Antioxidative and Photoprotective In Vitro Potential of Lavandula Angustifolium
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Raw Material
2.3. Preparation of the Extracts
2.4. Determination of Antioxidant Potential of the Lavender Extracts
2.5. GC-MS Analyses of Selected Extracts
2.6. Statistical Analysis of the Results
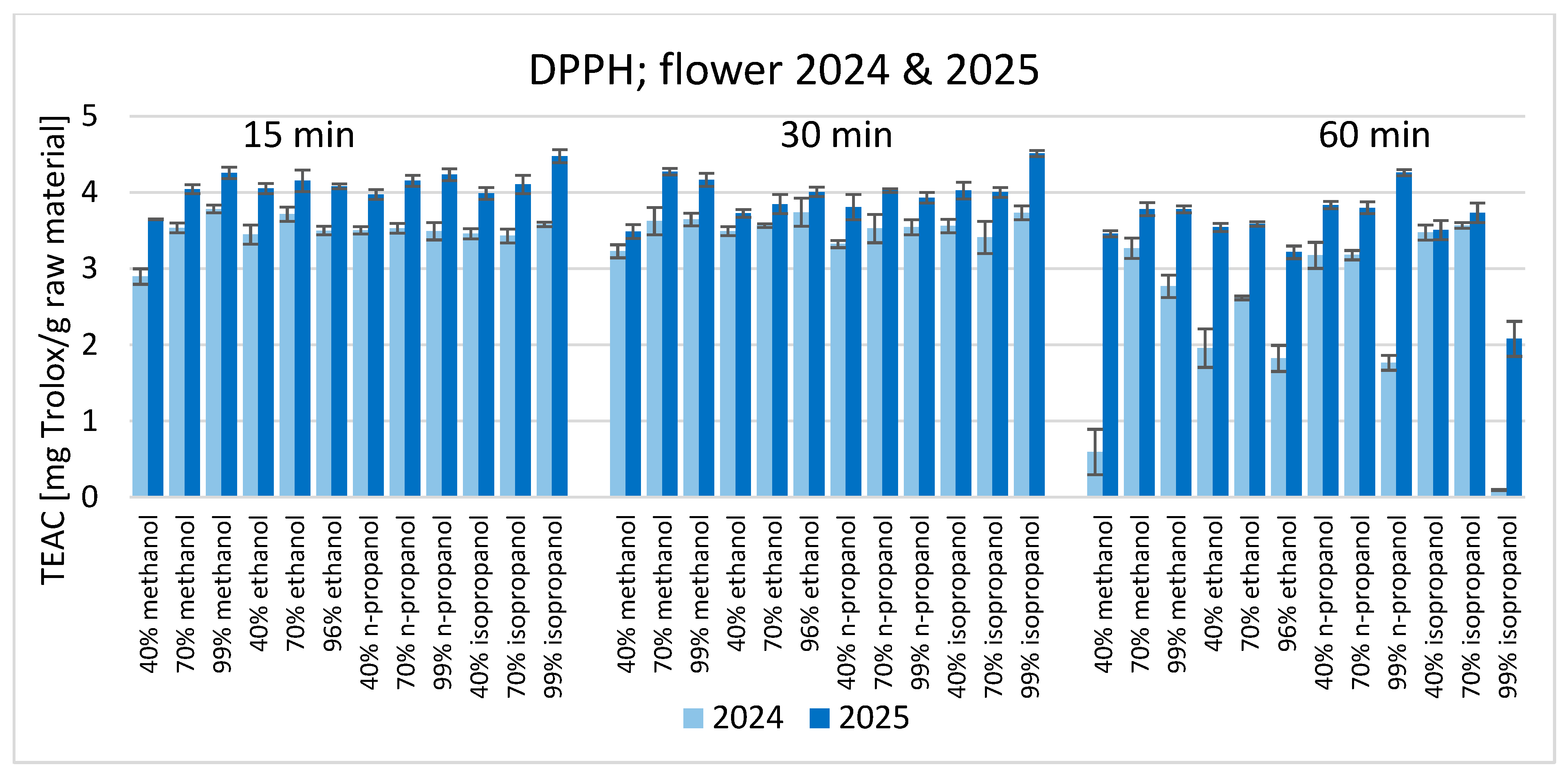
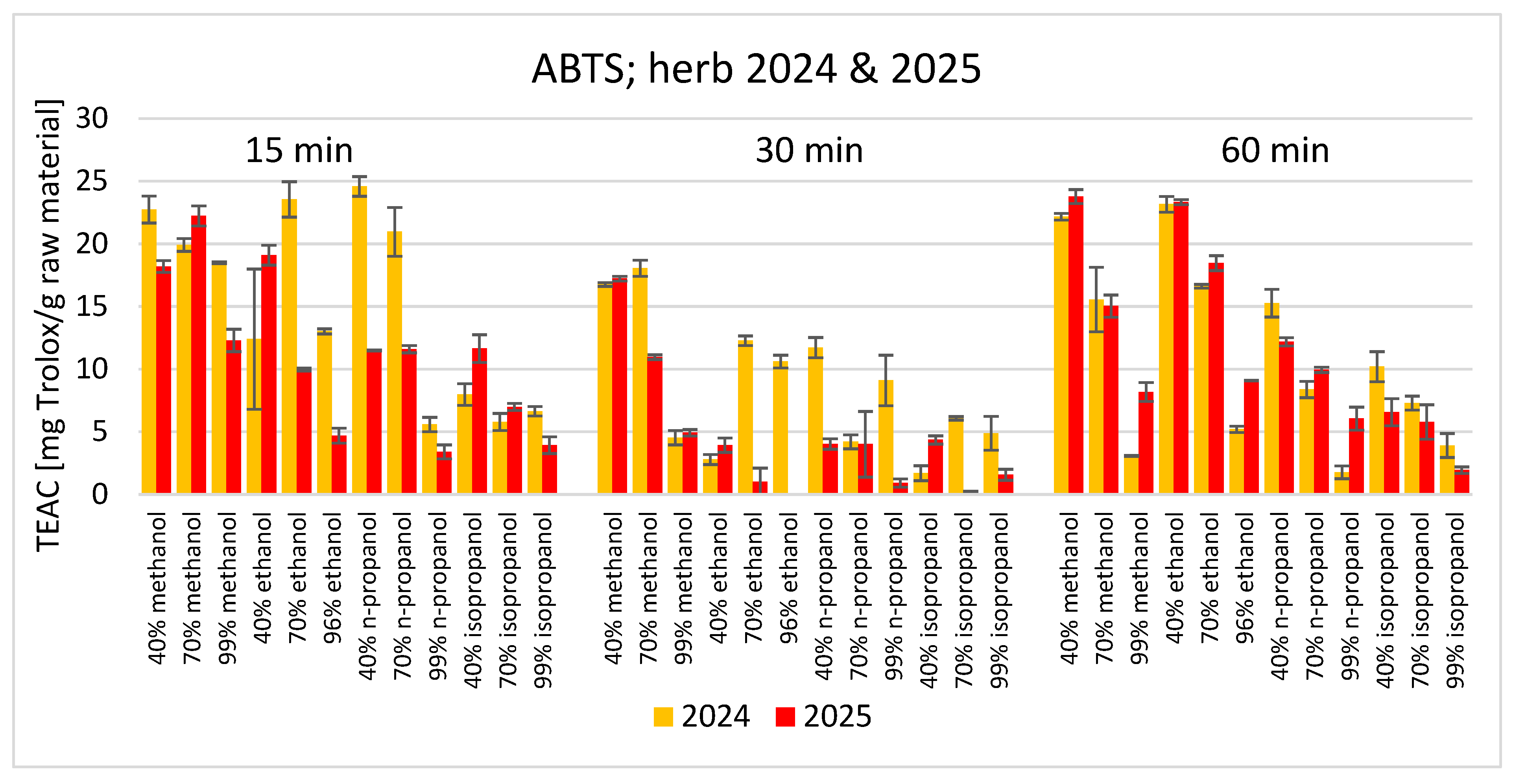
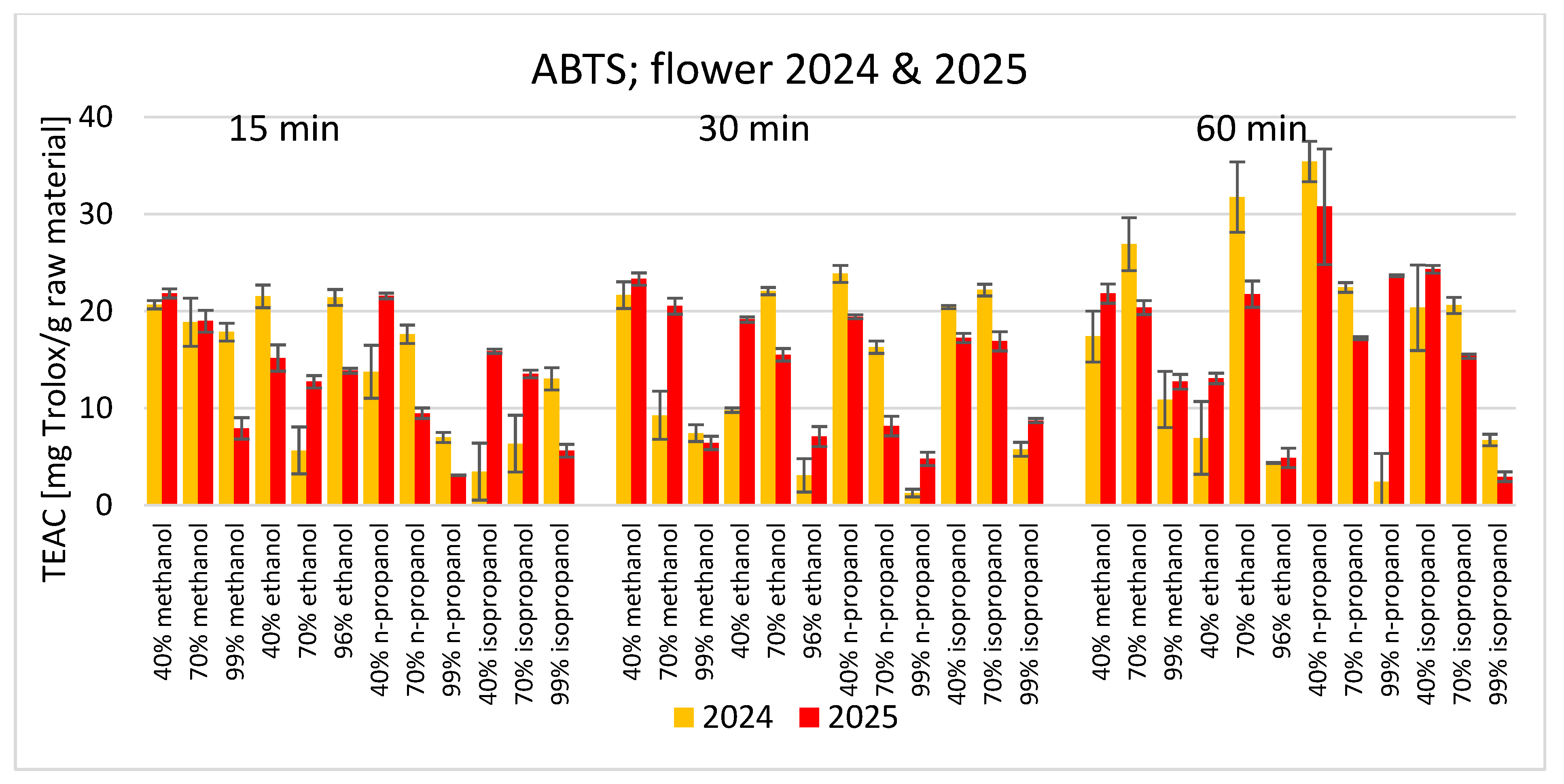
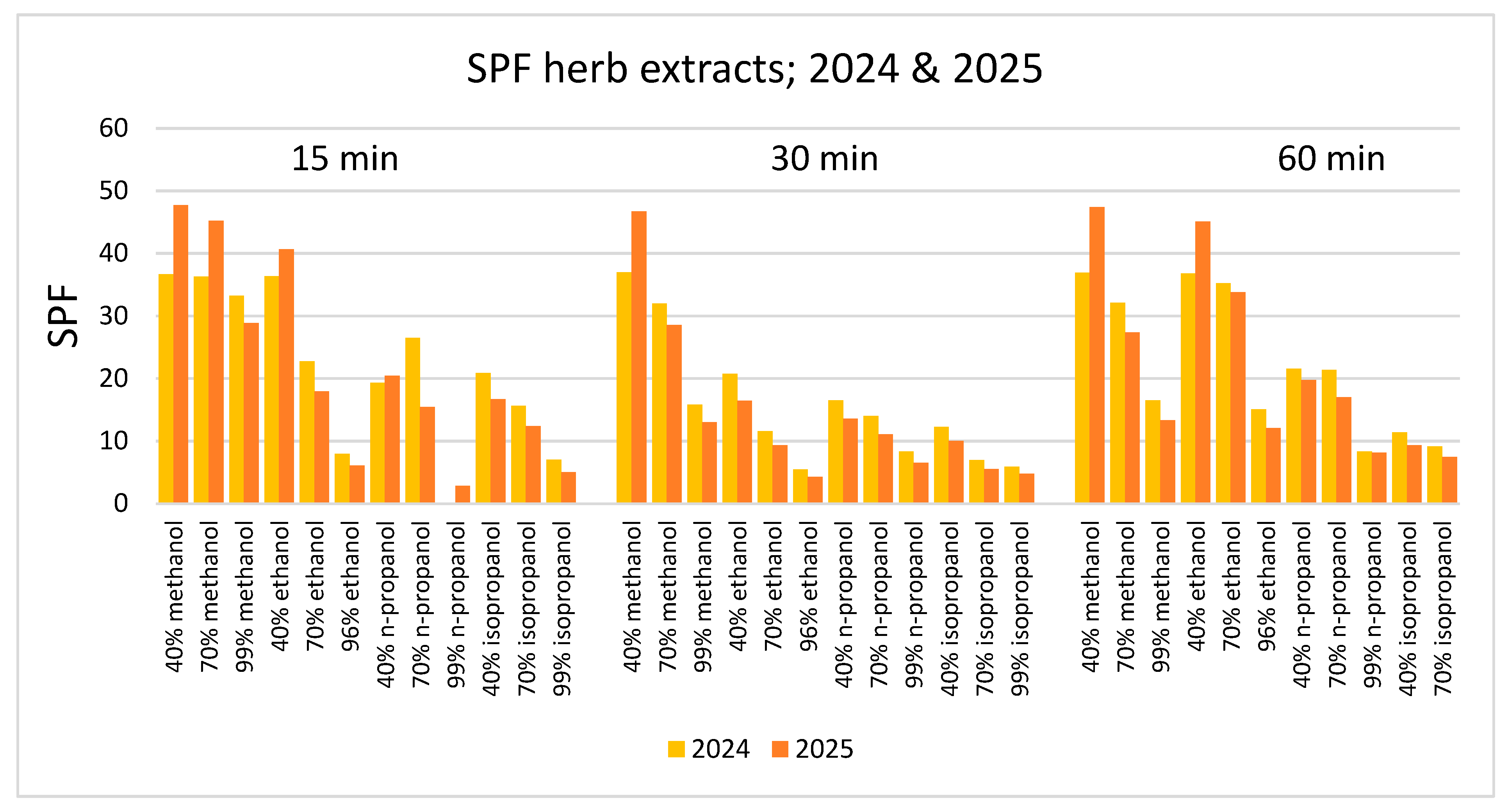
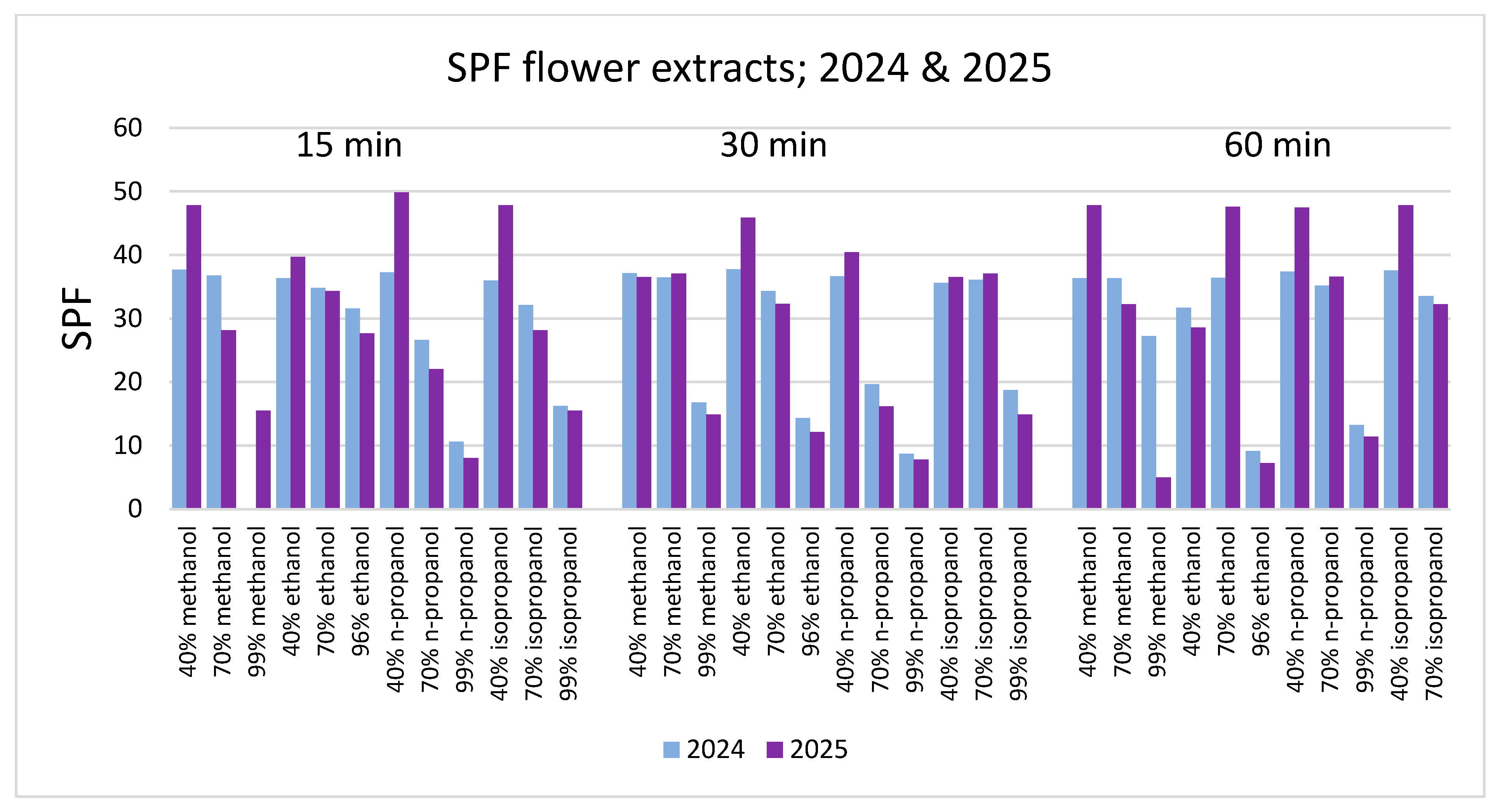
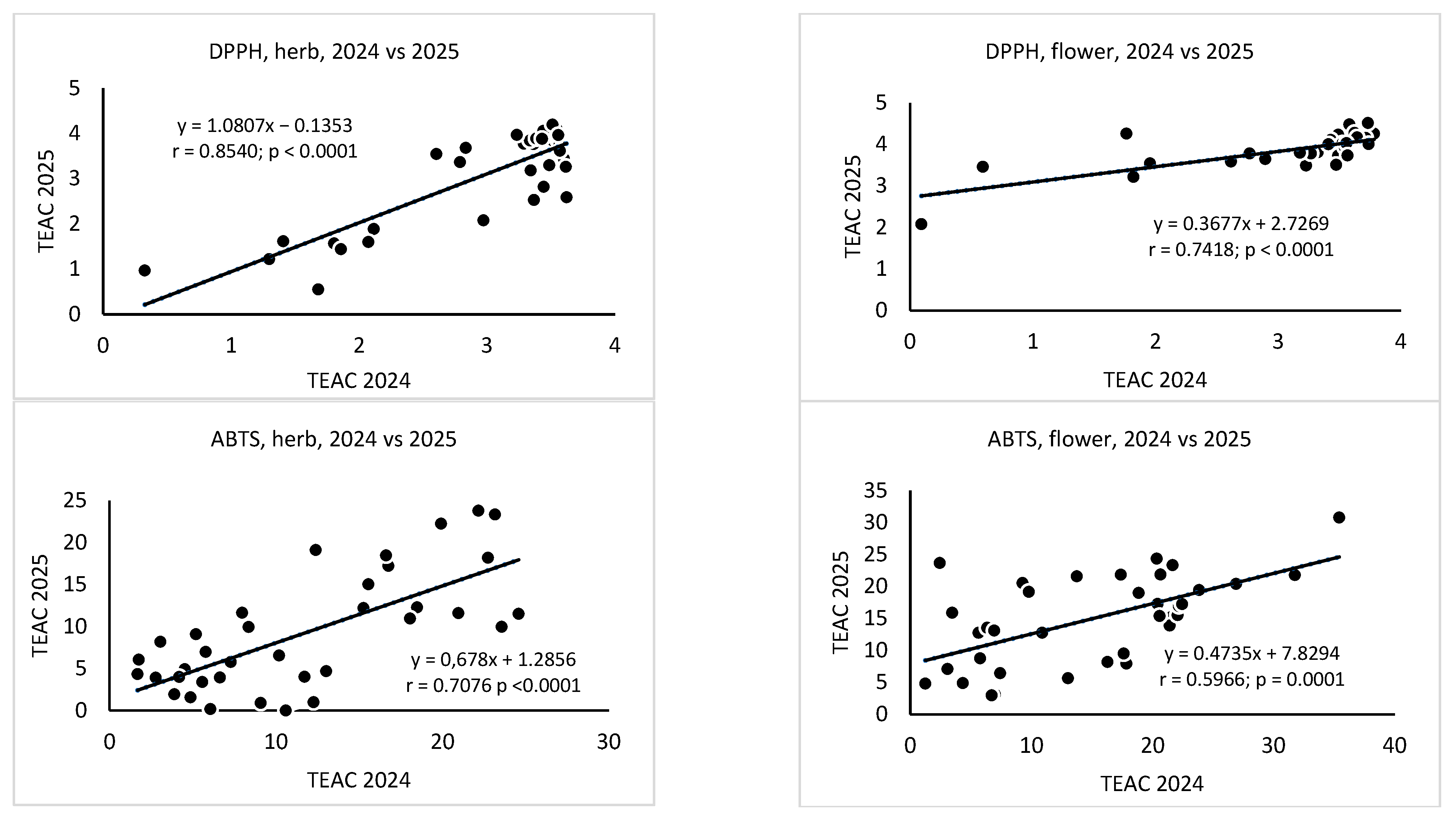
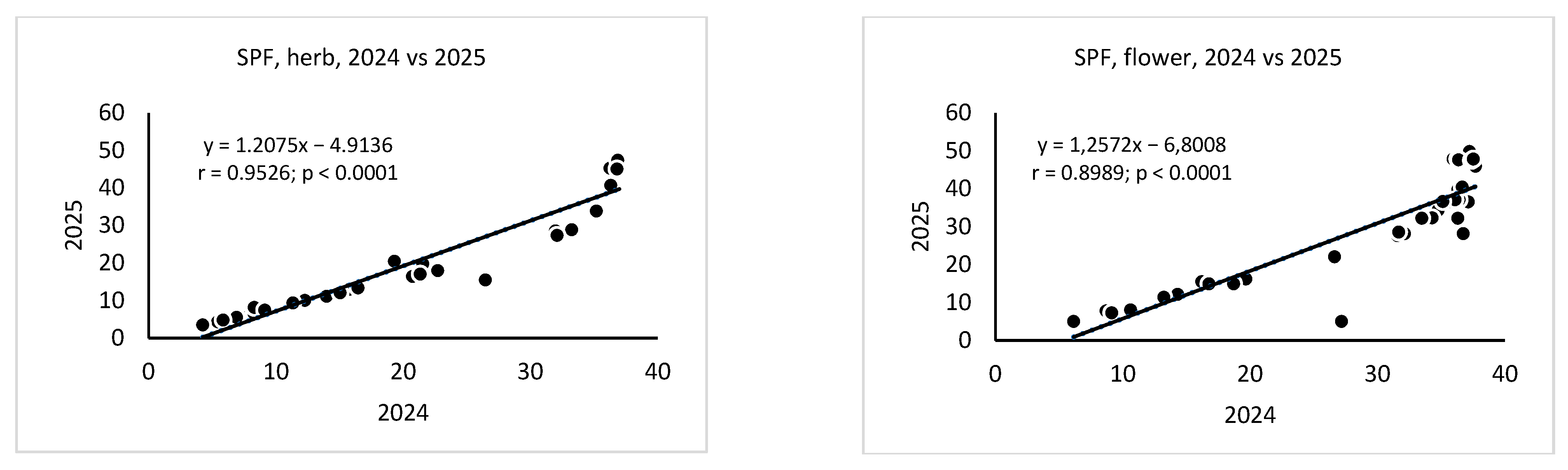
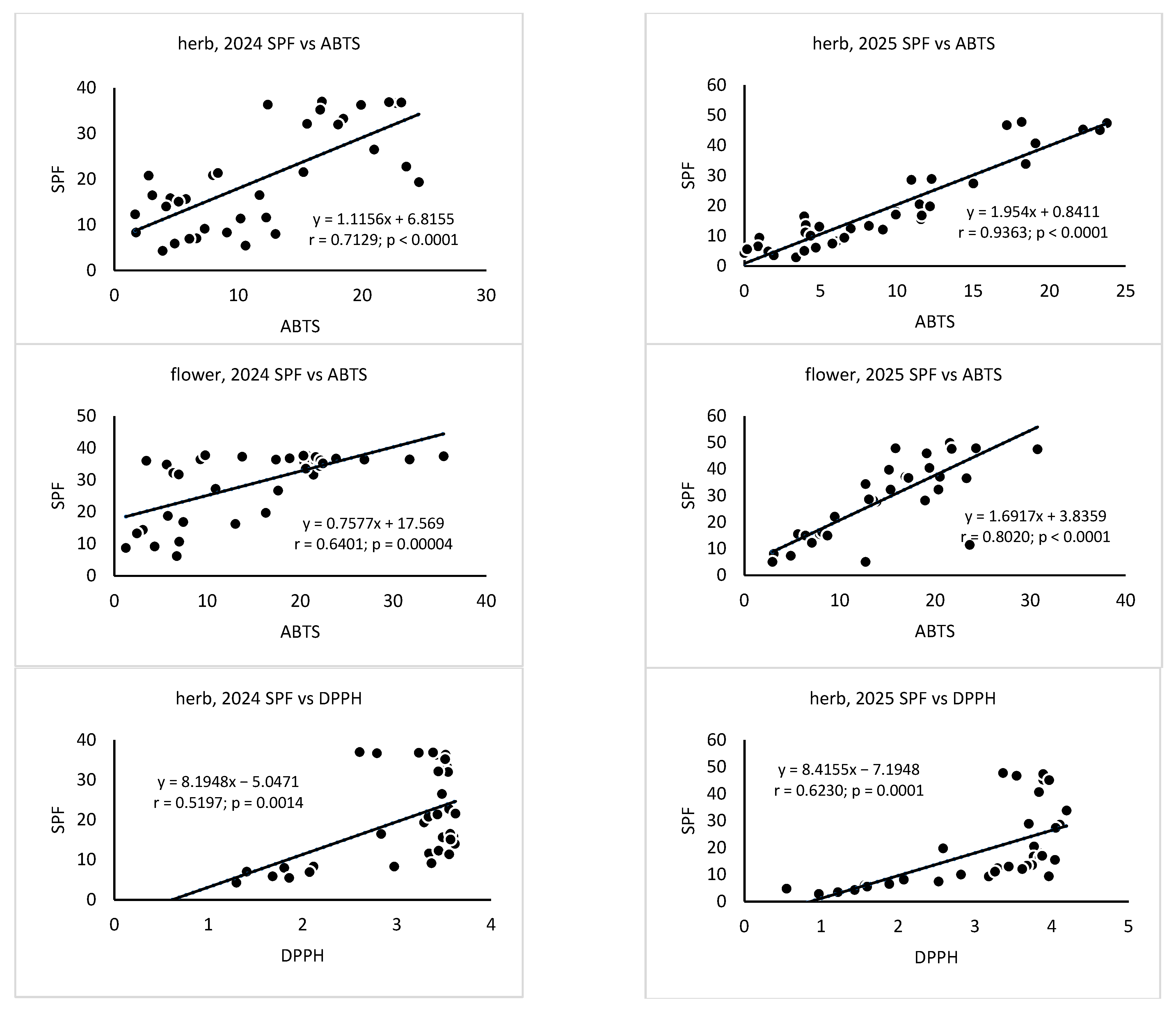
3. Results
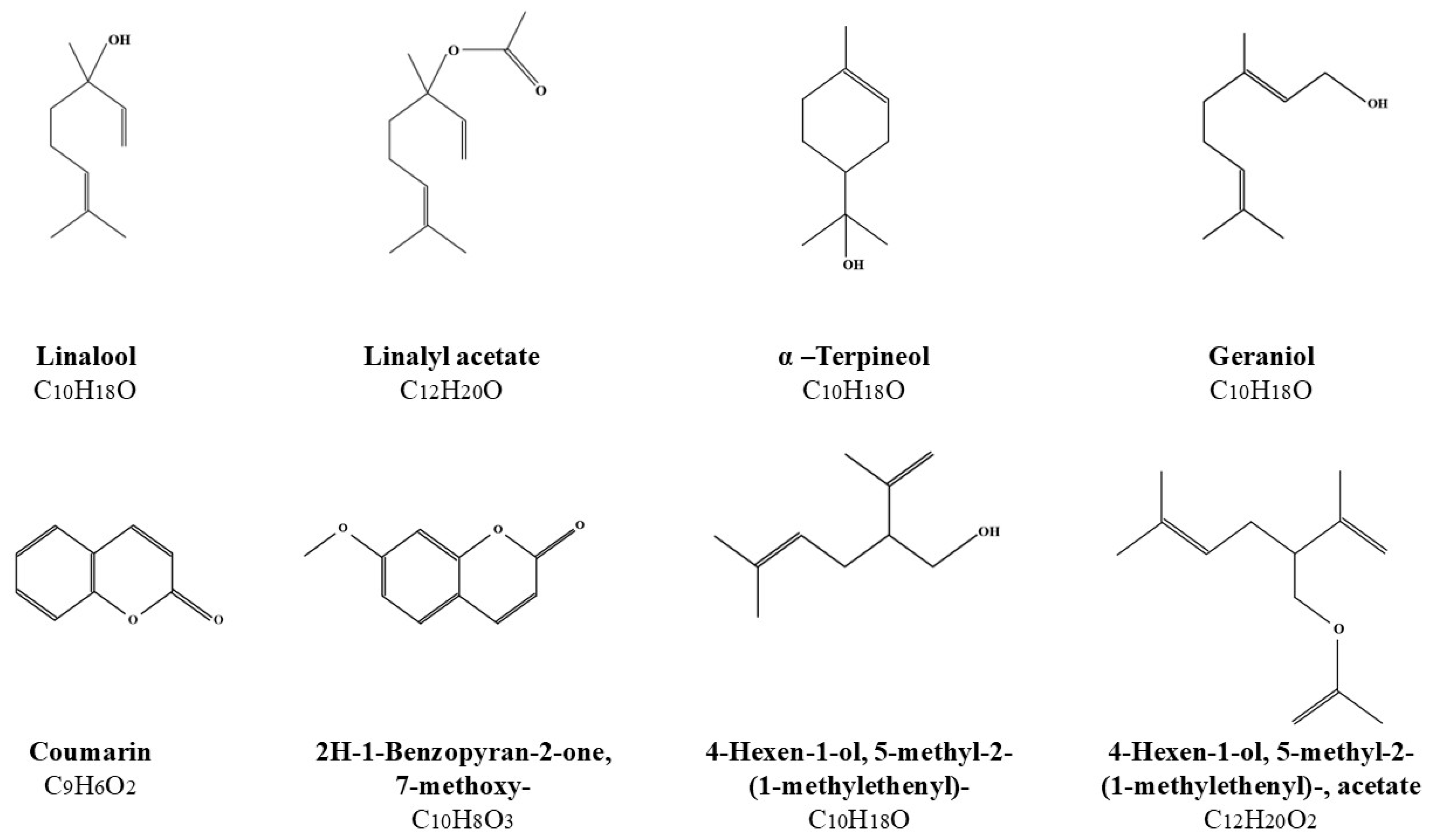
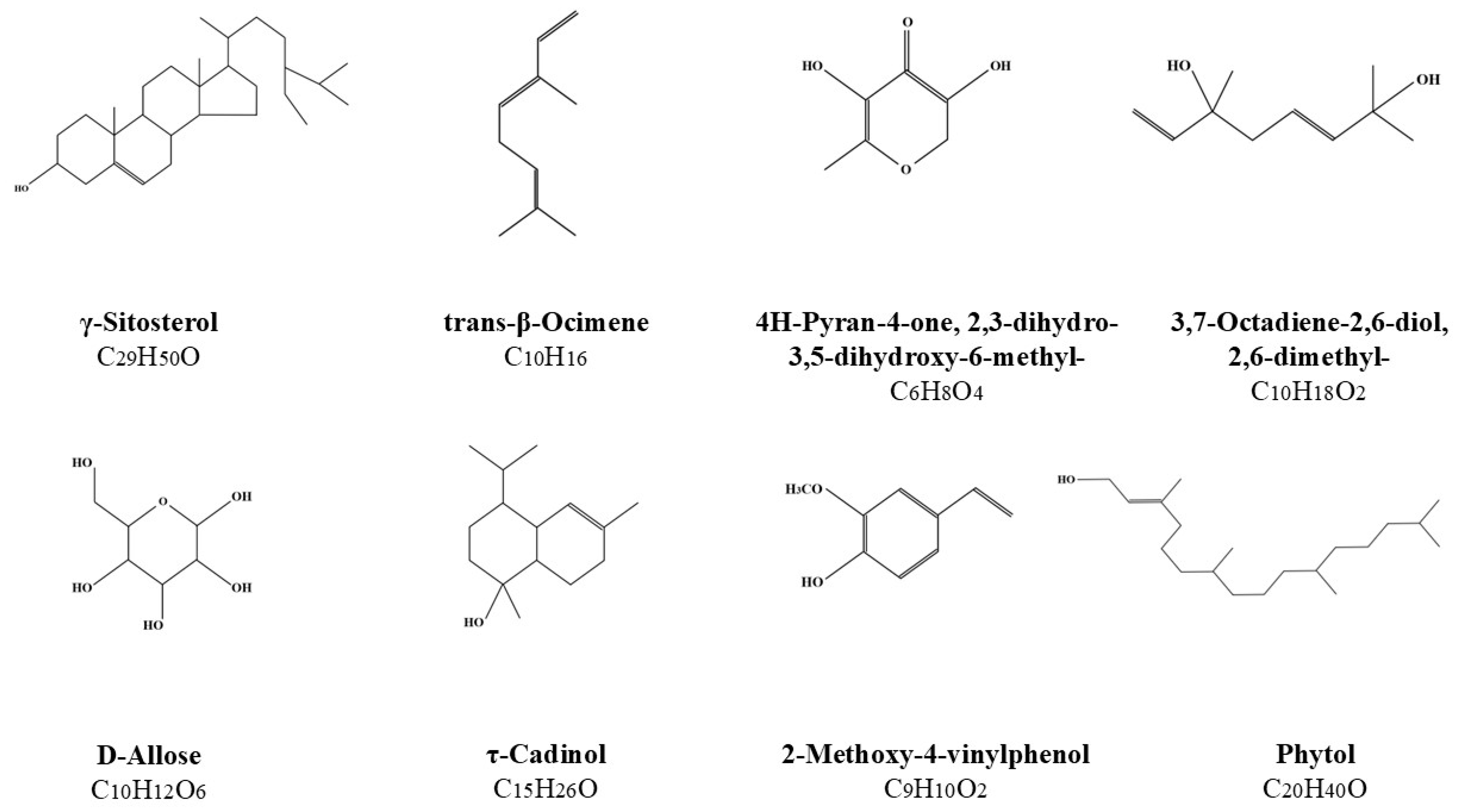
GC-MS Analysis of the Obtained Alcoholic Extracts by the Ultrasound-Assisted Extraction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | (2,2′-azino-bis(3-ehylbenzothiazline-6-sulphonic acid) diammonium salt |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| SD | Standard deviation |
| SPF | Sun protection factor |
| TEAC | Trolox equivalent antioxidant capacity |
References
- Mar, K.; Rivers, J.K. The mind body connection in dermatologic conditions: A literature review. J. Cutan. Med. Surg. 2023, 27, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Bouhout, S.; Aubert, A.; Vial, F.; Choquenet, B. Physiological benefits associated with facial skincare: Well-being from emotional perception to neuromodulation. Int. J. Cosm. Sci. 2023, 45, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.; Billick, S.B. Psychiatric consequences of skin conditions: Multiple case study analysis with literature review. Psychiat. Q. 2022, 93, 841–847. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Kıran, T.R.; Otlu, O.; Karabulut, A.B. Oxidative stress and antioxidants in health and disease. J. Lab. Med. 2023, 47, 1–11. [Google Scholar] [CrossRef]
- Houldsworth, A. Role of oxidative stress in neurodegenerative disorders: A review of reactive oxygen species and prevention by antioxidants. Brain Commun. 2024, 6, fcad356. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free radical properties, source and targets, antioxidant consumption and health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Martemucci, G.; Portincasa, P.; Di Ciaula, A.; Mariano, M.; Centonze, V.; D’Alessandro, A.G. Oxidative stress, aging, antioxidant supplementation and their impact on human health: An overview. Mech. Ageing Dev. 2022, 206, 111707. [Google Scholar] [CrossRef]
