One-Pot Synthesis of Magnetic Core-Shell Fe3O4@C Nanospheres with Pt Nanoparticle Immobilization for Catalytic Hydrogenation of Nitroarenes
Abstract
1. Introduction
2. Experimental
2.1. General Characterization
2.2. Synthesis of Fe3O4@C Nanospheres
2.3. Synthesis of Fe3O4@C–Pt Nanospheres
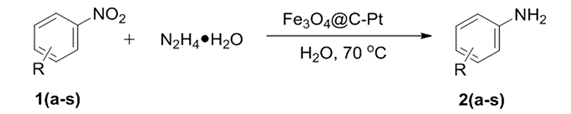
2.4. Catalytic Hydrogenation of Nitroarene Reactions Evaluation
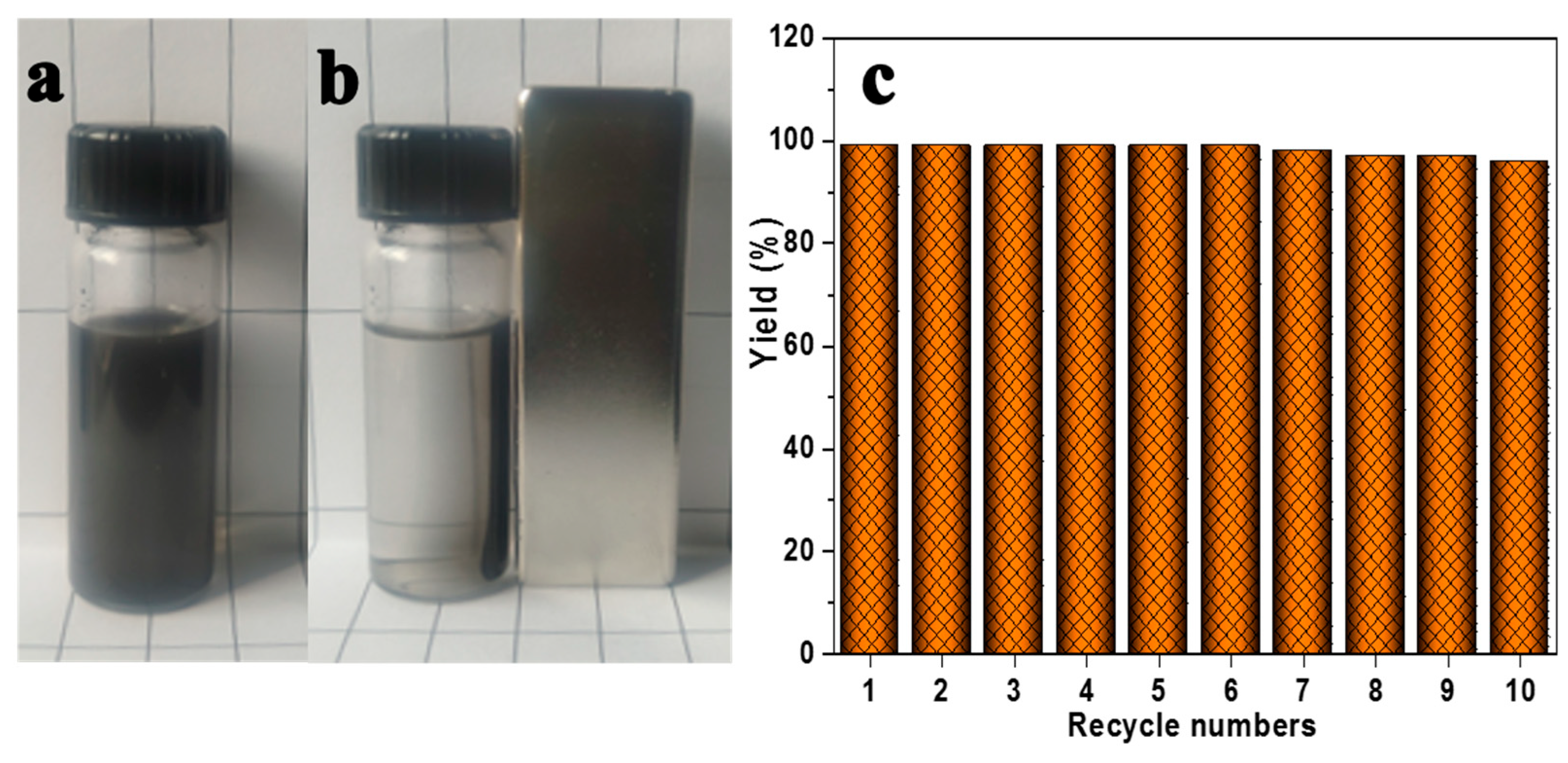
2.5. Catalyst Reused Evaluation
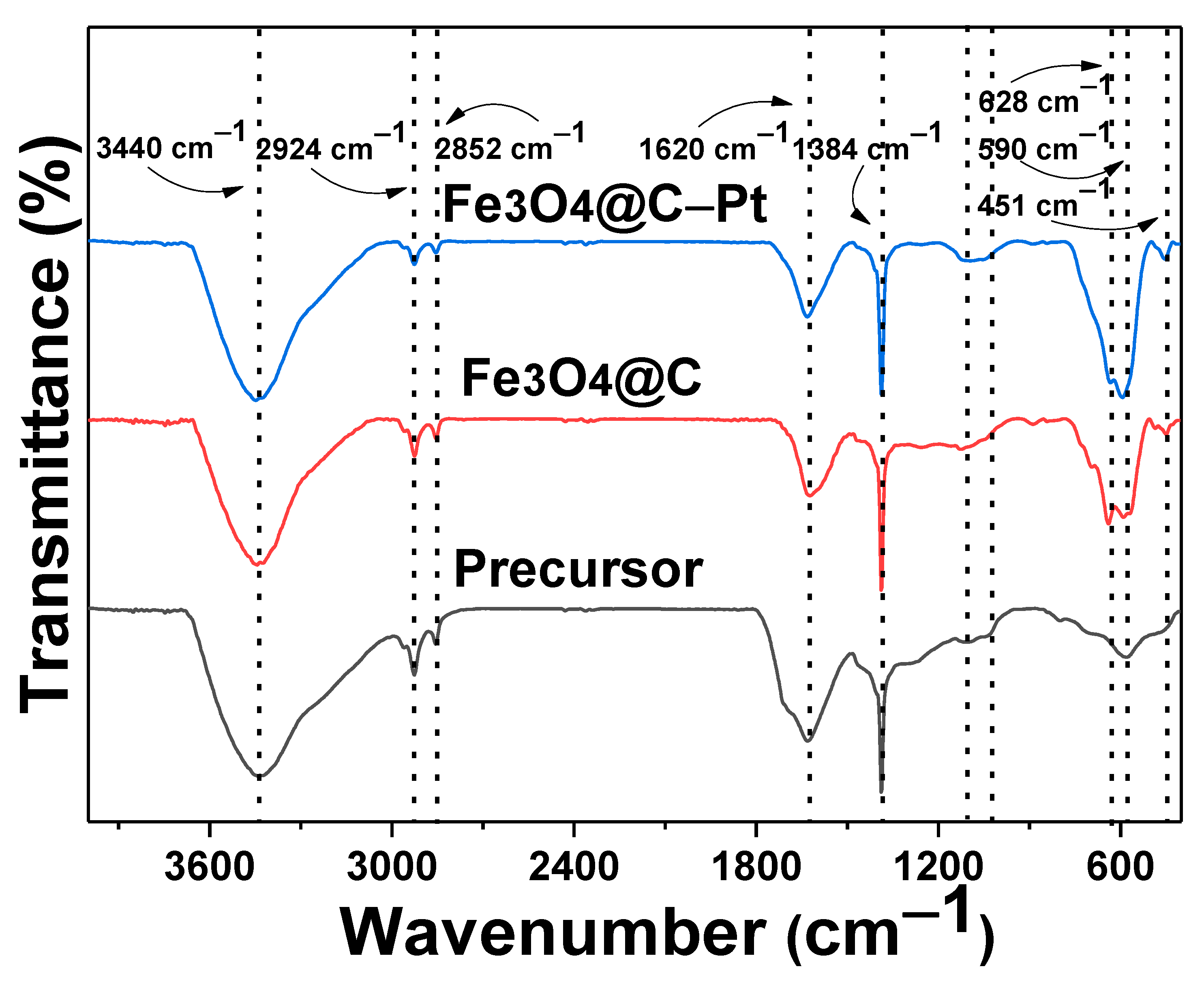
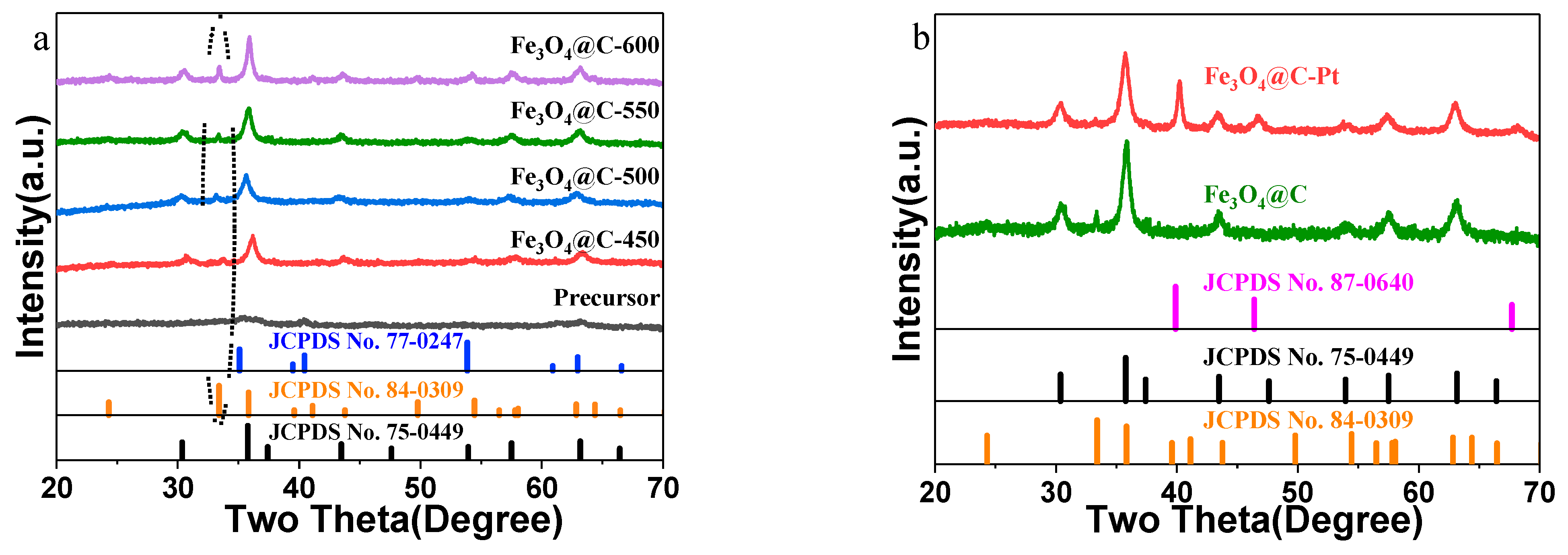
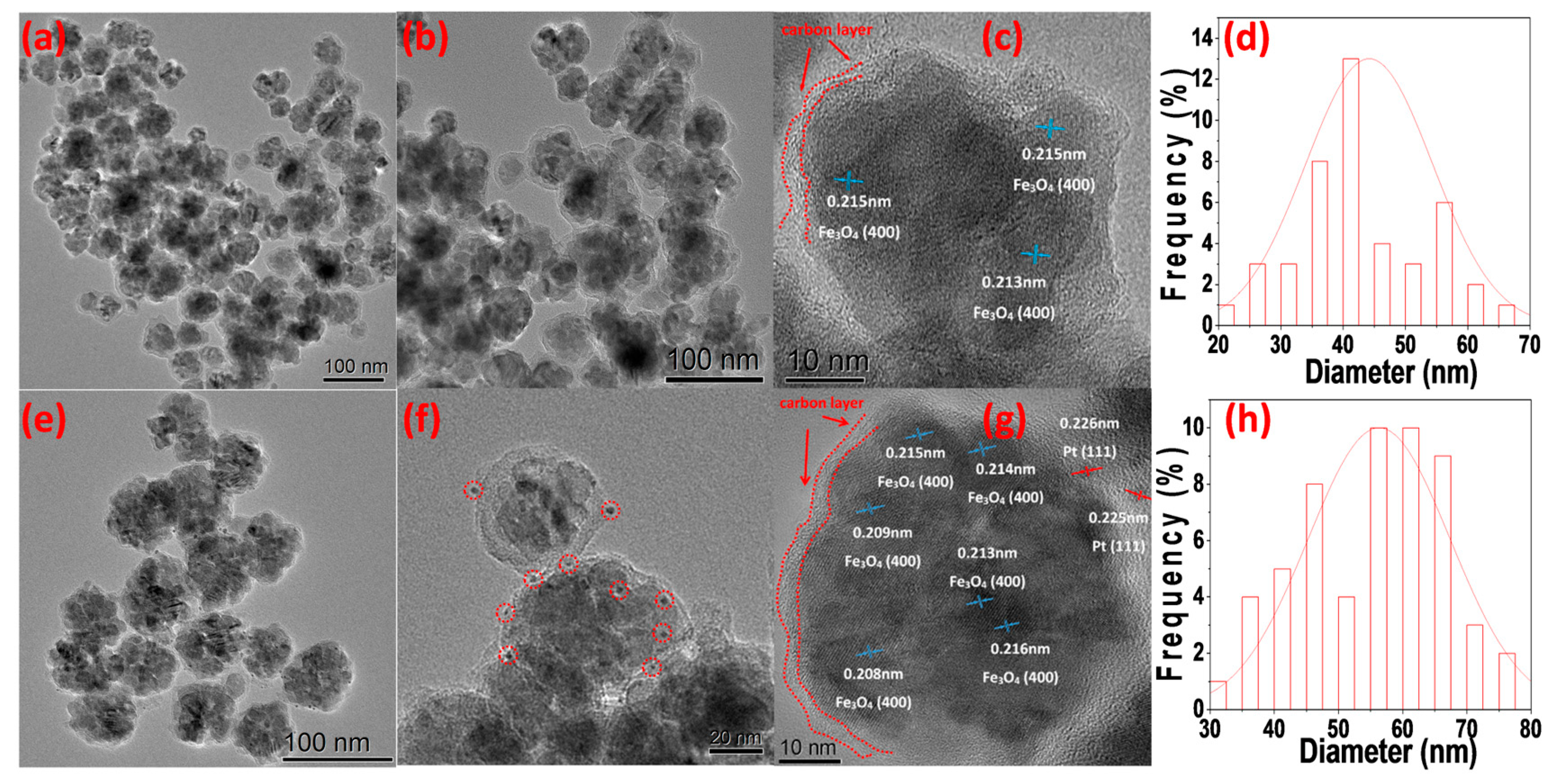
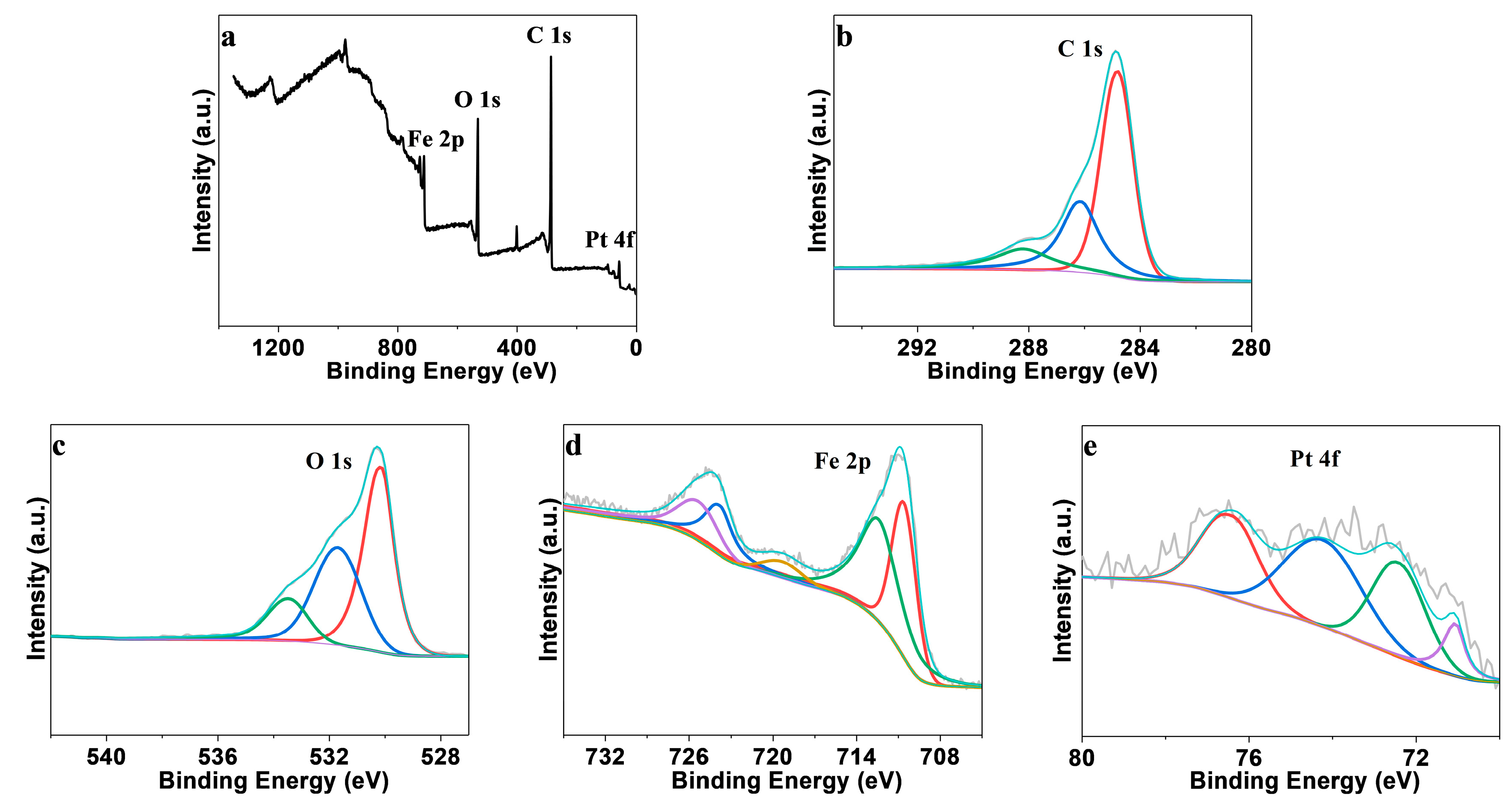
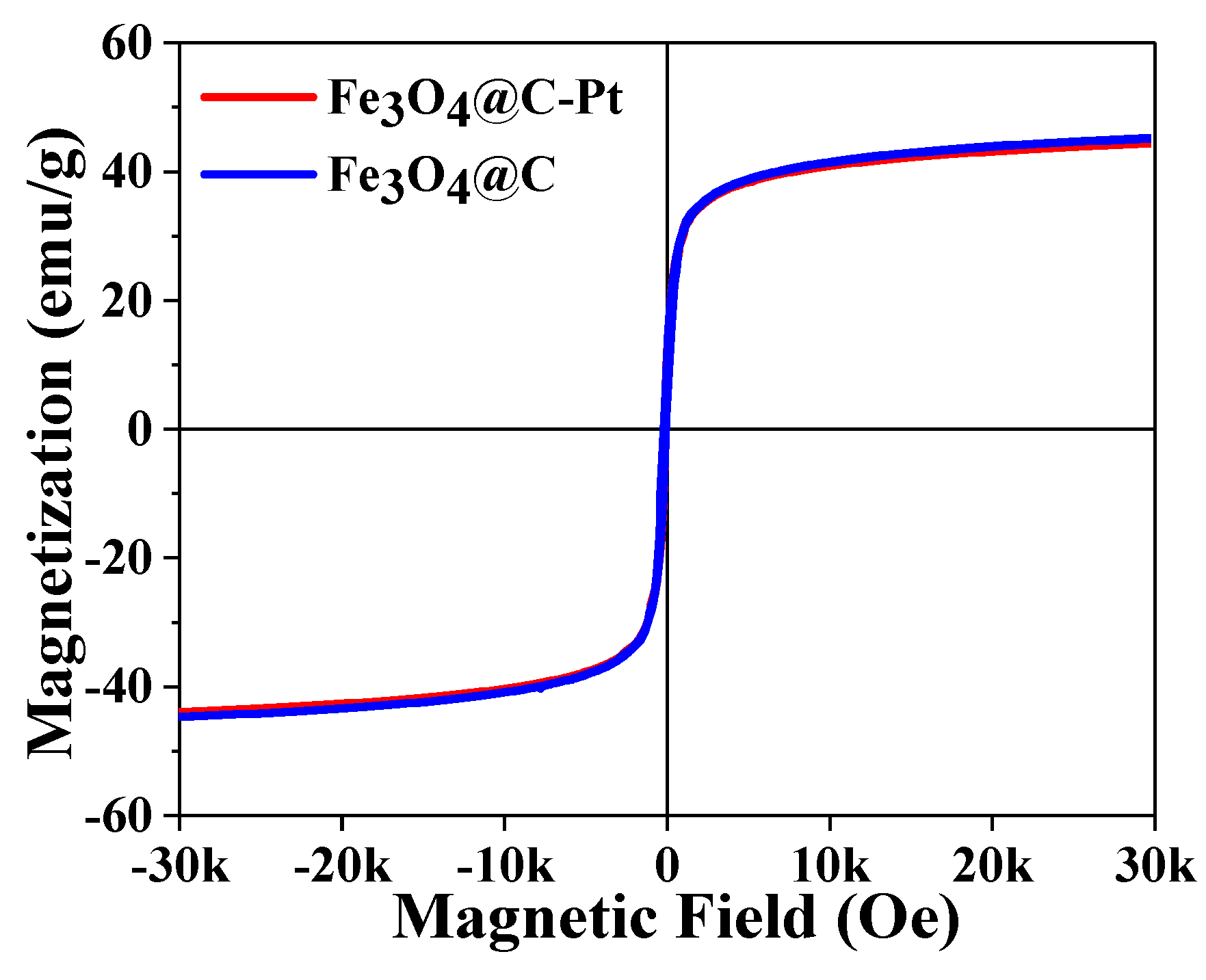
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ly, A.; Murphy, E.; Wang, H.; Huang, Y.; Ferro, G.; Guo, S.Y.; Asset, T.; Liu, Y.C.; Zenyuk, I.V.; Atanassov, P. Electrochemical trends of a hybrid platinum and metal–nitrogen–carbon catalyst library for the oxygen reduction reaction. EES Catal. 2024, 2, 624–637. [Google Scholar] [CrossRef]
- Chen, M.Y.; Li, Y.; Wu, H.R.; Lu, B.A.; Zhang, J.N. Highly stable Pt-based oxygen reduction electrocatalysts toward practical fuel cells: Progress and perspectives. Materials 2023, 16, 2590. [Google Scholar] [CrossRef]
- Linge, J.M.; Briega-Martos, V.; Hutzler, A.; Fritsch, B.; Erikson, H.; Tammeveski, K.; Cherevko, S. Stability of carbon supported silver electrocatalysts for alkaline oxygen reduction and evolution reactions. ACS Appl. Energy Mater. 2023, 6, 11497–11509. [Google Scholar] [CrossRef]
- Guo, S.Y.; Liu, Y.C.; Murphy, E.; Ly, A.; Xu, M.J.; Matanovic, I.; Pan, X.Q.; Atanassov, P. Robust palladium hydride catalyst for electrocatalytic formate formation with high CO tolerance. Appl. Catal. B-Environ. 2022, 316, 121659. [Google Scholar] [CrossRef]
- Medasani, B.; Park, Y.H.; Vasiliev, I. Theoretical study of the surface energy, stress, and lattice contraction of silver nanoparticles. Phys. Rev. B. 2007, 75, 235436. [Google Scholar] [CrossRef]
- Yang, X.F.; Wang, A.Q.; Qiao, B.T.; Li, J.; Liu, J.Y.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef]
- Nie, Y.; Deng, J.H.; Chen, S.G.; Wei, Z.D. Promoting stability and activity of PtNi/C for oxygen reduction reaction via polyaniline-confined space annealing strategy. Int. J. Hydrogen Energy 2019, 44, 5921–5928. [Google Scholar] [CrossRef]
