Nitrogen Metabolism in Two Flor Yeast Strains at Mid-Second Bottle Fermentation in Sparkling Wine Production
Abstract
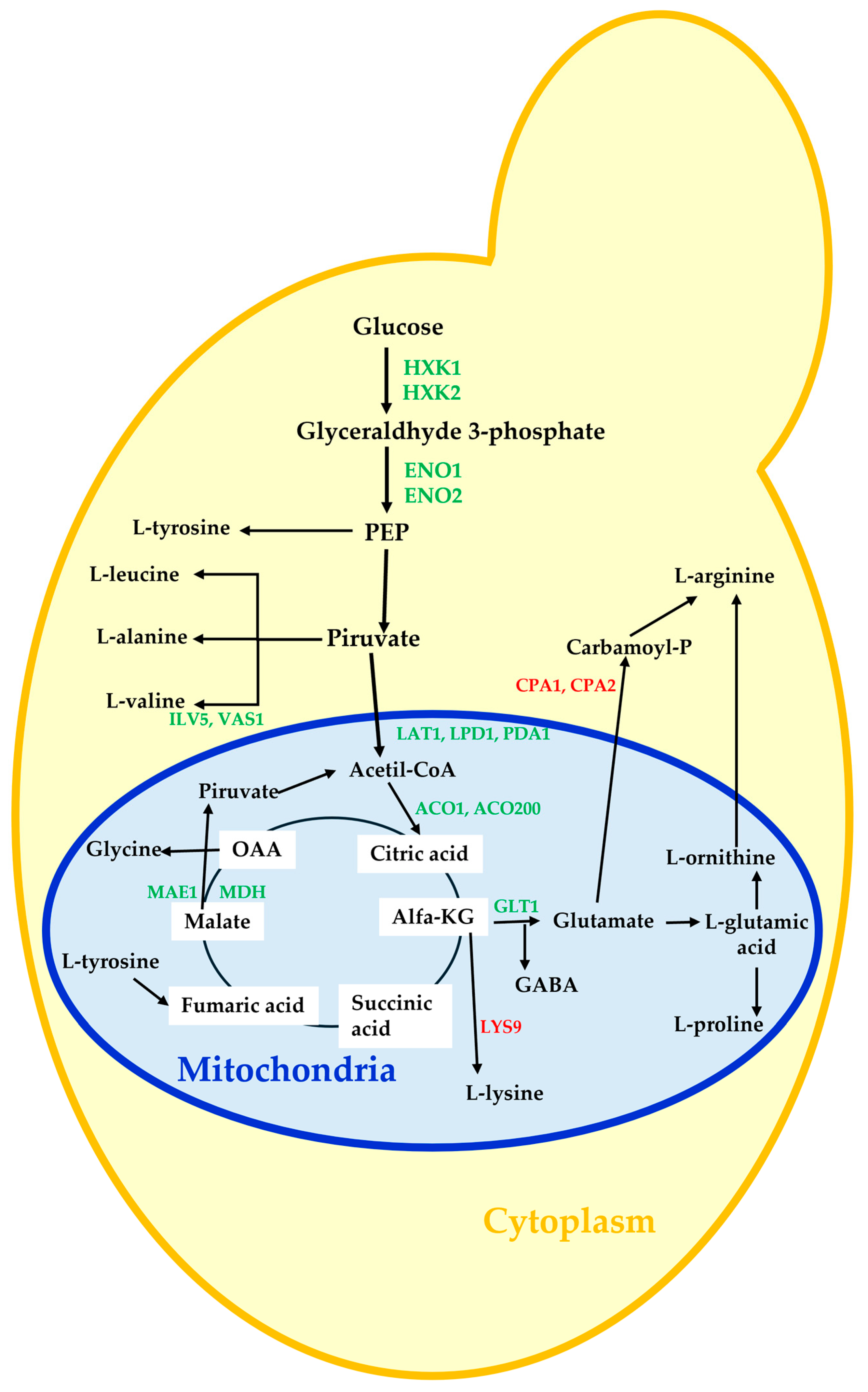
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Acclimation Process
2.2. Sparkling Wine Production
2.3. Nitrogenous Compound Analysis
2.4. Proteomic Analysis
2.4.1. Protein Extraction
2.4.2. LC-MS/MS Analysis
2.4.3. Protein Identification via Database Search
2.4.4. Statistical Procedures
3. Results
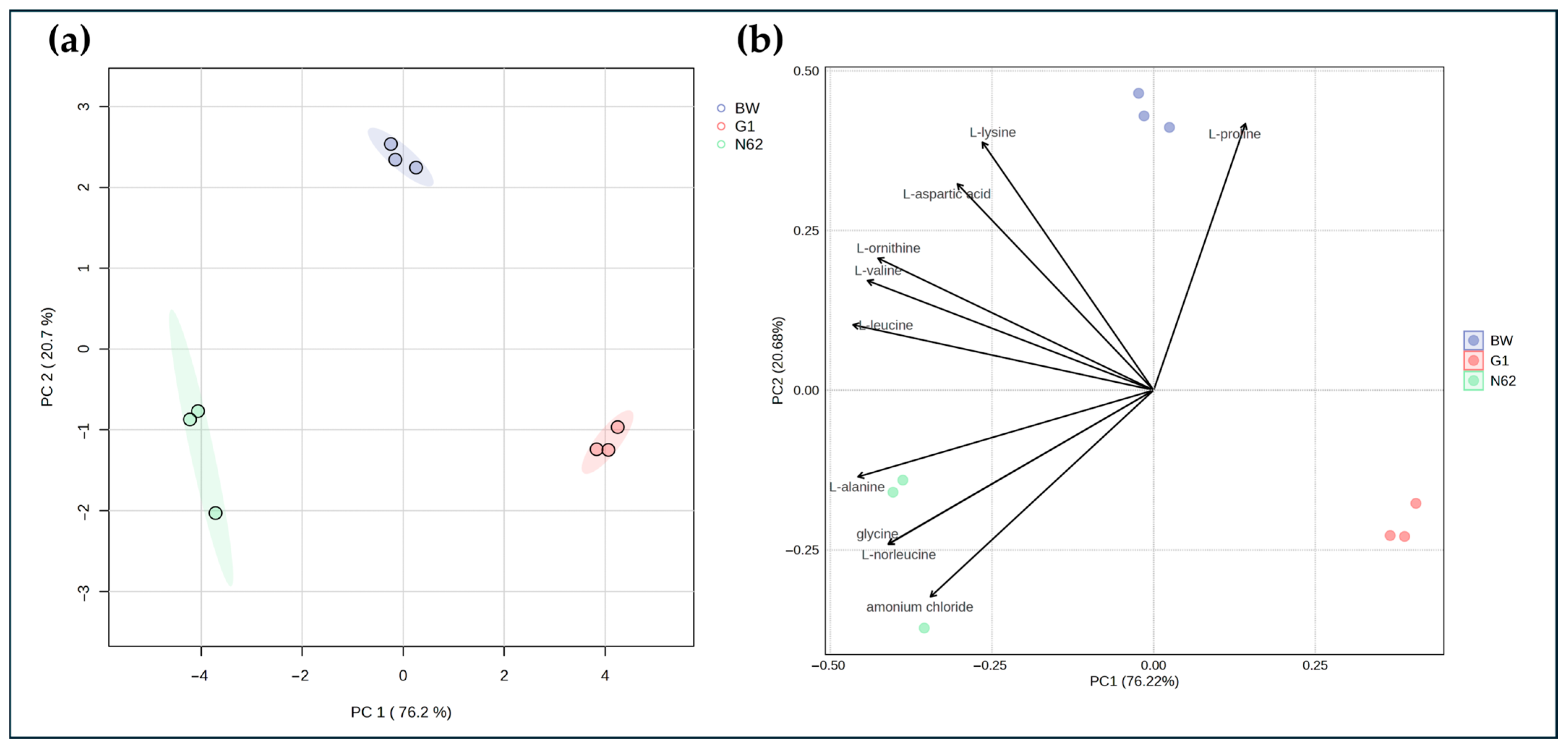
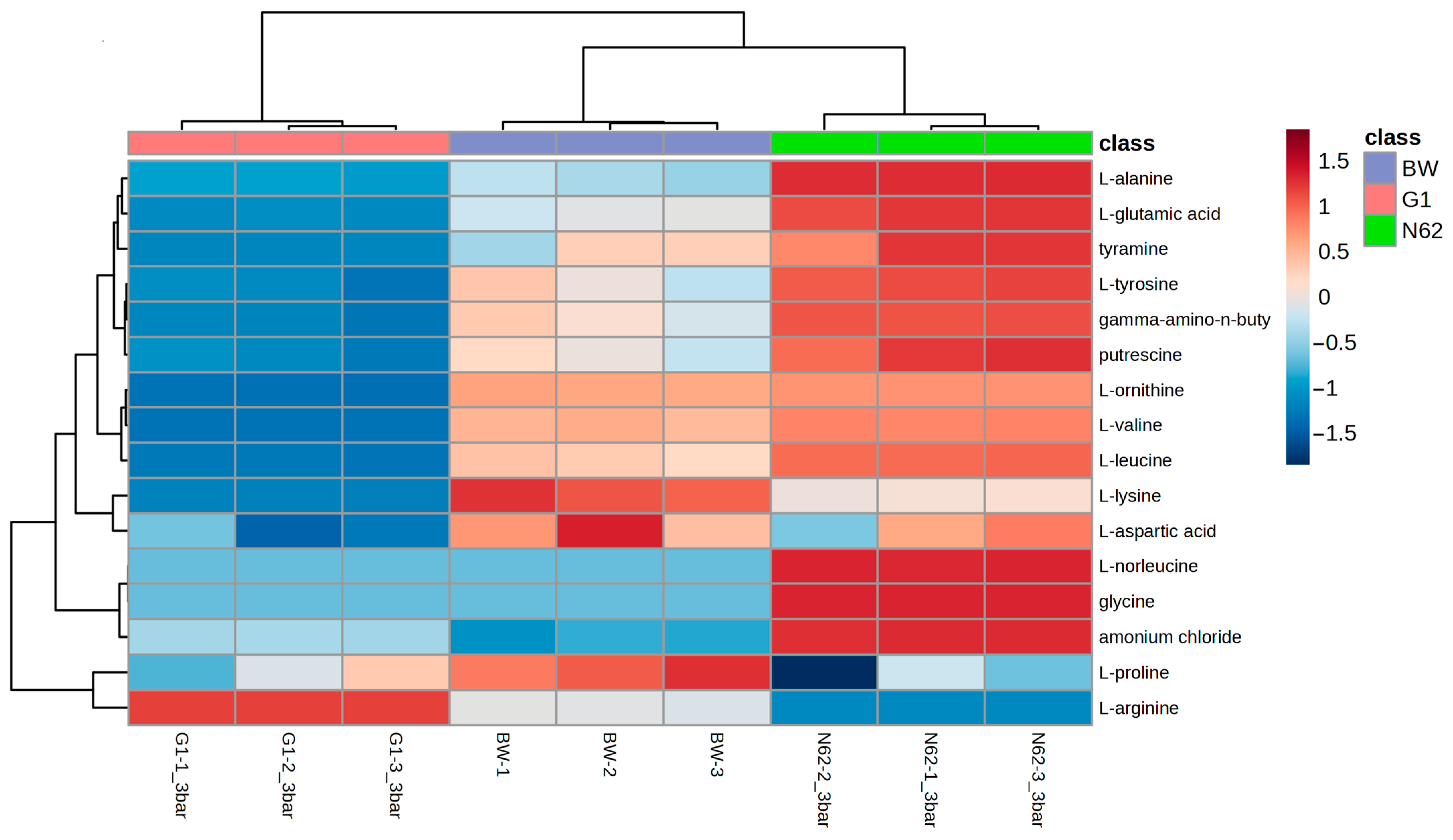
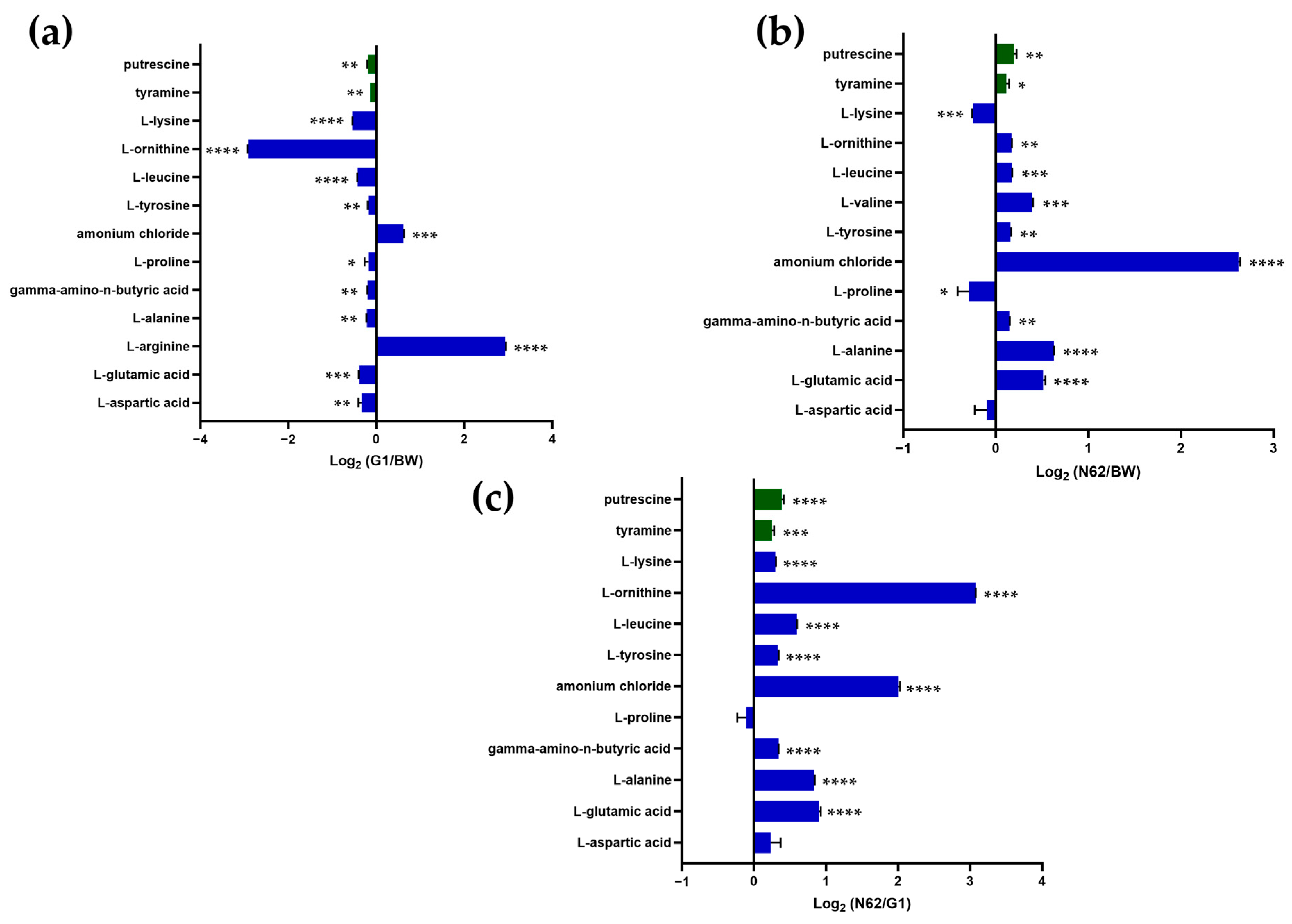
3.1. Comparison of the Nitrogen Compound Profiles of the Base Wine and Wines with Yeast Strains
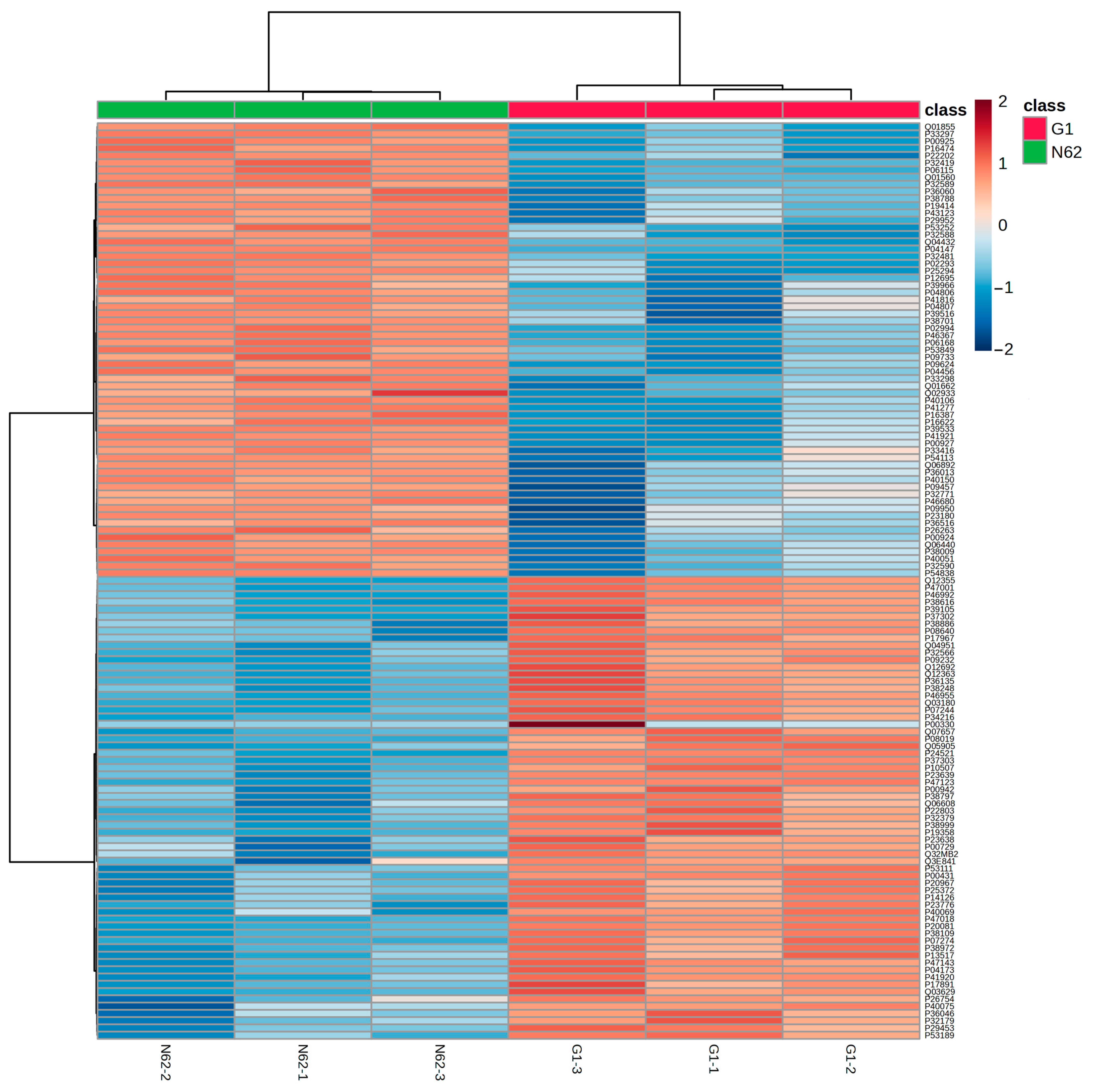
3.2. Comparison of the Proteomic Profiles of Two Yeast Strains at Three Bars of Pressure
3.2.1. Qualitative Proteomic Analysis
3.2.2. One-Way Analysis of Variance (ANOVA) of Proteins
3.3. Interaction Between Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Jiménez, M.D.C.; Mauricio, J.C.; Moreno-García, J.; Puig-Pujol, A.; Moreno, J.; García-Martínez, T. Differential response of the proteins involved in amino acid metabolism in two Saccharomyces cerevisiae strains during the second fermentation in a sealed bottle. Appl. Sci. 2021, 11, 12165. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.J.; Carrascosa, A.V.; Martín-Álvarez, P.J.; Moreno-Arribas, V.; Polo, M.C. Influence of the yeast strain on the changes of the amino acids, peptides and proteins during sparkling wine production by the traditional method. J. Ind. Microbiol. Biotechnol. 2002, 29, 314–322. [Google Scholar] [CrossRef]
- Prokes, K.; Baron, M.; Mlcek, J.; Jurikova, T.; Adamkova, A.; Ercisli, S.; Sochor, J. The influence of traditional and immobilized yeast on the amino-acid content of sparkling wine. Fermentation 2022, 8, 36. [Google Scholar] [CrossRef]
- Condé, B.C.; Bouchard, E.; Culbert, J.A.; Wilkinson, K.L.; Fuentes, S.; Howell, K.S. Soluble protein and amino acid content affects the foam quality of sparkling wine. J. Agric. Food Chem. 2017, 65, 9110–9119. [Google Scholar] [CrossRef]
