Abstract
Fibromyalgia (FM) is characterized by persistent widespread pain that severely impacts quality of life. Immersive virtual reality-based exercise (iVRE) is emerging as a therapeutic modality for chronic pain management. However, research on iVRE in FM patients has primarily focused on perceived pain intensity (PI), with limited exploration of underlying analgesic mechanisms. This study aims to explore the effects of iVRE on PI, considering risk of poor outcomes (RPO) stratification, and on mechanical pain sensitivity (MPS) in FM. A single-arm, uncontrolled, pre-post-test exploratory study was conducted in subjects with FM. The intervention included 2 weekly 15-min iVRE sessions for 6 weeks. PI (numeric rating scale [NRS]) and MPS (pressure pain thresholds [PPTs] at the upper trapezius, lumbar spine, and knee) were assessed at baseline, after the first session (to assess exercise-induced hypoalgesia), and postintervention. RPO was assessed using the Keele STarT MSK Tool. Eleven participants completed the study. No adverse effects were reported. Clinically important reductions were observed in PI (mean difference [MD]: −2.36, 95% CI: [−4.15, −0.58], d = 0.89; p < 0.05) with this effect being associated with baseline RPO. No observable changes were found in PPTs (all 95% CIs included 0, p > 0.05). In this sample, iVRE appears to reduce PI but not PPTs, suggesting the persistence of MPS and limitations in activating endogenous pain inhibitory mechanisms. Further randomized controlled trials with larger samples are needed to corroborate these results.
1. Introduction
Fibromyalgia (FM) is a syndrome characterized by persistent widespread pain [1], often accompanied by other symptoms such as fatigue, muscle tenderness, cognitive disturbances, mood and sleep disorders, as well as physical symptoms, like dizziness, constipation, and nausea [2,3]. The prevalence of FM varies widely, ranging from 1.4% to 13.4% in the general population [4,5]. It more frequently affects women, who represent over 90% of diagnosed cases and have a higher risk of developing severe symptoms [6]. Although potential links to inflammatory, immunological, genetic, and psychosocial factors have been suggested, the exact causes and etiopathogenesis of FM remain unclear [7].
The clinical presentation of FM can lead to disability and reduced quality of life, significantly impacting daily activities and overall well-being [8,9]. Moreover, compared to other rheumatic conditions, such as rheumatoid arthritis, spondyloarthritis, and Sjögren’s syndrome, FM has a more pronounced effect on quality of life; patients experience greater difficulties adapting to the condition, and often rely on less effective strategies to cope with pain [10].
Pain is one of the most common symptoms prompting FM patients to seek medical consultation due to its complexity, multidimensional nature, persistence, management challenges, and widespread impact [8]. These clinical characteristics of pain are associated with dysfunction in the neural circuits involved in the perception, transmission, and processing of nociceptive stimuli [7]. This dysfunction is reflected in a reduced ability to modulate pain through descending mechanisms, high concentrations of excitatory neurotransmitters in the nociceptive pathway, and changes in central and peripheral nervous system plasticity, contributing to sensitization within the nociceptive system, consistent with the concept of nociplastic pain [4,7,11].
While perceived pain intensity (PI) is commonly assessed using the numeric rating scale (NRS) [12], pressure pain thresholds (PPTs) provide a valuable tool for measuring mechanical pain sensitivity (MPS) in chronic pain conditions, such as FM [13]. Studies have shown that patients with FM exhibit significantly lower PPTs—indicative of mechanical hyperalgesia—compared to healthy controls [14,15]. This finding may be associated with increased pain sensitization [13,16], psychological alterations [17], and may also have a predictive relationship with PI [18].
Current clinical guidelines recommend a combination of pharmacological and non-pharmacological interventions, tailored to individual characteristics, to reduce symptom severity, improve quality of life, and help patients adapt to and coexist with a challenging and persistent condition [19,20]. Physical exercise is widely recommended as a first-line non-pharmacological therapeutic option for patients with FM, whether used alone or in conjunction with other interventions [20]. However, the literature indicates that exercise may initially worsen symptoms during the early stages of rehabilitation, potentially impacting adherence to physiotherapy protocols [20,21]. This issue is particularly pertinent given the complex interplay of factors, including a diminished hypoalgesic response to exercise [22], pain-avoidance behavior [23], and additional cognitive, motivational, and emotional challenges that FM patients must overcome to remain engaged in their rehabilitation [24].
Virtual reality (VR) has emerged as a promising therapeutic tool, offering various modalities, including non-immersive, semi-immersive, and fully immersive systems (iVR) [25,26,27]. Among these, iVR systems provide a more comprehensive multisensory experience by integrating visual, tactile, vestibular, and auditory stimuli, which enhances the sense of embodiment compared to less immersive systems [28]. Despite its potential, notable gaps remain in understanding how iVR influences pain management, particularly concerning the underlying pain mechanisms involved and its effectiveness relative to baseline prognostic risk [27]. Addressing these gaps is crucial for optimizing the therapeutic applications of iVR, especially in conditions, like FM [25], where effective pain management remains a significant challenge [20].
Immersive virtual reality-based exercise (iVRE) represents a novel therapeutic approach for chronic pain management, combining the well-established clinical benefits of physical exercise—such as pain reduction and functional improvement [29]—with the psychological advantages of immersive VR, including mood enhancement and distraction from pain [26,27]. While emerging evidence supports the clinical, cognitive, and motivational benefits of VR in FM, particularly when used as distraction therapy even in the absence of physical exercise [25,27,30], research specifically examining iVRE is extremely limited. To date, only one study has reported promising results regarding iVRE’s ability to reduce PI [31]. However, no previous research has explored its effects on variables related to endogenous analgesic mechanisms, such as exercise-induced hypoalgesia or MPS. This significant gap in the literature underscores the need for exploratory studies to better understand the potential mechanisms and therapeutic relevance of iVRE in FM treatment.
Considering the limited evidence on the effects of iVRE in FM and the need to understand its potential for managing chronic pain in this population, this exploratory study aims to assess the initial clinical impact of a 6-week iVRE program on PI and MPS. Additionally, it investigates the acute effects of a single iVRE session on PPTs as a marker of the exercise-induced hypoalgesia mechanism. Prognostic risk stratification is applied to analyze PI based on patients’ baseline risk of poor outcomes (RPO), and basic feasibility parameters are reported. These exploratory findings will not only address potential methodological issues but also lay the groundwork for future larger-scale studies to elucidate the mechanisms underlying the therapeutic benefits of iVRE on pain in FM.
2. Materials and Methods
2.1. Design
This exploratory study consisted of a single-arm, pre-post-test investigation, chosen due to the novelty of the intervention and the absence of prior studies evaluating the impact of iVRE on endogenous analgesic mechanisms, such as exercise-induced hypoalgesia and MPS, in FM. As the protocol, its dosage, and the outcome measures have not been previously tested in this context, a pragmatic, exploratory approach was deemed appropriate to assess feasibility and obtain preliminary insights into its potential effects. Recruitment was conducted by non-probability sampling according to defined participation criteria. All study participants signed an informed consent form. Afterwards, they completed a survey of sociodemographic variables, and the experimental protocols were coordinated. Due to the design of the study and the immersive nature of the intervention, neither the patients nor the evaluators could be blinded. However, the researchers who conducted the assessments and administered the intervention protocol were different and specifically trained for this purpose. Statistical analysis was performed by an independent researcher not involved in the evaluation or application of the intervention protocol.