- Gallon, B.J.; Kojima, R.W.; Kaner, R.B.; Diaconescu, P.L. Palladium Nanoparticles Supported on Polyaniline Nanofibers as a Semi-Heterogeneous Catalyst in Water. Angew. Chem. Int. Ed. 2007, 46, 7251–7254. [Google Scholar] [CrossRef]
- Deng, Y.H.; Qi, D.W.; Deng, C.H.; Zhang, X.M.; Zhao, D.Y. Superparamagnetic High-Magnetization Microspheres with an Fe3O4@SiO2 Core and Perpendicularly Aligned Mesoporous SiO2 Shell for Removal of Microcystins. J. Am. Chem. Soc. 2008, 130, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.P.; Zhang, Q.; Zhang, T.R.; Yin, Y.D. Core–Satellite Nanocomposite Catalysts Protected by a Porous Silica Shell: Controllable Reactivity, High Stability, and Magnetic Recyclability. Angew. Chem. Int. Ed. 2008, 47, 8924–8928. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Luque, R.; Fihri, A.; Zhu, H.B.; Bouhrara, M.; Basset, J.-M. Magnetically Recoverable Nanocatalysts. Chem. Rev. 2011, 111, 3036–3075. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Kostopoulou, A.; LaGrow, A.P. Magnetic Nanoparticle Composites: Synergistic Effects and Applications. Adv. Sci. 2021, 8, 2004951. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Wang, Y.S.; Lu, J.L.; Yan, X.; Liu, D.D.; Zhang, X.Q.; Huang, X.X.; Wen, G.W. Electromagnetic Absorption by Magnetic Oxide Nanomaterials: A Review. ACS Appl. Nano Mater. 2023, 6, 22611–22634. [Google Scholar] [CrossRef]
- Marcelo, L.R.; Santos de Gois, J.; Araujo da Silva, A.; Cesar, D.V. Synthesis of iron-based magnetic nanocomposites and applications in adsorption processes for water treatment: A review. Environ. Chem. Lett. 2021, 19, 1229–1274. [Google Scholar] [CrossRef]
- Du, Z.W.; Qiu, Y.; Niu, T.C.; Wang, W.C.; Ye, X.D.; Wang, J.; Zhang, W.L.; Choi, H.J.; Zeng, H.B. Bio-inspired passion fruit-like Fe3O4@C nanospheres enabling high-stability magnetorheological performances. Langmuir 2020, 36, 7706–7714. [Google Scholar] [CrossRef]
- Weber, D.; Rui, N.; Zhang, F.; Zhang, H.; Vovchok, D.; Wildy, M.; Arizapana, K.; Saporita, A.; Zhang, J.Z.; Senanayake, S.D.; et al. Carbon Nanosphere-Encapsulated Fe Core-Shell Structures for Catalytic CO2 Hydrogenation. ACS Appl. Nano Mater. 2022, 5, 11605–11616. [Google Scholar] [CrossRef]
- Liu, G.X.; Feng, K.; Cui, H.T.; Li, J.; Liu, Y.Y.; Wang, M.R. MOF derived in-situ carbon-encapsulated Fe3O4@C to mediate polysulfides redox for ultrastable Lithium-sulfur batteries. Chem. Eng. J. 2020, 381, 122652. [Google Scholar] [CrossRef]
