DART-HRMS for the Rapid Assessment of Bioactive Compounds in Ultrasound-Processed Rapeseed Meal By-Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Processing of the Rapeseed Meal By-Product and Experimental Design
2.2. Fingerprinting Analysis by DART-HRMS
2.3. Statistical Analysis
3. Results
3.1. Ambient Mass Spectrometry Spectra
3.2. Multivariate Statistical Analysis
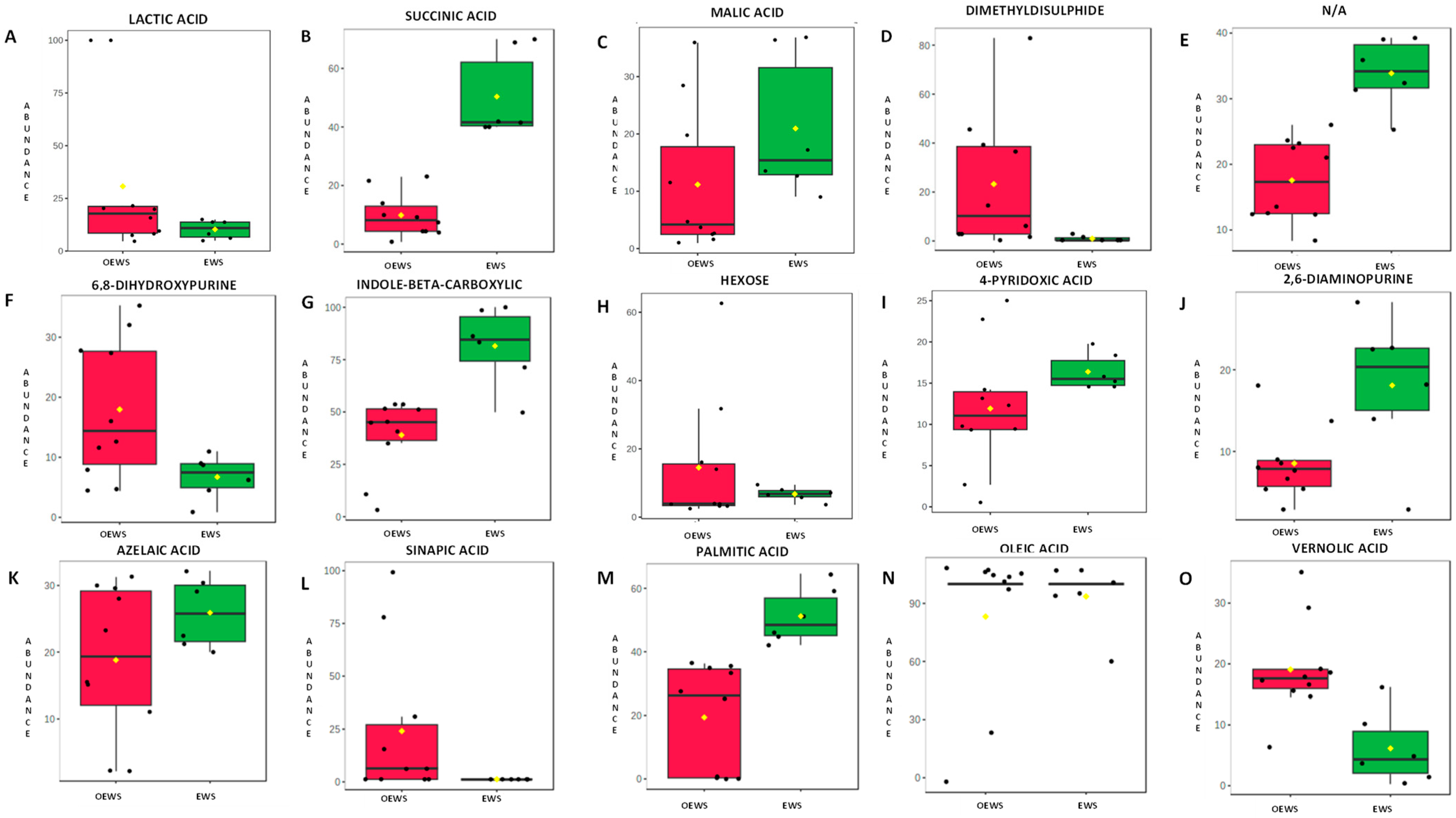
3.3. Chemical Changes Between EWS and OEWS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIMS | Ambient ionisation mass spectroscopy |
| CAP | Common agricultural policy |
| DART-HRMS | Direct analysis in real time–high resolution mass spectrometry |
| EWS | Ethanol-wash solutes |
| FA | Fatty acid |
| N/A | Not assigned |
| OEWS | Optimised EWS |
| PLS-DA | Partial least square–discriminant analysis |
| RM | Rapeseed meal |
| UA | Ultrasound-assisted |
References
- Fetzer, A.; Müller, K.; Schmid, M.; Eisner, P. Rapeseed proteins for technical applications: Processing, isolation, modification and functional properties—A review. Ind. Crop. Prod. 2020, 158, 112986. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Tse, T.J.; Reaney, M.J.T.; Tu, F.; Chen, Z.; Fang, H.; Kang, C.; Jiang, X.; Zhou, L.; et al. Determination of vanillin compounds in oilseed using solid-phase extraction with ultra-performance liquid chromatography–mass spectrometry. J. Food Compos. Anal. 2024, 133, 106388. [Google Scholar] [CrossRef]
- Marudova, M.; Viraneva, A.; Antova, G.; Nikolova, K.; Petkova, Z.; Teneva, O. Physico-Chemical Characterization of Sesame/Rapeseed Oil Mixtures. Appl. Sci. 2025, 15, 704. [Google Scholar] [CrossRef]
- Wongsirichot, P.; Gonzalez-Miquel, M.; Winterburn, J. Recent advances in rapeseed meal as alternative feedstock for industrial biotechnology. Biochem. Eng. J. 2022, 180, 108373. [Google Scholar] [CrossRef]
- Dygas, D.; Liszkowska, W.; Steglinska, A.; Sulyok, M.; Kregiel, D.; Berlowska, J. Rapeseed Meal Waste Biomass as a Single-Cell Protein Substrate for Nutritionally-Enhanced Feed Components. Processes 2023, 11, 1596. [Google Scholar] [CrossRef]
- Multescu, M.; Marinas, I.C.; Susman, I.E.; Belc, N. Byproducts (flour, meals, and groats) from the vegetable oil industry as as potential source of antioxidants. Foods 2022, 11, 253. [Google Scholar] [CrossRef]
