Abstract
Germination is a biotechnological process that activates enzymatic reactions and alters the chemical content of grain, improving its nutritional and functional value. However, the duration of the germination process significantly affects grain composition and properties. Prolonged and uncontrolled germination can lead to undesirable changes, such as excessive enzymatic activity, microbial contamination of sprouted grains, and loss of dry matter. While short-term germination is preferable for producing various food products, including whole-grain porridge, this article analyzed the impact of short-term germination of triticale grain (for 18, 20, 22, and 24 h) on its biochemical indicators and functional-technological properties. This research established that changes in biochemical values accompany the germination of triticale grain. The most significant increase in protein content was observed after 20 h of germination (GT 20), which increased by 4.43%. In contrast, after 24 h (GT 24), a decrease of 3.27% was noted compared to the original content in the whole grain (WGT). The contents of fats, carbohydrates, and ash tended to decrease. Germination promoted an increase in the amount of essential amino acids and improved the amino acid profile, particularly during the 20 and 22 h germination intervals. At 24 h of germination, the highest increase in total phenolic compounds (28.6%) and antioxidant activity (20.8%) was recorded. B-group vitamins, thiamine, and riboflavin, were detected in the sprouts only after 22 and 24 h of germination. The highest thiamine content (0.17 mg/kg) was observed at the 22nd hour, and the highest riboflavin content (2.51 mg/kg) was observed at the 24th hour. Niacin content showed a steady increase throughout the germination period. The maximum magnesium (0.200%) and molybdenum (0.589%) contents were recorded after 24 h of germination, while the calcium content increased at all germination intervals. The functional and technological properties of sprouted triticale grain improved, while the pasting (gelatinization) properties tended to decrease. Thus, it has been established that short-term germination enhances the biochemical indicators and functional-technological properties of triticale grains, indicating their potential for use in the production of whole-grain porridge.
1. Introduction
Triticale (Lat. ×Triticosecale, from Lat. Triticum-wheat and Lat. Secale-rye) is the first artificially created cereal crop obtained through the hybridization of wheat and rye, which combines the qualities of both parent species. Initially, triticale was utilized as a forage crop and for the production of ethyl alcohol; however, the growing demand for food resources has expanded its potential for the production of various food products [1,2].
Regarding its nutritional composition, triticale grain is more similar to wheat than to rye; it is characterized by a high protein content, a favorable amino acid profile, and an increased level of lysine, a limiting amino acid in many cereals [1,3,4]. In addition, it contains a significant amount of dietary fiber, vitamins, minerals, and bioactive phytochemicals [5,6,7], making it a valuable component of balanced nutrition and the production of functional food products [8,9,10].
Despite its high nutritional value, the use of triticale grain in the food industry remains limited. The main technological barriers include the low quality of its gluten network [1,2] and high amylolytic activity [11], which impairs rheological properties and destabilizes its structural and functional characteristics during processing. These factors reduce the technological suitability of triticale and hinder its widespread adoption in food production processes.
Germinated triticale grain is of significant scientific and practical interest due to its enhanced nutritional properties and potential applications in producing various food products [12,13,14]. Grain germination is considered an effective biotechnological method for modifying the structure of macromolecules, improving the bioavailability of nutrients, and partially compensating for the technological shortcomings of triticale [15,16]. During germination, metabolic processes are activated, leading to the hydrolysis of stored nutrients, degradation of anti-nutritional factors, and synthesis of secondary metabolites [17,18], ultimately optimizing the nutrient profile of the germinated grain [19,20]. Furthermore, when hydrolytic enzymatic activity is initiated, complex macromolecules, including proteins, fats, and carbohydrates, are broken down into simpler components, such as amino acids, simple sugars, and unsaturated fatty acids, thereby improving the bioavailability of nutrients in the grain. Similar changes have been noted in studies focusing on cereals and pseudocereals [21], legumes [22,23,24], and cereals [25].
The germination process activates the biosynthesis and accumulation of biologically active phytochemical compounds, mainly phenolic substances, with pronounced antioxidant potential. As a result, germinated grain exhibits significant therapeutic and preventive properties, including anti-diabetic, anticancer, and cardioprotective effects. Regular consumption of sprouted grain helps reduce the risk of cardiovascular diseases [26,27] and may play a role in preventing metabolic disorders and certain types of cancer [28,29].
Functional and technological properties, including water and oil absorption and foaming capacity, also affect germination due to structural transformations of the protein components [30]. The duration of the grain germination process must be considered, as it significantly affects the grain’s composition and properties. Prolonged and uncontrolled germination can result in undesirable changes, such as microbial contamination, loss of dry matter, and excessive enzymatic activity [25,31,32]. Increased amylolytic activity leads to the deterioration of rheological parameters [33,34].
Moreover, increased activity of lipase and lipoxygenase enzymes triggers intense lipid oxidation processes. As a result, the formation of undesirable low-molecular compounds increases, and their accumulation at high concentrations correlates with the development of undesirable organoleptic characteristics [35,36].
Previous studies on the germination of triticale grain [14,19] have been conducted over extended periods. Given the drawbacks of long-term germination and its impracticality for industrial processing, there is a growing interest in using short-term germination. In particular, as shown in the study by Waleed et al. [37], excessive germination duration leads to overactivation of enzymes, resulting in the degradation of starch and proteins, weakening of the gluten structure, and deterioration of rheological and baking properties.
Short-term germination was also explored by Sharma et al. [38], who showed that germinating barley for up to 12 h reduced the content of phenolic compounds, while increasing the duration to 24 h led to a significant increase in their levels and enhanced antioxidant activity.
At the same time, there is a lack of systematized data on the biochemical and functional-technological transformations occurring in triticale grain during short-term germination, which is considered a more promising approach for the food industry.
Considering triticale’s high amylolytic activity, studying short germination periods is essential for optimizing technological parameters, preventing undesirable changes, and improving the efficiency of industrial processing.
This study aimed to investigate the impact of short-term germination as one of the critical parameters capable of ensuring an optimal balance between improved nutritional value and preservation of the grain’s technological properties.
The obtained data will expand the potential use of triticale in producing functional food products, increase processing efficiency, and enhance its resilience to technological risks. This, in turn, will contribute to the broader adoption of triticale as a promising raw material in the food industry.
Therefore, given the increasing interest in using sprouted triticale grain in food products, this study aimed to examine the effect of short-term germination duration, as one of the critical parameters, on the biochemical indicators and functional-technological properties of triticale grain.
2. Materials and Methods
2.1. Materials, Chemicals, and Reagents Used
Triticale grain of the “Dauren” variety was provided by the North Kazakhstan Agricultural Experimental Station (Shagalaly, Republic of Kazakhstan).
All chemicals and reagents, including sulfuric acid (99.999%), sodium hydroxide (reagent grade, ≥98%), hydrochloric acid (ACS reagent, 37%), boric acid (ACS reagent, ≥99.5%), Folin-Ciocalteu’s phenol reagent, DPPH, Gallic acid, hydrogen peroxide solution (34.5–36.5%), and petroleum ether (ACS reagent), were of analytical grade and manufactured by Sigma-Aldrich (St. Louis, MO, USA).
2.2. Germination of Whole Triticale Grains
Germination of triticale grains was carried out according to [39] with experimental modifications. A total of 500 g of triticale grain was inspected, and contaminants and foreign substances were removed from the sample. The separated grain was washed three times in running water. Disinfection was performed for 15 min using a 5% hydrogen peroxide solution and rinsed with distilled water. Next, the grains were soaked in distilled water for 6 h, after which the water was drained using a sieve. The grains were then placed in containers with perforated bottoms, covered with filter paper, and topped with damp muslin cloth for germination.
The grains were germinated at a temperature of 20 ± 2 °C, with a relative air humidity of 85–90%, for short intervals of 18 h, 20 h, 22 h, and 24 h. These germination duration intervals were selected based on enzyme activation, the onset of metabolic processes, and improved organoleptic properties observed during short-term germination.
The obtained sprouted triticale grains were dried by sublimation using an SB 2 sublimator (SX Tekhnika, Kazan, Republic of Tatarstan, Russia) at 40 °C for 18 h under a pressure of 50–100 Pa.
2.3. Sample Preparation
Whole and sprouted triticale grain samples at 18 h, 20 h, 22 h, and 24 h were ground in a laboratory mill (Bastak 4000, Izmir, Turkey) into flour with a particle size of less than 60 mesh. Whole triticale grain samples were used as controls and labeled WGT (Whole-Grain Triticale). The triticale grain samples that were sprouted for 18 h, 20 h, 22 h, and 24 h, were labeled GT18, GT20, GT22, and GT24, respectively. All samples were hermetically sealed and stored in a refrigerator at 4 °C for further analysis.
2.4. Determination of Biochemical Indicators
The moisture, protein, fat, carbohydrate, and ash contents of the samples were determined according to official methods 44-15.02, 46-13.01, 30-25.01, 76-33.01, and 08-12.01, respectively, as outlined by the AACCI (2010) [40].
The samples’ color profiles were identified using a desktop spectrophotometer YS6060 (3nh Technology Co., Guangzhou, China) according to a previously described method [41].