2.2. Subjects
The study was conducted at the Physiotherapy Center of San Sebastian University in Concepción, Chile. A total of 11 patients of both sexes (male = 1 and female = 10) diagnosed with fibromyalgia (weight 76.06 ± 11.93 kg, height 160.27 ± 7.40 cm) participated in the study. They were recruited through social media posts and in cooperation with the Fibromyalgia Association of the same city. The inclusion criteria were: (i) age ≥ 18 years; (ii) having a medical diagnosis of FM according to the 2016 American College of Rheumatology criteria [2]; and (iii) not participating in another physical therapy program at the same time. Exclusion criteria were: (i) being pregnant or breastfeeding, having cancer pain or an uncontrolled metabolic disorder; (ii) presenting any physical or mental health condition that interfered with communication or the ability to participate in the intervention; and (iii) a history of conditions potentially contraindicated for VR exposure, including photosensitive epilepsy, severe vestibular disorders, known susceptibility to cybersickness, or other neurological conditions that could interfere with immersive VR use. The sociodemographic characteristics of the study population are shown in Table 1.

Table 1.
Sociodemographic characteristics of the sample.
2.3. Instruments for Outcomes Measurement and Stratification
2.3.1. Pain Intensity
The patient’s PI was assessed using the NRS [32]. Patients rated their average pain intensity over the past seven days on a scale from 0 (no pain) to 10 (worst possible pain). The NRS was selected because it is a simple, valid, and reliable tool widely used in clinical and research settings to evaluate pain in individuals with chronic conditions, including FM, as it is sensitive to change and facilitates the monitoring of pain trajectories in intervention studies [33]. PI was measured at three time points: before the intervention, at the start, and at the end of the program, following standardized administration guidelines [32].
2.3.2. Pressure Pain Threshold
PPT assessment provides an objective measure of MPS and quantifies generalized mechanical hyperalgesia in individuals with FM [34,35]. PPT is also a valid proxy to evaluate the presence of exercise-induced hypoalgesia, often impaired in FM patients [22]. To this end, PPTs were measured at three anatomical sites based on previous protocols: (i) upper trapezius—assessed at the center of the muscle belly, specifically at the midpoint between the C7 vertebra and the acromion [36]; (ii) the lumbar spine—assessed at the erector spinae muscle, 2 cm lateral to the interspinous space between the L4 and L5 vertebrae [37]; and (iii) knee—assessed at the joint line, 2 to 3 cm medial to the inferior pole of the patella [38]. PPTs were measured in kg/cm2 using a Wagner FPX 25 digital algometer (Wagner Instruments, Greenwich, CT, USA), which has a 1 cm diameter rubber probe. An experienced evaluator performed all measurements on the dominant side. PPT measurements were performed following previously validated protocols [37,38]: before each measurement, the evaluator gave the same instructions to the participants: “I am going to apply pressure progressively on your neck/back/knee using this instrument. I want you to let me know when the sensation changes from comfortable pressure to mildly unpleasant pain”. PPTs were measured by placing the algometer perpendicular to the skin surface of the area to be assessed. Pressure was increased at a continuous rate of 0.5 kg/cm2/s. The evaluator stopped the measurement when the subject notified. Each site was measured three times, with a 3-min rest between trials. The mean of the three measurements at each site was used for data analysis. PPTs were assessed before the intervention, immediately after the first iVRE session (to quantify the exercise-induced hypoalgesia mechanism) [22], and at the end of the protocol.
2.3.3. Risk of Poor Outcomes Associated with Pain
To stratify RPO, the Keele STarT MSK Tool (KSMT) was used [39,40]. The KSMT consists of ten items assessing physical and psychological risks factors that may worsen prognosis in patients with musculoskeletal pain. The KSMT score ranges from 0 to 12 points and the following categories were considered: “low risk” (0 to 4 points), “medium risk” (5 to 8 points) and “high risk” (9 to 12 points) [41]. The KSMT was administered before and after the intervention, following recommendations from the European Alliance of Rheumatology Associations (EULAR) for general populations and diseases associated with chronic musculoskeletal pain [42], to track changes in risk categorization.
2.3.4. Adverse Events Monitoring
To monitor potential adverse events related to the use of iVRE, the physical therapist supervising the intervention systematically documented any occurrences during each session. Additionally, participants who completed the iVRE program were queried again about adverse events following the final session, while those who discontinued the program were contacted via telephone to gather similar information. Specifically, the study assessed the presence of the most commonly reported symptoms of cybersickness [43], including general discomfort, dizziness, vertigo, nausea, cervical pain and headache. These symptoms were recorded using a dichotomous format (“present” or “absent”) for each session.
2.4. Intervention Program
2.4.1. Equipment
The iVRE intervention was delivered using the Oculus Quest 2™ VR device (Facebook Technologies, LLC. 1 Hacker Way, Menlo Park, CA 94025, USA) in combination with the FitXR application (version 1.10.0; FITAR LIMITED, London, UK). The Oculus Quest 2™ is a standalone VR device that provides high-quality experience without the need to be connected to a PC. It includes a head-mounted headset and two hand controllers, enabling real-time interaction with virtual environments. The Oculus Quest 2™ was selected for its portability, intuitive interface, and high-fidelity immersive capabilities, making it particularly suitable for therapeutic use in individuals with chronic pain conditions such as FM [44] (Figure 1A). Additionally, its wireless design minimizes movement restrictions, enabling a smoother exercise experience. The equipment was not modified for this study.

Figure 1.
(A) Fibromyalgia patient performing protocol with head-mounted Oculus Quest 2™ and handheld controllers. (B) Real-time image of the virtual environment experienced by the patient during the FitXR game.
The FitXR app is an immersive active video game selected for its intuitive interface, low user complexity, and its ability to provide a structured, consistent, and motivating exercise regimen, making it suitable for direct application in rehabilitation settings where patients are receiving treatment [32]. This boxing module was chosen for its goal-oriented, score-based activities and avatar-guided interactions, while its customizable, fixed virtual environments help mitigate potential adverse effects of iVR [45]. The game involves hitting incoming spheres when a two-glove symbol appears in front of the player, while simultaneously performing defensive and avoidance actions to navigate obstacles (Figure 1B). To achieve this, patients must perform upper limb movements (jabs, crosses, hooks, and uppercuts, depending on the color of the sphere and the avatar’s cues), torso bends, squats, lunges, and cover-ups, with minimal rotational movements to help prevent motion sickness [46]. Scoring was based on the number and accuracy of punches. At the time of the study design, the FitXR app had never been tested in patients with FM.
2.4.2. Patient Instructions
Before starting the intervention protocol, each patient underwent an induction session to become familiar with the VR equipment and the FitXR app. During this session, detailed and standardized instructions were provided on how to use the Oculus Quest 2™ headset and hand controllers. Patients learned to follow the game instructions, interact with the avatar, and perform the required movements, such as punching, squatting, and avoiding obstacles. Additionally, they were informed about possible symptoms that could occur during gameplay, such as dizziness or muscle fatigue, allowing them to recognize and respond to them appropriately. Safety measures were emphasized, including the option to stop the activity at any time if experiencing pain, fatigue, dizziness, or other symptoms. Patients were encouraged to ask questions and were instructed to inform the physical therapist immediately if any unexpected events or symptoms arose during the session. Patients could adjust the difficulty level of the session by selecting between beginner or intermediate levels based on their perceived effort after the familiarization session.
2.4.3. Intervention Protocol
Based on previously well-tolerated intervention protocols using VR in different health conditions, it was decided to conduct 12 intervention sessions, with two sessions per week on non-consecutive days over a period of 6 weeks [26,27]. A physiotherapist, specifically trained to administer the protocol, monitored patients throughout the iVRE intervention. During the sessions, the patient interacted with an avatar, and the game was simultaneously displayed on a laptop screen, allowing the physiotherapist to observe the patient in real-time. The physiotherapist provided guidance and ensured safety based only on the patient’s feedback but did not interact with the patient regarding the execution of the exercises, an action performed exclusively by the avatar and the iVRE interface. This physiotherapist only administered the protocol and did not participate in the evaluations.