- Petraru, A.; Amariei, S. Rapeseed—An Important Oleaginous Plant in the Oil Industry and the Resulting Meal a Valuable Source of Bioactive Compounds. Plants 2024, 13, 3085. [Google Scholar] [CrossRef]
- Di Lena, G.; Del Pulgar, J.S.; Lucarini, M.; Durazzo, A.; Ondrejíčková, P.; Oancea, F.; Frincu, R.M.; Aguzzi, A.; Nicoli, S.F.; Casini, I.; et al. Valorization potentials of rapeseed meal in a biorefinery perspective: Focus on nutritional and bioactive components. Molecules 2021, 26, 6787. [Google Scholar] [CrossRef]
- Egger, P.; Holzer, G.; Segato, S.; Werth, E.; Schwienbacher, F.; Peratoner, G.; Andrighetto, I.; Kasal, A. Effects of oilseed supplements on milk production and quality in dairy cows fed a hay-based diet. Ital. J. Anim. Sci. 2007, 6, 395–405. [Google Scholar] [CrossRef][Green Version]
- Lestingi, A. Alternative and sustainable protein sources in pig diet: A review. Animals 2024, 14, 310. [Google Scholar] [CrossRef]
- Sharaf Eldin, S.G.M.; Ziena, H.M.S.; Khair, S.T.M.; Rozan, M.A. Canola Seed Meal as a Potential Source of Natural Antioxidant. Alex. Sci. Exch. J. 2018, 39, 615–619. [Google Scholar] [CrossRef][Green Version]
- Pérez de Nanclares, M.; Marcussen, C.; Tauson, A.; Hansen, J.Ø.; Kjos, N.P.; Mydland, L.T.; Back Knudsen, K.E.; Øverland, M. Increasing levels of rapeseed expeller meal in diets for pigs: Effects on protein and energy metabolism. Animal 2019, 13, 273–282. [Google Scholar] [CrossRef]
- Hald, C.; Dawid, C.; Tressel, R.; Hofmann, T. Kaempferol 3-O-(2‴-O- Sinapoyl-β-sophoroside) Causes the Undesired Bitter Taste of Canola/Rapeseed Protein Isolates. J. Agric. Food Chem. 2019, 67, 372–378. [Google Scholar] [CrossRef]
- Manikandan, A.; Muthusamy, S.; Wang, E.S.; Ivarson, E.; Manickam, S.; Sivakami, R.; Narayanan, M.B.; Zhu, L.-H.; Rajasekaran, R.; Kanagarajan, S. Breeding and biotechnology approaches to enhance the nutritional quality of rapeseed byproducts for sustainable alternative protein sources—A critical review. Front. Plant Sci. 2024, 15, 1468675. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Tułodziecka, A. Antioxidant capacity of rapeseed extracts obtained by conventional and ultrasound-assisted extraction. J. Am. Oil Chem. Soc. 2014, 91, 2011–2019. [Google Scholar] [CrossRef]
