Removal of COD from Secondary Effluent Using Fenton Iron Sludge-Based Biochar/Fe(VI)/H2O2 Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Iron Sludge-Based Catalyst
2.3. Characterization
2.4. Catalytic Activity Measurements
2.5. The Removal Performance of COD in Secondary Effluent
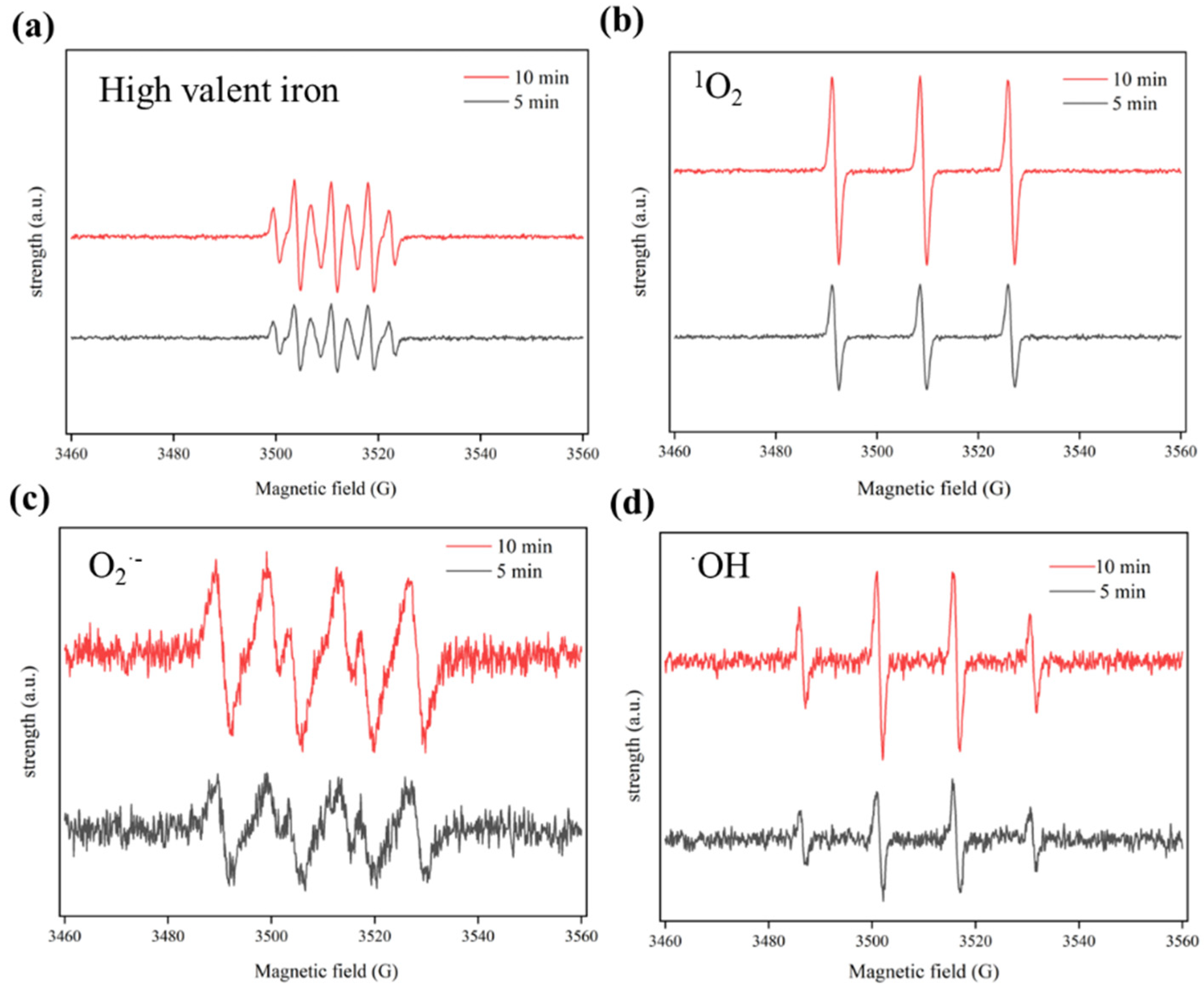
2.6. Reactive Oxygen Capture Assay
3. Results and Discussion
3.1. Characterization of Fenton Iron Peat Catalyst
3.2. Performance Testing
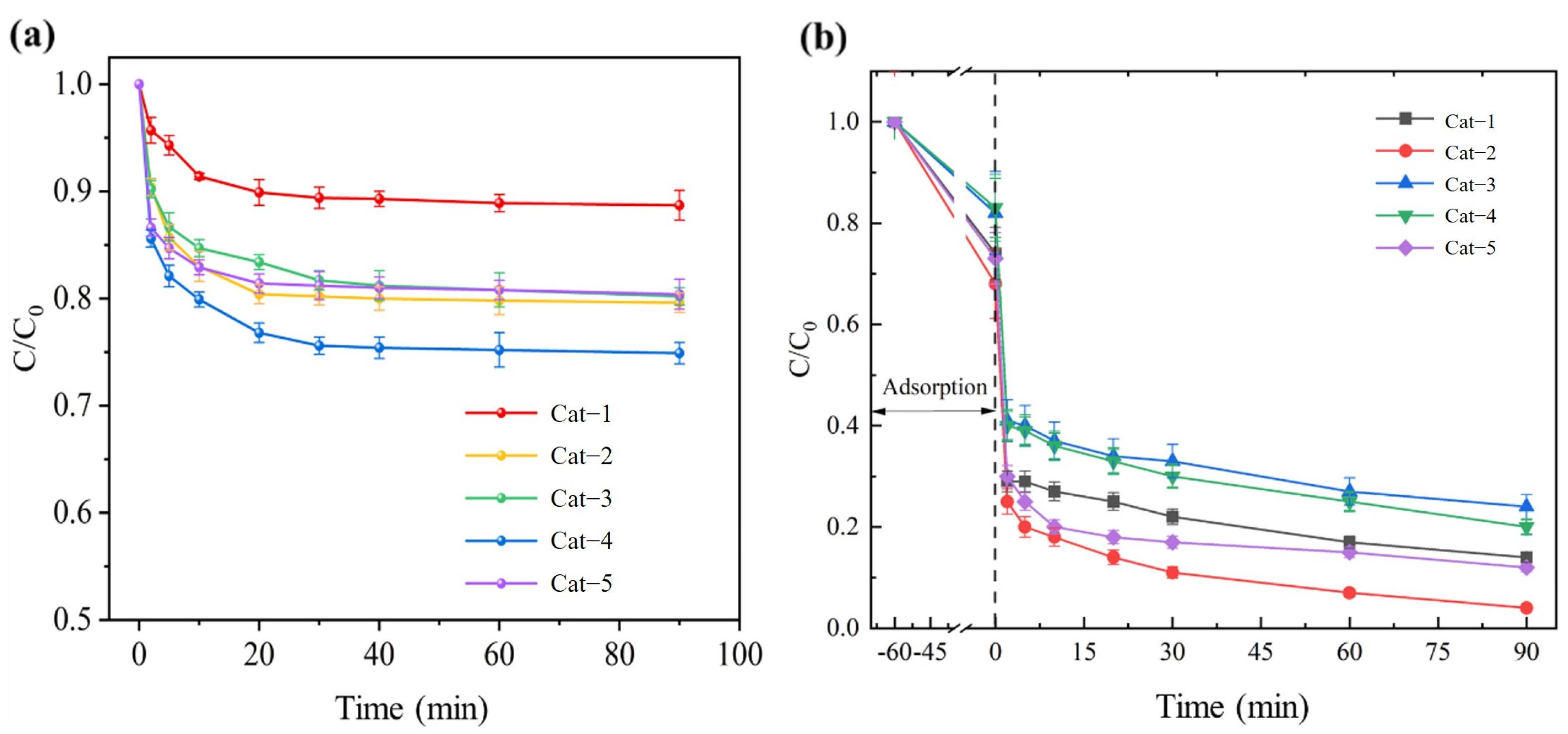
3.2.1. Effect of Pyrolysis Time on Catalyst Performance
3.2.2. COD Removal Performance in the Cat−2/H2O2 System
3.2.3. Effect of pH on COD Removal Performance in the Cat−2/H2O2/Fe(VI) System
3.2.4. Effect of Total Concentration of Fe(VI) and H2O2 on COD Removal Performance in the Cat−2/H2O2/Fe(VI) System
3.2.5. Effect of Molar Ratio of Fe(VI) and H2O2 on COD Removal Performance in the Cat−2/H2O2/Fe(VI) System
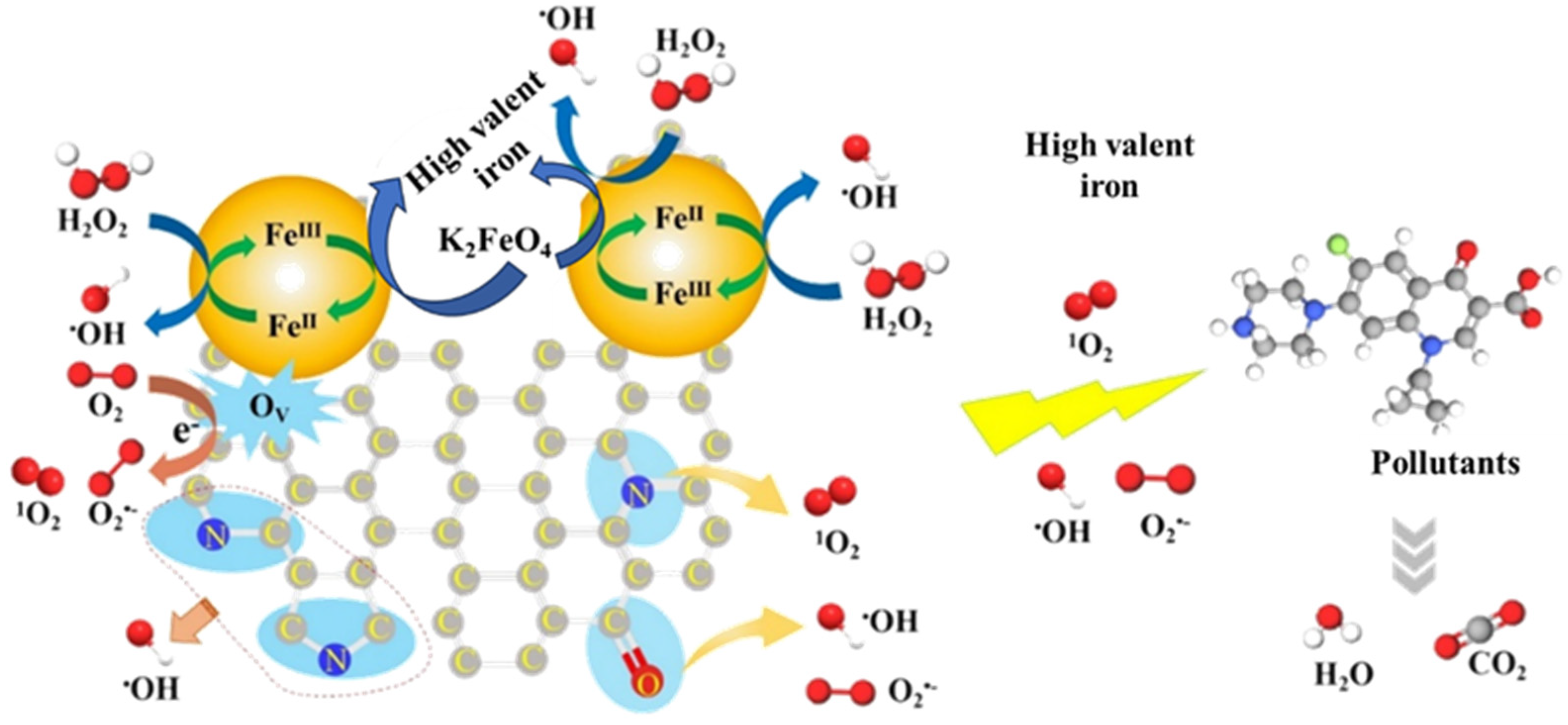
3.3. Catalytic Mechanisms in Cat−2/H2O2/Fe(VI) System
3.4. Comparison Between Cat−2/H2O2/Fe(VI) System and Fenton or Ozonation System
3.5. Small-Scale Continuous Flow Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castaldi, P.; Silvetti, M.; Enzo, S.; Melis, P. Study of Sorption Processes and FT-IR Analysis of Arsenate Sorbed onto Red Muds (a Bauxite Ore Processing Waste). J. Hazard. Mater. 