Estimation of Amino Acid and Tea Polyphenol Content of Tea Fresh Leaves Based on Fractional-Order Differential Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
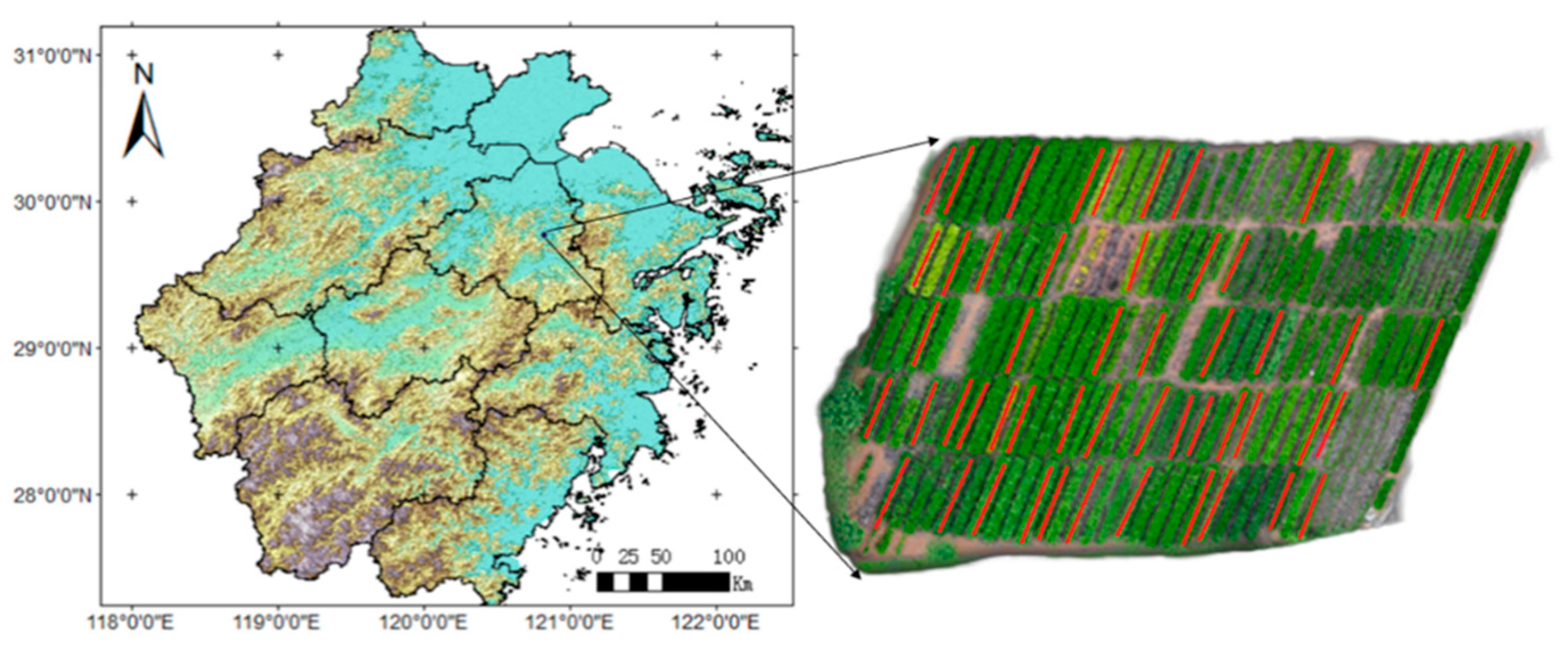
2.1. Research Area
2.2. Experiment
2.2.1. Sample Collection and Spectral Measurements
2.2.2. Chemical Determination of AA and TP Content
AA Content Measurement
TP Content Measurement
2.3. Method
2.3.1. FOD Spectral Data Processing
- denotes the fractional derivative of order of f(x);
- α ∈ R represents the fractional order of differentiation;
- represents a positive real number approaching zero;
- represents the generalized binomial coefficient, defined as follows:
2.3.2. CARS-PLSR Feature Band Selection
- is the response variable vector;
- is the predictor variable matrix;
- is the regression coefficient vector;
- is the error term vector.