- Yao, T.J.; Cui, T.Y.; Wu, J.; Chen, Q.Z.; Yin, X.J.; Cui, F.; Sun, K.N. Preparation of acid-resistant core/shell Fe3O4@C materials and their use as catalyst supports. Carbon 2012, 50, 2287–2295. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Xie, C.X.; Yu, S.T.; Liu, S.W. Synthesis of magnetic Ru/Fe3O4@C nanospheres with controlled carbon layer and its high selectivity to prepare cis-pinane. Chem. Eng. J. 2016, 303, 31–36. [Google Scholar] [CrossRef]
- Sun, W.Z.; Yang, W.Y.; Xu, Z.C.; Li, Q.; Shang, J.K. Synthesis of Superparamagnetic Core-Shell Structure Supported Pd Nanocatalysts for Catalytic Nitrite Reduction with Enhanced Activity, No Detection of Undesirable Product of Ammonium, and Easy Magnetic Separation Capability. ACS Appl. Mater. Interfaces 2016, 8, 2035–2047. [Google Scholar] [CrossRef]
- Li, L.; Li, R.M.; Gai, S.L.; He, F.; Yang, P.P. Facile fabrication and electrochemical performance of flower-like Fe3O4@C@layered double hydroxide (LDH) composite. J. Mater. Chem. A 2014, 2, 8758–8765. [Google Scholar] [CrossRef]
- Zhou, L.L.; Zhang, G.L.; Tian, J.; Wang, D.F.; Cai, D.Q.; Wu, Z.Y. Functionalized Fe3O4@C Nanospheres with Adjustable Structure for Efficient Hexavalent Chromium Removal. ACS Sustain. Chem. Eng. 2017, 5, 11042–11050. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Xu, S.H.; Xia, H.Y.; Zheng, F.C. Facile synthesis of Fe3O4@C hollow nanospheres and their application in polluted water treatment. Solid State Sci. 2016, 61, 16–23. [Google Scholar] [CrossRef]
- Jia, X.K.; Chen, X.; Liu, Y.; Zhang, B.L.; Zhang, H.P.; Zhang, Q.Y. Hydrophilic Fe3O4 nanoparticles prepared by ferrocene as high-efficiency heterogeneous Fenton catalyst for the degradation of methyl orange. Appl. Organomet. Chem. 2019, 33, 4826. [Google Scholar] [CrossRef]
- Liang, J.F.; Zhang, X.M.; Jing, L.Y.; Yang, H.Q. N-doped ordered mesoporous carbon as a multifunctional support of ultrafine Pt nanoparticles for hydrogenation of nitroarenes. Chin. J. Catal. 2017, 38, 1252–1260. [Google Scholar] [CrossRef]
- Song, M.X.; Song, Y.H.; Li, H.; Liu, P.Z.; Xu, B.S.; Wei, H.; Guo, J.J.; Wu, Y.C. Sucrose leavening-induced hierarchically porous carbon enhanced the hydrogen evolution reaction performance of Pt nanoparticles. Electrochim. Acta. 2019, 320, 134603. [Google Scholar] [CrossRef]