- Poljsak, B.; Kovač, V.; Milisav, I. Antioxidants, Food Processing and Health. Antioxidants 2021, 10, 433. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, T.; Yu, D.; Xiong, H.; Zhang, S. Current insights and future perspectives of ultraviolet radiation (UV) exposure: Friends and foes to the skin and beyond the skin. Environ. Int. 2024, 185, 108535. [Google Scholar] [CrossRef] [PubMed]
- Moskwa, J.; Bronikowska, M.; Socha, K.; Markiewicz-Żukowska, R. Vegetable as a source of bioactive compounds with photoprotective properties: Implication in the aging process. Nutrients 2023, 15, 3594. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef]
- Jesus, A.; Sousa, E.; Cruz, M.T.; Cidade, H.; Lobo, J.M.S.; Almeida, I.F. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef]
- Michalak, M. Plant-derived antioxidants: Significance in skin health and the ageing process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Mansuri, R.; Diwan, A.; Kumar, H.; Dangwal, K.; Yadav, D. Potential of natural compounds as sunscreen agents. Pharmacogn. Rev. 2021, 15, 47. [Google Scholar] [CrossRef]
- Milutinov, J.; Pavlović, N.; Ćirin, D.; Atanacković Krstonošić, M.; Krstonošić, V. The Potential of Natural Compounds in UV Protection Products. Molecules 2024, 29, 5409. [Google Scholar] [CrossRef]
- Verma, A.; Zanoletti, A.; Kareem, K.Y.; Adelodun, B.; Kumar, P.; Ajibade, F.O.; Silva, L.F.O.; Phillips, A.J.; Kartheeswaran, T.; Bontempi, E.; et al. Skin protection from solar ultraviolet radiation using natural compounds: A review. Environ. Chem. Lett. 2024, 22, 273–295. [Google Scholar] [CrossRef]
- Witaszczyk, A.; Klimowicz, A. Usefulness of aloe vera (Aloe vera) as a potential ingredient of cosmetic preparations. Pomeranian J. Life Sci. 2023, 69, 76–87. [Google Scholar] [CrossRef]
- Khan, S.U.; Hamza, B.; Mir, R.H.; Fatima, K.; Malik, F. Lavender plant: Farming and health benefits. Curr. Mol. Med. 2024, 24, 702–711. [Google Scholar] [CrossRef]
- Dobros, N.; Zawada, K.; Paradowska, K. Phytochemical Profile and Antioxidant Activity of Lavandula angustifolia and Lavandula x intermedia Cultivars Extracted with Different Methods. Antioxidants 2022, 11, 711. [Google Scholar] [CrossRef] [PubMed]