2.3.3. CC-PLSR Method
2.3.4. Model Evaluation
2.3.5. Comparative Preprocessing Treatments
3. Results
3.1. AA and TP Content in Fresh Tea Leaves
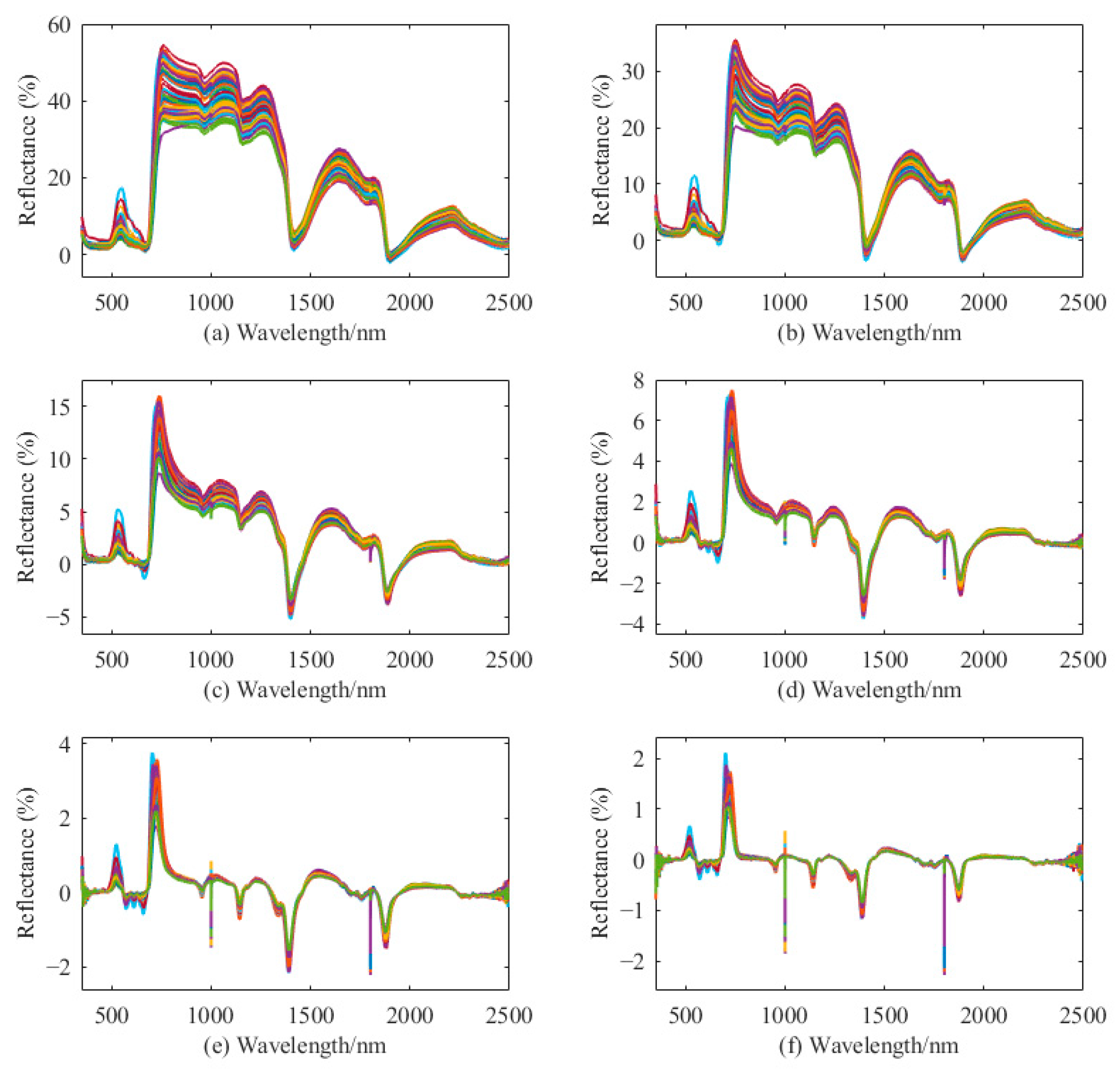
3.2. Hyperspectral Characteristics of AA and TP
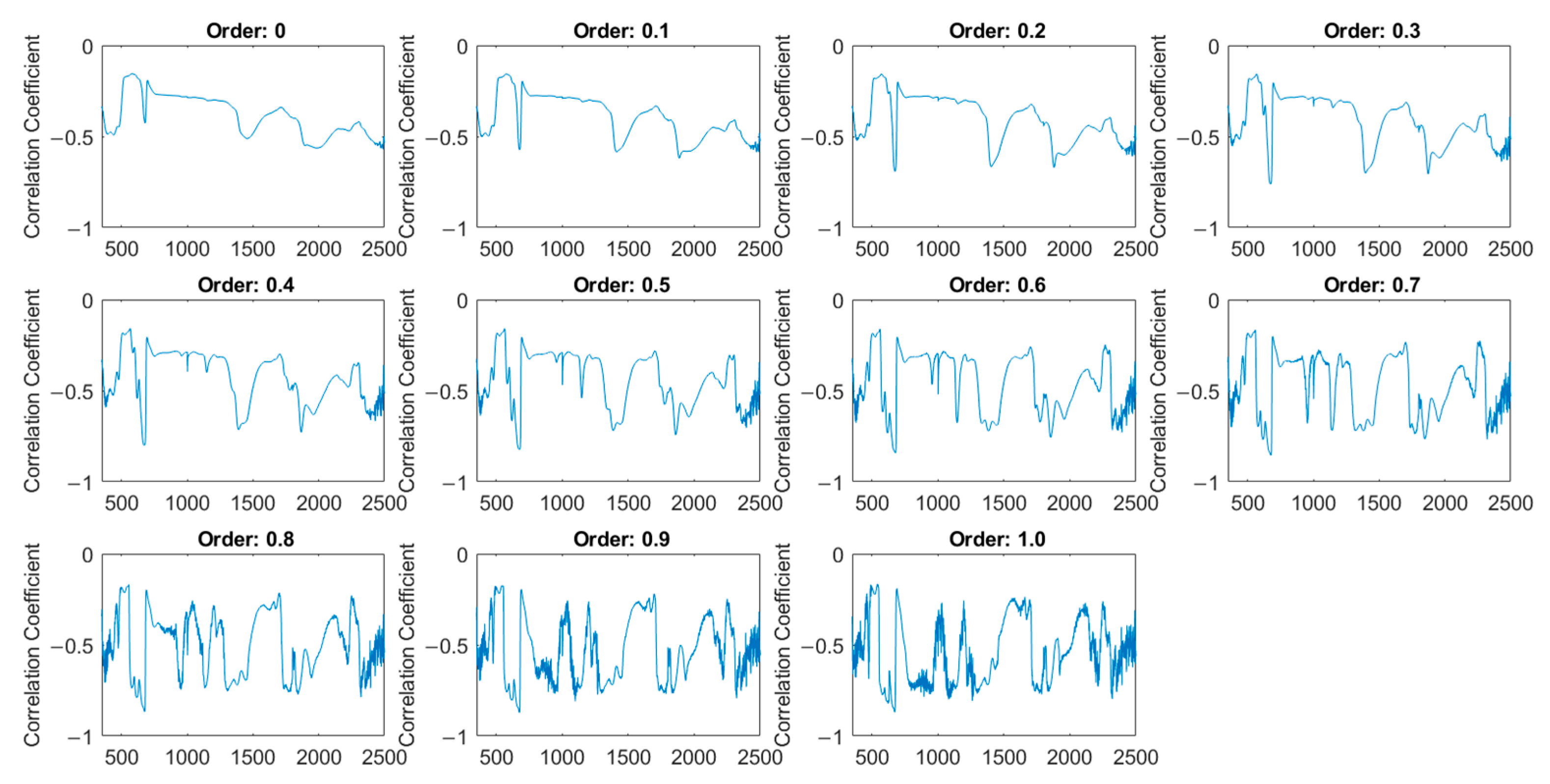
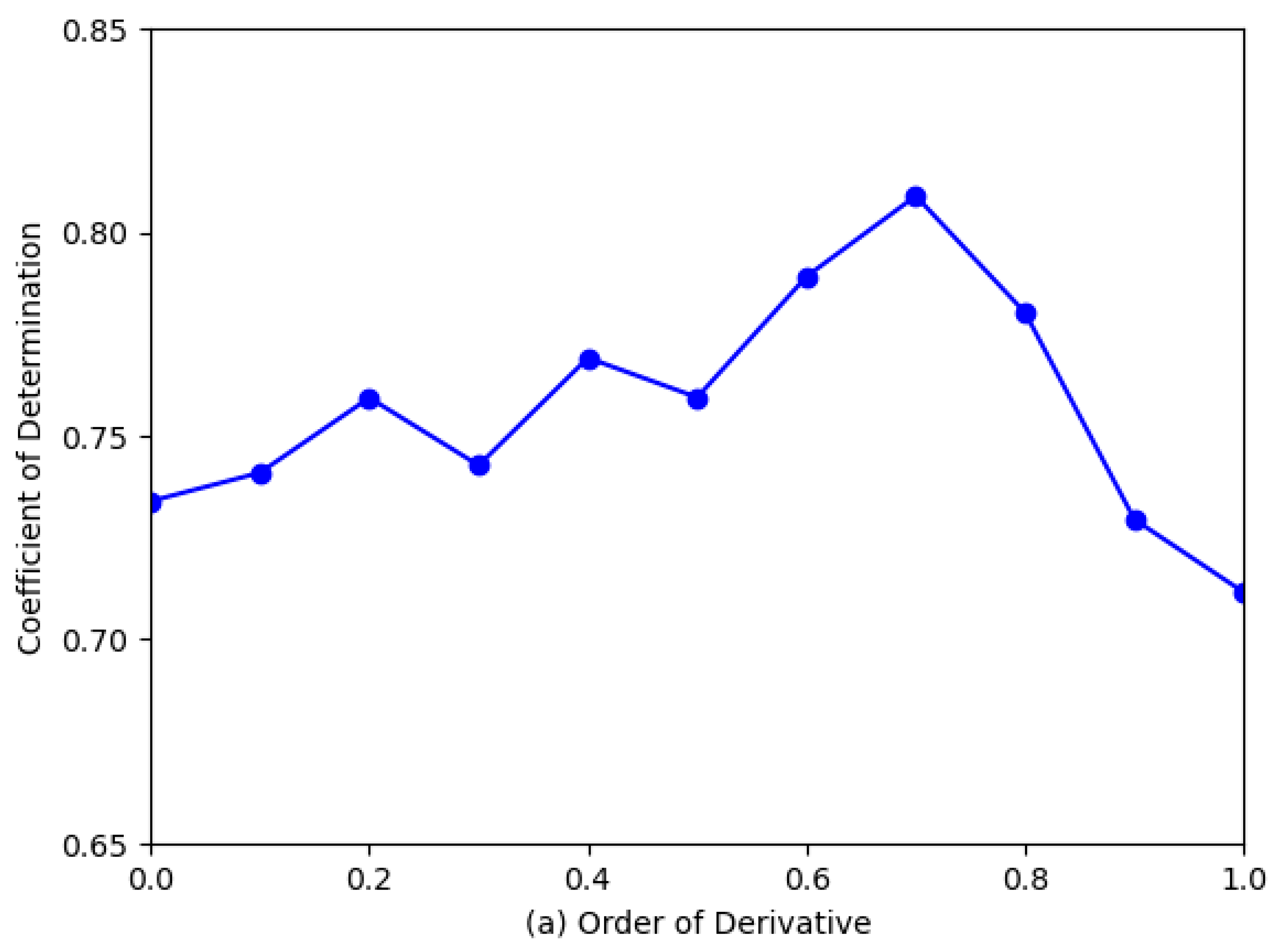
3.3. Determination of Optimal FOD Order
3.4. Analysis of CARS-Selected Feature Bands
3.5. Analysis of Prediction Based on MSC and SNV Preprocess
4. Discussion
4.1. Comparison of Integer-Order and Fractional-Order Differential Spectra
4.2. Optimal Fractional Order for Quality Prediction
4.3. Comparison of CARS and CC Feature Band Extraction
4.4. Evaluation of AA and TP Prediction Models and Future Direction
4.5. Comparison with Other Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raza, A.; Chaoqun, C.; Luo, L.; Asghar, M.A.; Li, L.; Shoaib, N.; Yin, C. Combined application of organic and chemical fertilizers improved the catechins and flavonoids biosynthesis involved in tea quality. Sci. Hortic. 