At the beginning of each session, patients performed a standardized 10-min warm-up on a cycle ergometer at a moderate intensity, rated 4 to 5 on the CR-10 subjective perception of exertion scale [47]. Subsequently, the iVRE intervention was started, lasting 15 min, in line with recommendations to prevent adverse effects in people with chronic pain and FM, and because previous studies using various immersive VR devices have reported high user satisfaction with protocols lasting between 10 and 20 min [27,31,44,48]. The iVRE intervention was designed to provide a gradual and consistent increase in physical activity, considering each patient’s tolerance to avoid adverse effects. The iVRE session was subdivided into three phases:
- Phase 1 (3 min): General joint mobility exercises for the spine and limbs, guided by the avatar in the FitXR app. This phase aimed to prepare the body for subsequent physical demands, promoting flexibility and blood circulation.
- Phase 2 (10 min): Interactive game, during which participants executed combinations of punches on virtual targets while also performing squats, lunges, and defensive actions. The patient followed on-screen prompts to determine which punches to execute. During this activity, the patient could skip or modify specific trunk or limb movements according to their preference if fatigue, discomfort, or pain occurred during execution, following the principle of preferred intensity [49].
- Phase 3 (2 min): Cool-down, which included slow breathing exercises and general mobility movements for the spine and limbs, again guided by the avatar. This phase aimed to facilitate recovery and reduce the risk of muscle pain or injury after the activity.
At the end of each session, patients could see their score and were encouraged to improve their score in the next session to improve adherence and motivation. Finally, the protocol included a 5- to 10-min cool-down period, which comprised slow breathing and free stretching as tolerated.
2.5. Data Analysis
Participants who did not complete the protocol as specified (12 sessions over 6 weeks, twice a week on non-consecutive days) were excluded from the final analysis to ensure that the outcomes accurately reflected full exposure to the intervention, as originally intended. Statistical analyses were performed using JASP software (version 0.18.3.0). Descriptive statistics, demographic variables, and outcomes were summarized using counts, percentages, means, and standard deviations (SD). Participants were stratified into high-, medium-, or low-risk categories based on their baseline scores on the KSMT. Responders to the iVRE treatment were identified using a 30% reduction from baseline NRS as the cut-off criterion [33]. Given the small sample size, mean differences (MD) and 95% confidence intervals for mean differences (CI-MD) were reported as primary measures. The assumption of normality for all variables was tested using the Shapiro–Wilk test before conducting pre-test (baseline) and post-test comparisons (15 min after the first session for PPTs and after six weeks of intervention for NRS and PPTs). As complementary analyses, paired Student’s t-tests were conducted to compare pre- and post-intervention NRS scores, while a repeated measures analysis of variance (ANOVA) was performed for PPTs. The significance level was set at p < 0.05. The effect size (ES) of the intervention, calculated using Cohen’s d, was categorized as small (0.20–0.49), medium (0.50–0.79), or large (>0.80) [50]. Finally, to assess the feasibility of this exploratory study, the following indicators were considered: recruitment rate, enrollment rate, and retention rate [51].
3. Results
3.1. General and Feasibility Results
A total of 26 patients were contacted and screened for eligibility (enrollment rate: 1.44 patients per week). Of these, 20 participants presented for evaluation, met the eligibility criteria, and entered the study (recruitment rate: 76.92%). Finally, 11 participants who entered the study completed all 12 sessions of the experimental protocol at the scheduled frequency (adherence rate: 100%) and were included in the final analysis (retention rate: 55%). Ten participants were female (90.9%), and the mean age of the sample was 40.55 ± 11.23 years (range 21 to 61 years). Except for one participant, all had a body mass index classified as overweight or higher.
The intervention plan was adequately executed and well tolerated by the patients, so no adjustments to the protocol were necessary. All patients who did not complete the protocol (n = 9) did so due to personal reasons unrelated to the study’s assessments or intervention protocols. These reasons included scheduling conflicts with patients’ work commitments (as the iVRE sessions were scheduled in the mornings, n = 1), accessibility issues due to weather conditions (with half of the study conducted during winter, n = 3), acute illnesses unrelated to FM (primarily seasonal respiratory conditions, n = 3), unforeseen family obligations (n = 1), and delays that could not be rescheduled (n = 1). No dropouts occurred due to adverse events related to the iVRE program. Additionally, none of the adverse events investigated were reported during the interventions or after completing the 12 sessions of iVRE.
3.2. Post-iVRE Results for Pain Intensity and Pressure Pain Thresholds
After six weeks of intervention, PI (NRS) showed a reduction (MD = −2.36; CI-MD [−4.15, −0.58]; Student’s t-test p < 0.05; ES = 0.89, large). The MD exceeded the minimum clinically important difference (MCID) described for the NRS (2 points) [33], suggesting clinical relevance. For PPTs after six weeks of intervention, the results were as follows: upper trapezius (MD = −0.38, CI-MD [−0.92, 0.17]), lumbar spine (MD = −0.04, CI-MD [−0.62, 0.54]), and knee (MD = −0.13, CI-MD [−0.78, 0.52]). Repeated measures ANOVA was conducted to evaluate changes in PPTs at the upper trapezius, lumbar spine, and knee at three time points: before the intervention, after the first iVRE session (to assess the exercise-induced hypoalgesia mechanism), and at the end of the protocol. The analysis revealed no significant differences in PPTs for any of the assessed regions at any of the three time points (p > 0.05). In summary, the iVRE intervention resulted in reductions in PI, surpassing the clinical importance threshold, while no changes were observed in PPTs in the assessed areas. Detailed results are presented in Table 2.

Table 2.
PI and PPTs pre- and post-iVRE intervention.
3.3. Stratification Based on Risk and Pain Intensity Behavior
Before the intervention, 27.27% (n = 3) of the patients were classified as medium-risk and 72.73% (n = 8) as high-risk according to the KSMT scale. No participants were classified in the low-risk category. After the intervention, 81.81% (n = 9) of the participants moved to a lower-risk category compared to baseline, while those who did not change their baseline stratification were all in the high-risk category (Figure 2).
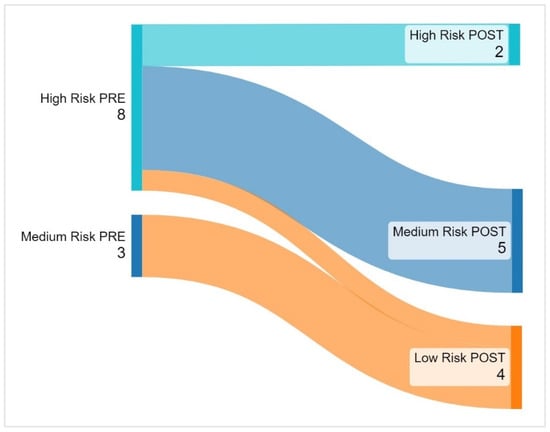
Figure 2.
Changes in risk groups post-iVRE intervention. Before the intervention, 8 participants (72.73%) were classified as high risk, and 3 (27.27%) as medium risk. After the intervention, 2 remained high risk, while 9 (81.81%) improved. 5 moved from high to medium risk, 1 from high to low risk, and all 3 initially medium risk shifted to the lowest category.
Using a 30% reduction threshold in the NRS [33], 63.63% (n = 7) of the patients were classified as responders to iVRE. Within the high-risk group, 50% (n = 4) demonstrated a positive response, while all medium-risk patients (n = 3) exhibited a positive response. Although PI was higher in high-risk individuals both before and after the intervention, the absolute and proportional reduction in NRS was greater in medium-risk patients. Details on PI changes based on risk stratification by KSMT are presented in Table 3.

Table 3.
NRS score changes post-iVRE intervention by risk stratification levels.