- Zhao, N.Q.; Wu, S.; He, C.N.; Wang, Z.Y.; Shi, C.S.; Liu, E.Z.; Li, J.J. One-pot synthesis of uniform Fe3O4 nanocrystals encapsulated in interconnected carbon nanospheres for superior lithium storage capability. Carbon 2013, 57, 130–138. [Google Scholar] [CrossRef]
- Yu, G.B.; Sun, B.; Pei, Y.; Xie, S.H.; Yan, S.R.; Qiao, M.H.; Fan, K.N.; Zhang, X.X.; Zong, B.N. FexOy@C Spheres as an Excellent Catalyst for Fischer-Tropsch Synthesis. J. Am. Chem. Soc. 2010, 132, 935–937. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, S.H.; Wang, H.; Chen, R.; Sun, P.C.; Chen, T.H. Platinum Nanoparticles Supported on Hierarchically Porous Aluminosilicate Nanospheres for Low-Temperature Catalytic Combustion of Volatile Organic Compounds. ACS Appl. Nano Mater. 2020, 3, 8472–8482. [Google Scholar] [CrossRef]
- Maruthupandy, M.; Muneeswaran, T.; Vennila, T.; Anand, M.; Cho, W.-S.; Quero, F. Development of chitosan decorated Fe3O4 nanospheres for potential enhancement of photocatalytic degradation of Congo red dye molecules. Spectrochim. Acta A 2022, 267, 120511. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Z.H.; Li, Q.; Wang, Y.B.; Shah, T.; Ahmad, M.; Zhang, Q.Y.; Zhang, B.L. Wrinkled Fe3O4@ C magnetic composite microspheres: Regulation of magnetic content and their microwave absorbing performance. J. Colloid Interface Sci. 2021, 601, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Y. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. 2004, 116, 607–611. [Google Scholar] [CrossRef]
- Straten, J.W.; Schleker, P.; Krasowska, M.; Veroutis, E.; Granwehr, J.; Auer, A.A.; Becker, S.; Schlögl, R.; Heumann, S. Nitrogen-functionalized hydrothermal carbon materials by using urotropine as the nitrogen precursor. Chem.–A Eur. J. 2018, 24, 12298–12317. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Jing, S.H.; Peng, X.W.; Chen, Z.H.; Hu, Y.J.; Zhuo, H.; Sun, R.C.; Zhong, L.X. Synthesizing green carbon dots with exceptionally high yield from biomass hydrothermal carbon. Cellulose 2020, 27, 415–428. [Google Scholar] [CrossRef]
- Vieira, L.H.S.; Sabino, C.M.S.; Júnior, F.H.S.; Rocha, J.S.; Castro, M.O.; Alencar, R.S.; da Costa, L.S.; Viana, B.C.; de Paula, A.J.; Soares, J.M.; et al. Strategic design of magnetic carbonaceous nanocomposites and its application as multifunctional adsorbent. Carbon 2020, 161, 758–771. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.H.; Wang, X.Z.; Deng, X.L. Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef]