2024, 337, 113518. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, B.; Yin, Z.; Gao, X.; Liu, M. Structure elucidation and anticancer activity of a heteropolysaccharide from white tea. Carbohydr. Polym. 2024, 333, 121976. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Wang, Y.; Shen, K.; Wang, Q.; Ahammed, G.J.; Han, W.; Jin, Z.; Li, X.; Shi, Y. Effects of Differential Shading on Summer Tea Quality and Tea Garden Microenvironment. Plants 2024, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Chen, M.; Bao, Q.; Wang, H.; Wang, Y.; Fu, H. The Effect of Intercropping with Different Leguminous Green Manures on the Soil Environment and Tea Quality in Tea Plantations. Microorganisms 2024, 12, 1721. [Google Scholar] [CrossRef]
- Ai, S.R.; Wu, R.M.; Yan, L.Y.; Wu, Y.H. Measurement of the Ratio of Tea Polyphenols to Amino Acids in Green Tea Infusion Based on near Infrared Spectroscopy. Adv. Mater. Res. 2011, 301–303, 1093–1097. [Google Scholar] [CrossRef]
- Wang, L.; Xu, R.; Hu, B.; Li, W.; Sun, Y.; Tu, Y.; Zeng, X. Analysis of free amino acids in Chinese teas and flower of tea plant by high performance liquid chromatography combined with solid-phase extraction. Food Chem. 2010, 123, 1259–1266. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Fan, K.; Mao, Y.; Shen, Y.; Ding, Z. Evaluation of important phenotypic parameters of tea plantations using multi-source remote sensing data. Front. Plant Sci. 2022, 13, 898962. [Google Scholar] [CrossRef]
- Moshou, D.; Bravo, C.; Wahlen, S.; West, J.; McCartney, A.; De Baerdemaeker, J.; Ramon, H. Simultaneous identification of plant stresses and diseases in arable crops using proximal optical sensing and self-organising maps. Precis. Agric. 2006, 7, 149–164. [Google Scholar] [CrossRef]
- Schulz, H.; Engelhardt, U.H.; Wegent, A.; Drews, H.-H.; Lapczynski, S. Application of Near-Infrared Reflectance Spectroscopy to the Simultaneous Prediction of Alkaloids and Phenolic Substances in Green Tea Leaves. J. Agric. Food Chem. 1999, 47, 5064–5067. [Google Scholar] [CrossRef]
- Dong, C.; Li, J.; Wang, J.; Liang, G.; Jiang, Y.; Yuan, H.; Yang, Y.; Meng, H. Rapid determination by near infrared spectroscopy of theaflavins-to-thearubigins ratio during Congou black tea fermentation process. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 227–234. [Google Scholar] [CrossRef]
- Schmitt, J.M. Fractional Derivative Analysis of Diffuse Reflectance Spectra. Appl. Spectrosc. 1998, 52, 840–846. [Google Scholar] [CrossRef]
- Zununjan, Z.; Turghan, M.A.; Sattar, M.; Kasim, N.; Emin, B.; Abliz, A. Combining the fractional order derivative and machine learning for leaf water content estimation of spring wheat using hyper-spectral indices. Plant Methods 2024, 20, 97. [Google Scholar] [CrossRef] [PubMed]
- GB/T 8313-2018; Determination of Total Polyphenols and Catechins Content in Tea (TEXT OF DOCUMENT IS IN CHINESE). Available online: https://webstore.ansi.org/standards/spc/gb83132018?srsltid=AfmBOoq3h9dEJLTeNYSe_yZwLh1Mb5yqiMcYz0ewqbOzJsv3Uxijuqp1 (accessed on 19 May 2025).