4. Discussion
This single-arm exploratory study investigated the immediate effects of a 6-week iVRE program on PI and PPTs, as well as the influence of a single bout of iVRE on PPTs in a sample of 11 patients with FM. Key findings include: (i) Improvements in PI by the end of the program, which were clinically and statistically significant in this sample; (ii) no observable changes in PPTs following either a single session or the 6-week intervention, implying that, in this sample, there would be no changes in MPS, nor would the exercise-induced hypoalgesia mechanism be activated; (iii) 81.81% of participants transitioned to a lower-risk category on the KSMT after the intervention; (iv) 63.63% of participants were classified as good responders to iVRE in terms of PI reduction; (v) medium-risk patients demonstrated greater absolute and proportional reductions in the NRS compared to high-risk patients; (vi) feasibility parameters showed an enrollment rate of 1.44 patients per week, a recruitment rate of 76.92%, and a retention rate of 55%; (vii) the proportion of women in the sample was 90.9%, which aligns with the epidemiological profile of FM, which predominantly affects females; and (viii) the intervention was safe, with no adverse events reported and no protocol modifications required.
In the present study, we used a head-mounted device that provides a high level of immersion, allowing exercises to be performed in a sensory-enriched virtual environment, similar to that used in a previous study conducted with patients with FM [31]. While these characteristics support the application of iVRE, it is important to consider potential adverse effects, such as nausea, motion sickness, headaches, and discomfort associated with head-mounted devices [29]. In our study, no adverse effects were reported during the application or after the iVRE protocol, which could be attributed to the duration of the intervention, the characteristics of the FitXR game, and the small sample size. Recent literature reviews [26,27] show that only two studies have previously used immersive VR-based interventions in individuals with FM, one of which combined it with cognitive–behavioral therapy (CBT), reporting very high satisfaction in 84% of cases and 96% of patients experiencing minimal or no adverse effects [44]. The other study, like our exploratory study, applied iVRE in FM but did not explicitly report the presence or absence of adverse effects [31]. Additionally, two previous studies on individuals with FM applied non-immersive VR for durations similar to those in our study: one associated with physical exercise [52] and the other with CBT [53], both reporting very high satisfaction and absence of adverse effects. Considering these results and those from the present preliminary study, there are indications that short-duration iVRE interventions may be well tolerated by patients with FM in the short term, which is consistent with previous recommendations [48]. Future controlled studies with larger samples should confirm these preliminary findings.
Although interventions involving iVRE in patients with chronic musculoskeletal conditions remain incipient [29], the incorporation of virtual reality-based physical exercise in rehabilitation processes is gaining attention due to its potential benefits on several clinically relevant variables, as well as its advantages over conventional physical therapy—such as increased enjoyment, motivation, and enhanced sensory feedback [54]. In the context of chronic pain management, the proposed mechanisms underlying the effects of VR include distraction, attentional engagement, and multisensory stimulation [30,55]. These mechanisms are thought to immerse the patient in a virtual environment, potentially leading to improvements in PI, PPTs, psychological factors (kinesiophobia, catastrophizing), and even temporal summation over the medium term [29]. Functional magnetic resonance imaging studies suggest that VR stimulates the insular and sensory cortices in ways comparable to opioids and may modulate pain perception through pathways that reduce conscious awareness of pain, potentially altering the behavior of the pain modulation system to decrease pain sensitivity [56,57]. However, given the exploratory nature of our study, caution is warranted in drawing definitive conclusions. Our findings reflect only the responses observed in a small sample and should not be generalized to the broader population. The response to iVRE appears to be complex and variable, as demonstrated by the contrasting trends observed between PI and PPTs following both a single session and the complete iVRE protocol. These findings underscore the complex nature of pain modulation in FM and highlight the need for further high-quality research to clarify the underlying mechanisms.
In our study, the iVRE intervention resulted in an observed improvement in the sample’s perceived PI. This finding aligns with a recent review focusing on the effects of VR-based exercise therapy in patients with chronic pain, which concluded that various VR modalities can reduce PI in this population [29]. However, that review did not specifically include iVRE interventions for patients with FM. A previous review focusing exclusively on FM patients [25], identified only one study that utilized an iVRE protocol [31]. In that study, Gulsen et al. reported a significant decrease in PI, measured using a visual analogue scale, and attributed the results to reduced fear of movement and increased adherence due to the high level of engagement associated with VR. However, Gulsen et al. did not explore the underlying analgesic mechanisms driving their patients’ pain responses, nor did they stratify participants according to baseline prognostic risk. Our study aimed to explore changes in PI, MPS, and exercise-induced hypoalgesia mechanisms following a 6-week iVRE program and examined how PI varied according to baseline risk in a sample of FM patients—an aspect not previously investigated. It is important to note that our findings are based on a small, uncontrolled sample and should not be extrapolated to the broader population. Nonetheless, these results provide a foundation for future controlled studies with larger samples to replicate this protocol and observe its effects on the studied variables. Such studies could enable a more in-depth analysis with stronger evidence to address current gaps in the literature, including the integration of innovative AI technologies for pain assessment [58].
Several studies have described the involvement of psychological variables as factors that can influence the perception of PI in individuals with FM [59,60]. In this context, therapeutic interventions using various modalities of VR have shown positive effects on pain-related outcomes in people with chronic pain and FM—particularly when integrated with other treatments that address the psychological aspects that influence PI [44,61]. In such cases, the improvements observed may result from the direct influence of VR on cognitive, emotional, and motivational factors [27,62,63]. However, its effect on the underlying analgesic mechanisms has not yet been clearly described. This study has explored the possibility of advancing in this line of research, presenting preliminary results that allow for the planning of future studies that, through appropriate designs and sample sizes, aim to address this gap.
All PPT values obtained by algometry in the sample evaluated in our study were lower than the standard values reported in previous literature [34,64,65]. This is consistent with evidence indicating that individuals with FM have lower PPT values compared to both healthy subjects [14] and subjects with other chronic pain conditions [35,66]. PPT measurement is a highly reliable mechanical sensory quantitative test, validated for assessing MPS of the nociceptive pathway [34,66]. Blumenstiel et al. (2011) and Goubert et al. (2017) found that the generalized mechanical hyperalgesia observed in subjects with FM, as measured by PPTs at different anatomical sites, was not present in individuals with varying degrees of chronic low back pain, in whom hyperalgesia is localized to the lumbar spine [35,66]. Our exploratory findings provide a foundation for future high-quality randomized controlled trials with larger sample sizes to address the gap in the literature and explore whether the response to iVRE is consistent across different chronic pain populations.
The results observed in our sample preliminarily imply that there were no observable changes in the measured PPTs (95% CI include the null value), both after the first session and after six weeks of intervention. However, it is important to consider that evidence suggests the pain mechanisms involved in FM may not always align with changes measured through PPTs [67]. This initial finding suggests that our protocol may not have effectively activated the descending endogenous pain inhibitory mechanisms in the participants, both in terms of acute hypoalgesia induced by exercise (measured after the first session) and following the full protocol [34,35]. These results could be influenced by factors such as the inherent limitations of the small sample size and single-arm design, the method used to measure the analgesic mechanism (more complex and comprehensive approaches exist) [68], the structure of the protocol, and the duration of the intervention [69]. In this regard, it seems that the frequency and the consistency of physical activity over time, rather than the specific type of exercise, primarily influence inflammatory modulation, cytokine regulation, and neuroimmune signaling, contributing to pain reduction in conditions, such as FM [69]. However, although it should be interpreted with extreme caution, our findings align with previous reports indicating that patients with FM may exhibit impairments in the exercise-induced hypoalgesia mechanism [70]. Considering this reasoning and the findings in the literature, it could be initially inferred that the iVRE program applied in this exploratory study with FM patients may have positively influenced perceived PI [25,26,71], which involves sensory, cognitive, and emotional processes but did not necessarily activate endogenous analgesic mechanisms efficiently, as measured by PPTs in both post-tests. Nevertheless, despite this reasoning being supported by evidence, it should be considered with extreme caution due to the methodological limitations of a small sample size in a pre-post, non-controlled exploratory study. Future high-quality research, including more extensive protocols and long-term follow-ups, is needed to confirm these preliminary findings and address the gap in the literature.