- Perović, A.B.; Karabegović, I.T.; Krstić, M.S.; Veličković, A.V.; Avramović, J.M.; Danilović, B.R.; Veljković, V.B. Novel hydrodistillation and steam distillation methods of essential oil recovery from lavender: A comprehensive review. Ind. Crops Prod. 2024, 211, 118244. [Google Scholar] [CrossRef]
- Héral, B.; Stierlin, É.; Fernandez, X.; Michel, T. Phytochemicals from the genus Lavandula: A review. Phytochem. Rev. 2021, 20, 751–771. [Google Scholar] [CrossRef]
- Dobros, N.; Zawada, K.D.; Paradowska, K. Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus. Molecules 2023, 28, 256. [Google Scholar] [CrossRef]
- Crisan, I.; Ona, A.; Vârban, D.; Muntean, L.; Vârban, R.; Stoie, A.; Mihăiescu, T.; Morea, A. Current Trends for Lavender (Lavandula angustifolia Mill.) Crops and Products with Emphasis on Essential Oil Quality. Plants 2023, 12, 357. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Teibo, J.O.; Wasef, L.; Shaheen, H.M.; Akomolafe, A.P.; Teibo, T.K.A.; Al-Kuraishy, H.M.; Al Garbeeb, A.I.; Alexiou, A.; Papadakis, M. A review of the bioactive components and pharmacological properties of Lavandula species. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 877–900. [Google Scholar] [CrossRef] [PubMed]
- Yoo, O.; Park, S.-A. Anxiety-Reducing Effects of Lavender Essential Oil Inhalation: A Systematic Review. Healthcare 2023, 11, 2978. [Google Scholar] [CrossRef]
- Marovska, G.I.; Hambarliyska, I.P.; Petkova, N.T.; Ivanov, I.G.; Vasileva, I.N.; Slavov, A.M. Chemical Composition and Antioxidant Activity of Ethanol Extracts Obtained from Lavender (Lavandula angustifolia Mill.). Philipp. J. Sci. 2023, 152, 861–870. [Google Scholar] [CrossRef]
- Talić, S.; Odak, I.; Boras, M.M.; Smoljan, A.; Bevanda, A.M. Essential oil and extracts from Lavandula angustifolia Mill. cultivated in Bosnia and Herzegovina: Antioxidant activity and acetylcholinesterase inhibition. Int. J. Plant Bas. Pharm. 2023, 3, 95–103. [Google Scholar] [CrossRef]
- Kaur, C.D.; Saraf, S. In vitro sun protection factor determination of herbal oils used in cosmetics. Pharmacogn. Res. 2010, 2, 22–25. [Google Scholar] [CrossRef]
- Malsawmtluangi, C.; Nath, D.K.; Jamatia, I.; Zarzoliana, E.; Pachuau, L. Determination of Sun Protection Factor (SPF) number of some aqueous herbal extracts. J. Appl. Pharm. Sci. 2013, 3, 150–151. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Belokurov, S.S.; Narkevich, I.A.; Flisyuk, E.V.; Kaukhova, I.E.; Aroyan, M.V. Modern extraction methods for medicinal plant raw material. Pharm. Chem. J. 2019, 53, 559–563. [Google Scholar] [CrossRef]