- Sun, N.; Zhao, L.; Liu, A.; Su, L.; Shi, K.; Zhao, H.; Liu, S. Role of amino acids in flavor profiles and foam characteristics of sparkling wines during aging. J. Food Compos. Anal. 2024, 126, 105903. [Google Scholar] [CrossRef]
- Sartor, S.; Burin, V.M.; Caliari, V.; Bordignon-Luiz, M.T. Profiling free amino acids in sparkling wines during over-lees aging and evaluation of sensory properties. LWT-Food Sci. Technol. 2021, 140, 110847. [Google Scholar] [CrossRef]
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F.; Lanciotti, R. Role of yeasts on the sensory component of wines. Foods 2022, 11, 1921. [Google Scholar] [CrossRef]
- Espinase Nandorfy, D.; Watson, F.; Likos, D.; Siebert, T.; Bindon, K.; Kassara, S.; Shellie, R.; Keast, R.; Francis, I.L. Influence of amino acids, and their interaction with volatiles and polyphenols, on the sensory properties of red wine. Aust. J. Grape Wine Res. 2022, 28, 621–637. [Google Scholar] [CrossRef]
- Charnock, H.M.; Pickering, G.J.; Kemp, B.S. The Maillard reaction in traditional method sparkling wine. Front. Microbiol. 2022, 13, 979866. [Google Scholar] [CrossRef]
- Restuccia, D.; Loizzo, M.R.; Spizzirri, U.G. Accumulation of biogenic amines in wine: Role of alcoholic and malolactic fermentation. Fermentation 2018, 4, 6. [Google Scholar] [CrossRef]
- Raymond Eder, M.L.; Rosa, A.L. Non-conventional grape varieties and yeast starters for first and second fermentation in sparkling wine production using the traditional method. Fermentation 2021, 7, 321. [Google Scholar] [CrossRef]
- Basile, T.; Debiase, G.; Mazzone, F.; Scarano, L.; Marsico, A.D.; Cardone, M.F. New sparkling wines from traditional grape varieties and native yeasts: Focusing on wine identity to address the industry’s crisis. Beverages 2025, 11, 25. [Google Scholar] [CrossRef]
- Martí-Raga, M.; Marullo, P.; Beltran, G.; Mas, A. Nitrogen modulation of yeast fitness and viability during sparkling wine production. Food Microbiol. 2016, 54, 106–114. [Google Scholar] [CrossRef]
