Abstract
This study aimed to assess the clinical value of three-dimensional printed personalised vascular models (3DPPVMs) in assisting with the pre-operative planning and simulation of endovascular interventions. CT angiographic images of four cases, namely, abdominal aorta aneurysm (AAA), carotid artery stenosis, coronary artery stenosis, and renal artery stenosis, were selected, and 3DPPVMs were obtained. A total of 21 clinicians specialising in interventional radiology and vascular surgery were invited to participate in the study, comprising 6 radiologists and 15 vascular surgeons. Of these, 66.7% had not used a 3DPPVM prior to their participation. Considering all areas of experience and all four models, it was observed that 75% of the participants gave a ranking of 7 or above out of 10 with regard to the recommendation of the use of the 3DPPVMs. The mean scores of the participants’ ranking of the models ranged from 3.2 to 4.3 out of 5. The AAA model was ranked the highest for realism (4.10 ± 0.89, p = 0.002), the planning of interventions and simulations (3.90 ± 1.12 and 4.05 ± 0.95), the development of haptic skills (3.56 ± 0.98), reducing the procedure time (3.47 ± 1.12), and clarifying the pathology to patients (4.33 ± 0.69, p all >0.05), indicating consistency amongst the participants. The carotid artery model was ranked the highest for accurately displaying anatomical structures (4.3 ± 0.73). All the 3DPPVMs enhanced the understanding of the disease demonstrated, with rankings between 3.8 and 3.95. All the models aided in elucidating the intervention procedure required and in the planning of vascular interventions, with rankings of 3.5 and 3.9. The highest rankings were given by qualified clinicians with 8 or more years of experience. This study shows the potential value of using 3D-printed vascular models in education for clinicians and patients, as well as for clinical training and the pre-surgical simulation of endovascular stent-grafting procedures.
1. Introduction
The challenges associated with diagnosing and treating vascular disease are many and diverse. The complexity of and variation in each patient’s anatomy and morphology, the tortuosity of access vessels, and hostile environments (such as thrombus and calcification deposition in blood vessels) make intervention multifaceted and risky. The current practice of endovascular aortic repair (EVAR) relies on two-dimensional (2D) and three-dimensional (3D) CT imaging visualisations, and these meet most of the current requirements for the planning of EVAR. However, they do not provide a realistic 3D representation of the relationship between complex vascular diseases and anatomical structures [1]. The implementation of 3D printing technology to produce patient-specific or personalised models has the potential to overcome the limitations of the currently used 2D and 3D visualisations, owing to its superiority in producing realistic 3D models of anatomical structures in relation to pathology.
It is purported that clinician skills can be optimised when 3D-printed models are introduced for training and procedural simulation [2]. Three-dimensional-printed cardiac models have been produced for teaching anaesthetic residents and fellows anatomy and pathology and for planning surgical interventions [3], while 3D-printed personalised vascular models (3DPPVMs) have been utilised for the pre-surgical planning and simulation of EVAR [4,5,6,7]. Previous studies have demonstrated the utility and clinical value of 3DPPVMs in vascular conditions, including aneurysm, dissection, extremity vascular disease, and other arterial diseases [2,3,4,5,8,9,10,11,12,13]. In a recent systematic and meta-analysis, Bernhard et al. analysed 107 studies with regard to the use of 3D-printed models in patient education, simulation, training, and procedural planning for cardiovascular interventions [14]. Most of the 3D-printed models were used in congenital heart disease (38 studies), left atrial appendage occlusion (11 studies), and aortic disease (10 studies). Of these 10 studies, the use of 3D-printed models was found to change the decision making in 20% of cases in patients with aortic aneurysm when compared to reviewing standard CT images [15]; reduce the fluoroscopy and intervention times (by 30% and 29%, respectively); and reduce the contrast medium (by 25%) in the training of residents in EVAR [16]. The current evidence confirms the accuracy, reliability, and potential clinical value of 3D-printed models in aortic and other vascular diseases, although further evidence is needed to support broader applications of 3D-printed vascular models.
There is a paradigm shift in surgical training, as it is moving from traditional methods to simulation-based approaches, with the aim of developing more effective learning environments. With the advancement of the materials used in 3D printing, it is possible to produce models with “tissue-mimicking properties” [17], which further enhances the applications of 3D printing technology in the cardiovascular field. Wu et al. [4] developed a personalised 3D-printed aortic dissection model and tested different materials. Their results showed that Agilus A30 had a tensile strength similar to normal human aortic tissue, with similar CT attenuation on both non-contrast and contrast-enhanced CT scans of aortic dissection. This prompted us to use this material to produce 3DPPVMs in our study, as we aimed to produce realistic vascular models for education and simulation purposes. Our study aimed to address the research gap by including common vascular diseases, such as aortic, carotid, coronary, and renal arterial diseases, with the development of corresponding 3D-printed models, as most previous studies are based on either isolated case reports or case series or a particular anatomical vascular region [14].