2.4.1. Amino Acid Composition
The amino acid composition was analyzed by high-performance liquid chromatography (HPLC) using an Agilent Technologies 1200 series (Santa Clara, CA, USA) system connected to a Kinetex Core-Shell RP-C18 column (150 × 4.6 mm, 100 Å, 5 μm) and a diode-array detector (DAD), according to the method described by [42].
2.4.2. Vitamins
Vitamins were determined using high-performance liquid chromatography (HPLC) on an Agilent Technologies 1200 series system (Santa Clara, CA, USA). A Phenomenex Luna 5 micron C18 100 Å LC column (150 × 4.6 mm) was used according to the standards EN 12822:2014 [43], EN 14122:2014 [44], EN 14152:2014 [45], EN 15652:2009 [46], MP MN 3008-2008 [47], EN 14663:2006 [48], and MP MN 2146-2004 [49].
2.4.3. Minerals
The mineral composition was analyzed using X-ray fluorescence (XRF) analysis on a Bruker S8 Tiger spectrometer (Billerica, MA, USA).
2.4.4. Total Phenols and Antioxidant Activity
The total phenol content was calculated as gallic acid equivalents, according to [50]. Measurements were performed using a Shimadzu UV-1800 Visible Spectrophotometer (Kyoto, Japan).
Antioxidant activity was quantified using the DPPH free radical-scavenging assay. Measurements were carried out using a Shimadzu UV-1800 Visible Spectrophotometer (Kyoto, Japan) [50].
2.5. Determination of Functional-Technological Properties
The water absorption capacity (WAC) and oil absorption capacity (OAC) were determined according to previously described methods [51,52]. One gram of the sample was weighed into a 50 mL centrifuge tube and thoroughly mixed for 1 min with water or vegetable oil (1:10 by mass/volume). The tubes were left to decant at 21 °C for 30 min. The samples were then centrifuged at 4000× g for 20 min, and the supernatant was removed. WAC and OAC were expressed in grams of water or oil absorbed per 100 g of flour.
The foaming capacity was determined using the method described in a previous study [53].
2.6. Viscosity
The viscosity of the sprouted triticale grains was tested using a Rapid Visco Analyser RVA 4500 (Perten Instruments, Hägersten, Sweden) equipped with Thermocline software for Windows, version 3.1, according to the AACC International method 76-21.01 [40].
To ensure reproducibility, the measurements were performed in triplicate for each sample (N = 3). Before the analysis, the RVA instrument was calibrated using a standard reference sample provided by the manufacturer, following the recommended calibration procedure described in the user manual.
Moisture content correction was conducted before viscosity analysis: the moisture content of each sample was determined using the AACC Method 44-15.02, and all viscosity values were adjusted to a standard 14% moisture basis to ensure comparability between the samples.
2.7. Statistical Analysis
All measurements were performed in triplicate for each experiment. Statistical analysis of the obtained results was carried out using the software Origin 2021. Pearson’s correlation analysis was performed to assess the linear relationships between the studied parameters. Duncan’s multiple range test (Post Hoc Duncan) was used in the IBM SPSS Statistics 27 software package to compare the mean values and determine statistically significant differences between them. Significantly different mean values are denoted by different superscript letters (p < 0.05, N = 3). To assess the significance of differences between the variants, a one-way analysis of variance (ANOVA) was conducted, followed by Duncan’s Multiple Range Test (DMRT) at a significance level of p < 0.05.
3. Results
3.1. Biochemical Indicators
Researchers have noted that changes in the biochemical indicators of sprouted grains are related to several factors, among which temperature and germination duration are the most significant [54,55,56].
The selection of short-term germination time intervals (18, 20, 22, and 24 h) was based on preliminary observations, which showed that the physiological activity of triticale grain, marked by the initial emergence of the embryo, begins at the 18th hour. From this point onward, key metabolic processes are activated, including enzymatic hydrolysis of macromolecules and biosynthesis of biologically active compounds. The chosen time range for the investigation was consistent with the findings reported by Sharma et al. [38].
Table 1 presents the results of the study on the influence of the germination duration of triticale grain (WGT and GT18, GT20, GT22, and GT24) on biochemical indicators.

Table 1.
Biochemical indicators of WGT and GTs.
The data presented in Table 1 show that the moisture content increases during germination at GT18, GT20, GT22, and GT24, which aligns with previous research results [57]. The authors described this process with the whole grain’s ability to absorb moisture during soaking, which supports the metabolic processes necessary for initiating germination, which in turn leads to structural changes in the grain.
The protein content in the germinated triticale grain also changed, showing slight fluctuations. For example, at GT18, there was an increase of 0.45%; at GT20, a rise of 4.43%, marking the highest growth; at GT22, an increase of 3.46%; while at GT24, there was a decrease of 3.27% compared to the initial content in the whole-grain triticale (WGT).
The observed increase in protein content during the early stages of germination is consistent with the findings of El-Safy F. et al. [58] and Ongol et al. [59]. Studies have demonstrated that germination activates enzymatic systems responsible for the breakdown of storage carbohydrates and proteins, leading to a relative increase in the proportion of protein within the dry matter of the grain. This phenomenon is associated with the intensification of anabolic processes [60,61,62] and the synthesis of proteins required for embryo development [63,64]. Apparent differences in protein concentration may reflect the loss of carbohydrate material during respiration or changes in the nitrogenous compounds present, rather than an actual increase in protein content [65].
However, the protein content of germinated grains decreases with prolonged germination. This is due to the enzymatic hydrolysis of proteins into amino acids, which are then utilized in metabolic processes, including the synthesis of new cellular structures, enzymes, and other functional proteins, as well as the redistribution of nutrients to different parts of the sprout [66,67,68].
For instance, Guardianelli et al. [69] reported that the protein content increased after 18 h of germination but declined after 24 h. These results are consistent with the current findings, showing a decrease in protein content after 24 h of germination. Overall, the changes in protein content during germination reflect the complex dynamics of the metabolic transformations occurring in sprouted grains.
Moreover, the obtained data align with previous studies that reported that during germination, the relative differences in protein content between whole and germinated grains, whether increasing or decreasing, were less than 10% [70,71,72].
The fat content in germinated grains decreased as the germination time increased, showing reductions of 7.34% at 18 h, 8.47% at 20 h, 10.73% at 22 h, and 11.86% at 24 h. These results are consistent with previous studies that explained the fat content reduction in sprouted grains of cereals, pseudocereals, and legumes [73,74], as well as barley and wheat [75,76], by increased lipolytic activity, which converts fats into fatty acids and glycerol.
The ash content in the germinated grains decreased with prolonged germination, with the most significant reductions observed at GT20 and GT22 (3.44% and 4.13%, respectively). Early studies have linked the reduction in ash content in germinated grains to leaching minerals during washing and the use of these minerals to activate metabolic processes [61,77,78].
The carbohydrate content in the germinated grains also showed a tendency to slightly decline. Studies on cereals and some legumes [79,80], as well as millet [81] and soybeans [82], have shown that the decrease in total carbohydrate content occurs due to their breakdown and utilization in energy processes that support the growth and development of sprouted seeds. Additionally, plants use simple sugars for respiration and biosynthetic processes, contributing to the reduction in their content.
3.1.1. Color Profile
The color profile affects the product’s attractiveness and sensory acceptability, and germination can significantly influence its color characteristics [83]. The results of the color profile study, presented in Figure 1, demonstrate changes in the color profile of the germinated triticale grain.
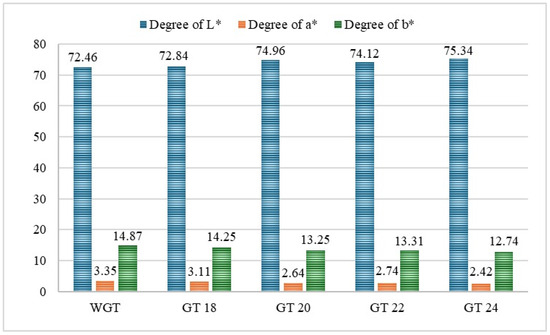
Figure 1.
The effect of germination time on the color characteristics of triticale samples. The Pearson correlation coefficient shows the relationship between changes in the color of flour from germinated triticale grain and the germination time of triticale grain: L* (lightness) r = 0.772, direct relationship, the strength of the relationship on the Chaddock scale is high; a* (redness) r = −0.861, strong inverse relationship; b* (yellowness) r = −0.886, strong inverse relationship.
The results obtained showed that the germination process significantly affected the color profile. The L* (lightness) value of the germinated triticale samples increased from 72.84% to 75.34% after germination, corresponding to other findings [84]. A similar increase in the L* value was linked to enzymatic activity that breaks down complex molecules, including colored pigments in the grain, which aligns with the results of Nefale et al. [85].
In contrast, the color a* and b* values decreased from 3.35 to 2.42 and 14.87 to 12.74, respectively. The reduction in the a* and b* color values during germination may be attributed to the breakdown of photosynthetic pigments, such as chlorophylls and carotenoids, as observed by Perveen et al. [86].
3.1.2. Amino Acid Composition
Amino acids are essential for human nutrition. Table 2 presents the amino acid compositions of WGT, GT18, GT20, GT22, and GT24.

Table 2.
Amino acid composition of triticale grain samples.