2010, 175, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, X.; Wang, M.; Wang, H.; Xian, P. Iron Recovery from the Leached Solution of Red Mud through the Application of Oxalic Acid. Int. J. Miner. Process. 2016, 157, 145–151. [Google Scholar] [CrossRef]
- Agrawal, S.; Dhawan, N. Evaluation of Red Mud as a Polymetallic Source—A Review. Miner. Eng. 2021, 171, 107084. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.M.; Tadé, M.O. Novel Applications of Red Mud as Coagulant, Adsorbent and Catalyst for Environmentally Benign Processes. Chemosphere 2008, 72, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xiang, J.; Liang, G.; Wang, J.; Ma, S.; He, C. Application of Common Industrial Solid Waste in Water Treatment: A Review. Environ. Sci. Pollut. Res. Int. 2023, 30, 111766–111801. [Google Scholar] [CrossRef]
- Brunori, C.; Cremisini, C.; Massanisso, P.; Pinto, V.; Torricelli, L. Reuse of a Treated Red Mud Bauxite Waste: Studies on Environmental Compatibility. J. Hazard. Mater. 2005, 117, 55–63. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A Review of Classic Fenton’s Peroxidation as an Advanced Oxidation Technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A Review on Fenton and Improvements to the Fenton Process for Wastewater Treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Soon, A.N.; Hameed, B.H. Heterogeneous Catalytic Treatment of Synthetic Dyes in Aqueous Media Using Fenton and Photo-Assisted Fenton Process. Desalination 2011, 269, 1–16. [Google Scholar] [CrossRef]