The primary aim of this study was to produce 3DPPVMs with materials that are flexible and responsive, with properties similar to arterial tissue, and to include models of abdominal aortic aneurysm, carotid artery stenosis, coronary artery, and renal artery stenosis based on selected CT angiographic images. The secondary aim of this study was to determine the clinical value of the 3DPPVMs in simulating interventional vascular procedures for the pre-surgical planning and treatment of these common vascular diseases.
2. Materials and Methods
2.1. Study Strategy
A qualitative/quantitative mixed study design was used to ascertain the applicability of 3DPPVMs by specialist clinicians. Ethical approval for this study was obtained from Curtin University Human Research Ethics Committee (HRE2023-0271). Computed tomography angiography (CTA) imaging data from four anonymised patients, comprising abdominal aortic aneurysm, left internal carotid artery stenosis, right coronary artery stenosis, and left renal artery stenosis, were selected for the development of 3DPPVMs.
2.2. Development of 3D-Printed Vascular Models
Four CT datasets with adequate contrast opacification were selected for the development of vascular models. These cases covered carotid arteries, coronary arteries, abdominal aorta, and renal arteries demonstrating vascular disease vividly (left internal carotid artery stenosis, right coronary artery stenosis, abdominal aortic aneurysm, and left renal artery stenosis). These cases represent common cardiovascular diseases that are usually treated using stenting or stent grafting procedures, and 2D or 3D CTA images are routinely used to diagnose them. The reason why we chose these cases is to produce 3DPPVMs based on CT images for the simulation of interventional procedures. These CTA images were segmented to remove the surrounding abdominal organs and tissues, thereby isolating the arteries of interest. The 3D Slicer (V5.2) was used to process the Digital Imaging and Communications in Medicine (DICOM) data. Smoothing and hollowing algorithms were applied to the virtual models, and a virtual cutting tool was applied to open the ends of the virtual models in order to prevent pressure build-up during printing. An appropriate wall thickness (2 mm) was added to the segmented models prior to printing in order to support the printing process and avoid printing errors. The models were saved as STL files (Standard Tessellation Language) (Figure 1).
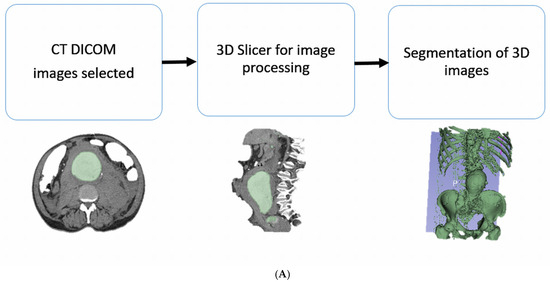
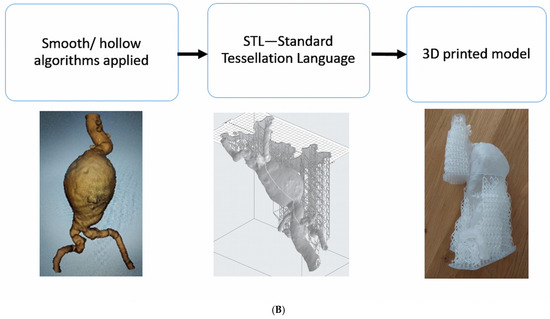
Figure 1.
Depicting the stages of the development of the 3DPPVMs. (A): Steps undertaken to segment the CT imaging data. (B): From the segmentation of volume data to the 3D printing process.
2.3. Material Selection
The STL files were printed using a variety of plastics and resins, and the models were shown to an interventional radiologist for informal discussion (Figure 2). All the models were CT-scanned in a 6% solution of Omnipaque and water to allow for the acquisition of contrast enhancement similar to standard CTA (CT attenuation close to 150–300 Hounsfield Units [HU]) [4]. The Hounsfield units measured on the models were compared to those measured on the original patient CT scans. After the measurements of the HU, Formlabs 50A elastic resin was selected for the final printing of the four 3DPPVMs chosen for the assessment (Figure 3). The files were exported for orientation and printing at a scale of 1:1 using a Formlabs 3D printer (Formlabs, Somerville, MA, USA).

Figure 2.
Variety of plastics and resins used for initial material selection. From left to right: V2 resin—draft; PETG—polyethylene terephthalate glycol; PLA—polylactic acid; HIPS—high-impact polystyrene; PMMA—polymethacrylate; TPU—thermoplastic polyurethane; 60C resin—tough white; TPU—polyurethane and rubber, soft and very soft.
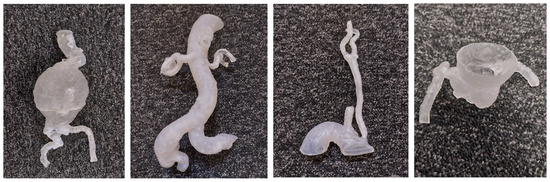
Figure 3.