The results of this research established a positive effect of germination on amino acid content. The quantities of essential amino acids, such as lysine, methionine, leucine, isoleucine, threonine, phenylalanine, and valine, were elevated during germination at various intervals. Specifically, increases in lysine and phenylalanine levels were observed during germination at 20 and 22 h, while leucine, isoleucine, and methionine levels increased between 18 and 22 h, and threonine levels increased between 18 and 24 h. Notably, the total amino acid content reached its maximum value during germination at 20 and 22 h, as this period corresponds to the active phase of enzymatic protein breakdown, which promotes the maximum accumulation of amino acids. This is consistent with the data from Wu et al. [87] and Guardianelli et al. [69], who showed an increase in lysine and other amino acids in cereal crops during germination.
The decrease in amino acid content at 24 h may be related to the fact that the accumulated amino acids could be used for other metabolic processes, such as the synthesis of new proteins or other biochemical reactions, which reduces their concentration [88,89].
The increase in amino acid content due to proteolytic activity during germination enhances the nutritional quality of the grain. The type of amino acid released varies according to the grain and germination conditions. In cereals, protein hydrolysis leads to the breakdown of prolamins, and the released amino acids, such as glutamic acid and proline, are converted into the limiting amino acid lysine, thus improving protein quality [90].
Among the essential amino acids, lysine deserves special attention due to its important role in maintaining human health. Lysine is an essential amino acid required for vital physiological functions, including calcium absorption, antibody synthesis, hormonal balance, muscle tissue development, and the production of carnitine, a compound involved in fatty acid metabolism. Lysine has high bioavailability, averaging 92% in various food products, which makes it an essential indicator of high-quality dietary protein [91]. Since the human body cannot independently synthesize lysine, it must be obtained from food in adequate amounts [92,93].
3.1.3. Vitamins
Vitamin composition is a crucial indicator of the nutritional value of germinated grains. Most vitamins, especially water-soluble ones, are not synthesized in the human body in sufficient quantities, making regular food intake physiologically necessary [94].
Germination of cereal crops such as wheat, barley, rice, and sorghum promotes an increase in the content of vitamins A and E (tocopherols) [95,96,97] and B vitamins—thiamine (B1), riboflavin (B2), and niacin (B3)—due to the activation of biosynthetic processes in the sprouts [98,99]. Table 3 presents the vitamin content of WGT, GT18, GT20, GT22, and GT24.

Table 3.
Vitamin content of WGT and GTs.
During the germination of triticale grain, a notable increase in the levels of several B vitamins was observed, indicating the activation of biosynthetic processes. For instance, the niacin (vitamin B3) content increased from 0.03 mg/kg in raw grain (WGT) to a peak of 0.52 mg/kg at the 22-h germination mark (GT22). Riboflavin (vitamin B2), which was absent in the initial sample, was detected only at the 22-h (GT22) and 24-h (GT24) germination stages, reaching a maximum of 2.51 mg/kg at the 24-h mark (GT24). A similar trend was noted for thiamine (vitamin B1), which was present only at the 22-h mark (GT22—0.17 mg/kg), followed by a decrease at the 24-h stage (GT24).
Vitamin B6 appeared at the 22-h germination stage (GT22), likely linked to the active metabolism of the grain and the synthesis of coenzymes. However, by the 24-h stage (GT24), the B6 level diminished, presumably due to the depletion of nutritional resources and environmental changes, such as oxygen levels. The maximum folic acid (vitamin B9) content was observed at the 18-h germination mark (GT18—1.66 mg/kg), followed by a noticeable decline. This decrease could be attributed to initial synthesis activation, followed by degradation or redistribution into the roots and sprouts [100]. These findings are consistent with studies on other crops, such as wheat [101], sorghum [102], and barley [103]. in which germination also contributes to elevated B vitamin levels. Moongngarm et al. [104] noted that some water-soluble vitamins may be partially lost during germination due to leaching from soaking. Nevertheless, the active synthesis of vitamins by young sprouts [96] significantly contributes to the increase in the B vitamin content of germinated grains.
In contrast, the level of vitamin E (tocopherol), a fat-soluble antioxidant, tended to decline at all germination stages. Specifically, vitamin E levels decreased by 38% after 18 h, 32% after 20 h, 31% after 22 h, and 23% after 24 h. Ferreira et al. [105] link the reduction in vitamin E levels to its protective role against lipid oxidation during germination, characterized by intensive metabolic and respiratory processes. Furthermore, tocopherols are consumed as part of the cell’s antioxidant defense, accounting for their reduction during germination. The germination of triticale grains leads to significant biochemical changes, notably the increase in B vitamins such as B1, B2, B3, B6, and B9, resulting from the activation of biosynthetic and enzymatic processes. Simultaneously, vitamin E levels are reduced, which is related to its antioxidant function in protecting lipids from oxidation. These findings underscore the potential nutritional value of germinated grains as a source of B vitamins, despite the partial loss of fat-soluble antioxidants.
Thus, the obtained vitamin levels, despite the partial loss of vitamin E, suggest that germinated triticale grain can be considered a functional ingredient enriched with physiologically essential micronutrients, capable of enhancing the nutritional value of products in healthy and preventive dietary regimens.
3.1.4. Minerals
Mineral elements are vital micronutrients necessary for maintaining human health, as they participate in key metabolic processes and the biosynthesis of macromolecules [79].
The study of mineral content in WGT and GT18h, GT20h, GT22h, and GT24h, as shown in Figure 2, revealed a relatively high content of potassium, molybdenum, and iron in the WGT samples, with concentrations of 1.51%, 0.540%, and 0.499%, respectively. During germination, both slight fluctuations in the concentration of certain minerals were observed depending on the germination time: potassium dropped from 1.51% (WGT) to 1.14% (GT18), 1.17% (GT20), 1.12% (GT22), and 1.13% (GT24); molybdenum remained stable at 0.540% (WGT) and GT18, decreased to 0.496% (GT20), 0.345% (GT22), and then significantly increased to 0.589% (GT24); iron reduced from 0.499% (WGT) to 0.432% (GT18), 0.450% (GT20), 0.401% (GT22), and 0.435% (GT24); calcium elevated from 0.202% (WGT) to 0.231% (GT18), 0.227% (GT20), 0.227% (GT22), and 0.229% (GT24); magnesium remained at 0.197% (WGT) and 0.197% (GT18), declined to 0.182% (GT20), 0.187% (GT22), and then escalated to 0.200% (GT24); manganese decreased from 0.276% (WGT) to 0.248% (GT18), 0.265% (GT20), 0.133% (GT22), and 0.269% (GT24).
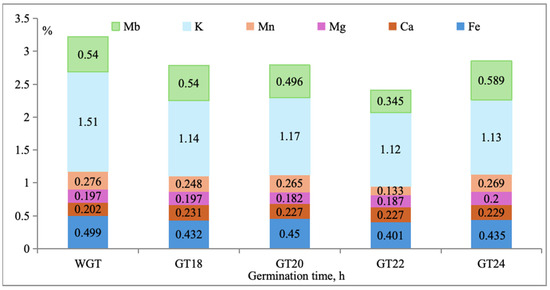
Figure 2.
Mineral content of WGT and GTs.
It is also noted that during germination at GT24, there was a significant increase in the content of magnesium and molybdenum to 0.200% and 0.589%, respectively, indicating the beneficial effect of this germination time on the enrichment of germinated grain with these essential trace elements.
The mineral composition results align with previous studies [106], which found an increase in calcium content during rice germination and a decrease in potassium content, consistent with the results obtained. Therefore, germination time significantly affects the mineral content and bioavailability in triticale grain. According to [107], partial mineral leaching may occur during soaking due to the water used. The use of minerals in other critical biochemical processes during germination is another reason for reduced mineral availability [108].
The increase in magnesium, molybdenum, and calcium content during triticale grain germination is associated with the enhanced bioavailability of these elements due to the activation of the enzyme, phytase. Activated during germination, phytase catalyzes the hydrolysis of phytic acid, facilitating the release of minerals bound to it and consequently improving their absorption. The observed differences in the bioavailability of minerals at different stages of germination may be due to variations in phytic acid content, phytase activity, and the strength of mineral binding in phytate complexes [109].
Additionally, minerals can be chelated or interact with proteins during germination, which contributes to their stabilization and accumulation [110]. According to Lemmens et al. [18], increased bioavailability of minerals is accompanied by a decrease in anti-nutritional factors. The increase in calcium content at all germination time intervals suggests that germinated triticale grains can be a good source of calcium, which is essential for bone and tooth formation. Therefore, germination is a promising biotechnology for enriching cereal raw materials and can be recommended for producing functional food products with improved mineral contents. In particular, the consumption of germinated cereals, such as yellow corn, may prevent deficiency conditions and promote health [111,112].
3.1.5. Total Phenolic Content and Antioxidant Activity
Phenolic compounds exhibit diverse biological activities, including significant antioxidant properties, due to their capacity to neutralize free radicals through various mechanisms [113,114].
The total phenolic content and antioxidant activity of the whole and germinated triticale grain samples are presented in Table 4.

Table 4.
Total phenolic content (TPC) and antioxidant activity (DPPH) in germinated triticale samples.