- Chen, Y.Q.; Petras, I.; Xue, D. Fractional order control—A tutorial. In Proceedings of the 2009 American Control Conference, St. Louis, MO, USA, 10–12 July 2009; pp. 1397–1411. [Google Scholar]
- Li, Z.; Liu, L.; Dehghan, S.; Chen, Y.; Xue, D. A review and evaluation of numerical tools for fractional calculus and fractional order controls. Int. J. Control. 2017, 90, 1165–1181. [Google Scholar] [CrossRef]
- Tolba, M.F.; Said, L.A.; Madian, A.H.; Radwan, A.G. FPGA Implementation of the Fractional Order Integrator/Differentiator: Two Approaches and Applications. IEEE Trans. Circuits Syst. I Regul. Pap. 2019, 66, 1484–1495. [Google Scholar] [CrossRef]
- Abbas, S.; Benchohra, M.; Lazreg, J.-E.; Zhou, Y. A survey on Hadamard and Hilfer fractional differential equations: Analysis and stability. Chaos, Solitons Fractals 2017, 102, 47–71. [Google Scholar] [CrossRef]
- Obeidat, N.A.; Bentil, D.E. New theories and applications of tempered fractional differential equations. Nonlinear Dyn. 2021, 105, 1689–1702. [Google Scholar] [CrossRef]
- Chen, L.; Xu, B.; Zhao, C.; Duan, D.; Cao, Q.; Wang, F. Application of Multispectral Camera in Monitoring the Quality Parameters of Fresh Tea Leaves. Remote. Sens. 2021, 13, 3719. [Google Scholar] [CrossRef]
- Koseoglu, M.; Deniz, F.N.; Alagoz, B.B.; Alisoy, H. An effective analog circuit design of approximate fractional-order derivative models of M-SBL fitting method. Eng. Sci. Technol. Int. J. 2022, 33, 101069. [Google Scholar] [CrossRef]
- Morais, J.; Zayed, H. Applications of differential subordination and superordination theorems to fluid mechanics involving a fractional higher-order integral operator. Alex. Eng. J. 2021, 60, 3901–3914. [Google Scholar] [CrossRef]
- Podlubny, I. Fractional Differential Equations: An Introduction to Fractional Deriva-Tives, Fractional Differential Equations, to Methods of Their Solution and Some of Their Applications; Academic Press: San Diego, CA, USA, 1998; Volume 198. [Google Scholar]
- Gao, S.; Xu, J.-H. Hyperspectral image information fusion-based detection of soluble solids content in red globe grapes. Comput. Electron. Agric. 2022, 196, 106822. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Shan, Y.; Su, D.; Ma, Q.; Wen, R.; Li, J. Qualitative and quantitative detection of honey adulterated with high-fructose corn syrup and maltose syrup by using near-infrared spectroscopy. Food Chem. 2017, 218, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xie, D.; Song, H.; Ma, J.; Zhou, Y.; Chen, J.; Yang, Y.; Huang, F. Estimation of chemical oxygen demand in different water systems by near-infrared spectroscopy. Ecotoxicol. Environ. Saf. 2022, 243, 113964. [Google Scholar] [CrossRef]