The four 3DPPVMs in elastic resin (Formlabs elastic resin 50A) for clinical assessment. From left to right: abdominal aortic aneurysm, left renal artery stenosis, left internal carotid artery stenosis, and right coronary artery stenosis.
2.4. Participant Recruitment
Participants were recruited from both public and private hospitals in Western Australia and Queensland, Australia, through convenience sampling, including snowball sampling via email invitations. Twenty-one radiology and surgical specialists were invited to participate in the study. They were asked to rank the usefulness and application of the four 3DPPVMs and their attributes for use in vascular intervention. The 3DPPVM assessment took 20 min, including the viewing of the CT DICOM images, which were made available on 3D Slicer software (V5.2), and operator guidance was provided. The segmented CT scans of the four models were made available to the participants, and they were viewable in coronal, axial, and sagittal reconstructions and 3D visualisations. The software “cutting tool” was used to demonstrate the opening of the virtual arterial vessel for luminal examination. Following the review of these CT images, the participants had the opportunity to practice simulation procedures, including the deployment of stents and stent grafts (prototyped device products) in the 3D-printed models. Afterwards, the participants were asked to complete a questionnaire focusing on the clinical applications of the 3DPPVMs (Figure 4), with answers ranking from 1 to 5, with 5 being the highest. The questions were designed to focus on the following aspects: whether the 3DPPVMs demonstrated realism, displayed anatomical structures with high accuracy, enhanced the planning of interventional procedures, aided in the development of haptic skills, reduced the procedure time, improved communication with clinicians, etc (Supplementary Material File S1). The limitations of the 3DPPVMs were also identified in one of the questions (Q18), and the participants were allowed to provide further comments or suggestions at the end. The participants also had the opportunity to rate their recommendation of the use of 3DPPVMs, ranking from 1 to 10, with 10 being highly recommended. The Likert scales used in this study were adapted from a recent study documenting the use of 3D-printed heart models for education and the pre-surgical planning of congenital heart surgery [18].
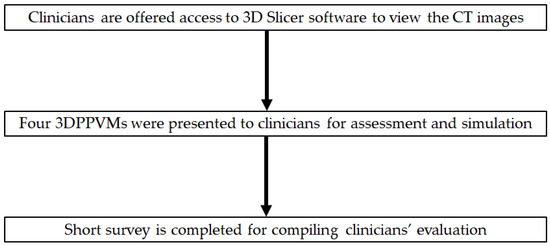
Figure 4.
Order of presentation of 3DPPVMs to clinicians.
2.5. Statistical Analysis
Quantitative and qualitative data were analysed using the IBM SPSS statistical package, version 29.0 (IBM Corporation, Armonk, NY, USA). The Shapiro–Wilk test was conducted to confirm the normality of the data, and statistical significance was established at a p-value of <0.05.
A two-way ANOVA was conducted to examine whether there were any significant differences in the distribution of the independent variables of medical specialty and years of experience against the dependent variables of 3DPPVM realism, anatomy, disease shown, procedure, planning, simulation, haptic feedback, time of surgery, pathology to patient, and pathology to health professional.
For the four 3DPPVMs, descriptive statistics were used to measure the mean, range, and standard deviation of the dependent variables of 3DPPVM realism, anatomy, disease shown, procedure, planning, simulation, haptic feedback, time of surgery, pathology to patient, and pathology to health professional. Descriptive statistics were also used to measure the minimum, maximum, mean, and standard deviation of the most suitable and most limited applications of the 3DPPVMs.
The qualitative responses provided by the participants were analysed thematically. T tests were conducted on the data, with a 95% confidence level. Using the data collected, frequencies were obtained, descriptive analyses were performed, and pie charts were constructed.
3. Results
A total of 21 participants were recruited in this study, comprising 6 radiologists and 15 surgeons. Figure 5 shows the demographics of the study participants with regard to their working experience.
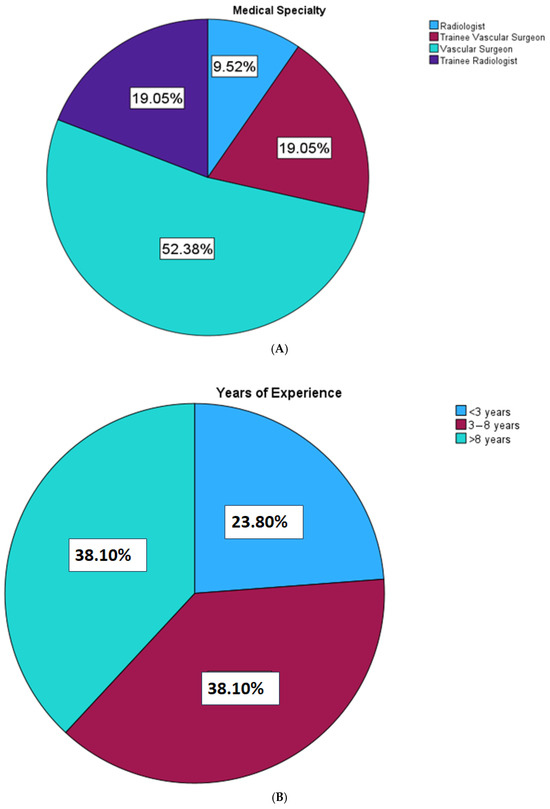
Figure 5.