- Chuo, S.C.; Nasir, H.M.; Mohd-Setapar, S.H.; Mohamed, S.F.; Ahmad, A.; Wani, W.A.; Muddassir, M.; Alarifi, A. A glimpse into the extraction methods of active compounds from plants. Crit. Rev. Anal. Chem. 2022, 52, 667–696. [Google Scholar] [CrossRef]
- Gajewska, S.; Siemak, J.; Bilska, J.; Nowak, A.; Klimowicz, A. Effect of storage on the antioxidant properties of Plantago lanceolata L. and Plantago major L. alcoholic extracts. Pomeranian J. Life Sci. 2021, 67, 52–56. [Google Scholar] [CrossRef]
- Kęsik, M.; Klimowicz, A. Antioxidant potential of extracts from different parts of Cichorium intybus L. Pomeranian J. Life Sci. 2024, 70, 59–63. [Google Scholar] [CrossRef]
- Oshetkova, D.; Klimowicz, A. Antioxidative and photoprotective activity of Pinus nigra, Pinus strobus and Pinus mugo. Appl. Sci. 2025, 15, 209. [Google Scholar] [CrossRef]
- Grzeszczak, J.; Wróblewska, A.; Klimowicz, A.; Gajewska, S.; Kucharski, Ł.; Koren, Z.C.; Janda-Milczarek, K. Antioxidant activities of ethanolic extracts obtained from α-pinene-containing plants and their use in cosmetic emulsions. Antioxidants 2024, 13, 811. [Google Scholar] [CrossRef]
- Nowak, A.; Duchnik, W.; Muzykiewicz-Szymańska, A.; Kucharski, Ł.; Zielonka-Brzezicka, J.; Nowak, A.; Klimowicz, A. The changes of antioxidant activity of three varieties of ‘Nalewka’, a traditional Polish fruit alcoholic beverage during long-term storage. Appl. Sci. 2023, 13, 1114. [Google Scholar] [CrossRef]
- Madanowska, A.; Kowalska-Baron, A. Application trial of a simple spectrophotometric method in determination of sun protection parameters of selected sunscreen cosmetics. Biotechnol. Food Sci. 2023, 85, 55–62. [Google Scholar]
- Herzog, B.; Mongiat, S.; Deshayes, C.; Neuhaus, M.; Sommer, K.; Mantler, A. In vivo and in vitro assessment of UVA protection by sunscreen formulations containing either butyl methoxy dibenzoyl methane, methylene bis-benzotriazolyl tetramethylbutylphenol, or microfine ZnO. Int. J. Cosm. Sci. 2002, 24, 170–185. [Google Scholar] [CrossRef]
- Sayre, R.M.; Agin, P.P.; Levee, G.J.; Marlowe, E. Comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Singh, I.; Gupta, M.; Singh, L.P.; Tiwari, G. Formulation and evaluation of herbal sunscreens: An assessment towards skin protection from ultraviolet radiation. Pharmacophore 2022, 13, 41–49. [Google Scholar] [CrossRef]