- García-García, J.C.; García-Martínez, T.; Román-Camacho, J.J.; Moreno, J.; Mauricio, J.C. Comparative proteomics of two flor yeasts in sparkling wine fermentation: First approach. Foods 2025, 14, 282. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Hermosín-Gutiérrez, I.; García-Romero, E. Simultaneous HPLC analysis of biogenic amines, amino acids, and ammonium ion as aminoenone derivatives in wine and beer samples. J. Agric. Food Chem. 2007, 55, 608–613. [Google Scholar] [CrossRef]
- Valero, E.; Millán, C.; Ortega, J.M.; Mauricio, J.C. Concentration of amino acids in wine after the end of fermentation by Saccharomyces cerevisiae strains. J. Sci. Food Agric. 2003, 83, 830–835. [Google Scholar] [CrossRef]
- Magasanik, B.; Kaiser, C.A. Nitrogen regulation in Saccharomyces cerevisiae. Gene 2002, 290, 1–18. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, W.; Ma, W.; Ma, W.; Zhou, J. Chromatin regulators Ahc1p and Eaf3p positively influence nitrogen metabolism in Saccharomyces cerevisiae. Front. Microbiol. 2022, 13, 883934. [Google Scholar] [CrossRef]
- Kumar, R.; Lahtvee, P.J. Proteome overabundance enables respiration but limitation onsets carbon overflow. BioRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, W.; Du, G.; Zhou, J.; Chen, J. Regulation of sensing, transportation, and catabolism of nitrogen sources in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2018, 82, 10–1128. [Google Scholar] [CrossRef]
- Kolkman, A.; Daran-Lapujade, P.; Fullaondo, A.; Olsthoorn, M.M.; Pronk, J.T.; Slijper, M.; Heck, A.J. Proteome analysis of yeast response to various nutrient limitations. Mol. Syst. Biol. 2006, 2, 2006–2026. [Google Scholar] [CrossRef]
- Burchacka, E.; Pięta, P.; Łupicka-Słowik, A. Recent advances in fungal serine protease inhibitors. Biomed. Pharmacother. 2022, 146, 112523. [Google Scholar] [CrossRef] [PubMed]
- Tian, E.; Shen, X.; Xiao, M.; Zhu, Z.; Yang, Y.; Yan, X.; Wang, P.; Zou, G.; Zhou, Z. An engineered Pichia pastoris platform for the biosynthesis of silk-based nanomaterials with therapeutic potential. Int. J. Biol. Macromol. 2024, 269, 131954. [Google Scholar] [CrossRef]