- Ghazani, S.M.; García-Llatas, G.; Marangoni, A.G. Micronutrient content of cold-pressed, hot-pressed, solvent extracted and RBD canola oil: Implications for nutrition and quality. Eur. J. Lipid Sci. Technol. 2014, 116, 380–387. [Google Scholar] [CrossRef]
- Hussain, S.; Rehman, A.U.; Obied, H.K.; Luckett, D.J.; Blanchard, C.L. Extraction, Chemical Characterization, In Vitro Antioxidant, and Antidiabetic Activity of Canola (Brassica napus L.) Meal. Separations 2022, 9, 38. [Google Scholar] [CrossRef]
- Mas, A.L.; Canalis, A.M.; Mattalloni, M.; Pasqualini, M.E.; Wunderlin, D.A.; Baroni, M.V. Sesame defatted flour: Antioxidant response and improvement in carbohydrate metabolism in high-fructose/high-saturated fatty acids diet-fed mice. J. Food Sci. Technol. 2025, 62, 644–653. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, L.; Wang, Y.; Yu, Y.; Liu, X.; Xia, J.; Yang, Y.; Wang, H.; Feng, B. Antihypertensive effect of sinapine extracted from rapeseed meal in 2K1C hypertensive rats. Sci. Rep. 2025, 15, 4133. [Google Scholar] [CrossRef]
- Kalaydzhiev, H.; Ivanova, P.; Stoyanova, M.; Pavlov, A.; Rustad, T.; Silva, C.L.M.; Chalova, V.I. Valorization of Rapeseed Meal: Influence of Ethanol Antinutrients Removal on Protein Extractability, Amino Acid Composition and Fractional Profile. Waste Biomass Valorization 2020, 11, 2709–2719. [Google Scholar] [CrossRef]
- Ruan, S.; Xiong, J.; Li, Y.; Huang, S.; Wang, X.; Ma, H. Improvement in enzymolysis efficiency and bioavailability of rapeseed meal protein concentrate by sequential dual frequency ultrasound pretreatment. Process Biochem. 2021, 102, 240–249. [Google Scholar] [CrossRef]
- Sezer Okur, P.; Okur, I. Recent Advances in the Extraction of Phenolic Compounds from Food Wastes by Emerging Technologies. Food Bioprocess Technol. 2024, 17, 4383–4404. [Google Scholar] [CrossRef]
- Zeb, A. A comprehensive review on different classes of polyphenolic compounds present in edible oils. Food Res. Int. 2021, 143, 110312. [Google Scholar] [CrossRef]
- Vaz, S. Analytical techniques for the chemical analysis of plant biomass and biomass products. Anal. Methods 2014, 6, 8094–8105. [Google Scholar] [CrossRef]