Analysis of the participants by specialisation and years of experience.
The mean ranks of the 3DPPVMs for each question are detailed in Table 1. The highest overall scores were found in accurately displaying anatomical structures (mean rank > 4.2 for all four models), clarifying pathology to patients (mean rank > 4.1 for all four models), and clarifying pathology to health professionals (mean rank of 4.0 for aorta, carotid, and coronary artery models and 3.83 for renal artery model). The lowest mean ranks were noted in reducing the procedure time and developing haptic skills, with values less than 3.5 and 3.5, respectively, when compared to the others. No significant difference was found with regard to the values of the 3DPPVMs in these areas, except for the demonstration of realism and enhancement of the planning of interventions, where the aorta model was ranked the highest.

Table 1.
The mean ranks of the 3DPPVMs in clinical applications based on the participants’ responses.
Further questions were asked regarding whether the use of 3DPPVMs enhances communication with interventional clinicians, whether the use of 3DPPVMs is feasible in a surgical setting, and whether the clinician would recommend the use of 3DPPVMs to their colleagues. Those that received Yes responses from the participants who completed the questionnaire are shown in Table 2.

Table 2.
Thematic analysis of clinicians’ qualitative data.
The participants were asked to rank the 3DPPVMs’ applications using a ranking scale of 1–5, with 1 being the most important application in areas including pre-interventional planning, pre-interventional simulation, interventional orientation, communication in medical practice, and education/training. The 3DPPVMs were found to have applications in all areas: education and training was measured as the most important, with a mean rank of 2.11, followed by pre-interventional planning, communication, and pre-intervention simulation, with interventional orientation being the least applicable, as shown in Table 3.

Table 3.
The mean ranks of the 3DPPVM applications.
The participants were also asked to provide their feedback on the 3D models’ limitations using a ranking scale of 1–5, with 1 being the most limited application. The most limiting factor was the lack of surrounding anatomy, with a mean rank of 2.20, followed by their static nature, softness, and orientation for simulation, with education and training being the least limiting factor (Table 4).

Table 4.
The mean ranks of the 3DPPVM limitations.
The clinicians ranked their recommendation of the use of 3DPPVMs on a scale of 1 to 10, with 10 being the highest. The lowest ranking was two, and the highest ranking was ten. A total of 30% of the participants ranked the models at an 8, and over 75% of the participants ranked the models over 7 out of 10 (Figure 6). No qualitative responses were given to indicate the reasons for the low rankings.
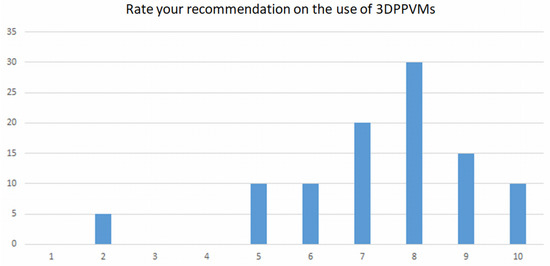
Figure 6.
Responses of participants on their recommendation of the use of 3DPPVMs.
Table 5 summarises the feedback or suggestions provided by the participants when they filled in the free-text sections of the questionnaires. Although a small number of the participants provided comments on the clinical value of 3DPPVMs, the overall response was quite positive with regard to their usefulness when compared to routine CTA images, and their limitations were highlighted, as shown in the table.

Table 5.
Thematic summary of clinicians’ responses on qualitative assessment.
4. Discussion
Our study shows that 3DPPVMs demonstrate good accuracy and realism, as the participants scored them more than 3.8 out of 5 in most application areas. The models developed in this study scored highly, with a mean score of 4.2 to 4.3 for closely replicating anatomy, a mean score of >4.2 for clarifying pathology to patients, and a mean score of 4.0 for clarifying pathology to health professionals. With the inclusion of four different vascular models comprising common vascular pathologies, this study’s findings contribute valuable information to the literature, reinforcing the usefulness of 3DPPVMs in the field of cardiovascular disease.