- Imran, M.; Zouli, N.; Ahamad, T.; Alshehri, S.M.; Chandan, M.R.; Hussain, S.; Aziz, A.; Dar, M.A.; Khan, A. Carbon-coated Fe3O4 core–shell super-paramagnetic nanoparticle-based ferrofluid for heat transfer applications. Nanoscale Adv. 2021, 3, 1962–1975. [Google Scholar] [CrossRef]
- Si, Y.; Shi, S.Y.; Jing, J.Y.; Bai, Y.; Wang, Q. Magnetization of amorphous FeOOH chrysanthemum-like nanosheets under ambient conditions. Chem. Commun. 2023, 59, 5701–5704. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, S.N.; Xi, S.B.; Ren, X.; Zhou, Y.; Zhang, G.L.; Yang, H.T.; Du, Y.H.; Xu, J.Z. Tailoring the Co3d-O2p covalency in LaCoO3 by Fe substitution to promote oxygen evolution reaction. Chem. Mater. 2017, 29, 10534–10541. [Google Scholar] [CrossRef]
- Pinto, I.S.; Pacheco, P.H.; Coelho, J.V.; Lorençon, E.; Ardisson, J.D.; Fabris, J.D.; de Souza, P.P.; Krambrock, K.W.H.; Oliveira, L.C.A.; Pereira, M.C. Nanostructured δ-FeOOH: An efficient Fenton-like catalyst for the oxidation of organics in water. Appl. Catal. B-Environ. 2012, 119, 175–182. [Google Scholar] [CrossRef]
- Qian, M.; Cheng, X.; Sun, T.; Tian, J.; Isimjan, T.T.; Shi, Z.; Yang, X. Synergistic catalytic effect of N-doped carbon embedded with CoFe-rich CoFe2O4 clusters as highly efficient catalyst towards oxygen reduction. J. Alloys Compd. 2020, 819, 153015. [Google Scholar] [CrossRef]
- Chai, K.; Yang, X.; Shen, R.; Chen, J.; Su, W.; Su, A. A high activity mesoporous Pt@KIT-6 nanocomposite for selective hydrogenation of halogenated nitroarenes in a continuous-flow microreactor. Nanoscale Adv. 2023, 5, 5649–5660. [Google Scholar] [CrossRef]
- ur Rehman, W.; Jiang, Z.; Qu, Z.; Wang, X.; Du, X.; Ghani, A.; Kabir, F.; Xu, Y. Porous carbon derived from cherry blossom leaves treatment with Fe3O4 enhanced the electrochemical performance of a lithium storage anode. Electrochim. Acta 2023, 455, 142426. [Google Scholar] [CrossRef]
- Qiao, Z.; Hwang, S.; Li, X.; Wang, C.; Samarakoon, W.; Karakalos, S.; Li, D.; Chen, M.; He, Y.; Wang, M.; et al. 3D porous graphitic nanocarbon for enhancing the performance and durability of Pt catalysts: A balance between graphitization and hierarchical porosity. Energy Environ. Sci. 2019, 12, 2830–2841. [Google Scholar] [CrossRef]
- Zhang, F.W.; Guo, H.F.; Liu, M.M.; Zhao, Y.; Hong, F.; Li, J.J.; Dong, Z.P.; Qiao, B.T. Enhancing the chemoselective hydrogenation of nitroarenes:Designing a novel surface-strained carbon-based Pt nanocatalyst. Chin. J. Catal. 2023, 48, 195–204. [Google Scholar] [CrossRef]