- Evangelista, M.; Mota, S.; Almeida, I.F.; Pereira, M.G. Usage patterns and self-esteem of female Consumers of Antiaging Cosmetic Products. Cosmetics 2022, 9, 49. [Google Scholar] [CrossRef]
- George, J.; Sneed, K.; Pathak, Y. The skin aging process and anti-aging strategies. Biomed. J. Sci. Tech. Res. 2022, 42, 33377–33386. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Magalhães, M.C.; Oliveira, R.; Sousa-Lobo, J.M.; Almeida, I.F. Trends in the use of botanicals in anti-aging cosmetics. Molecules 2021, 26, 3584. [Google Scholar] [CrossRef]
- Kowalski, S.; Karska, J.; Tota, M.; Skinderowicz, K.; Kulbacka, J.; Drąg-Zalesińska, M. Natural Compounds in Non-Melanoma Skin Cancer: Prevention and Treatment. Molecules 2024, 29, 728. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef] [PubMed]
- Aboalhaija, N.H.; Syaj, H.; Afifi, F.; Sunoqrot, S.; Al-Shalabi, E.; Talib, W. Chemical Evaluation, In Vitro and In Vivo Anticancer Activity of Lavandula angustifolia Grown in Jordan. Molecules 2022, 27, 5910. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Grande, F.; Occhiuzzi, M.A.; Sicari, V.; Loizzo, M.R.; Cappello, A.R. Lavandula angustifolia mill. (Lamiaceae) ethanol extract and its main constituents as promising agents for the treatment of metabolic disorders: Chemical profile, in vitro biological studies, and molecular docking. J. Enzym. Inhibit. Med. Chem. 2023, 38, 2269481. [Google Scholar] [CrossRef]
- Blažeković, B.; Vladimir-Knežević, S.; Brantner, A.; Štefan, M.B. Evaluation of antioxidant potential of Lavandula x intermedia Emeric ex Loisel. ‘Budrovka’: A comparative study with L. angustifolia Mill. Molecules 2010, 15, 5971–5987. [Google Scholar] [CrossRef]
- Ozsefil, I.C.; Ziylan-Yavas, A. Green approach for polyphenol extraction from waste tea biomass: Single and hybrid application of conventional and ultrasound-assisted extraction. Environ. Res. 2023, 235, 116703. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Taher, Z.M.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Rather, G.A.; Nanda, A.; Raj, E.; Mathivanan, N.; Thiruvengadam, K.; Sofi, M.A.; Nayak, B.K. Determination of Phytochemicals, in vitro Antioxidant and Antibacterial activity of Lavandula angustifolia Mill. Res. J. Pharm. Technol. 2023, 16, 1161–1166. [Google Scholar] [CrossRef]