- Mosegaard, K.; Tarantola, A. Monte Carlo sampling of solutions to inverse problems. J. Geophys. Res. 1995, 100, 12431–12447. [Google Scholar] [CrossRef]
- Li, X.; Sun, C.; Luo, L.; He, Y. Determination of tea polyphenols content by infrared spectroscopy coupled with iPLS and random frog techniques. Comput. Electron. Agric. 2015, 112, 28–35. [Google Scholar] [CrossRef]
- Ren, G.; Wang, S.; Ning, J.; Xu, R.; Wang, Y.; Xing, Z.; Wan, X.; Zhang, Z. Quantitative analysis and geographical traceability of black tea using Fourier transform near-infrared spectroscopy (FT-NIRS). Food Res. Int. 2013, 53, 822–826. [Google Scholar] [CrossRef]
- Cozzolino, D. The Role of Visible and Infrared Spectroscopy Combined with Chemometrics to Measure Phenolic Compounds in Grape and Wine Samples. Molecules 2015, 20, 726–737. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Saimaiti, A.; Huang, S.-Y.; Xiong, R.-G.; Wu, S.-X.; Zhou, D.-D.; Yang, Z.-J.; Luo, M.; Gan, R.-Y.; Li, H.-B. Antioxidant Capacities and Polyphenol Contents of Kombucha Beverages Based on Vine Tea and Sweet Tea. Antioxidants 2022, 11, 1655. [Google Scholar] [CrossRef]
- Guo, P.; Li, T.; Gao, H.; Chen, X.; Cui, Y.; Huang, Y. Evaluating Calibration and Spectral Variable Selection Methods for Predicting Soil Nutrients Using Vis-NIR Spectroscopy. Remote. Sens. 2021, 13, 4000. [Google Scholar] [CrossRef]
- Jahani, T.; Kashaninejad, M.; Ziaiifar, A.M. Effect of selected pre-processing methods by PLSR to predict low-fat mozzarella texture measured by hyperspectral imaging. J. Food Meas. Charact. 2024, 18, 5060–5072. [Google Scholar] [CrossRef]
- Xiao, Q.; Tang, W.; Zhang, C.; Zhou, L.; Feng, L.; Shen, J. Spectral preprocessing combined with deep transfer learning to evaluate chlorophyll content in cotton leaves. Plant Phenomics 2022, 2022, 9813841. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, J.; Li, Y.; Meng, Y.; Zhang, Z. Modeling and prediction of soil organic matter content based on visible-near-infrared spectroscopy. Forests 2021, 12, 1809. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Wang, W.; Wang, F.; Liu, Q.; Yan, L. Research on Rapid and Low-Cost Spectral Device for the Estimation of the Quality Attributes of Tea Tree Leaves. Sensors 2023, 23, 571. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Skidmore, A.K.; Schlerf, M.; Wang, T.; Liu, Y.; Zeng, R.; Fei, T. Predicting foliar biochemistry of tea (Camellia sinensis) using reflectance spectra measured at powder, leaf and canopy levels. ISPRS J. Photogramm. Remote Sens. 2013, 78, 148–156. [Google Scholar] [CrossRef]