- Ramachandran, G.; Chidambaram, R.; Nachiappan, V. FSH1 encodes lysophospholipase activity in Saccharomyces cerevisiae. Biotechnol. Lett. 2021, 43, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Hoh, K.L.; Mu, B.; See, T.; Ng, A.Y.E.; Ng, A.Q.E.; Zhang, D. VAP-mediated membrane-tethering mechanisms implicate ER-PM contact function in pH homeostasis. Cell Rep. 2024, 43, 114592. [Google Scholar] [CrossRef] [PubMed]
- Luqueño-Bocardo, O.I.; Pardo, J.P.; Guerra-Sánchez, G.; González, J.; Matus-Ortega, G.; Romero-Aguilar, L. The isoprenoids as biofuel source. Tip Rev. Espec. Cienc. Quím.-Biol. 2021, 24, 1–11. [Google Scholar]
- Gobert, A.; Tourdot-Maréchal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of nitrogen status in wine alcoholic fermentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef]
- Jordá, T.; Puig, S. Regulation of ergosterol biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef]
- Vidal, E.E.; de Billerbeck, G.M.; Simões, D.A.; Schuler, A.; François, J.M.; de Morais, M.A., Jr. Influence of nitrogen supply on the production of higher alcohols/esters and expression of flavour-related genes in cachaça fermentation. Food Chem. 2013, 138, 701–708. [Google Scholar] [CrossRef]
- Esposito, F.; Montuori, P.; Schettino, M.; Velotto, S.; Stasi, T.; Romano, R.; Cirillo, T. Level of biogenic amines in red and white wines, dietary exposure, and histamine-mediated symptoms upon wine ingestion. Molecules 2019, 24, 3629. [Google Scholar] [CrossRef]
- Moreira, L.; Milheiro, J.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Exploring factors influencing the levels of biogenic amines in wine and microbiological strategies for controlling their occurrence in winemaking. Food Res. Int. 2024, 190, 114558. [Google Scholar] [CrossRef] [PubMed]
- Visciano, P.; Schirone, M. Update on biogenic amines in fermented and non-fermented beverages. Foods 2022, 11, 353. [Google Scholar] [CrossRef]
- Yang, P.; Liu, W.; Chen, Y.; Gong, A.D. Engineering the glyoxylate cycle for chemical bioproduction. Front. Bioeng. Biotechnol. 2022, 10, 1066651. [Google Scholar] [CrossRef]