- Tölgyesi, Á.; Tóth, E.; Farkas, T.; Simon, A.; Dernovics, M.; Bálint, M. Determination of Aminophosphonate Herbicides in Glutamate Loaded Spice Mix by LC-IDMS and Method Extension to Other Food Matrices. Food Anal. Methods 2022, 15, 2012–2025. [Google Scholar] [CrossRef]
- Hurtaud-Pessel, D.; Jagadeshwar-Reddy, T.; Verdon, E. Developing a new screening method for the detection of antibiotic residues in muscle tissues in using liquid chromatography and high resolution mass spectrometry with a LC-LTQ-Orbitrap instrument. Food Addit. Contam. Part A 2011, 28, 1340–1351. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, H.; Chingin, K.; Xiong, J.; Fang, X.; Chen, H. Ambient mass spectrometry for food science and industry. Trends Anal. Chem. 2018, 107, 99–115. [Google Scholar] [CrossRef]
- Wang, Y. Recent advances in the application of direct analysis in real time-mass spectrometry (DART-MS) in food analysis. Food Res. Int. 2024, 188, 114488. [Google Scholar] [CrossRef]
- Schmauder, F.; Creydt, M.; Fischer, M. Novel DART-MS approach for rapid and environmentally friendly determination of the geographical origin of hazelnuts (Corylus avellana L.). Food Chem. 2025, 467, 142265. [Google Scholar] [CrossRef]
- Zacometti, C.; Khazzar, S.; Massaro, A.; Tata, A.; Riuzzi, G.; Piro, R.; Novelli, E.; Segato, S.; Balzan, S. DART-HRMS reveals metabolic changes of whey through microparticulation and fermentations. Appl. Food Res. 2024, 4, 100443. [Google Scholar] [CrossRef]
- Lippolis, V.; De Angelis, E.; Fiorino, G.M.; Di Gioia, A.; Arlorio, M.; Logrieco, A.F.; Monaci, L. Geographical Origin Discrimination of Monofloral Honeys by Direct Analysis in Real Time Ionization-High Resolution Mass Spectrometry (DART-HRMS). Foods 2020, 9, 1205. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Fang, P.; Jiang, J.; Zhang, F.; Yong, W.; Liu, J.; Dong, Y. Rapid screening and quantification of residual pesticides and illegal adulterants in red wine by direct analysis in real time mass spectrometry. J. Chromatogr. A 2016, 1471, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Sun, J.; Chen, P.; Frazier, J.; Benefield, V.; Zhang, M. Chemical analysis and classification of black pepper (Piper nigrum L.) based on their country of origin using mass spectrometric methods and chemometrics. Food Res. Int. 2021, 140, 109877. [Google Scholar] [CrossRef] [PubMed]