- Betlej, I.; Andres, B.; Cebulak, T.; Kapusta, I.; Balawejder, M.; Jaworski, S.; Lange, A.; Kutwin, M.; Pisulewska, E.; Kidacka, A.; et al. Antimicrobial Properties and Assessment of the Content of Bioactive Compounds Lavandula angustifolia Mill. Cultivated in Southern Poland. Molecules 2023, 28, 6416. [Google Scholar] [CrossRef]
- Dvorackova, E.; Snoblova, M.; Hrdlicka, P. Content of phenolic compounds in herb used in the Czech Republic. Int. Food Res. J. 2014, 21, 1495–1500. [Google Scholar]
- Dong, G.; Bai, X.; Aimila, A.; Aisa, H.A.; Maiwulanjiang, M. Study on lavender essential oil chemical compositions by GC-MS and improved pGC. Molecules 2020, 25, 3166. [Google Scholar] [CrossRef] [PubMed]
- Zagorcheva, T.; Rusanov, K.; Stanev, S.; Atanassov, I. A simple procedure for comparative GC-MS analysis of lavender (Lavandula angustifolia Mill.) flower volatile composition. IOSR J. Pharm. Biol. Sci. 2016, 11, 9–14. [Google Scholar] [CrossRef]
- Caprari, C.; Fantasma, F.; Monaco, P.; Divino, F.; Iorizzi, M.; Ranalli, G.; Fasano, F.; Saviano, G. Chemical Profiles, In Vitro Antioxidant and Antifungal Activity of Four Different Lavandula angustifolia L. EOs. Molecules 2023, 28, 392. [Google Scholar] [CrossRef]
- Jabir, M.S.; Taha, A.A.; Sahib, S.I. Antioxidant activity of Linalool. Eng. Technol. J. 2018, 36 Pt B, 64–67. [Google Scholar] [CrossRef]
- Kıvrak, S. Essential oil composition and antioxidant activities of eight cultivars of Lavender and Lavandin from western Anatolia. Ind. Crops Prod. 2018, 117, 88–96. [Google Scholar] [CrossRef]
- Sancheti, S.; Sancheti, S.; Seo, S.-Y. Ameliorative effects of 7-methylcoumarin and 7-methoxycoumarin against CCl4-induced hepatotoxicity in rats. Drug Chem. Toxicol. 2013, 36, 42–47. [Google Scholar] [CrossRef]

| Wavelength [nm] | EE(λ) · I(λ) |
|---|---|
| 290 | 0.0150 |
| 295 | 0.0817 |
| 300 | 0.2874 |
| 305 | 0.3278 |
| 310 | 0.1864 |
| 315 | 0.0839 |
| 320 | 0.0180 |
| Compared Parameters | Z-Value | p | |
|---|---|---|---|
| Antioxidant activity evaluated with DPPH method | |||
| herb assayed in 2024 | herb assayed in 2025 | −1.7909 | 0.0733 (NS) |
| flower assayed in 2024 | flower assayed in 2025 | −5.7612 | <0.00001 |
| herb assayed in 2024 | flower assayed in 2024 | −1.3517 | 0.1765 (NS) |