- De Iseppi, A.; Rocca, G.; Marangon, M.; Corich, V.; Arrigoni, G.; Porcellato, D.; Curioni, A. Characterization and identification of yeast peptides released during model wine fermentation and lees contact. J. Agric. Food Chem. 2024, 72, 24749–24761. [Google Scholar] [CrossRef]
- Guo, X.; Wu, X.; Ma, H.; Liu, H.; Luo, Y. Yeast: A platform for the production of L-tyrosine derivatives. Yeast 2023, 40, 214–230. [Google Scholar] [CrossRef]
- Garrigós, V.; Picazo, C.; Matallana, E.; Aranda, A. Activation of the yeast retrograde response pathway by adaptive laboratory evolution with S-(2-aminoethyl)-L-cysteine reduces ethanol and increases glycerol during winemaking. Microb. Cell Fact. 2024, 23, 231. [Google Scholar] [CrossRef] [PubMed]
- Vion, C.; Yeramian, N.; Hranilovic, A.; Masneuf-Pomarède, I.; Marullo, P. Influence of yeasts on wine acidity: New insights into Saccharomyces cerevisiae. OENO One 2024, 58. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Luchian, C.E.; Cioroiu, I.B.; Trincă, L.C.; Cotea, V.V. Increasing amino acids content of white wines with enzymes treatments. Agronomy 2022, 12, 1406. [Google Scholar] [CrossRef]
- Labuschagne, P.W.J.; Divol, B. Thiamine: A key nutrient for yeasts during wine alcoholic fermentation. Appl. Microbiol. Biotechnol. 2021, 105, 953–973. [Google Scholar] [CrossRef]
- Mandl, K.; Silhavy-Richter, K.; Korntheuer, K.; Prinz, M.; Patzl-Fischerleitner, E.; Eder, R. Influence of different yeasts on the amino acid pattern of rosé wine. In BIO Web of Conferences; EDP Sciences: Sofia, Bulgaria, 2017; Volume 9, p. 02014. [Google Scholar]
- Lu, Y.; Qin, Y.; Sun, Y.; Joseph, C.L.; Bisson, L.F.; Song, Y.; Liu, Y. Insight into the growth and metabolic characteristics of indigenous commercial S. cerevisiae NX11424 at high and low levels of yeast assimilable nitrogen based on metabolomic approach. Food Microbiol. 2024, 124, 104593. [Google Scholar] [CrossRef]
- Crépin, L.; Truong, N.M.; Bloem, A.; Sanchez, I.; Dequin, S.; Camarasa, C. Management of multiple nitrogen sources during wine fermentation by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2017, 83, e02617-16. [Google Scholar] [CrossRef] [PubMed]