- Feider, C.L.; Krieger, A.; Dehoog, R.J.; Eberlin, L.S. Ambient Ionization Mass Spectrometry: Recent Developments and Applications. Anal. Chem. 2019, 91, 4266–4290. [Google Scholar] [CrossRef]
- Georgiev, R.; Ivanov, I.G.; Ivanova, P.; Tumbarski, Y.; Kalaydzhiev, H.; Dincheva, I.N.; Badjakov, I.K.; Chalova, V.I. Phytochemical Profile and Bioactivity of Industrial Rapeseed Meal Ethanol-Wash Solutes. Waste Biomass Valorization 2021, 12, 5051–5063. [Google Scholar] [CrossRef]
- Cisneros-Yupanqui, M.; Chalova, V.I.; Kalaydzhiev, H.R.; Mihaylova, D.; Krastanov, A.I.; Lante, A. Ultrasound-assisted extraction of antioxidant bioactive compounds from wastes of rapeseed industry and their application in delaying rapeseed oil oxidation. Environ. Technol. Innov. 2023, 30, 103081. [Google Scholar] [CrossRef]
- Song, L.; Chuah, W.C.; Lu, X.; Remsen, E.; Bartmess, J.E. Ionization Mechanism of Positive-Ion Nitrogen Direct Analysis in Real Time. J. Am. Soc. Mass Spectrom. 2018, 29, 640–650. [Google Scholar] [CrossRef]
- Tata, A.; Massaro, A.; Riuzzi, G.; Lanza, I.; Bragolusi, M.; Negro, A.; Novelli, E.; Piro, R.; Gottardo, F.; Segato, S. Ambient mass spectrometry for rapid authentication of milk from Alpine or lowland forage. Sci. Rep. 2022, 12, 7360. [Google Scholar] [CrossRef]
- Zacometti, C.; Lante, A.; Cisneros, M.; Massaro, A.; Mihaylova, D.; Chalova, V.; Krastanov, A.; Kalaydzhiev, H.; Riuzzi, G.; Tata, A.; et al. Rapid assessment of metabolomic fingerprinting of recycled sunflower by-products via DART-HRMS. Molecules 2024, 29, 4092. [Google Scholar] [CrossRef]
- An, Z.Y.; Jin, J.M.; Tan, Z.J.; Wang, Y.F.; Wang, T.; Dong, Z.K.; Chen, J.J.; Xiong, Y.C.; Jin, W.L. Lanzhou lily as nutraceuticals: Identification of active metabolites via the UHPLC-Q-exactive orbitrap mass spectrometer. Microchem. J. 2025, 213, 113626. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Yuan, M.; Xu, Y.; Xu, H. Gout and Diet: A Comprehensive Review of Mechanisms and Management. Nutrients 2022, 14, 3525. [Google Scholar] [CrossRef] [PubMed]
