04 |
| 2019 | 1.78 b ± 0.05 | 15.3 A ± 0.59 | 1.14 ± 0.13 | 0.95 B ± 0.05 | |
| 2020 | 1.73 b ± 0.05 | 13.4 B ± 0.41 | 1.35 ± 0.04 | 0.81 B ± 0.05 | |
| Interaction (t × y) | s 1 | ns 2 | ns | ns | |
| Treatment (t) | pH | N-NH3 1 g∙kg−1 TN 13 | LA 2 | AA 3 | PA 4 | BA 5 | VFA 6 | Et 7 | LA:AA 8 | UFL 9 | UFV 10 | PDIN 11 | PDIE 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g∙kg−1 DM 14 | ||||||||||||||
| Total average | 4.41 ± 0.04 | 45.0 ± 2.02 | 97.9 ± 4.12 | 27.9 ± 1.88 | 0.05 ± 0.02 | 1.86 ± 0.26 | 30.4 ± 1.63 | 1.17 ± 0.32 | 2.72 ± 0.44 | 0.91 ± 0.05 | 0.81 ± 0.05 | 48.1 ± 2.05 | 64.1 ± 3.46 | |
| 1. Zero-fert | 4.46 ± 0.04 | 46.6 ± 3.01 | 98.9 ± 6.79 | 30.1 A ± 1.41 | 0.070 ± 0.02 | 2.83 ± 0.39 | 33.3 a ± 1.45 | 1.00 ± 0.32 | 3.34 ± 0.39 | 0.91 ± 0.05 | 0.80 ± 0.04 | 33.5 ± 2.36 | 61.3 B ± 4.58 | |
| 2. Inorganic NPK | 4.38 ± 0.04 | 41.5 ± 3.93 | 97.1 ± 9.04 | 28.4 AB ± 1.85 | 0.047 ± 0.01 | 1.48 ± 0.58 | 30.4 ab ± 1.36 | 1.05 ± 0.25 | 3.48 ± 0.85 | 0.92 ± 0.06 | 0.81 ± 0.05 | 49.6 ± 3.38 | 66.8 Aa ± 5.78 | |
| 3. 1.0 Mg MBM + N79 | 4.38 ± 0.08 | 45.8 ± 2.54 | 86.7 ± 5.52 | 27.1 B ± 1.32 | 0.059 ± 0.01 | 1.57 ± 0.54 | 29.2 ab ± 1.87 | 1.16 ± 0.35 | 3.22 ± 0.54 | 0.92 ± 0.07 | 0.81 ± 0.05 | 44.3 ± 2.45 | 65.3 A ± 4.32 | |
| 4. 1.5 Mg MBM + N40 | 4.39 ± 0.06 | 44.4 ± 2.36 | 102.6 ± 7.32 | 26.1 B ± 1.69 | 0.045 ± 0.01 | 1.93 ± 0.46 | 29.0 b ± 1.39 | 1.31 ± 0.41 | 3.93 ± 0.41 | 0.92 ± 0.05 | 0.82 ± 0.05 | 41.3 ± 2.48 | 64.5 AB ± 4.25 | |
| 5. 2.0 Mg MBM | 4.47 ± 0.06 | 46.8 ± 2.02 | 104.2 ± 4.91 | 28.2 AB ± 2.32 | 0.047 ± 0.01 | 1.46 ± 0.46 | 30.1 ab ± 1.58 | 1.31 ± 0.35 | 3.75 ± 0.54 | 0.90 ± 0.06 | 0.80 ± 0.06 | 71.4 ± 2.78 | 62.8 Bb ± 4.81 | |
| Annual mean (y) | 2014 | 4.35 b ± 0.06 | 43.7 ± 2.52 | 100.8 ± 6.78 | 28.8 a ± 1.58 | 0.010 b ± 0.01 | 3.28 a ± 0.32 | 33.1 A ± 1.97 | 1.04 ± 0.41 | 3.56 ± 0.41 | 0.97 A ± 0.09 | 0.88 A ± 0.07 | 50.9 ± 3.14 | 70.0 A ± 3.34 |
| 2019 | 4.51 a ± 0.04 | 46.8 ± 1.94 | 96.4 ± 3.92 | 28.6 a ± 1.59 | 0.065 a ± 0.02 | 1.61 b ± 0.35 | 30.5 B ± 1.32 | 1.24 ± 0.26 | 3.39 ± 0.61 | 0.87 Bb ± 0.04 | 0.76 Bb ± 0.05 | 58.9 ± 1.59 | 61.4 B ± 4.45 | |
| 2020 | 4.38 b ± 0.02 | 44.5 ± 2.06 | 96.6 ± 5.09 | 26.5 b ± 2.12 | 0.086 a ± 0.03 | 0.68 b ± 0.23 | 27.5 C ± 1.58 | 1.22 ± 0.31 | 1.22 ± 0.32 | 0.90 Ba ± 0.03 | 0.79 Ba ± 0.04 | 34.4 ± 1.96 | 60.9 B ± 3.23 | |
| Interaction (t × y) | ns 15 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogalska, A.; Borsuk-Stanulewicz, M.; Purwin, C.; Nogalski, Z. Quality of Maize Silage After Using Meat Bone Meal as a Phosphorus Fertilizer in a Field Experiment. Appl. Sci. 2025, 15, 6129. https://doi.org/10.3390/app15116129
Nogalska A, Borsuk-Stanulewicz M, Purwin C, Nogalski Z. Quality of Maize Silage After Using Meat Bone Meal as a Phosphorus Fertilizer in a Field Experiment. Applied Sciences. 2025; 15(11):6129. https://doi.org/10.3390/app15116129
Chicago/Turabian StyleNogalska, Anna, Marta Borsuk-Stanulewicz, Cezary Purwin, and Zenon Nogalski. 2025. "Quality of Maize Silage After Using Meat Bone Meal as a Phosphorus Fertilizer in a Field Experiment" Applied Sciences 15, no. 11: 6129. https://doi.org/10.3390/app15116129
APA StyleNogalska, A., Borsuk-Stanulewicz, M., Purwin, C., & Nogalski, Z. (2025). Quality of Maize Silage After Using Meat Bone Meal as a Phosphorus Fertilizer in a Field Experiment. Applied Sciences, 15(11), 6129. https://doi.org/10.3390/app15116129