- Songdech, P.; Butkinaree, C.; Yingchutrakul, Y.; Promdonkoy, P.; Runguphan, W.; Soontorngun, N. Increased production of isobutanol from xylose through metabolic engineering of Saccharomyces cerevisiae overexpressing transcription factor Znf1 and exogenous genes. FEMS Yeast Res. 2024, 24, foae006. [Google Scholar] [CrossRef] [PubMed]
- Chandler, M.; Stanley, G.A.; Rogers, P.; Chambers, P. A genomic approach to defining the ethanol stress response in the yeast Saccharomyces cerevisiae. Ann Microbiol. 2004, 54, 427–454. [Google Scholar]
- Jansen, M.L.A.; Van De Graaf, M.J.; Verwaal, R.U.S. Dicarboxylic Acid Production. Process. Patent No. 9,353,387, 31 May 2016. [Google Scholar]
- Ogawa, M.; Moreno-García, J.; Joseph, L.C.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Metabolic changes by wine flor-yeasts with gluconic acid as the sole carbon source. Metabolites 2021, 11, 150. [Google Scholar] [CrossRef]
- Huang, G.; Ren, H.; Wang, A.; Wan, X.; Wu, Z.; Zhong, X. iTRAQ-based proteomic analysis reveals the molecule mechanism of reducing higher alcohols in Chinese rice wine by nitrogen compensation. Ann. Microbiol. 2021, 71, 1–13. [Google Scholar] [CrossRef]
- Li, J.; Liu, B.; Feng, X.; Zhang, M.; Ding, T.; Zhao, Y.; Wang, C. Comparative proteome and volatile metabolome analysis of Aspergillus oryzae 3.042 and Aspergillus sojae 3.495 during koji fermentation. Food Res. Int. 2023, 165, 112527. [Google Scholar] [CrossRef]
- Li, M.; Deng, M.; Chen, Y.; Fan, H.; Huang, Y.; Huang, Y.; Wan, G.; Fu, G. Exploring the stress mechanism of tannic acid on Saccharomyces cerevisiae based on transcriptomics. Food Biosc. 2023, 56, 103365. [Google Scholar] [CrossRef]
- Torres-Guardado, R.; Rozès, N.; Esteve-Zarzoso, B.; Reguant, C.; Bordons, A. Succinic acid production by wine yeasts and the influence of GABA and glutamic acid. Int. Microbiol. 2024, 27, 505–512. [Google Scholar] [CrossRef]
- Bailey, P.S.; Ortmann, B.M.; Martinelli, A.W.; Houghton, J.W.; Costa, A.S.; Burr, S.P.; Antrobus, R.; Frezza, C.; Nathan, J.A. ABHD11 maintains 2-oxoglutarate metabolism by preserving functional lipoylation of the 2-oxoglutarate dehydrogenase complex. Nat. Commun. 2020, 11, 4046. [Google Scholar] [CrossRef]