| herb assayed in 2025 | flower assayed in 2025 | −3.8693 | 0.0001 |
| Antioxidant activity evaluated with ABTS method | |||
| herb assayed in 2024 | herb assayed in 2025 | −1.6724 | 0.0944 (NS) |
| flower assayed in 2024 | flower assayed in 2025 | −0.0056 | 0.9955 (NS) |
| herb assayed in 2024 | flower assayed in 2024 | −1.4247 | 0.1542 (NS) |
| herb assayed in 2025 | flower assayed in 2025 | −3.3230 | 0.0009 |
| Sun protection factor | |||
| herb assayed in 2024 | herb assayed in 2025 | −1.0755 | 0.2821 (NS) |
| flower assayed in 2024 | flower assayed in 2025 | −0.0169 | 0.9865 (NS) |
| herb assayed in 2024 | flower assayed in 2024 | −2.9901 | 0.0028 |
| herb assayed in 2025 | flower assayed in 2025 | −2.8935 | 0.0038 |
| EXTRACTS of Lavandula angustifolia (60 min) | AREA [%] | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIN | 4-HEX | α-TER | GER | COU | 2H-1 | LIA | 4-HEA | ɣ-SIT | t-β-OCI | 4H-PYR | 3,7-OCT | D-ALL | τ-CAD | 2-MET | PHY | |
| flower 40% (v/v) MeOH | 25.32 | 5.96 | 3.52 | 2.22 | 4.9 | 5.9 | - | - | - | - | 1.31 | 1.17 | - | 0.74 | - | - |
| flower 70% (v/v) MeOH | 28.6 | 1.5 | 3.25 | - | 3.97 | 4.88 | 9 | 7.6 | - | - | - | 0.99 | - | 1.76 | - | - |
| flower 99% (v/v) MeOH | 20.88 | 0.66 | - | - | 2.53 | 3.3 | 31.65 | 10.05 | - | 1.19 | - | 1.32 | - | 1.34 | - | 0.57 |
| flower 40% (v/v) EtOH | 24.86 | 3.19 | 3.94 | - | 3.24 | 4.54 | 13.29 | 5.65 | - | - | - | 0.86 | - | 1.21 | - | - |
| flower 70% (v/v) EtOH | 24.47 | 5.19 | 2.38 | - | 3.89 | 5.1 | 18.29 | 3.63 | - | - | - | 1.92 | - | 1.77 | - | - |
| flower 96% (v/v) EtOH | 19.5 | 0.49 | - | - | 1.09 | 0.92 | 32.42 | 8.48 | 1.18 | 2.38 | - | 0.62 | - | 1.24 | - | - |
| flower 40% (v/v) n-ProOH | 28.76 | 7.74 | 6.48 | - | 3.46 | 4.12 | - | - | 2.76 | - | - | 1.27 | - | 1.43 | - | - |
| flower 70% (v/v) n-ProOH | 20.69 | 1.79 | 0.64 | - | 2.86 | 4.02 | 26.95 | 6.64 | - | 1.31 | - | 0.86 | - | 1.1 | - | - |
| flower 99% (v/v) n-ProOH | 17.85 | - | - | - | 1.02 | 1.4 | 30.52 | 8.68 | - | 2.19 | - | 0.91 | - | 1.21 | - | - |
| flower 40% (v/v) isoProOH | 25.54 | 7.84 | 4.44 | - | 4.29 | 4.89 | 13.23 | 0.91 | - | - | - | 0.85 | - | 1.4 | - | - |
| flower 70% (v/v) isoProOH | 20.59 | 5.28 | 0.92 | - | 3.11 | 3.87 | 26.94 | 3.63 | - | - | - | 1.68 | - | 1.4 | - | 0.68 |
| flower 99% (v/v) isoProOH | 19.13 | 0.5 | - | - | - | - | 32.17 | 8.99 | - | 2.41 | - | 2.76 | - | 1.28 | - | - |