Elucidating the therapeutic prognosis for FM patients is challenging due to the condition’s unknown etiology, its profound impact on quality of life, and the variability in therapeutic options, which, while promising, exhibit differing levels of efficacy [72]. FM patients face a high RPO, influenced by the intricate interplay of clinical characteristics and prognostic factors, including physical (e.g., pain, disability), psychological (e.g., depression, anxiety, catastrophizing), and social factors (e.g., employment, economic strain, isolation) [73,74,75]. FM itself is associated with reduced satisfaction, increased pain, poorer functional outcomes, higher opioid use, and an increase in post-orthopedic surgery complications [74,76]. Our preliminary study is the first to explore the use of the KSMT to stratify FM patients based on baseline risk, as EULAR promotes its generic use [42]. Interestingly, only 18% of participants in our sample did not show a positive change in risk scores after the iVRE protocol, all of whom had a high baseline RPO. Future well-designed studies should validate KSMT as a reliable predictive tool for treatment response in FM and explore its potential in guiding personalized therapeutic approaches. Additionally, our study opens the door for future research to investigate whether iVRE can positively influence other key prognostic indicators, further expanding treatment options for FM.
While the primary focus of this study was to explore the preliminary clinical outcomes of the intervention protocol, feasibility indicators were also reported. The enrollment rate was 1.44 patients per week, the recruitment rate was 76.92%, and the retention rate was 55%. The relatively low retention rate was attributed to unavoidable non-clinical circumstances, including personal reasons, scheduling conflicts, and difficulties in rescheduling, all consistent with the study’s protocol requirements. Importantly, no adverse events were reported throughout the study. Although the intervention was well tolerated and executed as planned, suggesting no necessary protocol adjustments, future studies could enhance retention by offering more flexible scheduling options (such as evening or weekend sessions), implementing contingency plans for rescheduling missed sessions, and considering similar apps with audible stimuli (e.g., music) or remote delivery options to address motivational, weather-related, or accessibility barriers. These adaptations would help minimize non-compliance and improve overall participant retention.
The limitations of the study include: (i) the absence of a control group, which introduces potential confounding bias in the results; (ii) the sample size was not probabilistically estimated, implying a potential limitation in statistical power; (iii) due to the small sample size, exploratory nature, and single-arm design, statistical inferences and external validity cannot be assured, limiting the generalizability of the results. However, these design choices were based on the novelty of the intervention and the lack of prior studies evaluating iVRE protocols targeting endogenous analgesic mechanisms or prognostic stratification in FM. Additionally, the low retention rate of 55% may influence the internal validity of the study; (iv) despite no reported adverse health events, the low retention rate highlights the need for more flexible scheduling to reduce dropout; (v) the study lacked long-term follow-up; (vi) although a survey format was used to assess the most frequently reported adverse events in the literature, the tool employed has not been validated; (vii) psychosocial factors such as kinesiophobia, catastrophizing, and motivation were not considered in this exploratory study, which may have influenced results and participant engagement; (viii) quantitative sensory testing was not employed to further assess pain mechanisms; and (ix) there were selection biases that may affect the generalizability of the findings. Adherence bias was present, as only participants who completed the intervention were analyzed. Additionally, recruitment from a patient association may limit the representativeness of the sample, and volunteer bias could introduce baseline differences or more favorable outcome expectations. Nevertheless, the main strengths of the study are: (i) its exclusive focus on FM patients, a condition requiring targeted clinical attention; (ii) the innovative intervention proposal based on iVRE, which has been minimally explored previously in FM patients to date; (iii) the measurement of PPTs in studies with iVRE interventions, as an indicator of MPS, which is linked to the mechanisms involved in analgesic response; and (iv) the stratification of patients based on the RPO using the KSMT.
Considering the study’s limitations, strengths, and preliminary results, we believe it is crucial to advance knowledge through future randomized controlled trials employing blinding strategies to evaluate the effectiveness of iVRE in FM patients. These studies should ensure appropriate bias control, adequate statistical power, and incorporate more comprehensive tools to assess pain inhibitory mechanisms as well as psychological factors influencing pain experience in FM patients undergoing iVRE. These limitations should be considered when interpreting the results. Future research should be conducted on a larger scale, with representative samples, broader recruitment strategies, long-term follow-up assessments, and intention-to-treat analyses to improve external validity and enhance the generalizability of the findings.
5. Conclusions
As an exploratory study, our findings suggest that a 6-week iVRE program may have a potential effect in reducing PI in our sample of FM patients, with this effect being associated with baseline RPO. However, it is important to note that this iVRE program did not lead to measurable changes in PPTs across various body sites, suggesting the persistence of MPS and potential limitations in activating endogenous pain inhibitory mechanisms in our sample. Moreover, a single bout of iVRE did not appear to produce notable changes in PPTs, which, according to existing evidence, may indicate that our sample of FM patients is unlikely to exhibit a substantial exercise-induced hypoalgesic response. These findings highlight that, while iVRE effectively reduced perceived pain in our sample of FM patients, it did not induce changes in their PPTs. Given the exploratory nature of these results, they should be interpreted with caution due to the inherent methodological limitations of a small sample size and an uncontrolled design. Although our study provides initial insights, it underscores the need for further validation through randomized controlled trials with larger samples to comprehensively evaluate the effects of iVRE on pain-related outcomes and to explore other factors that may influence these responses in this population. Such future research is crucial to confirm our preliminary observations and determine the potential of iVRE as a therapeutic approach for FM.