- Zhong, Y.; Gao, Y.; Zhou, D.; Ma, X.; Chen, H.; Xu, Y.; Yang, W.; Yu, X. Structural basis for the activity and regulation of human α-ketoglutarate dehydrogenase revealed by Cryo-EM. Biochem. Biophys. Res. Commun. 2022, 602, 120–126. [Google Scholar] [CrossRef]
- Brambilla, M. Rewiring yeast nitrogen and mRNA metabolism for eliciting industrially relevant phenotypes. In The Saccharomyces cerevisiae Glutamate Synthase (Glt1) and the Poly (A) Binding Protein (Pab1) as Case Studies; Tesi di Dottorato, Università Degli Studi di Milano-Bicocca: Milan, Italy, 2018. [Google Scholar]
- Gong, J.; Huang, J.; Xiao, G.; You, Y.; Yuan, H.; Chen, F.; Liu, S.; Mao, J.; Li, B. Determination of γ-aminobutyric acid in Chinese rice wines and its evolution during fermentation. J. Inst. Brew. 2017, 123, 417–422. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; Ortega-Heras, M.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B. Multivariate analysis for the differentiation of sparkling wines elaborated from autochthonous Spanish grape varieties: Volatile compounds, amino acids and biogenic amines. Eur. Food Res. Technol. 2013, 236, 827–841. [Google Scholar] [CrossRef]
- Minebois, R.; Lairón-Peris, M.; Barrio, E.; Pérez-Torrado, R.; Querol, A. Metabolic differences between a wild and a wine strain of Saccharomyces cerevisiae during fermentation unveiled by multi-omic analysis. Environ. Microbiol. 2021, 23, 3059–3076. [Google Scholar] [CrossRef]
- Deng, H.; Ji, L.; Han, X.; Wu, T.; Han, B.; Li, C.; Zhan, J.; Huang, W.; You, Y. Research progress on the application of different controlling strategies to minimizing ethyl carbamate in grape wine. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1495–1516. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Yang, S.; Zhang, Z.; Tang, C.; Kan, Y.; Yao, L. Prolyl aminopeptidases: Reclassification, properties, production and industrial applications. Process Biochem. 2022, 118, 121–132. [Google Scholar] [CrossRef]