- Bautista, P.; Mohedano, A.F.; Casas, J.A.; Zazo, J.A.; Rodriguez, J.J. An Overview of the Application of Fenton Oxidation to Industrial Wastewaters Treatment. J. Chem. Technol. Biotechnol. 2008, 83, 1323–1338. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, L.; Dai, H.; Li, N.; Song, Y.; Yan, B.; Chen, G.; Hou, L. Activation of Peroxymonosulfate by Food Waste Digestate Derived Biochar for Sulfamethoxazole Degradation: Performance and Mechanism. Sep. Purif. Technol. 2023, 327, 124935. [Google Scholar] [CrossRef]
- Nie, X.; Li, G.; Li, S.; Luo, Y.; Luo, W.; Wan, Q.; An, T. Highly Efficient Adsorption and Catalytic Degradation of Ciprofloxacin by a Novel Heterogeneous Fenton Catalyst of Hexapod-like Pyrite Nanosheets Mineral Clusters. Appl. Catal. B 2022, 300, 120734. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Ou, C.; Faheem; Shen, J.; Yu, H.; Jiao, Z.; Han, W.; Sun, X.; Li, J.; et al. Reuse of Fenton Sludge as an Iron Source for NiFe2O4 Synthesis and Its Application in the Fenton-Based Process. J. Environ. Sci. 2017, 53, 1–8. [Google Scholar] [CrossRef]
- Lei, Y.; Yu, Y.; Lei, X.; Liang, X.; Cheng, S.; Ouyang, G.; Yang, X. Assessing the Use of Probes and Quenchers for Understanding the Reactive Species in Advanced Oxidation Processes. Environ. Sci. Technol. 2023, 57, 5433–5444. [Google Scholar] [CrossRef]