The initial total phenolic content in whole triticale grain (WGT) was 42 mg GAE/100 g, and the antioxidant activity (DPPH) was 380 mg/100 g. As the germination time increased, the total phenolic content increased, reaching a maximum of 54 mg GAE/100 g after 24 h of germination, representing a 28.6% increase. The antioxidant activity (DPPH) also increased, reaching a peak of 459 mg/100 g at 24 h, representing a 20.8% increase.
The dynamics of these changes suggest enhanced antioxidant properties in the germinated triticale grain compared to the whole grain. As noted in studies by [115,116], the increase in total phenolic content is likely due to the release of free and bound phenolic compounds during germination, resulting from the enzymatic degradation of the grain’s cell walls. In this study, the antioxidant activity was directly correlated with the total phenolic content.
In a study by [39], the effects of germination on the total phenolic content and antioxidant activity of hull-less barley and triticale were investigated. The results revealed an increase in phenolic compound concentration and enhancement of antioxidant activity.
Gawlik-Dziki et al. [117] found that the germination process increased the levels of phenolic compounds in Polish wheat. Gao et al. [118] found that the germination of brown rice increased the content of free and bound phenols and raised antioxidant activity.
3.2. Functional and Technological Indicators
3.2.1. Water and Oil Absorption Capacities
The water absorption capacity (WAC) and oil absorption capacity (OAC) of triticale are presented in Figure 3. Both WAC and OAC increased as germination progressed from WGT to GT20. The maximum water and oil absorption capacities were observed at GT20 germination, with 0.69 g/g and 0.91 g/g, respectively. After 20 h of germination, both values showed a slight decrease: WAC decreased to 0.65–0.66 g/g, and OAC to 0.88–0.90 g/g. This may be related to the enzymatic degradation of macromolecules (particularly proteins and cellular structures), which reduces the material’s ability to retain moisture and oil.
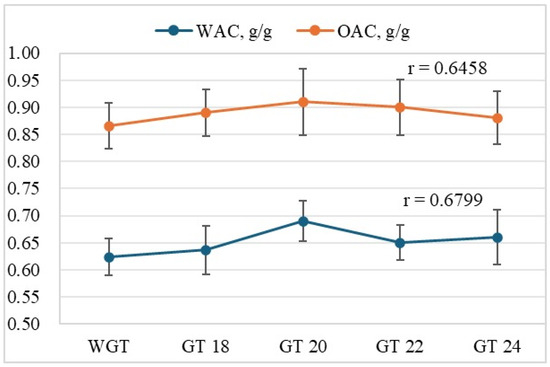
Figure 3.
The effects of germination time on the water absorption capacity (WAC) and oil absorption capacity (OAC) of triticale samples (WGT and GTs) are shown. The values are presented as mean ± standard deviation (n = 3). A moderately strong positive correlation was found between germination time and WAC, with r = 0.6799 on the Chedok scale. A similar relationship was observed for OAC, with r = 0.6458, indicating the influence of germination on the grain’s ability to retain water and oil.
Based on the data obtained in Figure 3, we propose the following hypothesis: the optimal germination time for triticale, GT 20, activates metabolic processes and increases the grain’s water and oil absorption capacities. However, with further extension of the germination time, a slight decrease in both parameters was observed, which limits the grain’s ability to retain water and oil.
The analysis of the dynamics of water and oil absorption capacity depending on germination time showed a consistent increase in the parameters up to 20 h, followed by a moderate decrease. All the studied time points (GT18, GT20, GT22, and GT24) corresponded to this trend: an increase in WAC and OAC in the early stages of germination and a decline in these parameters after 20 h. No deviations from this pattern were noted. However, considering the close values of WAC and OAC at GT 20–24 stages and the possible overlap of standard deviations, the decrease after 20 h may be statistically insignificant. This indicates the relative stability of the functional properties of the grain in the 20–24 h range, which could be helpful in selecting the optimal germination window in production practices.
The obtained results align with previous studies [12,52], which reported an increase in water absorption capacity due to the formation of compounds with high water retention ability, such as soluble sugars, which increase the number of available hydrophilic centers interacting with water molecules [119,120].
The observed increase in water retention capacity during the early stages of germination can be associated with the accumulation of soluble sugars, which have high hydrophilicity, and increased hydration of cellular components [52]. During this process, protein structures unfold, exposing additional hydrophilic sites capable of binding water [121,122]. Similar changes have been previously recorded for cereals in general [123], which is consistent with the results of this study. However, by the time 22 h of germination was reached, the water retention capacity began to decrease, likely due to the subsequent degradation of macromolecules and their metabolic redistribution, a reduction in polysaccharide content, and partial loss of water-binding components.
Earlier studies attributed the increased oil absorption capacity in germinated grains [52,78] to the partial disruption of the native protein structure during germination. This disruption facilitates the release of lipophilic proteins and hydrophobic amino acids associated with the hydrocarbon side chains of lipids, thereby increasing the oil absorption capacity of the grain.
The research revealed that the oil absorption capacity of germinated triticale grain varies dynamically in response to germination time, resulting from structural modifications of protein molecules. In particular, this parameter increased to 0.68% at 22 h of germination in GT22, which aligns with previously published data [52,78] and results from studies on wheat and its germinated form, triticale, and brown rice [80,124]. During germination, partial denaturation of proteins occurs, leading to the release of lipophilic fragments and hydrophobic amino acids that interact with lipids, thereby increasing the oil absorption capacity.
The obtained data also demonstrated an increase in this parameter at 20 and 22 h of germination in GT20 (0.64%) and GT22 (0.69%), respectively, which can be linked to the activation of protein structural changes at these stages. A higher oil absorption capacity is desirable as it imparts better sensory properties, enhances taste, and improves mouthfeel, which are fat characteristics [125]. A decrease in oil absorption capacity was observed at the later stage of germination, for example, after 24 h in the GT24 sample (0.66% compared to the previous stage), which may indicate the completion of key protein structural transformations and stabilization of lipid components in the grain.
3.2.2. Foam Formation Ability and Foam Stability
Germination affected the foam formation capacity and stability of the germinated triticale samples, as shown in Figure 4, with values ranging from 2.1% to 2.8% for foam formation capacity and 1.1% to 1.9% for foam stability. An increase in foam formation capacity improves the softness and texture of food products [126]. Its growth is accompanied by inflated amphiphilic properties, which are linked to changes in protein structure during germination [12].
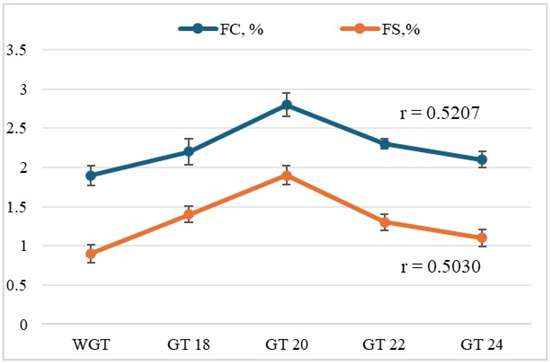
Figure 4.
The effect of germination time on the foam formation capacity and foam stability of triticale samples (WGT and GTs) is shown. The values are presented as the mean ± standard deviation (n = 3). Moderately positive correlations according to Cheddock’s scale were established between germination time and FC (r = 0.5207) and FS (r = 0.5030), indicating the potential of germination to improve the functional properties related to foaming.
Previous studies have also observed increased foaming capacity in cereals [127,128]. These changes were attributed by Siddiqua et al. [123] to the activation of endogenous enzymes, primarily proteases, which cause partial hydrolysis of proteins, resulting in the formation of soluble peptides and amino acids.
Additionally, denaturation of the tertiary structure of proteins increases the exposure of hydrophobic and hydrophilic regions of the molecule, enhancing their surface activity [129,130], which explains the increase in foaming capacity at 20–22 h of germination. Similar findings were noted in the studies by Elkhalifa et al. [52], Singh et al. [78], and Dhillon et al. [80], which correlated with the optimal functional properties of proteins being achieved at intermediate stages of germination.
3.3. Determination of Pasting Properties of Whole and Germinated Triticale Grain
Table 5 and Figure 5 present the samples’ pasting properties and curves for whole (WGT) and germinated triticale grain (GT 18, GT 20, GT 22, and GT 24).

Table 5.
Pasting properties of whole and germinated triticale grain samples.
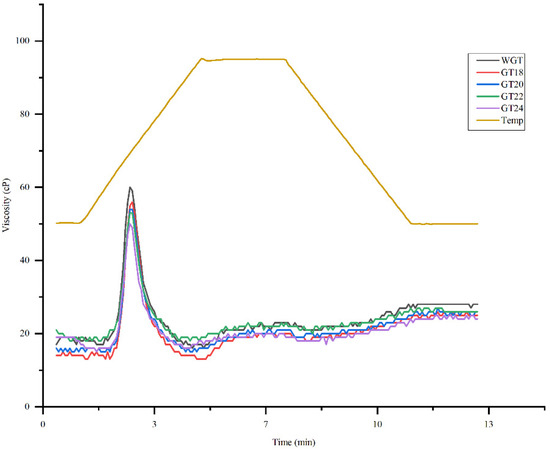
Figure 5.
Pasting property curves of whole and germinated triticale grain samples.