- Bardin, T.; Richette, P. Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.; Gray, S.G.; Meaney, S.; Finn, S.; Kenny, O.; Hayes, M. Sinapinic and protocatechuic acids found in rapeseed: Isolation, characterisation and potential benefits for human health as functional food ingredients. Ir. J. Agric. Food Res. 2017, 56, 104–119. [Google Scholar] [CrossRef]
- Koski, A.; Pekkarinen, S.; Hopia, A.; Wähälä, K.; Heinonen, M. Processing of rapeseed oil: Effects on sinapic acid derivative content and oxidative stability. Eur. Food Res. Technol. 2003, 217, 110–114. [Google Scholar] [CrossRef]
- Cairone, F.; Allevi, D.; Cesa, S.; Fabrizi, G.; Goggiamani, A.; Masci, D.; Iazzetti, A. Valorisation of Side Stream Products through Green Approaches: The Rapeseed Meal Case. Foods 2023, 12, 3286. [Google Scholar] [CrossRef]
- Ansari, M.A.; Raish, M.; Ahmad, A.; Alkharfy, K.M.; Ahmad, S.F.; Attia, S.M.; Alsaad, A.M.S.; Bakheet, S.A. Sinapic acid ameliorate cadmium-induced nephrotoxicity: In vivo possible involvement of oxidative stress, apoptosis, and inflammation via NF-κB downregulation. Environ. Toxicol. Pharmacol. 2017, 51, 100–107. [Google Scholar] [CrossRef]
- Musa, W.J.A.; Bialangi, N.; Kilo, A.K.; Situmeang, B.; Susparini, N.T.; Rusydi, I.D. Antioxidant, cholesterol lowering activity, and analysis of Caesalpinia bonducella seeds extract. Pharmacia 2023, 70, 97–103. [Google Scholar] [CrossRef]
- Brenna, E.; Colombo, D.; Di Lecce, G.; Gatti, F.G.; Ghezzi, M.C.; Tentori, F.; Tessaro, D.; Viola, M. Conversion of oleic acid into azelaic and pelargonic acid by a chemo-enzymatic route. Molecules 2020, 25, 1882. [Google Scholar] [CrossRef]
- Todea, A.; Deganutti, C.; Spennato, M.; Asaro, F.; Zingone, G.; Milizia, T.; Gardossi, L. Azelaic Acid: A Bio-Based Building Block for Biodegradable Polymers. Polyners 2021, 13, 4091. [Google Scholar] [CrossRef]
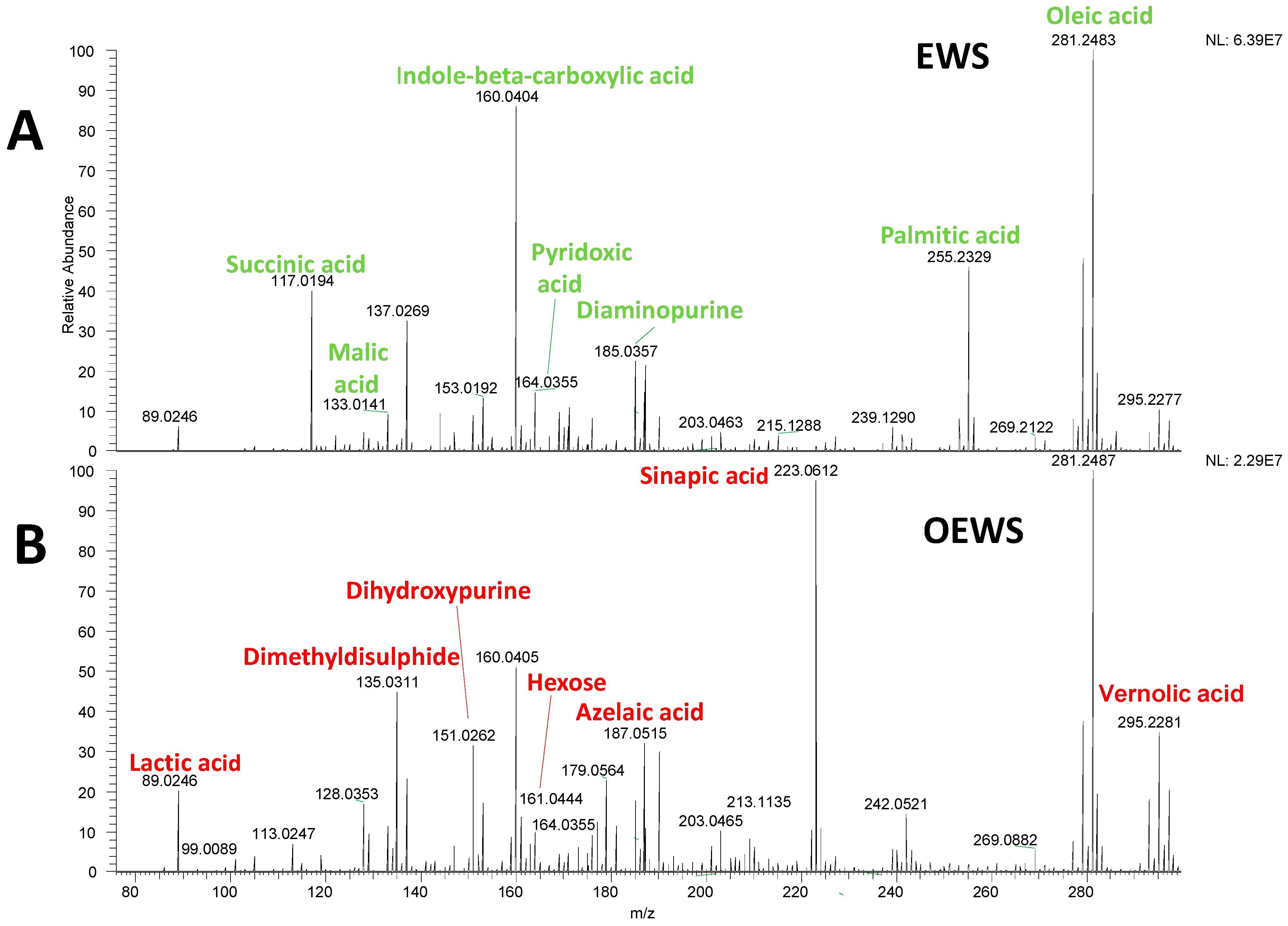

| Observed m/z | Theoretical m/z | Error (ppm) | Predicted Molecular Formula | Ion Type | Tentative Annotation |
|---|---|---|---|---|---|
| EWS | |||||
| 117.0194 | 117.0193 | 0.9 | C4H6O4 | [M−H]− | Succinic acid |
| 133.0141 | 133.0142 | −0.8 | C4H6O5 | [M−H]− | Malic acid |
| 137.0269 | − | − | − | − | N/A |