Author Contributions
Conceptualization, C.C.-P. and G.A.-Á.; methodology, C.C.-P. and F.G.-R.; software, C.C.-P., G.A.-Á. and F.G.-R.; validation, C.C.-P., G.A.-Á., D.U.-D., L.R.-V., O.A.-R., F.G.-R. and J.G.P.-G.; formal analysis, C.C.-P., G.A.-Á., F.G.-R. and J.G.P.-G.; investigation, C.C.-P. and G.A.-Á.; resources, C.C.-P. and G.A.-Á.; data curation, C.C.-P. and G.A.-Á.; writing—original draft preparation, C.C.-P., G.A.-Á., D.U.-D., L.R.-V., O.A.-R., F.G.-R. and J.G.P.-G.; writing—review and editing, C.C.-P., G.A.-Á., D.U.-D., L.R.-V., O.A.-R., F.G.-R. and J.G.P.-G.; visualization, C.C.-P., G.A.-Á., D.U.-D., L.R.-V., O.A.-R. and F.G.-R.; supervision, C.C.-P., G.A.-Á., and J.G.P.-G.; project administration, C.C.-P. and G.A.-Á.; funding acquisition, C.C.-P. and G.A.-Á. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Collaborative Project (ID3003) of the Vice-Rector’s Office for University Outreach (VCM) at the Universidad San Sebastián, Concepción, Chile.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, approved by the Ethics Committee of Universidad San Sebastián (protocol code No. 151-23), and registered on the ReBEC platform (ID: RBR-9t9j3hs).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The research team extends their gratitude to the Fibromyalgia Association of Concepción for their collaboration in conducting the study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| FM | Fibromyalgia |
| PI | Pain Intensity |
| NRS | Numerical Rating Scale |
| PPTs | Pressure Pain Thresholds |
| MPS | Mechanical Pain Sensitivity |
| VR | Virtual Reality |
| iVR | Immersive Virtual Reality |
| iVRE | Immersive Virtual Reality-Based Exercise |
| RPO | Risk of Poor Outcomes |
| KSMT | Keele STarT MSK Tool |
| EULAR | European Alliance of Rheumatology Associations |
| CBT | Cognitive–Behavioral Therapy |
References
- Giorgi, V.; Bazzichi, L.; Batticciotto, A.; Pellegrino, G.; Di Franco, M.; Sirotti, S.; Atzeni, F.; Alciati, A.; Salaffi, F.; Sarzi Puttini, P. Fibromyalgia: One Year in Review 2023. Clin. Exp. Rheumatol. 2023, 41, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 Fibromyalgia Diagnostic Criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Elgueta-Aguilera, N.; Guede-Rojas, F.; Mendoza, C.; Carvajal-Parodi, C.; Jerez-Mayorga, D.; Elgueta-Aguilera, N.; Guede-Rojas, F.; Mendoza, C.; Carvajal-Parodi, C.; Jerez-Mayorga, D. Self-Perceived Cognitive Function and Neuropsychological Performance in Women with Fibromyalgia. Rev. Médica Chile 2022, 150, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, B.F.; Akyol, A. Fibromyalgia Syndrome: Epidemiology, Diagnosis and Treatment. Reumatologia 2022, 60, 413–421. [Google Scholar] [CrossRef]
- Bawazir, Y. Prevalence of Fibromyalgia Syndrome in Saudi Arabia: A Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2023, 24, 692. [Google Scholar] [CrossRef]
- Wolfe, F.; Walitt, B.; Perrot, S.; Rasker, J.J.; Häuser, W. Fibromyalgia Diagnosis and Biased Assessment: Sex, Prevalence and Bias. PLoS ONE 2018, 13, e0203755. [Google Scholar] [CrossRef]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Weinstein, J.; Rosero, S.; Seabury, J.; Varma, A.; Engebrecht, C.; Khosa, S.; Heatwole, J.; Dilek, N.; Kaat, A.; Matallana, L.K.; et al. Patient-Reported Impact of Symptoms in Fibromyalgia (PRISM-FM). J. Rheumatol. 2024, 51, 628–636. [Google Scholar] [CrossRef]
- Fernandez-Feijoo, F.; Samartin-Veiga, N.; Carrillo-de-la-Peña, M.T. Quality of Life in Patients with Fibromyalgia: Contributions of Disease Symptoms, Lifestyle and Multi-Medication. Front. Psychol. 2022, 13, 924405. [Google Scholar] [CrossRef]
- Bucourt, E.; Martaillé, V.; Goupille, P.; Joncker-Vannier, I.; Huttenberger, B.; Réveillère, C.; Mulleman, D.; Courtois, A.R. A Comparative Study of Fibromyalgia, Rheumatoid Arthritis, Spondyloarthritis, and Sjögren’s Syndrome; Impact of the Disease on Quality of Life, Psychological Adjustment, and Use of Coping Strategies. Pain Med. Malden Mass 2021, 22, 372–381. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Guo, X.; Zhang, J.; Jiang, R. Pain Characteristics of Patients with Fibromyalgia: A Comparison Between Gender and Different Emotional States. Pain Physician 2024, 27, E109–E118. [Google Scholar]
- Amiri, M.; Alavinia, M.; Singh, M.; Kumbhare, D. Pressure Pain Threshold in Patients with Chronic Pain: A Systematic Review and Meta-Analysis. Am. J. Phys. Med. Rehabil. 2021, 100, 656–674. [Google Scholar] [CrossRef] [PubMed]
- Maquet, D.; Croisier, J.-L.; Demoulin, C.; Crielaard, J.-M. Pressure Pain Thresholds of Tender Point Sites in Patients with Fibromyalgia and in Healthy Controls. Eur. J. Pain 2004, 8, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Petzke, F.; Harris, R.E.; Williams, D.A.; Clauw, D.J.; Gracely, R.H. Differences in Unpleasantness Induced by Experimental Pressure Pain between Patients with Fibromyalgia and Healthy Controls. Eur. J. Pain 2005, 9, 325–335. [Google Scholar] [CrossRef]
- Arroyo-Fernandez, R.; Bravo-Esteban, E.; Domenech-Garcia, V.; Ferri-Morales, A. Pressure-Induced Referred Pain as a Biomarker of Pain Sensitivity in Fibromyalgia. Pain Physician 2020, 23, E353–E362. [Google Scholar]
- Weber, T.; Tatzl, E.; Kashofer, K.; Holter, M.; Trajanoski, S.; Berghold, A.; Heinemann, A.; Holzer, P.; Herbert, M.K. Fibromyalgia-Associated Hyperalgesia Is Related to Psychopathological Alterations but Not to Gut Microbiome Changes. PLoS ONE 2022, 17, e0274026. [Google Scholar] [CrossRef]
- Staud, R.; Weyl, E.E.; Price, D.D.; Robinson, M.E. Mechanical and Heat Hyperalgesia Highly Predict Clinical Pain Intensity in Patients with Chronic Musculoskeletal Pain Syndromes. J. Pain 2012, 13, 725–735. [Google Scholar] [CrossRef]
- El Miedany, Y.; Gadallah, N.; Mohasseb, D.; Gaballah, N.M.; El Zohiery, A.K.; Hassan, M.; El Gaafary, M.; Hassan, W.; Mortada, M.; Eissa, M.; et al. Consensus Evidence-Based Clinical Practice Recommendations for the Management of Fibromyalgia. Egypt. Rheumatol. Rehabil. 2022, 49, 30. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR Revised Recommendations for the Management of Fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef]
- Busch, A.J.; Webber, S.C.; Brachaniec, M.; Bidonde, J.; Bello-Haas, V.D.; Danyliw, A.D.; Overend, T.J.; Richards, R.S.; Sawant, A.; Schachter, C.L. Exercise Therapy for Fibromyalgia. Curr. Pain Headache Rep. 2011, 15, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.; Nijs, J.; Kosek, E.; Wideman, T.; Hasenbring, M.I.; Koltyn, K.; Graven-Nielsen, T.; Polli, A. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J. Pain 2019, 20, 1249–1266. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Mira, M.-A.; López-Roig, S.; Martínez-Zaragoza, F.; León, E.; Abad, E.; Lledó, A.; Peñacoba, C. Goal Preferences, Affect, Activity Patterns and Health Outcomes in Women with Fibromyalgia. Front. Psychol. 2019, 10, 1912. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Mira, M.-Á.; López-Roig, S.; Martínez-Zaragoza, F.; Toribio, E.; Nardi-Rodríguez, A.; Peñacoba, C. Motivational Determinants of Objective Physical Activity in Women with Fibromyalgia Who Attended Rehabilitation Settings. J. Clin. Med. 2021, 10, 5547. [Google Scholar] [CrossRef]