- Zhang, N.; Yi, Y.; Lian, J.; Fang, Z. Effects of Ce Doping on the Fenton-like Reactivity of Cu-Based Catalyst to the Fluconazole. Chem. Eng. J. 2020, 395, 124897. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Z.; Zhang, Y. Disposal of Fenton Sludge with Anaerobic Digestion and the Roles of Humic Acids Involved in Fenton Sludge. Water Res. 2019, 163, 114900. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, T.; Chen, D.; Zhang, Z.-X.; Zhang, Q.; Ye, X.; Wan, J.; Zou, J.-P. Fenton-like Catalysis by MnO2 Membrane Reactor with Oxygen Vacancies for Carbamazepine Degradation. Appl. Catal. B Environ. 2024, 353, 124106. [Google Scholar] [CrossRef]
- Yu, Y.; Li, N.; Wang, C.; Cheng, Z.; Yan, B.; Chen, G.; Hou, L.; Wang, S. Iron Cobalt and Nitrogen Co-Doped Carbonized Wood Sponge for Peroxymonosulfate Activation: Performance and Internal Temperature-Dependent Mechanism. J. Colloid Interface Sci. 2022, 619, 267–279. [Google Scholar] [CrossRef]
- Cheng, Z.; Luo, S.; Li, X.; Zhang, S.; Thang Nguyen, T.; Guo, M.; Gao, X. Ultrasound-Assisted Heterogeneous Fenton-like Process for Methylene Blue Removal Using Magnetic MnFe2O4/Biochar Nanocomposite. Appl. Surf. Sci. 2021, 566, 150654. [Google Scholar] [CrossRef]