| 160.0404 | 160.0404 | 0.0 | C9H7NO2 | [M−H]− | Indole-β-carboxylic acid |
| 164.0355 | 164.0348 | 4.3 | C8H9NO4 | [M−H2O−H]− | 4-Pyridoxic acid |
| 185.0357 | 185.0348 | 4.9 | C5H6N6 | [M+Cl]− | 2,6-Diaminopurine |
| 255.2335 | 255.2330 | 2.0 | C16H32O2 | [M−H]− | Palmitic acid |
| 281.2486 | 281.2486 | 0.0 | C18H34O2 | [M−H]− | Oleic acid |
| OEWS | |||||
| 89.0242 | 89.0244 | −2.2 | C3H6O3 | [M−H]− | Lactic acid |
| 135.0305 | 135.0308 | −2.2 | C2H6S2 | [M−H]− | Dimethyldisulphide |
| 151.026 | 151.0261 | −0.7 | C5H4N4O2 | [M−H]− | 6,8-Dihydroxypurine |
| 187.0974 | 187.0976 | −1.1 | C9H16O4 | [M−H]− | Azelaic acid |
| 161.0444 | 161.0450 | −3.7 | C6H12O6 | [M−H2O−H]− | Hexose |
| 223.0612 | 223.0612 | 0.0 | C11H12O5 | [M−H]− | Sinapic acid |
| 295.2279 | 295.2279 | 0.0 | C18H32O3 | [M−H]− | Vernolic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lante, A.; Massaro, A.; Zacometti, C.; Mihaylova, D.; Chalova, V.; Krastanov, A.; Kalaydzhiev, H.; Cisneros, M.; Morbin, G.; Riuzzi, G.; et al. DART-HRMS for the Rapid Assessment of Bioactive Compounds in Ultrasound-Processed Rapeseed Meal By-Product. Appl. Sci. 2025, 15, 5952. https://doi.org/10.3390/app15115952
Lante A, Massaro A, Zacometti C, Mihaylova D, Chalova V, Krastanov A, Kalaydzhiev H, Cisneros M, Morbin G, Riuzzi G, et al. DART-HRMS for the Rapid Assessment of Bioactive Compounds in Ultrasound-Processed Rapeseed Meal By-Product. Applied Sciences. 2025; 15(11):5952. https://doi.org/10.3390/app15115952
Chicago/Turabian StyleLante, Anna, Andrea Massaro, Carmela Zacometti, Dasha Mihaylova, Vesela Chalova, Albert Krastanov, Hristo Kalaydzhiev, Miluska Cisneros, Greta Morbin, Giorgia Riuzzi, and et al. 2025. "DART-HRMS for the Rapid Assessment of Bioactive Compounds in Ultrasound-Processed Rapeseed Meal By-Product" Applied Sciences 15, no. 11: 5952. https://doi.org/10.3390/app15115952
APA StyleLante, A., Massaro, A., Zacometti, C., Mihaylova, D., Chalova, V., Krastanov, A., Kalaydzhiev, H., Cisneros, M., Morbin, G., Riuzzi, G., Segato, S., & Tata, A. (2025). DART-HRMS for the Rapid Assessment of Bioactive Compounds in Ultrasound-Processed Rapeseed Meal By-Product. Applied Sciences, 15(11), 5952. https://doi.org/10.3390/app15115952