Three-dimensional-printed vascular models are showing great promise in medical education and the pre-surgical planning and simulation of complex endovascular procedures [8,9,10,11,12,13,14,15,16,19,20]. Three-dimensional-printed models are not only highly accurate but also possess high haptic and fluoroscopic fidelity, which makes them suitable for training and simulation purposes. Coles-Black et al. performed a systematic review of nine studies focusing on the development of 3D-printed templates to guide fenestrated physician-modified stent grafts [19]. Although the review included studies with design limitations (five case reports, one technical report, and one letter to the editor, with only two prospective trials), it showed the potential value of using 3D-printed templates to assist with the placement of fenestrated stent grafts in patients with complex or challenging aortic aneurysms, as it could lead to a significant reduction in costs and increase the accuracy of fenestration alignment. This was also confirmed by a recent review on the application of 3D-printed models in the planning of complex aortic aneurysm repair [21]. By comparing a 3D printing-guided group with a non-3D printing-guided group, it was found that the cardiopulmonary bypass time (170 vs. 181 min) and total circulatory arrest time (12 vs. 13.5 min) were reduced; however, no significant differences were found between the two groups in other areas, such as the total operative time, hospitalisation duration, and intensive care unit admission duration [21,22]. The authors also indicated the importance of incorporating artificial intelligence into image processing and segmentation, as AI-driven 3D printing will significantly reduce the time and effort required for printing such models [22,23].
Our study followed the previous study design with regard to the process that each participant undertook, first viewing the original DICOM images, followed by the presentation of 3DPPVMs, and then completing a short questionnaire [18,24]. Consistent with previous studies, the 3DPPVMs were scored higher in demonstrating anatomical structures and pathology, which is similar to two reports documenting the value of 3D-printed models in the appreciation of complex anatomy and pathology (congenital heart defects), communication with patients and health professionals, and pre-operative/intra-operative planning. In Lee’s study, 3D-printed models and virtual reality were considered the preferred tools in medical education, while 3D-printed models were ranked as the best communication tool in comparison to other methods [24]. These previous studies mainly focused on one particular disease, namely, congenital heart disease, with the development of personalised models, while our study included four different vascular diseases, thus contributing valuable information to the literature with regard to the clinical application of 3D printing in vascular disease.
There is increasing evidence proving the value of using 3D-printed models in the simulation-based training and planning of endovascular procedures. A number of review/systematic review articles have reported promising applications of 3D printing in cardiovascular/vascular diseases [20,21,25,26,27,28,29]. Early reports based on cases or case series showed the potential of using 3D-printed models in the training and planning of endovascular surgical procedures, indicating a paradigm shift [9,15,16,30,31]. Recent studies have further validated these findings based on analyses of more cases [10,32,33]. Tong et al. used patient-specific 3D-printed models to guide on-site physician-modified fenestrated stent grafts in 34 patients with thoracoabdominal aortic disease (107 fenestrations). Their results showed that, with aid of 3D-printed templates, fenestrated stent grafts were accurately positioned in all cases, achieving good short-to-mid-term outcomes [10]. The same group further expanded the application of 3D printing technology to 44 cases involving aortic arch aneurysm and dissection, and they used 3D-printed models to guide the fabrication of fenestrated stent grafts. A total of 132 branches were successfully reconstructed with 100% technical success, rapid postoperative recovery, and fewer complications [32]. Karkkainen et al. proved the feasibility of connecting a 3D-printed abdominal aortic aneurysm model to a fluid pump, and they used this approach for vascular surgical trainees to perform EVAR simulation procedures. All 22 EVAR procedures were performed with high fidelity [33]. Unlike these previous reports, our 3D-printed models were based on common vascular diseases and did not include complex aortic disease scenarios. However, our study’s findings contribute valuable information to the literature, as we covered a range of vascular diseases rather than focusing on one particular vascular disease. Thus, our results align well with those of previous studies, although further evidence is needed to validate the findings, as well as the inclusion of more cases with peri-operative and post-operative follow-up data.
Although our models were printed with soft material, the static nature of the 3DPPVMs was recognised by some of the participants as one of the limitations that could influence their use in surgical simulation. The lack of surrounding structures was scored as the most limiting factor by most of the participants. Ideally, 3D-printed models should be placed in an environment with surrounding anatomical structures, as shown in a study by Kaschwich et al. [34]. The authors developed a patient-specific 3D-printed vascular anatomy simulator, which was connected to a pumping system simulating an authentic environment during interventional procedures. Furthermore, all of the main abdominal arteries were drained to simulate blood flow, representing realistic physiological conditions. Despite the inclusion of different vascular diseases in our 3DPPVMs, this limitation should be addressed in future studies, as most of the currently used 3D-printed models lack a surrounding anatomical environment. In their study, Silberstein et al. presented the feasibility of a 3D-printed chest phantom with the simulation of various anatomical structures, including the skin, fat and muscle layers, bones, lungs, heart and pulmonary vascular structures, and lung nodules [35]. Although their study focused on low-dose computed tomography (CT) imaging protocols, they did present the feasibility of printing a realistic chest phantom with the use of different materials, with some of them presenting similar CT attenuations to the original images. This study lays the foundation for future research to develop more realistic 3D-printed models with the inclusion of surrounding anatomical structures.