- Cortés-Pérez, I.; Zagalaz-Anula, N.; Ibancos-Losada, M.D.R.; Nieto-Escámez, F.A.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Virtual Reality-Based Therapy Reduces the Disabling Impact of Fibromyalgia Syndrome in Women: Systematic Review with Meta-Analysis of Randomized Controlled Trials. J. Pers. Med. 2021, 11, 1167. [Google Scholar] [CrossRef]
- Goudman, L.; Jansen, J.; Billot, M.; Vets, N.; De Smedt, A.; Roulaud, M.; Rigoard, P.; Moens, M. Virtual Reality Applications in Chronic Pain Management: Systematic Review and Meta-Analysis. JMIR Serious Games 2022, 10, e34402. [Google Scholar] [CrossRef]
- Wong, K.P.; Tse, M.M.Y.; Qin, J. Effectiveness of Virtual Reality-Based Interventions for Managing Chronic Pain on Pain Reduction, Anxiety, Depression and Mood: A Systematic Review. Healthcare 2022, 10, 2047. [Google Scholar] [CrossRef]
- Brady, N.; McVeigh, J.G.; McCreesh, K.; Rio, E.; Dekkers, T.; Lewis, J.S. Exploring the Effectiveness of Immersive Virtual Reality Interventions in the Management of Musculoskeletal Pain: A State-of-the-Art Review. Phys. Ther. Rev. 2021, 26, 262–275. [Google Scholar] [CrossRef]
- Bilika, P.; Karampatsou, N.; Stavrakakis, G.; Paliouras, A.; Theodorakis, Y.; Strimpakos, N.; Kapreli, E. Virtual Reality-Based Exercise Therapy for Patients with Chronic Musculoskeletal Pain: A Scoping Review. Healthcare 2023, 11, 2412. [Google Scholar] [CrossRef]
- Slitzky, M.; Yong, R.J.; Lo Bianco, G.; Emerick, T.; Schatman, M.E.; Robinson, C.L. The Future of Pain Medicine: Emerging Technologies, Treatments, and Education. J. Pain Res. 2024, 17, 2833–2836. [Google Scholar] [CrossRef]
- Gulsen, C.; Soke, F.; Eldemir, K.; Apaydin, Y.; Ozkul, C.; Guclu-Gunduz, A.; Akcali, D.T. Effect of Fully Immersive Virtual Reality Treatment Combined with Exercise in Fibromyalgia Patients: A Randomized Controlled Trial. Assist. Technol. 2022, 34, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Birckhead, B.; Khalil, C.; Liu, X.; Conovitz, S.; Rizzo, A.; Danovitch, I.; Bullock, K.; Spiegel, B. Recommendations for Methodology of Virtual Reality Clinical Trials in Health Care by an International Working Group: Iterative Study. JMIR Ment. Health 2019, 6, e11973. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.T.; Young, J.P.; LaMoreaux, L.; Werth, J.L.; Poole, R.M. Clinical Importance of Changes in Chronic Pain Intensity Measured on an 11-Point Numerical Pain Rating Scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Tahara, S.; Mitsuda, M.; Izumi, H.; Ikeda, S.; Seki, K.; Nishida, N.; Funaba, M.; Imajo, Y.; Yukata, K.; et al. Current Concept of Quantitative Sensory Testing and Pressure Pain Threshold in Neck/Shoulder and Low Back Pain. Healthcare 2022, 10, 1485. [Google Scholar] [CrossRef]
- Goubert, D.; Danneels, L.; Graven-Nielsen, T.; Descheemaeker, F.; Meeus, M. Differences in Pain Processing Between Patients with Chronic Low Back Pain, Recurrent Low Back Pain, and Fibromyalgia. Pain Physician 2017, 20, 307–318. [Google Scholar]
- Beltran-Alacreu, H.; López-de-Uralde-Villanueva, I.; Calvo-Lobo, C.; Fernández-Carnero, J.; La Touche, R. Clinical Features of Patients with Chronic Non-Specific Neck Pain per Disability Level: A Novel Observational Study. Rev. Assoc. Médica Bras. 2018, 64, 700–709. [Google Scholar] [CrossRef]
- Waller, R.; Straker, L.; O’Sullivan, P.; Sterling, M.; Smith, A. Reliability of Pressure Pain Threshold Testing in Healthy Pain Free Young Adults. Scand. J. Pain 2015, 9, 38–41. [Google Scholar] [CrossRef]
- Burrows, N.J.; Booth, J.; Sturnieks, D.L.; Barry, B.K. Acute Resistance Exercise and Pressure Pain Sensitivity in Knee Osteoarthritis: A Randomised Crossover Trial. Osteoarthr. Cartil. 2014, 22, 407–414. [Google Scholar] [CrossRef]
- Dunn, K.M.; Campbell, P.; Lewis, M.; Hill, J.C.; van der Windt, D.A.; Afolabi, E.; Protheroe, J.; Wathall, S.; Jowett, S.; Oppong, R.; et al. Refinement and Validation of a Tool for Stratifying Patients with Musculoskeletal Pain. Eur. J. Pain 2021, 25, 2081–2093. [Google Scholar] [CrossRef]
- Nativ, N.; Pincus, T.; Hill, J.; Ben Ami, N. Predicting Persisting Disability in Musculoskeletal Pain Patients with the STarT MSK Screening Tool: Results from a Prospective Cohort Study. Musculoskelet. Care 2023, 21, 1005–1010. [Google Scholar] [CrossRef]
- Campbell, P.; Hill, J.C.; Protheroe, J.; Afolabi, E.K.; Lewis, M.; Beardmore, R.; Hay, E.M.; Mallen, C.D.; Bartlam, B.; Saunders, B.; et al. Keele Aches and Pains Study Protocol: Validity, Acceptability, and Feasibility of the Keele STarT MSK Tool for Subgrouping Musculoskeletal Patients in Primary Care. J. Pain Res. 2016, 9, 807–818. [Google Scholar] [CrossRef] [PubMed]
- EULAR Outcome Measures Library. Available online: https://oml.eular.org/index.cfm (accessed on 27 December 2024).
- Weech, S.; Kenny, S.; Barnett-Cowan, M. Presence and Cybersickness in Virtual Reality Are Negatively Related: A Review. Front. Psychol. 2019, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Darnall, B.D.; Krishnamurthy, P.; Tsuei, J.; Minor, J.D. Self-Administered Skills-Based Virtual Reality Intervention for Chronic Pain: Randomized Controlled Pilot Study. JMIR Form. Res. 2020, 4, e17293. [Google Scholar] [CrossRef]
- Park, S.; Lee, G. Full-Immersion Virtual Reality: Adverse Effects Related to Static Balance. Neurosci. Lett. 2020, 733, 134974. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, B.; Hecht, H. Axis Rotation and Visually Induced Motion Sickness: The Role of Combined Roll, Pitch, and Yaw Motion. Aviat. Space Environ. Med. 2011, 82, 1023–1029. [Google Scholar] [CrossRef]
- Soriano-Maldonado, A.; Ruiz, J.R.; Álvarez-Gallardo, I.C.; Segura-Jiménez, V.; Santalla, A.; Munguía-Izquierdo, D. Validity and Reliability of Rating Perceived Exertion in Women with Fibromyalgia: Exertion-Pain Discrimination. J. Sports Sci. 2015, 33, 1515–1522. [Google Scholar] [CrossRef]
- Son, H.; Ross, A.; Mendoza-Tirado, E.; Lee, L.J. Virtual Reality in Clinical Practice and Research: Viewpoint on Novel Applications for Nursing. JMIR Nurs. 2022, 5, e34036. [Google Scholar] [CrossRef]
- Newcomb, L.W.; Koltyn, K.F.; Morgan, W.P.; Cook, D.B. Influence of Preferred versus Prescribed Exercise on Pain in Fibromyalgia. Med. Sci. Sports Exerc. 2011, 43, 1106–1113. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and Reporting Effect Sizes to Facilitate Cumulative Science: A Practical Primer for t-Tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Ying, X.; Ehrhardt, S. Pilot Trial Characteristics, Postpilot Design Modifications, and Feasibility of Full-Scale Trials. JAMA Netw. Open 2023, 6, e2333642. [Google Scholar] [CrossRef]