- Chanda, S.; Sing, D.; Majumder, S.; Nag, S.; Karmakar, S.; Upadhyay, A.K.; Hazarika, A.K.; Sabhapondit, S.; Tasrin, S. NIR spectroscopy with grey wolf optimization algorithm for prediction of polyphenol content in inward tea leaves. In Proceedings of the 2017 IEEE Calcutta Conference (CALCON), Kolkata, India, 2–3 December 2017; pp. 392–396. [Google Scholar]
- Bian, M.; Skidmore, A.K.; Schlerf, M.; Fei, T.; Liu, Y.; Wang, T. Reflectance Spectroscopy of Biochemical Components as Indicators of Tea (Camellia sinensis) Quality. Photogramm. Eng. Remote. Sens. 2010, 76, 1385–1392. [Google Scholar] [CrossRef]
- Zareef, M.; Arslan, M.; Hassan, M.; Ahmad, W.; Chen, Q. Comparison of Si-GA-PLS and Si-CARS-PLS build algorithms for quantitation of total polyphenols in black tea using the spectral analytical system. J. Sci. Food Agric. 2023, 103, 7914–7920. [Google Scholar] [CrossRef]





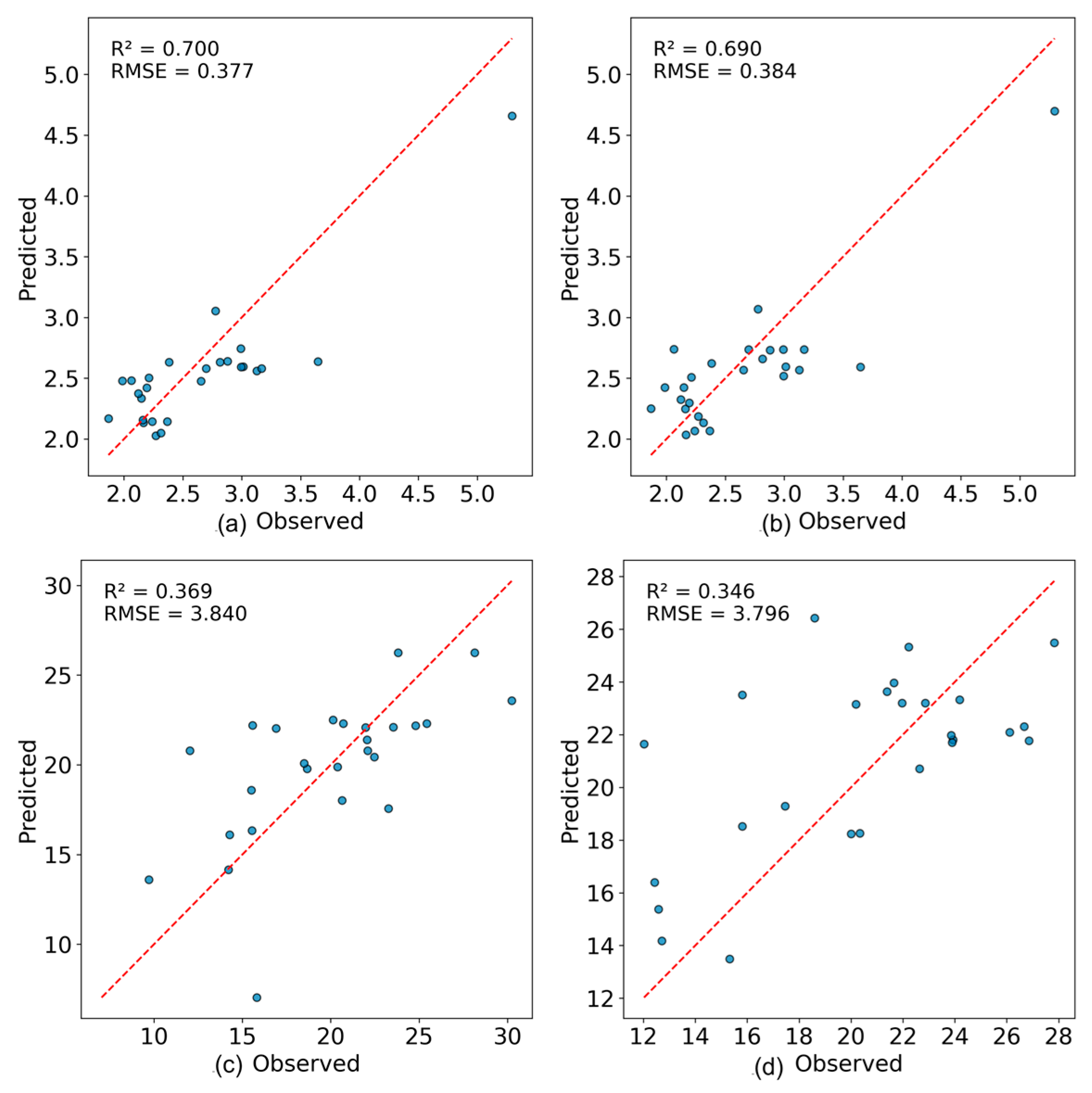
| Quality Indicator | Modeling Method | FOD-Order | R2 (Train) | RMSE (Train) | R2 (Test) | RMSE (Test) |
|---|---|---|---|---|---|---|
| AA | CC+PLSR | 0.7 | 0.67 | 0.51 | 0.63 | 0.47 |
| CARS+PLSR | 0.7 | 0.86 | 0.3 | 0.8 | 0.25 | |
| TP | CC+PLSR | 0.1 | 0.48 | 3.21 | 0.39 | 4.57 |
| CARS+PLSR | 0.1 | 0.7 | 2.79 | 0.42 | 3.96 |
| Quality Indicator | Modeling Method | Number of Bands | Characteristic Wavelengths (nm) |
|---|---|---|---|
| AA | CARS+PLSR | 19 | 372, 406, 515, 553, 641, 643, 676, 1207, 1415, 1892, 1893, 2103, 2413, 2430, 2443, 2456, 2465, 2470, 2496 |
| TP | CARS+PLSR | 22 | 354, 362, 368, 418, 645, 1021, 1194, 1428, 1541, 1651, 1658, 1678, 1685, 2136, 2273, 2430, 2449, 2458, 2476, 2483, 2488 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Sun, R.; Li, X.; Li, Y.; Zhao, L.; Huang, X.; Xu, Y. Estimation of Amino Acid and Tea Polyphenol Content of Tea Fresh Leaves Based on Fractional-Order Differential Spectroscopy. Appl. Sci. 2025, 15, 5792. https://doi.org/10.3390/app15115792
Li S, Sun R, Li X, Li Y, Zhao L, Huang X, Xu Y. Estimation of Amino Acid and Tea Polyphenol Content of Tea Fresh Leaves Based on Fractional-Order Differential Spectroscopy. Applied Sciences. 2025; 15(11):5792. https://doi.org/10.3390/app15115792
Chicago/Turabian StyleLi, Shirui, Rui Sun, Xin Li, Yang Li, Liang Zhao, Xinyu Huang, and Yufei Xu. 2025. "Estimation of Amino Acid and Tea Polyphenol Content of Tea Fresh Leaves Based on Fractional-Order Differential Spectroscopy" Applied Sciences 15, no. 11: 5792. https://doi.org/10.3390/app15115792
APA StyleLi, S., Sun, R., Li, X., Li, Y., Zhao, L., Huang, X., & Xu, Y. (2025). Estimation of Amino Acid and Tea Polyphenol Content of Tea Fresh Leaves Based on Fractional-Order Differential Spectroscopy. Applied Sciences, 15(11), 5792. https://doi.org/10.3390/app15115792