Some of the limitations of this study should be acknowledged. First, this small cohort of participants could be expanded to include more clinicians from different specialties, such as interventional cardiologists and vascular residents. Second, as previously highlighted, our 3D-printed models lack the simulation of hemodynamic flow, thus restricting their application to some extent when performing endovascular stent-grafting procedures. This should be considered in future studies in order to optimise the training outcomes, as operators would be able to experience a realistic haptic feeling when placing guide wires or catheters into the vascular system. Third, our selected cases represent common pathologies, such as abdominal aortic aneurysm and artery stenosis; these are simple cases and do not reflect more complex or challenging vascular anomalies. Future studies should include more complex or challenging cases, such as aortic aneurysm or aortic dissection involving the aortic arch, as these difficult cases will benefit more from the guidance of 3D-printed models. Furthermore, it is necessary to consider the inclusion of differing degrees of plaque and thrombus to make the models more realistic in their pliability and conformation when being evaluated for stent graft access. Major factors affecting the advancement of the delivery systems include the tortuosity of the vessel and how variances in calcification density will be included in future model designs. Other future directions include enhancing patient education with the use of 3D-printed models to improve their understanding of cardiovascular anatomy and pathology, as confirmed by recent studies [36,37], and incorporating the use of 3D-printed models into the medical curriculum to enhance anatomy education among medical students or residents [1].
5. Conclusions
In this study, we developed personalised 3DPPVMs that replicate patient anatomy and disease morphology with high accuracy. The results show that 3DPPVMs can serve as educational tools in medical education and training, as well as in the pre-operative simulation of endovascular procedures. With further improvements in model design and the inclusion of complex cases involving aortic and other vascular regions, 3D-printed personalised models will increase clinicians’ practical skills and technical competencies when performing interventional procedures, thus leading to outcome improvements (reduction in procedure-related risks or peri-operative and post-operative complications) in patients undergoing endovascular treatment for vascular diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15105695/s1, File S1: Survey questions used in the study.
Author Contributions
Conceptualisation: D.L.D. and Z.S.; writing—original and draft preparation, D.L.D.; project administration, Z.S.; project supervision, Z.S.; writing—review and editing, D.L.D. and Z.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Royal Perth Hospital Imaging Grant (3-2/0622), Perth, Australia.
Institutional Review Board Statement
This research was approved by the Human Research Ethics Committee of Curtin University (approval number: HRE2023-0271) and carried out in accordance with the guidelines of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all participants involved in this study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Guy Tomlinson from Rockingham Hospital, Western Australia, for assisting with the CT scanning of models, and Carl Lares from Curtin University for printing the 3D vascular models. The authors are grateful for Werner Ducke from Cook Medical Australia for his kind assistance in providing the prototyped stents/stent grafts used in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to resolve spelling errors. This change does not affect the scientific content of the article.
References
- Yaprak, F.; Ozer, M.A.; Govsa, F.; Cinkooglu, A.; Pinar, Y.; Gokmen, G. Prespecialist perceptions of three-dimensional heart models in anatomical education. Surg. Radiol. Anat. 2023, 45, 1165–1175. [Google Scholar] [CrossRef]
- Bonvini, S.; Raunig, I.; Demi, L.; Spadoni, N.; Tasselli, S. Unsuspected limitations of 3D printed model in planning of complex aortic aneurysm endovascular treatment. Vasc. Endovasc. Surg. 2024, 58, 645–650. [Google Scholar] [CrossRef]