| herb 40% (v/v) MeOH | - | - | - | - | 15.15 | 29.72 | - | - | - | - | 2.46 | - | - | - | 1.83 | - |
| herb 70% (v/v) MeOH | 0.61 | - | - | - | 13.74 | 28.79 | - | - | - | - | 0.75 | - | 2.42 | 2.85 | 1.72 | 1.25 |
| herb 99% (v/v) MeOH | - | - | - | - | 10.75 | 17.04 | 0.84 | - | - | - | - | - | 2.88 | 3.44 | 2.2 | 1.4 |
| herb 40% (v/v) EtOH | 0.67 | - | - | - | 16.25 | 30.43 | - | - | 1.92 | - | 1.62 | - | - | 1.69 | 2.72 | - |
| herb 70% (v/v) EtOH | 0.57 | - | - | - | 13.19 | 28.22 | 0.45 | - | - | - | 0.48 | - | 1.78 | 3.41 | 1.66 | 2.32 |
| herb 96% (v/v) EtOH | - | - | - | - | 9.43 | 16.38 | 0.95 | - | 1.75 | - | - | - | 2.07 | 2.76 | 1.55 | 2.35 |
| herb 40% (v/v) n-ProOH | 0.54 | - | - | - | 10.2 | 20.33 | 0.34 | - | - | - | - | - | - | 2.93 | 1 | 3.64 |
| herb 70% (v/v) n-ProOH | - | - | - | - | 8.39 | 18.22 | 0.48 | - | 1.58 | - | - | - | 1.25 | 2.87 | 1.22 | 2.11 |
| herb 99% (v/v) n-ProOH | 0.75 | - | - | - | 4.44 | 7.28 | 0.95 | - | 1.66 | - | - | - | - | 3.69 | 0.66 | 1.82 |
| herb 40% (v/v) isoProOH | - | - | - | - | 5.74 | 11.12 | - | - | 4.72 | - | - | - | 1.42 | 2.22 | 0.83 | 1.79 |
| herb 70% (v/v) isoProOH | 0.78 | - | - | - | 5.48 | 8.13 | 0.74 | - | 1.86 | - | - | - | - | 2.73 | - | 1.4 |
| herb 99% (v/v) isoProOH | 1.06 | - | - | - | 1.46 | 2.97 | 2.11 | - | 2.16 | - | - | - | - | 3.62 | - | 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelmach, M.; Klimowicz, A.; Wróblewska, A.; Oshetkova, D.; Gajewska, S.; Siemak, J. Antioxidative and Photoprotective In Vitro Potential of Lavandula Angustifolium. Appl. Sci. 2025, 15, 6004. https://doi.org/10.3390/app15116004
Stelmach M, Klimowicz A, Wróblewska A, Oshetkova D, Gajewska S, Siemak J. Antioxidative and Photoprotective In Vitro Potential of Lavandula Angustifolium. Applied Sciences. 2025; 15(11):6004. https://doi.org/10.3390/app15116004
Chicago/Turabian StyleStelmach, Magdalena, Adam Klimowicz, Agnieszka Wróblewska, Daria Oshetkova, Sylwia Gajewska, and Joanna Siemak. 2025. "Antioxidative and Photoprotective In Vitro Potential of Lavandula Angustifolium" Applied Sciences 15, no. 11: 6004. https://doi.org/10.3390/app15116004
APA StyleStelmach, M., Klimowicz, A., Wróblewska, A., Oshetkova, D., Gajewska, S., & Siemak, J. (2025). Antioxidative and Photoprotective In Vitro Potential of Lavandula Angustifolium. Applied Sciences, 15(11), 6004. https://doi.org/10.3390/app15116004