- Luo, X.; Zhu, R.; Zhao, L.; Gong, X.; Zhang, L.; Fan, L.; Liu, Y. Defective Nitrogen Doped Carbon Material Derived from Nano-ZIF-8 for Enhanced in-Situ H2O2 Generation and Tetracycline Hydrochloride Degradation in Electro-Fenton System. Environ. Res. 2024, 251, 118644. [Google Scholar] [CrossRef] [PubMed]
- da Silveira Salla, J.; Dotto, G.L.; Hotza, D.; Landers, R.; da Boit Martinello, K.; Foletto, E.L. Enhanced Catalytic Performance of CuFeS2 Chalcogenide Prepared by Microwave-Assisted Route for Photo-Fenton Oxidation of Emerging Pollutant in Water. J. Environ. Chem. Eng. 2020, 8, 104077. [Google Scholar] [CrossRef]
- Cai, F.; Sun, C.; Sun, Z.; Lai, Y.; Ding, H. Sulfur-Functionalized CoMn2O4 as a Fenton-like Catalyst for the Efficient Rhodamine B Degradation. Appl. Surf. Sci. 2023, 623, 157044. [Google Scholar] [CrossRef]
- Chen, F.; Wu, X.; Shi, C.; Lin, H.; Chen, J.; Shi, Y.; Wang, S.; Duan, X. Molecular Engineering toward Pyrrolic N-Rich M-N4 (M = Cr, Mn, Fe, Co, Cu) Single-Atom Sites for Enhanced Heterogeneous Fenton-Like Reaction. Adv. Funct. Mater. 2021, 31, 110725. [Google Scholar] [CrossRef]
- Chen, S.; Ran, X.; Ren, G.; Wei, Z.; Wang, Z.; Rao, T.; Li, R.; Ma, X. Comparison of Fenton and Ozone Oxidation for Pretreatment of Petrochemical Wastewater: COD Removal and Biodegradability Improvement Mechanism. Separations 2022, 9, 179. [Google Scholar] [CrossRef]