- Arango, S.; Gorbaty, B.; Brigham, J.; Iaizzo, P.A.; Perry, T.E. A role for ultra-high resolution three-dimensional printed human heart models. Echocardiography 2023, 40, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.A.; Squelch, A.; Sun, Z. Investigation of three-dimensional printing materials for printing aorta model replicating type B aortic dissection. Curr. Med. Imaging 2021, 17, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Rynio, P.; Kazimierczak, A.; Jedrzejczak, T.; Gutowski, P. A 3-dimensional printed aortic arch template to facilitate the creation of physician-modified stent-grafts. J. Endovasc. Ther. 2018, 25, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Rynio, P.; Wojtun, M.; Wojcik, K.; Kawa, M.; Falkowski, A.; Gutowski, P.; Kazimierczak, A. The accuracy and reliability of 3D printed aortic templates: A comprehensive three-dimensional analysis. Quant. Imaging Med. Surg. 2022, 12, 1385–1396. [Google Scholar] [CrossRef]
- Tang, F.; Hu, C.; Huang, S.; Long, W.; Wang, Q.; Xu, G.; Liu, S.; Qang, B.; Zhang, L.; Li, L. An innovative customized stent graft manufacture system assisted by three-dimensional printing technology. Ann. Thorac. Surg. 2021, 112, 308–314. [Google Scholar] [CrossRef]
- Zheng, R.; Huayuan, X.; Zhu, F.; Cheng, C.; Huang, W.; Zhang, H.; He, X.; Shen, K.; Liu, Y.; Lu, Q.; et al. Clinical comparative analysis of 3D printing-assisted extracorporeal pre-fenestration and Castor integrated branch stent techniques in treating type B aortic dissections with inadequate proximal landing zones. BMC. Cardiovasc. Dis. 2024, 24, 124. [Google Scholar] [CrossRef]
- Huang, J.; Lo, G.; Wang, W.; Wu, K.; Le, T. 3D printing guiding stent graft fenestration: A novel technique for fenestration in endovascular aneurysm repair. Vascular 2017, 25, 442–446. [Google Scholar] [CrossRef]
- Tong, Y.H.; Yu, T.; Zhou, M.J.; Liu, C.; Zhou, M.; Jiang, Q.; Liu, C.J.; Li, X.Q.; Liu, Z. Use of 3D printing to guide creation of fenestrations in physician-modified stent grafts for treatment of thoracoabdominal aortic disease. J. Endovasc. Ther. 2020, 27, 385–393. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, J.; Wang, X.; Shu, Y. Personalized 3D-print-covered stent grafts for endovascular treatment of complicated abdominal aortic dissection with Marfan syndrome. Asian J. Surg. 2023, 46, 1387–1389. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, G.; Anggrahini, D.W.; Rismawanti, R.I.; Fatimah, V.A.N.; Hakim, A.; Hidayah, R.N.; Gharini, P.P.R. 3D-Printing-Based Fluoroscopic Coronary Angiography Simulator Improves Learning Capability Among Cardiology Trainees. Adv. Med. Educ. Pract. 2023, 14, 763–771. [Google Scholar] [CrossRef]
- Nasr, B.; Lareyre, F.; Guigo, S.; Bellenger, K.; Raffort, J.; Goueffic, Y. 3-dimensional printing in vascular disease: From manufacturer to clinical use. Semin. Vasc. Surg. 2024, 37, 326–332. [Google Scholar] [CrossRef]
- Bernhard, B.; Illi, J.; Gloeckler, M.; Pilgrim, T.; Praz, F.; Windecker, S.; Haeberlin, A.; Grani, C. Imaging-Based, Patient-Specific Three-Dimensional Printing to Plan, Train, and Guide Cardiovascular Interventions: A Systematic Review and Meta-Analysis. Heart Lung. Circ. 2022, 31, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Tam, M.D.; Latham, T.R.; Lewis, M.; Khanna, K.; Zaman, A.; Parker, M.; Grunwald, I.Q. A pilot study assessing the impact of 3-D printed models of aortic aneurysms on management decisions in EVAR planning. Vasc. Endovascular. Surg. 2016, 50, 4–9. [Google Scholar] [CrossRef]
- Torres, I.O.; Luccia, N.D. A simulator for training in endovascular aneurysm repair: The use of three-dimensional printers. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.K.F.; Cestai, I.N.; de Sales, E.; Mazzetto, M.; Cestai, I.A. Metamaterial design for aortic aneurysm simulation using 3D printing. 3D Print. Med. 2024, 10, 29. [Google Scholar] [CrossRef]
- Lau, I.; Gupta, A.; Ihdayhid, A.; Sun, Z. Clinical applications of mixed reality and 3D printing in congenital heart disease. Biomolecules 2022, 12, 1548. [Google Scholar] [CrossRef] [PubMed]
- Coles-Black, J.; Barber, T.; Bolton, D.; Chuen, J. A systematic review of three-dimensional printed template-assisted physician-modified stent grafts for fenestrated endovascular aneurysm repair. J. Vasc. Surg. 2021, 74, 296–306. [Google Scholar] [CrossRef]
- Coles-Black, J.; Bolton, D.; Chuen, J. Accessing 3D printed vascular phantoms for procedural simulation. Front. Surg. 2021, 7, 626212. [Google Scholar] [CrossRef]