The results showed that the pasting properties gradually decreased as the germination time increased. An analysis of the peak viscosity parameter showed that the values for whole grain (WGT-60.00) were significantly higher than those for the germinated grain at 24 h (GT24-50.00), indicating a significant reduction in the starch’s ability to swell and retain water due to the enzymatic modification occurring during germination [98]. Germination triggers hydrolytic enzymes like α-amylase, which quickly lowers the viscosity of starch suspensions by breaking them down into simple sugars and oligosaccharides, modifying the rheological properties of germinated flours [33].
A similar trend was observed for the final viscosity, which reflects the structural changes in the starch gel during cooling and characterizes the starch’s ability to retrograde after thermal treatment. The value for the whole-grain sample (WGT) was 28.00, while for the sample germinated for 24 h (GT24), it decreased to 24.00. This decrease was statistically significant (p < 0.05) and indicated a reduction in the starch’s ability to retrograde due to the enzymatic modification occurring during germination.
However, despite the observed decrease in final viscosity, the starch in the germinated samples still partially retained its ability to retrograde after thermal treatment, as evidenced by the residual values of this parameter (e.g., 24.00 for GT24). This suggests that the structural elements responsible for retrogradation, particularly amylose fragments, did not undergo complete degradation but only partial breakdown. Stable retrogradation in starches is essential for producing products like vermicelli, instant porridge, and noodles, as it results in increased hardness and reduced stickiness of the functional product [131]. Nutritionally, retrograded starches are significant because they are digested slowly, which leads to more stable postprandial blood glucose levels, offering potential benefits for glycemic control and sustained energy release. A similar trend of decreasing viscosity parameters was observed in previous studies [33,132].
The breakdown viscosity, which refers to the stability of the starch gel to thermal and mechanical stress, also decreased from 39.00 in the whole grain to 33.00 in the grain germinated for 24 h, indicating reduced destruction of the gel structure during thermal processing. As germination progressed, the setback viscosity, which represents the difference between the final and minimum viscosities and characterizes the degree of starch retrogradation, changed insignificantly. The reduction in the viscosity values of the germinated triticale grain samples indicates moderate amylolytic activity associated with short-term germination.
4. Conclusions
The short-term germination of triticale grain resulted in favorable changes in its biochemical composition and functional-technological properties. The most significant increase in protein content was observed after 20 h of germination, accompanied by an improved amino acid profile and a higher proportion of essential amino acids, indicating enhanced nutritional value and protein quality. Throughout the germination period, a reduction in the content of fats, carbohydrates, and ash elements was noted. The maximum content of phenolic compounds and antioxidant activity were recorded at 24 h of germination, alongside increased levels of B-group vitamins and key minerals such as magnesium, molybdenum, and calcium, indicating functional value and potential health benefits.
Germination also improved the grain’s technological properties, including water and oil absorption capacities and foaming ability. The decrease in pasting properties suggests moderate amylolytic activity, which is favorable for further processing. These changes in the composition and properties of germinated triticale grain offer opportunities to improve the quality of whole-grain products. In particular, the use of germinated whole-grain flour in the baking industry can significantly enhance the nutritional value of products, improve their amino acid composition, and increase their antioxidant activity, making them appealing for healthy nutrition.
The limitations of this study include the lack of data on the impact of germination on the sensory characteristics of the final products, the shelf life of germinated flour, and the stability of bioactive compounds during thermal processing. However, these aspects require further investigation.
Author Contributions
Conceptualization, G.O.; methodology, I.T.; software, D.K. and M.M.; validation, S.T., A.A. and S.S.; formal analysis, S.T.; investigation, I.T. and D.T.; resources, S.S. and M.M.; data curation, A.Z. and D.T.; writing—original draft preparation, I.T.; writing—review and editing, G.O.; visualization, D.T. and D.K.; supervision, G.O.; project administration, G.O.; funding acquisition, G.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan, grant number BR21882327, “Development of new technologies for organic production and processing of agricultural products”.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, G.O., e-mail: bulashevag@mail.ru, upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| MP MN | Measurement Procedure Metrological Norm |
| GT | Germination Time |
| WGT | Whole-Grain Triticale |
| EN | European Norm |
| AACCI | American Association of Cereal Chemists International |
| n/d | Not detected |
References
- McGoverin, C.M.; Snyders, F.; Muller, N.; Botes, W.; Fox, G.; Manley, M. A review of triticale uses and the effect of growth environment on grain quality. J. Sci. Food Agric. 2011, 91, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Triticale: Nutritional composition and food uses. Food Chem. 2018, 241, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Fraś, A.; Gołębiewska, K.; Gołębiewski, D.; Mańkowski, D.R.; Boros, D.; Szecówka, P. Variability in the chemical composition of triticale grain, flour and bread. J. Cereal Sci. 2016, 71, 66–72. [Google Scholar] [CrossRef]
- Camerlengo, F.; Kiszonas, A.M. Genetic factors influencing triticale quality for food. J. Cereal Sci. 2023, 113, 103744. [Google Scholar] [CrossRef]
- Buchholz, M.; Drotleff, A.M.; Ternes, W. Thiamin (vitamin B1) and thiamin phosphate esters in five cereal grains during maturation. J. Cereal Sci. 2012, 56, 109–114. [Google Scholar] [CrossRef]
- Hosseinian, F.; Mazza, G. Triticale bran and straw: Potential new sources of phenolic acids, proanthocyanidins, and lignans. J. Funct. Foods 2009, 1, 57–64. [Google Scholar] [CrossRef]
- Makowska, A.; Szwengiel, A.; Kubiak, P.; Tomaszewska-Gras, J. Characteristics and structure of starch isolated from triticale. Starch-Stärke 2014, 66, 895–902. [Google Scholar] [CrossRef]
- Messina, V.; Cano, J.; Silvio, A.; Pattison, A.L.; Roberts, T.H. Wholegrain triticale sourdough: Effects of triticale: Wheat flour ratio and hydration level on bread quality. Food Sci. Nutr. 2024, 12, 3910–3919. [Google Scholar] [CrossRef]
- Kaszuba, J.; Jaworska, G.; Krochmal-Marczak, B.; Kogut, B.; Kuźniar, P. Effect of bran addition on rheological properties of dough and quality of triticale bread. J. Food Process. Preserv. 2021, 45, e15093. [Google Scholar] [CrossRef]
- Langó, B.; Bóna, L.; Ács, E.; Tömösközi, S. Nutritional features of triticale as affected by genotype, crop year, and location. Acta Aliment. 2017, 46, 238–245. [Google Scholar] [CrossRef]
- Dennett, A.L.; Wilkes, M.A.; Trethowan, R.M. Characteristics of modern triticale quality: The relationship between carbohydrate properties, α-amylase activity, and falling number. Cereal Chem. 2013, 90, 594–600. [Google Scholar] [CrossRef]
- Sibian, M.S.; Saxena, D.C.; Riar, C.S. Effect of germination on chemical, functional and nutritional characteristics of wheat, brown rice and triticale: A comparative study. J. Sci. Food Agric. 2017, 97, 4643–4651. [Google Scholar] [CrossRef]