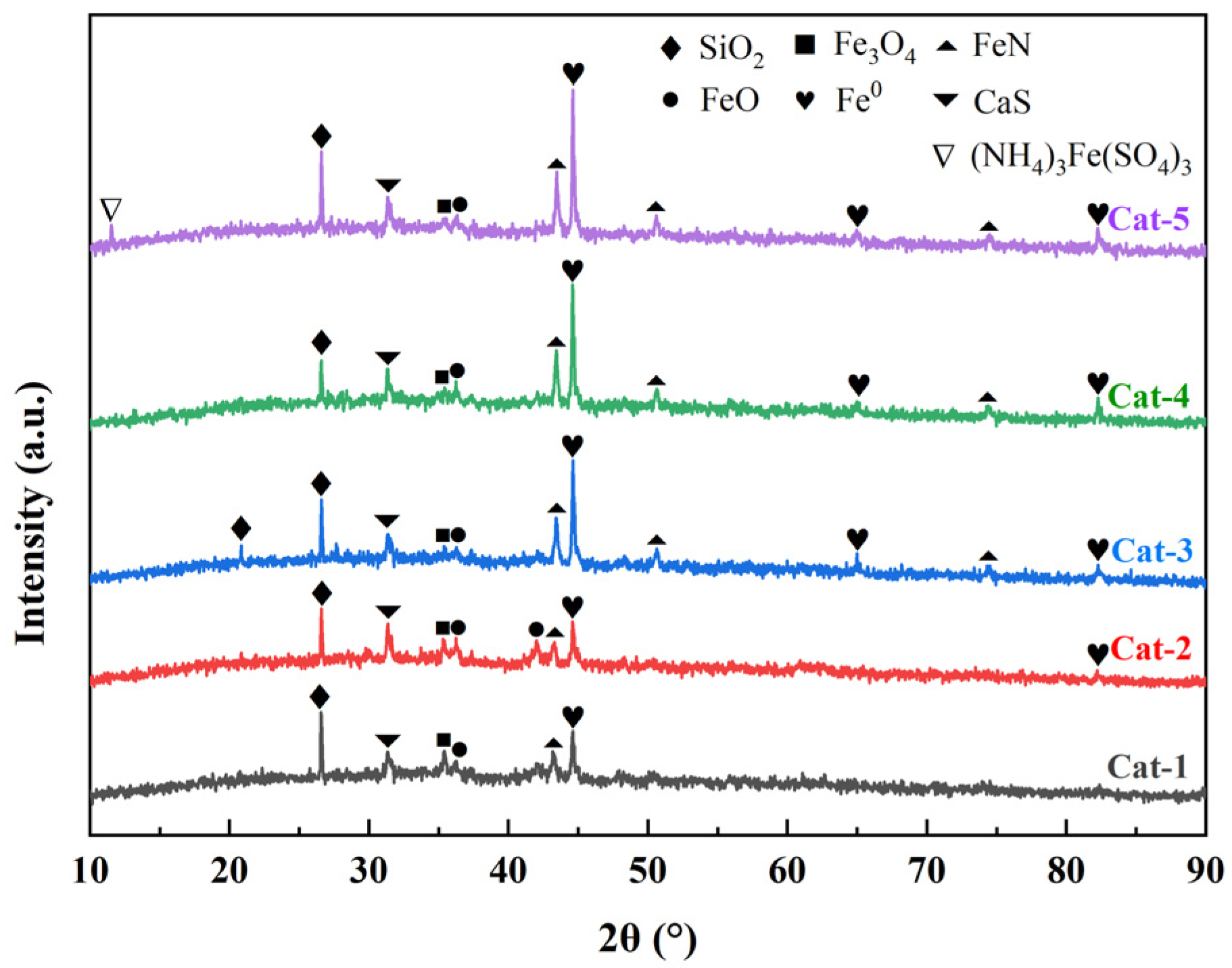
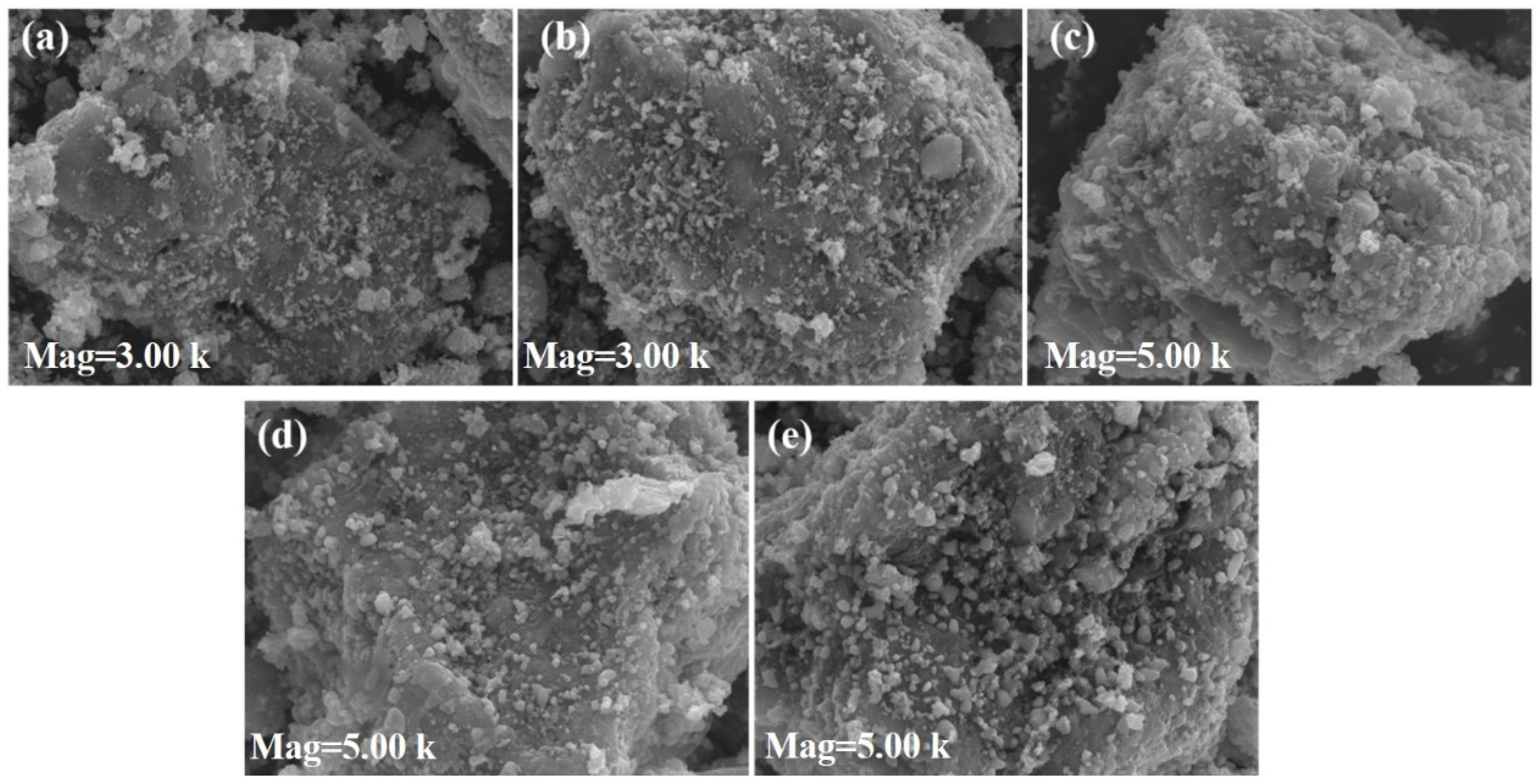
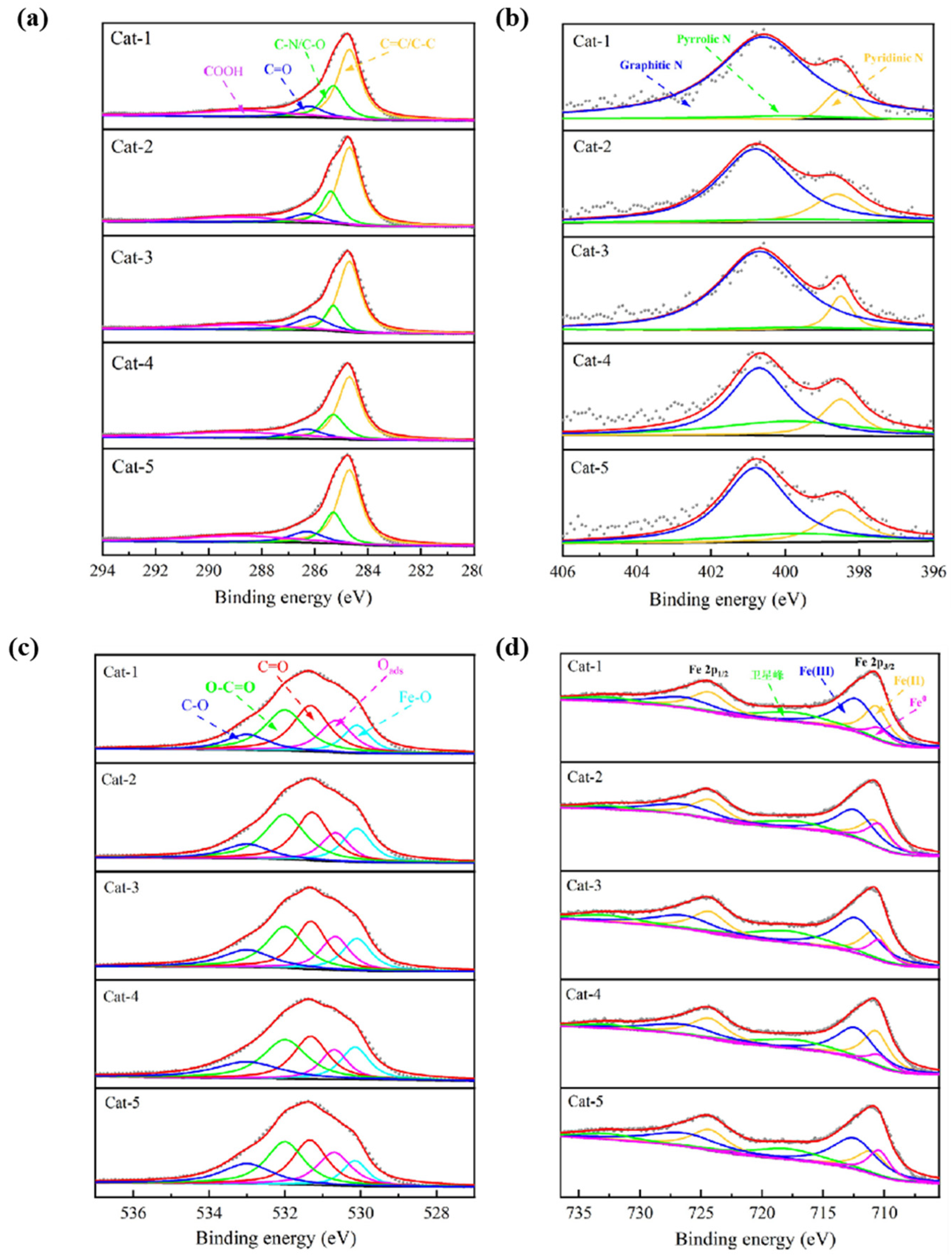
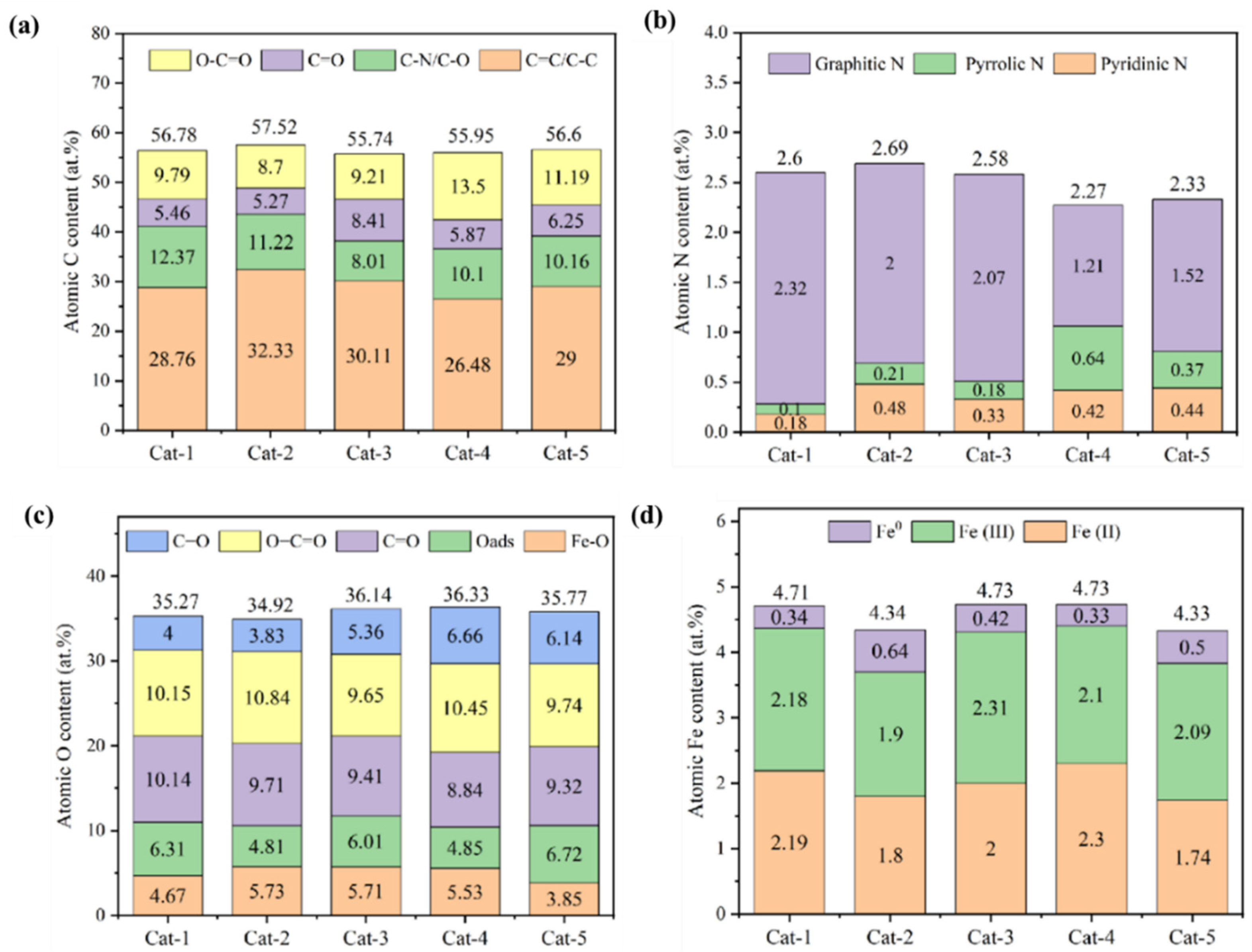


| Elemental Composition | Proportion (wt%) | Elemental Composition | Proportion (wt%) |
|---|---|---|---|
| C | 49.70 | S | 1.45 |
| Fe | 35.40 | Al | 1.03 |
| N | 3.43 | P | 0.89 |
| Ca | 3.33 | Na | 0.61 |
| Si | 2.98 |
| pH | Catalyst Concentration (g/L) | [H2O2] (mM) | Removal Rate (%) | COD (mg/L) (±1) |
|---|---|---|---|---|
| 4 | 0.5 | 1.0 | 33.0 | 48 |
| 5 | 0.5 | 1.0 | 13.6 | 60 |
| 4 | 0.5 | 1.5 | 16.5 | 58 |
| 4 | 2.0 | 1.0 | 29.1 | 50 |
| pH | Removal Rate | COD (mg/L) (±1) |
|---|---|---|
| 4.0 | 7.7% | 65 |
| 5.0 | 0.0% | 70 |
| 6.0 | 0.0% | 70 |
| 7.5 | 11.8% | 62 |
| 8.1 | 31.3% | 48 |
| pH | [H2O2] + Fe(VI)](mM) | [H2O2]:[Fe(VI)] | Removal Rate (%) |
|---|---|---|---|
| 8.1 | 0.9 | 5:1 | 31.8 |
| 8.1 | 1.5 | 5:1 | 32.8 |
| 8.1 | 1.8 | 5:1 | 34.4 |
| Quencher | k (min−1) |
|---|---|
| TBA | 0.0240 |
| CF | 0.0216 |
| FFA | 0.0055 |
| black | 0.0533 |
| Catalyst (g) | Wastewater COD (mg/L) | Flow Rate (mL/min) | pH (±0.20) | [H2O2] (mM) | [Fe(VI)] (mM) | Reflux Ratio | Outlet COD (mg/L) |
|---|---|---|---|---|---|---|---|
| Lower layer 60, Middle layer 50, Upper layer 30 | 71 | 40.12 | 8.10 | 0.8 | 0.1 | - | 57 |
| 69 | 40.12 | 8.10 | 0.8 | 0.1 | 1:2 | 48 | |