- Shu, C.Y.; Yang, X.D.; Chen, Y.Z.; Fang, Y.; Zhou, Y.P.; Liu, Y.N. Nano-Fe3O4 Grown on Porous Carbon and its Effect on Oxygen Reduction Reaction for DMFCs with Polymer Fiber Membrane. RSC Adv. 2016, 6, 37012–37017. [Google Scholar] [CrossRef]
- Tong, Z.Y.; Liao, Z.J.; Liu, Y.Y.; Ma, M.L.; Bi, Y.X.; Huang, W.B.; Ma, Y.; Qiao, M.T.; Wu, G.L. Hierarchical Fe3O4/Fe@C@MoS2 core-shell nanofibers for efficient microwave absorption. Carbon 2021, 179, 646–654. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Tian, H.L.; Xu, R.X.; Men, W.W.; Su, T.; Qu, Y.G.; Zhao, W.; Liu, D. Magnetic crystallite-decorated hollow multi-cavity carbon nanosheet spheres for superior electromagnetic absorption. Carbon 2023, 205, 138–150. [Google Scholar] [CrossRef]
- Shu, R.W.; Li, X.H.; Tian, K.H.; Shi, J.J. Fabrication of bimetallic metal-organic frameworks derived Fe3O4/C decorated graphene composites as high-efficiency and broadband microwave absorbers. Compos. Part B-Eng. 2022, 228, 109423. [Google Scholar] [CrossRef]
- Zhang, P.; Li, R.; Huang, Y.M.; Chen, Q.W. A Novel Approach for the in situ Synthesis of Pt-Pd Nanoalloys Supported on Fe3O4@C Core–shell Nanoparticles with Enhanced Catalytic Activity for Reduction Reactions. ACS Appl. Mater. Interfaces 2014, 6, 2671–2678. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reino, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Seaton, N.A.; Walton, J.P.R.B.; Quirke, N. A New Analysis Method for the Determination of the Pore Size Distribution of Porous Carbons from Nitrogen Adsorption Measurements. Carbon 1989, 27, 853–861. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, T.; Zheng, K.; Zhou, E.M.; Shen, C.; Jia, A.Q.; Zhang, Q.F. Magnetically Reusable Fe3O4@NC@Pt Catalyst for Selective Reduction of Nitroarenes. Catalysts 2021, 11, 1219. [Google Scholar] [CrossRef]
- Deng, X.; Qin, B.; Liu, R.Z.; Qin, X.T.; Dai, W.L.; Wu, G.J.; Guan, N.J.; Ma, D.; Li, L.D. Zeolite-encaged isolated platinum ions enable heterolytic dihydrogen activation and selective hydrogenations. J. Am. Chem. Soc. 2021, 143, 20898–20906. [Google Scholar] [CrossRef]