- Martínez-Moreno, R.; Quirós, M.; Morales, P.; Gonzalez, R. New insights into the advantages of ammonium as a winemaking nutrient. Int. J. Food Microbiol. 2014, 177, 128–135. [Google Scholar] [CrossRef]
- Lleixà, J.; Martín, V.; Giorello, F.; Portillo, M.C.; Carrau, F.; Beltran, G.; Mas, A. Analysis of the NCR mechanisms in Hanseniaspora vineae and Saccharomyces cerevisiae during winemaking. Front. Genet. 2019, 9, 747. [Google Scholar] [CrossRef]
- Luchian, C.E.; Grosaru, D.; Scutarasu, E.C.; Colibaba, L.C.; Scutarasu, A.; Cotea, V.V. Advancing sparkling wine in the 21st century: From traditional methods to modern innovations and market trends. Fermentation 2025, 11, 174. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, J.C.; G-García, M.E.; Carbonero-Pacheco, J.; Santos-Dueñas, I.M.; Mauricio, J.C.; Alcalá-Jiménez, M.T.; Moreno, J.; García-Martínez, T. Nitrogen Metabolism in Two Flor Yeast Strains at Mid-Second Bottle Fermentation in Sparkling Wine Production. Appl. Sci. 2025, 15, 5579. https://doi.org/10.3390/app15105579
García-García JC, G-García ME, Carbonero-Pacheco J, Santos-Dueñas IM, Mauricio JC, Alcalá-Jiménez MT, Moreno J, García-Martínez T. Nitrogen Metabolism in Two Flor Yeast Strains at Mid-Second Bottle Fermentation in Sparkling Wine Production. Applied Sciences. 2025; 15(10):5579. https://doi.org/10.3390/app15105579
Chicago/Turabian StyleGarcía-García, Juan Carlos, Miguel E. G-García, Juan Carbonero-Pacheco, Inés M. Santos-Dueñas, Juan Carlos Mauricio, María Trinidad Alcalá-Jiménez, Juan Moreno, and Teresa García-Martínez. 2025. "Nitrogen Metabolism in Two Flor Yeast Strains at Mid-Second Bottle Fermentation in Sparkling Wine Production" Applied Sciences 15, no. 10: 5579. https://doi.org/10.3390/app15105579
APA StyleGarcía-García, J. C., G-García, M. E., Carbonero-Pacheco, J., Santos-Dueñas, I. M., Mauricio, J. C., Alcalá-Jiménez, M. T., Moreno, J., & García-Martínez, T. (2025). Nitrogen Metabolism in Two Flor Yeast Strains at Mid-Second Bottle Fermentation in Sparkling Wine Production. Applied Sciences, 15(10), 5579. https://doi.org/10.3390/app15105579