| 69 | 40.12 | 8.10 | 0.8 | 0.1 | 1:4 | 43 |
| Catalyst (g) | Wastewater COD (mg/L) | Flow Rate (mL/min) | pH (±0.20) | [H2O2] (mM) | [Fe(VI)] (mM) | Reflux Ratio | Outlet COD (mg/L) |
|---|---|---|---|---|---|---|---|
| Lower layer 60, Middle layer 50, Upper layer 30 | 69 | 40.12 | 8.10 | 0.8 | 0.1 | 1:2 | 47 |
| 70 | 40.12 | 8.10 | 1.2 | 0.15 | 1:2 | 45 | |
| 68 | 32.98 | 8.10 | 0.8 | 0.1 | - | 45 | |
| 68 | 32.98 | 8.10 | 1.2 | 0.15 | - | 44 |
| Catalyst (g) | Wastewater COD (mg/L) | Flow Rate (mL/min) | pH (±0.20) | [H2O2] (mM) | [Fe(VI)] (mM) | Reflux Ratio | Outlet COD (mg/L) |
|---|---|---|---|---|---|---|---|
| Lower layer 60, Middle layer 50, Upper layer 30 | 62 | 32.98 | 8.10 | 0.8 | 0.1 | 1:3 | 48 |
| 62 | 26.01 | 8.10 | 0.8 | 0.1 | 1:3 | 43 | |
| 67 | 40.12 | 8.10 | 1.2 | 0.15 | - | 42 | |
| 68 | 32.98 | 8.10 | 1.2 | 0.15 | - | 44 |
| Catalyst (g) | Wastewater COD (mg/L) | Flow Rate (mL/min) | pH (±0.20) | [H2O2] (mM) | [Fe(VI)] (mM) | Reflux Ratio | Outlet COD (mg/L) | |
|---|---|---|---|---|---|---|---|---|
| Lower layer Middle layer Upper layer | 60 | 61 | 26.01 | 8.10 | 1.0 | 0.125 | 1:4 | 42 |
| 50 | ||||||||
| 30 | ||||||||
| Lower layer Middle layer Upper layer (Aeration) | 60 | 61 | 26.01 | 8.10 | 1.0 | 0.125 | 1:4 | 41 |
| 50 | ||||||||
| 30 | ||||||||
| Lower layer Middle layer Upper layer | 120 | 62 | 26.01 | 8.10 | 1.0 | 0.125 | 1:4 | 39 |