- Byun, S.; Song, Y.; Kim, B.M. Heterogenized Bimetallic Pd-Pt-Fe3O4 Nanoflakes as Extremely Robust, Magnetically Recyclable Catalysts for Chemoselective Nitroarene Reduction. ACS Appl. Mater. Interfaces 2016, 8, 14637–14647. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, X.G.; Xing, Z.K.; Chen, X.B.; Zou, X.J.; Lu, X.G. Highly active and chemoselective reduction of halogenated nitroarenes catalyzed by ordered mesoporous carbon supported platinum nanoparticles. ACS Sustainable Chem. Eng. 2019, 7, 8908–8916. [Google Scholar] [CrossRef]
- Wu, Z.S.; Wang, S.; Gao, D.W.; Hu, R.M.; Jiang, X.C.; Chen, G.Z. Unveiling the Origin of Enhanced Catalytic Performance of CePO4/Pt for Selective Hydrogenation of Nitroarenes. J. Catal. 2024, 430, 115313. [Google Scholar] [CrossRef]
- Jing, P.; Gan, T.; Qi, H.; Zheng, B.; Chu, X.F.; Yu, G.Y.; Yan, W.F.; Zou, Y.C.; Zhang, W.X.; Liu, G. Synergism of Pt nanoparticles and iron oxide support for chemoselective hydrogenation of nitroarenes under mild conditions. Chin. J. Catal. 2019, 40, 214–222. [Google Scholar] [CrossRef]
- Li, J.F.; Ding, S.L.; Wang, F.S.; Zhao, H.C.; Kou, J.F.; Akram, M.; Xu, M.H.; Gao, W.; Liu, C.; Yang, H.R.Y.; et al. Platinum clusters anchored on sulfur-doped ordered mesoporous carbon for chemoselective hydrogenation of halogenated nitroarenes. J. Colloid Interface Sci. 2022, 625, 640–650. [Google Scholar] [CrossRef]
- Gao, M.M.; Kou, J.F.; Xu, M.H.; Yuan, K.; Li, M.Y.; Dong, Z.P. Electron-rich Pt anchored on covalent triazine frameworks for the selective hydrogenation of halogenated nitrobenzenes. Green Chem. 2024, 26, 3884–3902. [Google Scholar] [CrossRef]
- Michalke, J.; Haas, M.; Krisch, D.; Bögl, T.; Bartling, S.; Rockstroh, N.; Schöfberger, W.; Topf, C. Generation of Cobalt-Containing Nanoparticles on Carbon via Pyrolysis of a Cobalt Corrole and Its Application in the Hydrogenation of Nitroarenes. Catalysts 2022, 12, 11. [Google Scholar] [CrossRef]


 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Product (2a–2s) | Time (h) | Yield b (%) | |
| 1 | 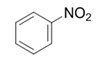 | 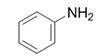 | 2a | 3 h | 99 |
| 2 | 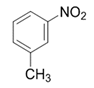 | 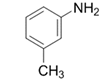 | 2b | 3 h | 96 |
| 3 | 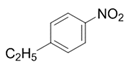 | 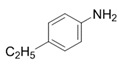 | 2c | 3 h | 99 |
| 4 | 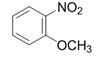 | 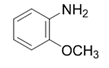 | 2d | 6 h | 94 |
| 5 | 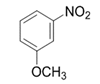 | 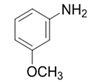 | 2e | 3 h | 97 |