- Polat, M.; Kahveci, A.; Muci, B.; Günendi, Z.; Kaymak Karataş, G. The Effect of Virtual Reality Exercises on Pain, Functionality, Cardiopulmonary Capacity, and Quality of Life in Fibromyalgia Syndrome: A Randomized Controlled Study. Games Health J. 2021, 10, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Palacios, A.; Herrero, R.; Vizcaíno, Y.; Belmonte, M.A.; Castilla, D.; Molinari, G.; Baños, R.M.; Botella, C. Integrating Virtual Reality with Activity Management for the Treatment of Fibromyalgia: Acceptability and Preliminary Efficacy. Clin. J. Pain 2015, 31, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, A.; Samad-Soltani, T.; Salahzadeh, Z.; Rezaei-Hachesu, P. Effectiveness of Virtual Reality-Based Exercise Therapy in Rehabilitation: A Scoping Review. Inform. Med. Unlocked 2021, 24, 100562. [Google Scholar] [CrossRef]
- Pretat, T.; Koller, C.; Hügle, T. Virtual Reality as a Treatment for Chronic Musculoskeletal Pain Syndromes. Jt. Bone Spine 2025, 92, 105769. [Google Scholar] [CrossRef]
- Ahern, M.M.; Dean, L.V.; Stoddard, C.C.; Agrawal, A.; Kim, K.; Cook, C.E.; Narciso Garcia, A. The Effectiveness of Virtual Reality in Patients with Spinal Pain: A Systematic Review and Meta-Analysis. Pain Pract. 2020, 20, 656–675. [Google Scholar] [CrossRef]
- Viderman, D.; Tapinova, K.; Dossov, M.; Seitenov, S.; Abdildin, Y.G. Virtual Reality for Pain Management: An Umbrella Review. Front. Med. 2023, 10, 1203670. [Google Scholar] [CrossRef]
- Cascella, M.; Shariff, M.N.; Bianco, G.L.; Monaco, F.; Gargano, F.; Simonini, A.; Ponsiglione, A.M.; Piazza, O. Employing the Artificial Intelligence Object Detection Tool YOLOv8 for Real-Time Pain Detection: A Feasibility Study. J. Pain Res. 2024, 17, 3681–3696. [Google Scholar] [CrossRef]
- Varallo, G.; Suso-Ribera, C.; Ghiggia, A.; Veneruso, M.; Cattivelli, R.; Guerrini Usubini, A.; Franceschini, C.; Musetti, A.; Plazzi, G.; Fontana, J.M.; et al. Catastrophizing, Kinesiophobia, and Acceptance as Mediators of the Relationship Between Perceived Pain Severity, Self-Reported and Performance-Based Physical Function in Women with Fibromyalgia and Obesity. J. Pain Res. 2022, 15, 3017–3029. [Google Scholar] [CrossRef]
- Paschali, M.; Lazaridou, A.; Paschalis, T.; Napadow, V.; Edwards, R.R. Modifiable Psychological Factors Affecting Functioning in Fibromyalgia. J. Clin. Med. 2021, 10, 803. [Google Scholar] [CrossRef]
- Botella, C.; Garcia-Palacios, A.; Vizcaíno, Y.; Herrero, R.; Baños, R.M.; Belmonte, M.A. Virtual Reality in the Treatment of Fibromyalgia: A Pilot Study. Cyberpsychol. Behav. Soc. Netw. 2013, 16, 215–223. [Google Scholar] [CrossRef]
- Herrero, R.; García-Palacios, A.; Castilla, D.; Molinari, G.; Botella, C. Virtual Reality for the Induction of Positive Emotions in the Treatment of Fibromyalgia: A Pilot Study over Acceptability, Satisfaction, and the Effect of Virtual Reality on Mood. Cyberpsychol. Behav. Soc. Netw. 2014, 17, 379–384. [Google Scholar] [CrossRef]
- Wiederhold, B.K.; Gao, K.; Sulea, C.; Wiederhold, M.D. Virtual Reality as a Distraction Technique in Chronic Pain Patients. Cyberpsychol. Behav. Soc. Netw. 2014, 17, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Geri, T.; Botticchio, A.; Rossettini, G.; Pournajaf, S.; Pellicciari, L.; Di Antonio, S.; Castaldo, M. Pressure Pain Threshold of the Upper Trapezius Trigger Point: A Systematic Review with Meta-Analysis of Baseline Values and Their Modification after Physical Therapy. J. Clin. Med. 2022, 11, 7243. [Google Scholar] [CrossRef]
- Pelfort, X.; Torres-Claramunt, R.; Sánchez-Soler, J.F.; Hinarejos, P.; Leal-Blanquet, J.; Valverde, D.; Monllau, J.C. Pressure Algometry Is a Useful Tool to Quantify Pain in the Medial Part of the Knee: An Intra- and Inter-Reliability Study in Healthy Subjects. Orthop. Traumatol. Surg. Res. 2015, 101, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Blumenstiel, K.; Gerhardt, A.; Rolke, R.; Bieber, C.; Tesarz, J.; Friederich, H.-C.; Eich, W.; Treede, R.-D. Quantitative Sensory Testing Profiles in Chronic Back Pain Are Distinct from Those in Fibromyalgia. Clin. J. Pain 2011, 27, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E.; Gracely, R.H.; McLean, S.A.; Williams, D.A.; Giesecke, T.; Petzke, F.; Sen, A.; Clauw, D.J. Comparison of Clinical and Evoked Pain Measures in Fibromyalgia. J. Pain 2006, 7, 521–527. [Google Scholar] [CrossRef]
- Wodehouse, T.; Poply, K.; Ramaswamy, S.; Snidvongs, S.; Bourke, J.; Tahir, H.; Ullrich, K.; Mehta, V. A Pilot Study Investigating Whether Quantitative Sensory Testing Alters after Treatment in Patients with Fibromyalgia. Br. J. Pain 2018, 12, 250–256. [Google Scholar] [CrossRef]
- Sluka, K.A.; Law, L.F.; Bement, M.H. Exercise-Induced Pain and Analgesia? Underlying Mechanisms and Clinical Translation. Pain 2018, 159, S91–S97. [Google Scholar] [CrossRef]
- Vaegter, H.B.; Jones, M.D. Exercise-Induced Hypoalgesia after Acute and Regular Exercise: Experimental and Clinical Manifestations and Possible Mechanisms in Individuals with and without Pain. Pain Rep. 2020, 5, e823. [Google Scholar] [CrossRef]
- Brea-Gómez, B.; Torres-Sánchez, I.; Ortiz-Rubio, A.; Calvache-Mateo, A.; Cabrera-Martos, I.; López-López, L.; Valenza, M.C. Virtual Reality in the Treatment of Adults with Chronic Low Back Pain: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Int. J. Environ. Res. Public Health 2021, 18, 11806. [Google Scholar] [CrossRef]
- Ram, P.R.; Jeyaraman, M.; Jeyaraman, N.; Nallakumarasamy, A.; Khanna, M.; Gupta, A.; Yadav, S. Beyond the Pain: A Systematic Narrative Review of the Latest Advancements in Fibromyalgia Treatment. Cureus 2023, 15, e48032. [Google Scholar] [CrossRef] [PubMed]
- Ghavidel-Parsa, B.; Bidari, A.; Amir Maafi, A.; Ghalebaghi, B. The Iceberg Nature of Fibromyalgia Burden: The Clinical and Economic Aspects. Korean J. Pain 2015, 28, 169–176. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, A.; Roorda, L.D.; Otten, R.H.J.; van der Leeden, M.; Dekker, J.; Steultjens, M.P.M. Predictors of Multidisciplinary Treatment Outcome in Fibromyalgia: A Systematic Review. Disabil. Rehabil. 2013, 35, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Roussel, N.; Van Oosterwijck, J.; De Kooning, M.; Ickmans, K.; Struyf, F.; Meeus, M.; Lundberg, M. Fear of Movement and Avoidance Behaviour toward Physical Activity in Chronic-Fatigue Syndrome and Fibromyalgia: State of the Art and Implications for Clinical Practice. Clin. Rheumatol. 2013, 32, 1121–1129. [Google Scholar] [CrossRef]
- D’Onghia, M.; Ciaffi, J.; McVeigh, J.G.; Di Martino, A.; Faldini, C.; Ablin, J.N.; Meliconi, R.; Ursini, F. Fibromyalgia Syndrome—A Risk Factor for Poor Outcomes Following Orthopaedic Surgery: A Systematic Review. Semin. Arthritis Rheum. 2021, 51, 793–803. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).