- Meija, L.; Havensone, G.; Lejnieks, A. Postprandial glycaemic and insulinaemic responses after consumption of activated wheat and triticale grain flakes. J. Nutr. Metab. 2019, 2019, 6594896. [Google Scholar] [CrossRef] [PubMed]
- Piazza, I.; Carnevali, P.; Faccini, N.; Baronchelli, M.; Terzi, V.; Morcia, C.; Ghizzoni, R.; Patrone, V.; Morelli, L.; Cervini, M. Combining native and malted triticale flours in biscuits: Nutritional and technological implications. Foods 2023, 12, 3418. [Google Scholar] [CrossRef]
- Allai, F.M.; Azad, Z.; Gul, K.; Dar, B. Wholegrains: A review on the amino acid profile, mineral content, physicochemical, bioactive composition and health benefits. Int. J. Food Sci. Technol. 2022, 57, 1849–1865. [Google Scholar] [CrossRef]
- Xu, M.; Jin, Z.; Simsek, S.; Hall, C.; Rao, J.; Chen, B. Effect of germination on the chemical composition, thermal, pasting, and moisture sorption properties of flours from chickpea, lentil, and yellow pea. Food Chem. 2019, 295, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-España, M.; Figueroa-Hernández, C.Y.; de Dios Figueroa-Cárdenas, J.; Rayas-Duarte, P.; Hernández-Estrada, Z.J. Effects of germination and lactic acid fermentation on nutritional and rheological properties of sorghum: A graphical review. Curr. Res. Food Sci. 2022, 5, 807–812. [Google Scholar] [CrossRef]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.A.; Van den Broeck, H.C.; Brouns, F.J.; De Brier, N. Impact of cereal seed sprouting on its nutritional and technological properties: A critical review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, S. Bioactive components and functional properties of biologically activated cereal grains: A bibliographic review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3051–3071. [Google Scholar] [CrossRef]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Sangronis, E.; Machado, C. Influence of germination on the nutritional quality of Phaseolus vulgaris and Cajanus cajan. LWT-Food Sci. Technol. 2007, 40, 116–120. [Google Scholar] [CrossRef]
- Frias, J.; Miranda, M.L.; Doblado, R.; Vidal-Valverde, C. Effect of germination and fermentation on the antioxidant vitamin content and antioxidant capacity of Lupinus albus L. var. Multolupa. Food Chem. 2005, 92, 211–220. [Google Scholar] [CrossRef]
- Ghavidel, R.A.; Prakash, J. The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT-Food Sci. Technol. 2007, 40, 1292–1299. [Google Scholar] [CrossRef]
- Guzmán-Ortiz, F.A.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Mora-Escobedo, R.; Rojas-León, A.; Rodríguez-Marín, M.L.; Falfán-Cortés, R.N.; Román-Gutiérrez, A.D. Enzyme activity during germination of different cereals: A review. Food Rev. Int. 2019, 35, 177–200. [Google Scholar] [CrossRef]
- Nguyen, B.C.Q.; Shahinozzaman, M.; Tien, N.T.K.; Thach, T.N.; Tawata, S. Effect of sucrose on antioxidant activities and other health-related micronutrients in gamma-aminobutyric acid (GABA)-enriched sprouting Southern Vietnam brown rice. J. Cereal Sci. 2020, 93, 102985. [Google Scholar] [CrossRef]
- Sahai, V.; Kumar, V. Anti-diabetic, hepatoprotective and antioxidant potential of Brassica oleracea sprouts. Biocatal. Agric. Biotechnol. 2020, 25, 101623. [Google Scholar] [CrossRef]
- Nelson, K.; Mathai, M.L.; Ashton, J.F.; Donkor, O.N.; Vasiljevic, T.; Mamilla, R.; Stojanovska, L. Effects of malted and non-malted whole-grain wheat on metabolic and inflammatory biomarkers in overweight/obese adults: A randomised crossover pilot study. Food Chem. 2016, 194, 495–502. [Google Scholar] [CrossRef]
- Kadiri, O. A review on the status of the phenolic compounds and antioxidant capacity of the flour: Effects of cereal processing. Int. J. Food Prop. 2017, 20, S798–S809. [Google Scholar] [CrossRef]
- Chinma, C.E.; Adewuyi, O.; Abu, J.O. Effect of germination on the chemical, functional and pasting properties of flour from brown and yellow varieties of tigernut (Cyperus esculentus). Food Res. Int. 2009, 42, 1004–1009. [Google Scholar] [CrossRef]
- Ding, H.; Fu, T.J.; Smith, M.A. Microbial contamination in sprouts: How effective is seed disinfection treatment? J. Food Sci. Technol. 2013, 78, R495–R501. [Google Scholar] [CrossRef]
- Malik, I.O.M.; Yousif, S.A.; Ali, A.E.; Hamadnalla, H.M. Effect of germination on proximate composition of three grains from Sudan. J. Nutr. Food Sci. 2021, 3, 104–109. [Google Scholar]
- Baranzelli, J.; Kringel, D.H.; Colussi, R.; Paiva, F.F.; Aranha, B.C.; de Miranda, M.Z.; da Rosa Zavareze, E.; Dias, A.R.G. Changes in enzymatic activity, technological quality and gamma-aminobutyric acid (GABA) content of wheat flour as affected by germination. LWT 2018, 90, 483–490. [Google Scholar] [CrossRef]
- Marti, A.; Cardone, G.; Pagani, M.A.; Casiraghi, M.C. Flour from sprouted wheat as a new ingredient in bread-making. LWT 2018, 89, 237–243. [Google Scholar] [CrossRef]
- Dong, L.; Piao, Y.; Zhang, X.; Zhao, C.; Hou, Y.; Shi, Z. Analysis of volatile compounds from a malting process using headspace solid-phase micro-extraction and GC–MS. Food Res. Int. 2013, 51, 783–789. [Google Scholar] [CrossRef]
- Wu, F.; Yang, N.; Chen, H.; Jin, Z.; Xu, X. Effect of germination on flavor volatiles of cooked brown rice. Cereal Chem. 2011, 88, 497–503. [Google Scholar] [CrossRef]
- Waleed, A.-A.; Fadhl, J.A.; Abdullah, A.B.; Al-Adeeb, A.; Mahdi, A.A.; Al-Maqtari, Q.A.; Mushtaq, B.S.; Fan, M.; Li, Y.; Qian, H. Effect of highland barely germination on thermomechanical, rheological, and micro-structural properties of wheat-oat composite flour dough. Food Biosci. 2023, 53, 102521. [Google Scholar]
- Sharma, P.; Gujral, H.S. Antioxidant and polyphenol oxidase activity of germinated barley and its milling fractions. Food Chem. 2010, 120, 673–678. [Google Scholar] [CrossRef]
- Kruma, Z.; Tomsone, L.; Ķince, T.; Galoburda, R.; Senhofa, S.; Sabovics, M.; Straumite, E.; Sturite, I. Effects of germination on total phenolic compounds and radical scavenging activity in hull-less spring cereals and triticale. Agron. Res. 2016, 14, 1372–1383. [Google Scholar]
- AACC Intemational. Approved methods of analysis. In Rheological Behavior of Flour by Farinograph: Constant Flour Weight Procedure; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Alviola, J.N.A.; Monterde, V.G. Physicochemical and Functional Properties of Wheat (Triticum aestivum) and Selected Local Flours in the Philippines. Philipp. J. Sci. 2018, 147, 419–430. [Google Scholar]
- Lohdip, A.; Jikmyan, M. Proximate and amino acid analyses of the stem of Sesamum indicum L. (Black Seed Specie) Pedaliaceae. J. Chem. Soc. Niger. 2019, 44, 65–70. [Google Scholar]
- EN 12822:2014; Foodstuffs—Determination of Vitamin E by High Performance Liquid Chromatography—Measurement of α-, β-, γ- and δ-Tocopherol. Comité Européen de Normalisation (CEN): Brussels, Belgium, 2014.
- EN 14122:2014; Foodstuffs—Determination of Vitamin B1 by High Performance Liquid Chromatography. Comité Européen de Normalisation (CEN): Brussels, Belgium, 2014.
- EN 14152:2014; Foodstuffs—Determination of Vitamin B2 by High Performance Liquid Chromatography. Comité Européen de Normalisation (CEN): Brussels, Belgium, 2014.
- EN 15652:2009; Foodstuffs—Determination of Niacin by HPLC. Comité Européen de Normalisation (CEN): Brussels, Belgium, 2009.
- MP MN 3008-2008; Method for Determining the Mass Fraction of Pantothenic Acid in Specialized Food Products and Dietary Supplements. SI RSPC of Hygiene, MH RB: Minsk, Republic of Belarus, 2008.
- EN 14663:2006; Foodstuffs—Determination of Vitamin B6 (Including Its Glycosylated Forms) by HPLC. Comité Européen de Normalisation (CEN): Brussels, Belgium, 2006.
- MP MN 2146-2004; Method for the Determination of Folic Acid in Fortified Food Products. SI RSPC of Hygiene, MH RB: Minsk, Republic of Belarus, 2004.