- Patel, H.; Choi, P.; Ku, J.C.; Vergara, R.; Malgor, R.; Patel, D.; Li, Y. Application of three-dimensional printing in the planning and execution of aortic aneurysm repair. Front. Cardiovasc. Med. 2025, 11, 1485267. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.; Park, S.J.; Kim, T.; Kim, N.; Yang, D.H.; Kim, J.B. Pre-sewn multi-branched aortic graft and 3D-printing guidance for Crawford extent II or III thoracoabdominal aortic aneurysm repair. Semin. Thorac. Cardiovasc. Surg. 2021, 34, 816–822. [Google Scholar] [CrossRef]
- Raffort, J.; Adam, C.; Carrier, M.; Ballaith, A.; Coscas, R. Jen-Baptise, E; Hassen-Khodja, R.; Chakfé, N.; Lareyre, F. Artificial intelligence in abdominal aortic aneurysm. J. Vasc. Surg. 2020, 72, 321–323.E1. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Squelch, A.; Sun, Z. Investigation of the clinical value of four visualization modalities for congenital heart disease. J. Cardiovasc. Dev. Dis. 2024, 11, 278. [Google Scholar] [CrossRef]
- Ganguli, A.; Pagan-Diaz, G.J.; Grant, L.; Cvetkovic, C.; Bramlet, M.; Vozenilek, J.; Kesavadas, T.; Bashir, R. 3D printing for preoperative planning and surgical training. A review. Biomed. Microdevices 2018, 20, 65. [Google Scholar] [CrossRef]
- Sun, Z.; Wee, C. 3D printed models in cardiovascular disease: An exciting future to deliver personalized medicine. Micromachines 2022, 13, 1575. [Google Scholar] [CrossRef] [PubMed]
- Catasta, A.; Martini, C.; Mersanne, A.; Foresti, R.; Bianchini Massoni, C.; Freyrie, A.; Perini, P. Systematic review on the use of 3D-printed models for planning, training and simulation in vascular surgery. Diagnostics 2024, 14, 1658. [Google Scholar] [CrossRef]
- McGuire, L.S.; Fuentes, A.; Alaraj, A. Three-dimensional modelling in training, simulation, and surgical planning in open vascular and endovascular neurosurgery: A systematic review of the literature. World Neurosurg. 2021, 154, 53–63. [Google Scholar] [CrossRef]
- El Sabbagh, A.; Eleid, M.F.; Al-Hijji, M.; Anavekar, N.S.; Holmes, D.R.; Nkomo, V.T.; Oderich, G.S.; Cassiv, S.D.; Said, S.M.; Rihal, C.S.; et al. The various applications of 3D printing in cardiovascular diseases. Curr. Cardiol. Rep. 2018, 20, 7. [Google Scholar] [CrossRef]
- Gomes, E.N.; Dias, R.R.; Rocha, B.A.; Santiago, J.A.D.; de Souza Dinato, F.J.; Saadi, E.K.; Gomes, W.J.; Jatene, F.B. Use of 3D printing in preoperative planning and training for aortic endovascular repair and aortic valve disease. Braz. J. Cardiovasc. Surg. 2018, 33, 490–495. [Google Scholar] [CrossRef]
- Measanne, A.; Foresti, R.; Martini, C.; Malvezzi, C.C.; Rossi, G.; Fornasari, A.; De Filippo, M.; Freyrie, A.; Perini, P. In-house fabrication and validation of 3D-printed custom-made medical devices for planning and simulation of peripheral endovascular therapies. Diagnostics 2024, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.S.; Jin, Y.; Zhao, Z.H.; Wang, C.; Shi, Y.H.; Zhou, M.J.; Zhao, J.X.; Liu, C.; Qiao, T.; Liu, C.J.; et al. Three-dimensional printing to guide fenestrated/branched TEVAR in triple aortic arch branch reconstruction with curative effect analysis. J. Endovasc. Ther. 2024, 31, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, J.M.; Sandri, G.; Tenorio, E.R.; Alexander, A.; Bjellum, K.; Matsumoto, J.; Morris, J.; Mendes, B.C.; DeMartino, R.R.; Oderich, G.S. Simulation of endovascular aortic repair using 3D printed abdominal aortic aneurysm model and fluid pump. Cardiovasc. Interv. Radiol. 2019, 42, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Kaschwich, M.; Sieren, M.; Matysiak, F.; Bouchagiar, J.; Dell, A.; Bayer, A.; Ernst, F.; Ellebrecht, D.; Kleemann, M.; Horn, M. Feasibility of an endovascular training and research environment with exchangeable patient specific 3D printed vascular anatomy: Simulator with exchangeable patient-specific 3D-printed vascular anatomy for endovascular training and research. Ann. Anat. 2020, 231, 151519. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, J.; Tran, S.; Wong, Y.H.; Yeong, C.H.; Sun, Z. Development of a 3D-printed chest phantom with simulation of lung nodules for studying ultra-low-dose computed tomography protocols. Appl. Sci. 2025, 15, 309. [Google Scholar] [CrossRef]
- Kargul, M.; Skorka, P.; Gutowski, P.; Kazimierczak, A.; Rynio, P. Empowering EVAR: Revolutionizing patient understanding and qualification with 3D Printing. J. Cardiovasc. Dev. Dis. 2024, 11, 365. [Google Scholar] [CrossRef]
- Grab, M.; Hundertmark, F.; Thierfelder, N.; Fairchild, M.; Mela, P.; Hagl, C.; Grefen, L. New perspectives in patient education for cardiac surgery using 3D-printing and virtual reality. Front. Cardiovasc. Med. 2023, 10, 1092007. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).