| 6 | 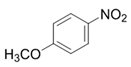 |  | 2f | 3 h | 99 |
| 7 | 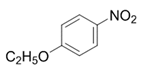 | 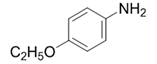 | 2g | 3 h | 99 |
| 8 | 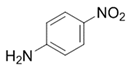 |  | 2h | 3 h | 99 |
| 9 | 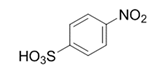 | 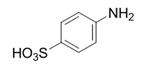 | 2i | 3 h | 98 |
| 10 |  | 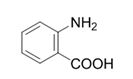 | 2j | 6 h | 94 |
| 11 | 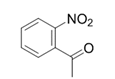 | 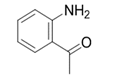 | 2k | 6 h | 95 |
| 12 | 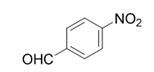 | 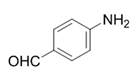 | 2l | 9 h | 95 |
| 13 |  | 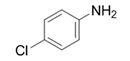 | 2m | 3 h | 99 |
| 14 | 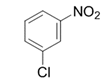 |  | 2n | 6 h | 95 |
| 15 |  | 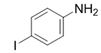 | 2o | 9 h | 93 |
| 16 | 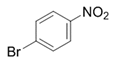 | 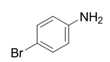 | 2p | 6 h | 95 |
| 17 |  | 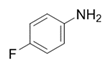 | 2q | 6 h | 99 |
| 18 | 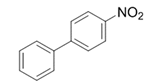 |  | 2r | 3 h | 98 |
| 19 |  | 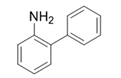 | 2s | 6 h | 95 |
| 20 |  | 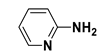 | 2t | 9 h | 94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, J.; Gao, Y.; Zheng, K.; Shen, C.; Jia, A.; Zhang, Q. One-Pot Synthesis of Magnetic Core-Shell Fe3O4@C Nanospheres with Pt Nanoparticle Immobilization for Catalytic Hydrogenation of Nitroarenes. Appl. Sci. 2025, 15, 5773. https://doi.org/10.3390/app15105773
Qiao J, Gao Y, Zheng K, Shen C, Jia A, Zhang Q. One-Pot Synthesis of Magnetic Core-Shell Fe3O4@C Nanospheres with Pt Nanoparticle Immobilization for Catalytic Hydrogenation of Nitroarenes. Applied Sciences. 2025; 15(10):5773. https://doi.org/10.3390/app15105773
Chicago/Turabian StyleQiao, Jun, Yang Gao, Kai Zheng, Chao Shen, Aiquan Jia, and Qianfeng Zhang. 2025. "One-Pot Synthesis of Magnetic Core-Shell Fe3O4@C Nanospheres with Pt Nanoparticle Immobilization for Catalytic Hydrogenation of Nitroarenes" Applied Sciences 15, no. 10: 5773. https://doi.org/10.3390/app15105773
APA StyleQiao, J., Gao, Y., Zheng, K., Shen, C., Jia, A., & Zhang, Q. (2025). One-Pot Synthesis of Magnetic Core-Shell Fe3O4@C Nanospheres with Pt Nanoparticle Immobilization for Catalytic Hydrogenation of Nitroarenes. Applied Sciences, 15(10), 5773. https://doi.org/10.3390/app15105773