- Ragaee, S.; Abdel-Aal, E.-S.M.; Noaman, M. Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 2006, 98, 32–38. [Google Scholar] [CrossRef]
- Marchini, M.; Carini, E.; Cataldi, N.; Boukid, F.; Blandino, M.; Ganino, T.; Vittadini, E.; Pellegrini, N. The use of red lentil flour in bakery products: How do particle size and substitution level affect rheological properties of wheat bread dough? LWT 2021, 136, 110299. [Google Scholar] [CrossRef]
- Abd Elmoneim, O.E.; Bernhardt, R. Influence of grain germination on functional properties of sorghum flour. Food Chem. 2010, 121, 387–392. [Google Scholar]
- Coffmann, C.; Garciaj, V. Functional properties and amino acid content of a protein isolate from mung bean flour. Int. J. Food Sci. Technol. 1977, 12, 473–484. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Lu, H.; Shu, Q.; Zhang, Y.; Chen, Q. New perspectives on physiological, biochemical and bioactive components during germination of edible seeds: A review. Trends Food Sci. Technol. 2022, 123, 187–197. [Google Scholar] [CrossRef]
- Singkhornart, S.; Ryu, G.-H.J.P.N.; Science, F. Effect of soaking time and steeping temperature on biochemical properties and γ-aminobutyric acid (GABA) content of germinated wheat and barley. Prev. Nutr. Food Sci. 2011, 16, 67–73. [Google Scholar] [CrossRef]
- Kruma, Z.; Kince, T.; Galoburda, R.; Tomsone, L.; Straumite, E.; Sabovics, M.; Sturite, I.; Kronberga, A. Influence of germination temperature and time on phenolic content and antioxidant properties of cereals. In Proceedings of the Baltic Conference on Food Science and Technology: Conference Proceedings, Jelgava, Latvia, 2–3 May 2019. [Google Scholar]
- Ahmed, W.E.; Ragab, I.; Gadallah, M.G.; Alhomaid, R.M.; Almujaydil, M.S. Effect of sprouting whole wheat grain on the sensory quality, physicochemical properties, and antioxidant activity of cupcakes. Appl. Food Res. 2024, 4, 100412. [Google Scholar] [CrossRef]
- El-Safy, F.; Salem, R.; Mukhtar, E.Y. The impact of soaking and germination on chemical composition, carbohydrate fractions, digestibility, antinutritional factors and minerals content of some legumes and cereals grain seeds. Alex. Sci. Exch. J. 2013, 34, 499–513. [Google Scholar]
- Ongol, M.P.; Niyonzima, E.; Gisanura, I.; Vasanthakaalam, H. Effect of germination and fermentation on nutrients in maize flour. Pak. J. Food Sci. 2013, 23, 183–188. [Google Scholar]
- Erba, D.; Angelino, D.; Marti, A.; Manini, F.; Faoro, F.; Morreale, F.; Pellegrini, N.; Casiraghi, M.C. Effect of sprouting on nutritional quality of pulses. Int. J. Food Sci. Nutr. 2019, 70, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Afify, A.E.-M.M.; El-Beltagi, H.S.; Abd El-Salam, S.M.; Omran, A.A. Protein solubility, digestibility and fractionation after germination of sorghum varieties. PLoS ONE 2012, 7, e31154. [Google Scholar] [CrossRef]
- Tizazu, S.; Urga, K.; Abuye, C.; Retta, N. Improvement of energy and nutrient density of sorghumbased complementary foods using germination. Afr. J. Food Agric. Nutr. Dev. 2010, 10, 2927–2942. [Google Scholar] [CrossRef]
- Oser, B.L. An integrated essential amino acid index for predicting the biological value of proteins. In Protein and Amino Acid Nutrition; Elsevier: Amsterdam, The Netherlands, 1959; pp. 281–295. [Google Scholar]
- Steve, I.O. Influence of germination and fermentation on chemical composition, protein quality and physical properties of wheat flour (Triticum aestivum). J. Cereals Oil Seeds 2012, 3, 35–47. [Google Scholar]
- Lemar, L.E.; Swanson, B.G. Nutritive value of sprouted wheat flour. J. Food Sci. 1976, 41, 719–720. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.; Nonogaki, H.; Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.; Nonogaki, H. Mobilization of stored reserves. In Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 183–246. [Google Scholar]
- Pal, R.; Bhartiya, A.; Yadav, P.; Kant, L.; Mishra, K.; Aditya, J.; Pattanayak, A. Effect of dehulling, germination and cooking on nutrients, anti-nutrients, fatty acid composition and antioxidant properties in lentil (Lens culinaris). J. Food Sci. Technol. 2017, 54, 909–920. [Google Scholar] [CrossRef]
- Yang, B.; Yin, Y.; Liu, C.; Zhao, Z.; Guo, M. Effect of germination time on the compositional, functional and antioxidant properties of whole wheat malt and its end-use evaluation in cookie-making. Food Chem. 2021, 349, 129125. [Google Scholar] [CrossRef]
- Guardianelli, L.M.; Salinas, M.V.; Puppo, M.C. Chemical and thermal properties of flours from germinated amaranth seeds. J. Food Meas. Charact. 2019, 13, 1078–1088. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.; El-Beltagi, H.S.; Abd El-Salam, S.M.; Omran, A.A. Effect of soaking, cooking, germination and fermentation processing on proximate analysis and mineral content of three white sorghum varieties (Sorghum bicolor L. Moench). Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 92–98. [Google Scholar]
- Teixeira, C.; Nyman, M.; Andersson, R.; Alminger, M. Effects of variety and steeping conditions on some barley components associated with colonic health. J. Sci. Food Agric. 2016, 96, 4821–4827. [Google Scholar] [CrossRef]
- Donkor, O.; Stojanovska, L.; Ginn, P.; Ashton, J.; Vasiljevic, T. Germinated grains–Sources of bioactive compounds. Food Chem. 2012, 135, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, S.; Parimalavalli, R. Effect of processing methods on proximate composition of cereal and legume flours. J. Hum. Nutr. Food Sci. 2014, 2, 1051. [Google Scholar]
- Jan, R.; Saxena, D.; Singh, S. Physico-chemical, textural, sensory and antioxidant characteristics of gluten–Free cookies made from raw and germinated Chenopodium (Chenopodium album) flour. LWT-Food Sci. Technol. 2016, 71, 281–287. [Google Scholar] [CrossRef]
- Kubicka, E.; Grabska, J.; Jedrychowski, L.; Czyz, B. Changes of specific activity of lipase and lipoxygenase during germination of wheat and barley. Int. J. Food Sci. Nutr. 2000, 51, 301. [Google Scholar] [PubMed]
- Poudel, R.; Finnie, S.; Rose, D.J. Effects of wheat kernel germination time and drying temperature on compositional and end-use properties of the resulting whole wheat flour. J. Cereal Sci. 2019, 86, 33–40. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, A.; Gupta, K.; Gat, Y.; Kumar, V. Assessment of germination time of finger millet for value addition in functional foods. Curr. Sci. 2021, 120, 406–413. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S.; Singh, B. Effect of germination time and temperature on the functionality and protein solubility of sorghum flour. J. Cereal Sci. 2017, 76, 131–139. [Google Scholar] [CrossRef]
- Gunathunga, C.; Senanayake, S.; Jayasinghe, M.A.; Brennan, C.S.; Truong, T.; Marapana, U.; Chandrapala, J. Germination effects on nutritional quality: A comprehensive review of selected cereals and pulses changes. J. Food Compos. Anal. 2024, 128, 106024. [Google Scholar] [CrossRef]
- Dhillon, B.; Choudhary, G.; Sodhi, N.S. A study on physicochemical, antioxidant and microbial properties of germinated wheat flour and its utilization in breads. J. Food Sci. Technol. 2020, 57, 2800–2808. [Google Scholar] [CrossRef]
- Sharma, S.; Saxena, D.C.; Riar, C.S. Analysing the effect of germination on phenolics, dietary fibres, minerals and γ-amino butyric acid contents of barnyard millet (Echinochloa frumentaceae). Food Biosci. 2016, 13, 60–68. [Google Scholar] [CrossRef]
- Suda, M.; Watanabe, T.; Kobayashi, M.; Matsuda, K. Changes in starch content and related enzyme activities during the growth of germinating soybeans. Agric. Biol. Chem. 1986, 50, 3195–3196. [Google Scholar]
- Enujiugha, V.N.; Badejo, A.A.; Iyiola, S.O.; Oluwamukomi, M.O. Effect of germination on the nutritional and functional properties of African oil bean (Pentaclethra macrophylla Benth) seed flour. J. Food Agric. Environ. 2003, 1, 72–75. [Google Scholar]
- Murungweni, K.T.; Ramashia, S.E.; Mashau, M.E. Effect of malting on physicochemical, antioxidant, and microstructural properties of finger millet (Eleusine coracana) flours. Food Sci. Nutr. 2024, 12, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Nefale, F.E.; Mashau, M.E. Effect of germination period on the physicochemical, functional and sensory properties of finger millet flour and porridge. Asian J. Appl. Sci. 2018, 6, 360–367. [Google Scholar] [CrossRef]
- Perveen, S.; Akhtar, S.; Ismail, T.; Qamar, M.; Saeed, W.; Younis, M.; Esatbeyoglu, T. Comparison of nutritional, antioxidant, physicochemical, and rheological characteristics of whole and sprouted wheat flour. LWT 2024, 209, 116679. [Google Scholar] [CrossRef]
- Wu, Y.V. Lysine content of triticale protein increased by germination. J. Agric. Food Chem. 1982, 30, 820–823. [Google Scholar] [CrossRef]
- Smith, D. The amino acid composition of barley grain protein during development and germination. J. Agric. Sci. 1972, 78, 265–273. [Google Scholar] [CrossRef]
- Li, Q.; Xu, J.-G. Changes in nutritive value and in vitro digestibility of proteins from naked oats during germination. Eur. J. Food Sci. Technol. 2015, 3, 49–57. [Google Scholar] [CrossRef]
- Kamjijam, B.; Bednarz, H.; Suwannaporn, P.; Jom, K.N.; Niehaus, K. Localization of amino acids in germinated rice grain: Gamma-aminobutyric acid and essential amino acids production approach. J. Cereal Sci. 2020, 93, 102958. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Bains, K.; Moughan, P.J. Available lysine and digestible amino acid contents of proteinaceous foods of India. Br. J. Nutr. 2012, 108, S59–S68. [Google Scholar] [CrossRef]