| 120 | ||||||||
| 100 | ||||||||
| Lower layer Middle layer Upper layer | 120 | 62 | 26.01 | 8.10 | 1.0 | 0.125 | 1:4 | 36 |
| 120 | ||||||||
| 120 | ||||||||
| Lower layer Middle layer Upper layer | 140 | 62 | 26.01 | 8.10 | 1.0 | 0.125 | 1:4 | 37 |
| 140 | ||||||||
| 140 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; He, X.; Liang, L.; Wang, Y.; Yan, B.; Chen, G.; Li, N.; Hou, L. Removal of COD from Secondary Effluent Using Fenton Iron Sludge-Based Biochar/Fe(VI)/H2O2 Process. Appl. Sci. 2025, 15, 5945. https://doi.org/10.3390/app15115945
Wang L, He X, Liang L, Wang Y, Yan B, Chen G, Li N, Hou L. Removal of COD from Secondary Effluent Using Fenton Iron Sludge-Based Biochar/Fe(VI)/H2O2 Process. Applied Sciences. 2025; 15(11):5945. https://doi.org/10.3390/app15115945
Chicago/Turabian StyleWang, Lia, Xu He, Lan Liang, Yanshan Wang, Beibei Yan, Guanyi Chen, Ning Li, and Li’an Hou. 2025. "Removal of COD from Secondary Effluent Using Fenton Iron Sludge-Based Biochar/Fe(VI)/H2O2 Process" Applied Sciences 15, no. 11: 5945. https://doi.org/10.3390/app15115945
APA StyleWang, L., He, X., Liang, L., Wang, Y., Yan, B., Chen, G., Li, N., & Hou, L. (2025). Removal of COD from Secondary Effluent Using Fenton Iron Sludge-Based Biochar/Fe(VI)/H2O2 Process. Applied Sciences, 15(11), 5945. https://doi.org/10.3390/app15115945