- Singh, M.; Rao, D.M.; Pande, S.; Battu, S.; Mahalakshmi, K.; Dutt, K.R.; Ramesh, M. Medicinal uses of L-lysine: Past and future. Int. J. Res. Pharm. Sci. 2011, 2, 637–642. [Google Scholar]
- Sánchez, A.; Rubano, D.A.; Shavlik, G.W.; Hubbard, R.; Horning, M.C. Cholesterolemic effects of the lysine/arginine ratio in rabbits after initial early growth. Arch. Latinoam. Nutr. 1988, 38, 229–238. [Google Scholar]
- Majzoobi, M.; Wang, Z.; Teimouri, S.; Pematilleke, N.; Brennan, C.S.; Farahnaky, A. Unlocking the potential of sprouted cereals, pseudocereals, and pulses in combating malnutrition. Foods 2023, 12, 3901. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Hwang, I.G.; Kim, T.M.; Woo, K.S.; Park, D.S.; Kim, J.H.; Kim, D.J.; Lee, J.; Lee, Y.R.; Jeong, H.S. Chemical and functional components in different parts of rough rice (Oryza sativa L.) before and after germination. Food Chem. 2012, 134, 288–293. [Google Scholar] [CrossRef]
- Žilić, S.; Delić, N.; Basić, Z.; Ignjatović-Micić, D.; Janković, M.; Vančetović, J. Effects of alkaline cooking and sprouting on bioactive compounds, their bioavailability and relation to antioxidant capacity of maize flour. J. Food Nutr. Res. 2015, 54, 155. [Google Scholar]
- Okorie, S.; Ekwe, C. The comparative analysis of sprouted legume and cereal based composite diet. J. Appl. Biotechnol. Bioeng. 2017, 4, 554–561. [Google Scholar]
- Chaves-López, C.; Rossi, C.; Maggio, F.; Paparella, A.; Serio, A. Changes occurring in spontaneous maize fermentation: An overview. Fermentation 2020, 6, 36. [Google Scholar] [CrossRef]
- Rico, D.; Peñas, E.; García, M.d.C.; Martínez-Villaluenga, C.; Rai, D.K.; Birsan, R.I.; Frias, J.; Martín-Diana, A.B. Sprouted barley flour as a nutritious and functional ingredient. Foods 2020, 9, 296. [Google Scholar] [CrossRef]
- Kariluoto, S.; Liukkonen, K.-H.; Myllymäki, O.; Vahteristo, L.; Kaukovirta-Norja, A.; Piironen, V. Effect of germination and thermal treatments on folates in rye. J. Agric. Food Chem. 2006, 54, 9522–9528. [Google Scholar] [CrossRef]
- Plaza, L.; de Ancos, B.; Cano, P.M. Nutritional and health-related compounds in sprouts and seeds of soybean (Glycine max), wheat (Triticum aestivum. L) and alfalfa (Medicago sativa) treated by a new drying method. Eur. Food Res. Technol. 2003, 216, 138–144. [Google Scholar] [CrossRef]
- Malleshi, N.; Klopfenstein, C. Nutrient composition, amino acid and vitamin contents of malted sorghum, pearl millet, finger millet and their rootlets. Int. J. Food Sci. Nutr. 1998, 49, 415–422. [Google Scholar] [CrossRef]
- Hucker, B.; Wakeling, L.; Vriesekoop, F. Investigations into the thiamine and riboflavin content of malt and the effects of malting and roasting on their final content. J. Cereal Sci. 2012, 56, 300–306. [Google Scholar] [CrossRef]
- Moongngarm, A.; Saetung, N. Comparison of chemical compositions and bioactive compounds of germinated rough rice and brown rice. Food Chem. 2010, 122, 782–788. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Bubolz, V.K.; da Silva, J.; Dittgen, C.L.; Ziegler, V.; de Oliveira Raphaelli, C.; de Oliveira, M. Changes in the chemical composition and bioactive compounds of chickpea (Cicer arietinum L.) fortified by germination. LWT 2019, 111, 363–369. [Google Scholar] [CrossRef]
- Steve Ijarotimi, O.; Ruth Esho, T. Comparison of nutritional composition and anti-nutrient status of fermented, germinated and roasted bambara groundnut seeds (vigna subterranea). Food Chem. 2009, 111, 376–386. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.; El-Beltagi, H.S.; Abd El-Salam, S.M.; Omran, A.A. Bioavailability of iron, zinc, phytate and phytase activity during soaking and germination of white sorghum varieties. PLoS ONE 2011, 6, e25512. [Google Scholar] [CrossRef]
- Hübner, F.; O’Neil, T.; Cashman, K.D.; Arendt, E.K. The influence of germination conditions on beta-glucan, dietary fibre and phytate during the germination of oats and barley. Eur. Food Res. Technol. 2010, 231, 27–35. [Google Scholar] [CrossRef]
- Egli, I.; Davidsson, L.; Juillerat, M.; Barclay, D.; Hurrell, R. The influence of soaking and germination on the phytase activity and phytic acid content of grains and seeds potentially useful for complementary feedin. J. Food Sci. 2002, 67, 3484–3488. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Yassin, E.; Eisa, Y.; Abou El-Enien, N.F. Effect of infestation with acaridid mite, Tyrophagus putrescentiae (Schrank) on germination rate of maize grains. Egypt. Acad. J. Biol. Sci. A Entomol. 2013, 6, 81–85. [Google Scholar] [CrossRef]
- Rosique-Esteban, N.; Guasch-Ferré, M.; Hernández-Alonso, P.; Salas-Salvadó, J. Dietary magnesium and cardiovascular disease: A review with emphasis in epidemiological studies. Nutrients 2018, 10, 168. [Google Scholar] [CrossRef]
- Sadawarte, S.; Pawar, V.; Sawate, A.; Thorat, P.; Shere, P.; Surendar, J. Effect of germination on vitamin and mineral content of horse gram and green gram malt. IJCS 2018, 6, 1761–1764. [Google Scholar]
- Agidew, M.G.; Dubale, A.A.; Atlabachew, M.; Abebe, W. Fatty acid composition, total phenolic contents and antioxidant activity of white and black sesame seed varieties from different localities of Ethiopia. Chem. Biol. Technol. Agric. 2021, 8, 14. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Tian, S.; Nakamura, K.; Kayahara, H. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J. Agric. Food Chem. 2004, 52, 4808–4813. [Google Scholar] [CrossRef] [PubMed]
- Lemus, C.; Angelis, A.; Halabalaki, M.; Skaltsounis, A.L. γ-Oryzanol: An attractive bioactive component from rice bran. In Wheat and Rice in Disease Prevention and Health; Elsevier: Amsterdam, The Netherlands, 2014; pp. 409–430. [Google Scholar]
- Gawlik-Dziki, U.; Dziki, D.; Nowak, R.; Świeca, M.; Olech, M.; Pietrzak, W. Influence of sprouting and elicitation on phenolic acids profile and antioxidant activity of wheat seedlings. J. Cereal Sci. 2016, 70, 221–228. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, X.; Liu, Y.; Zhang, M.; Zhang, R.; Abbasi, A.M.; You, L.; Li, T.; Liu, R.H. Comparative assessment of phytochemical profile, antioxidant capacity and anti-proliferative activity in different varieties of brown rice (Oryza sativa L.). LWT 2018, 96, 19–25. [Google Scholar] [CrossRef]
- Gernah, D.; Ariahu, C.; Ingbian, E. Effects of malting and lactic fermentation on some chemical and functional properties of maize (Zea mays). Am. J. Food Technol. 2011, 6, 404–412. [Google Scholar] [CrossRef]
- Adedeji, O.; Oyinloye, O.; Ocheme, O. Effects of germination time on the functional properties of maize flour and the degree of gelatinization of its cookies. Afr. J. Food Sci. 2014, 8, 42–47. [Google Scholar] [CrossRef]
- Akaerue, B.I.; Onwuka, G.I. The functional properties of the dehulled and undehulled mung bean (Vigna radiata (l.) Wilczek) flours as influenced by processing treatments. J. Agric. Vet. Sci. 2010, 2, 1–28. [Google Scholar]
- Offia Olua, B.; Onwuzuruike, U.; Nwankpa, M. The effect of different processing treatments on the proximate composition and functional properties of maize-mung bean composite flours. J. Food Stab. 2020, 3, 12–25. [Google Scholar] [CrossRef]
- Siddiqua, A.; Ali, M.; Ahmed, S. Functional properties of germinated and non-germinated cereals: A comparative study. Bangladesh J. Sci. Ind. Res. 2019, 54, 383–390. [Google Scholar] [CrossRef]
- Hussain, I.; Uddin, M.B. Optimization effect of germination on functional properties of wheat flour by response surface methodology. Int. Res. J. Plant Sci. 2012, 3, 31–37. [Google Scholar]
- Hung, S.; Zayas, J. Protein solubility, water retention, and fat binding of corn germ protein flour compared with milk proteins. J. Food Sci. 1992, 57, 372–376. [Google Scholar] [CrossRef]
- Wang, X.-H.; Tai, Z.-J.; Song, X.-J.; Li, Z.-J.; Zhang, D.-J. Effects of germination on the structure, functional properties, and in vitro digestibility of a black bean (Glycine max (L.) Merr.) protein isolate. Foods 2024, 13, 488. [Google Scholar] [CrossRef]
- Ocheme, O.; Adedeji, O.; Lawal, G.; Zakari, U. Effect of germination on functional properties and degree of starch gelatinization of sorghum flour. J. Food Res. 2015, 4, 159. [Google Scholar]
- Eltayeb, A.; Ali, A.O.; Abou-Arab, A.A.; Abu-Salem, F.M. Chemical composition and functional properties of flour and protein isolate extracted from Bambara groundnut (Vigna subterranean). Afr. J. Food Sci. 2011, 5, 82–90. [Google Scholar]
- Pork, B.; Yoon, K.Y. Functional properties of enzymatic hydrolysate and peptide fractions from perilla seed meal protein. Pol. J. Food Nutr. Sci. 2019, 69, 119–127. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Luck, P.; Davis, J.P. Factors determining the physical properties of protein foams. Food Hydrocoll. 2006, 20, 284–292. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Fan, L.; Ma, S. A review of wheat starch analyses: Methods, techniques, structure and function. Int. J. Biol. Macromol. 2022, 203, 130–142. [Google Scholar] [CrossRef]
- Cardone, G.; Grassi, S.; Scipioni, A.; Marti, A. Bread-making performance of durum wheat as affected by sprouting. LWT 2020, 134, 110021. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).