Cross-Sectional Study on Proportions of Type 2 Diabetic Patients Presenting with Oral Candidal Lesions
Abstract
Featured Application
Abstract
1. Introduction
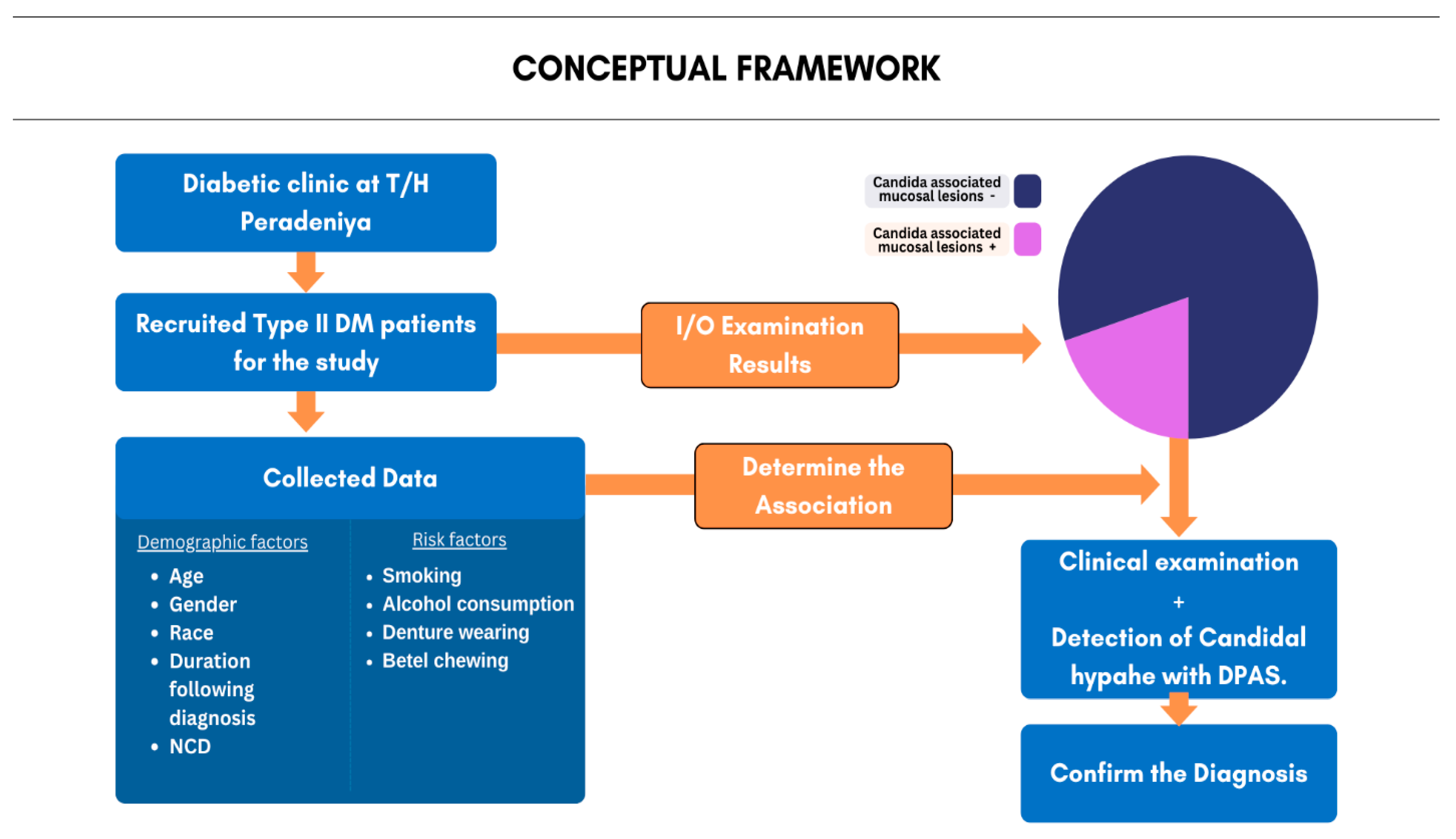
2. Materials and Methods
2.1. Study Design, Study Setting, and Sample Selection
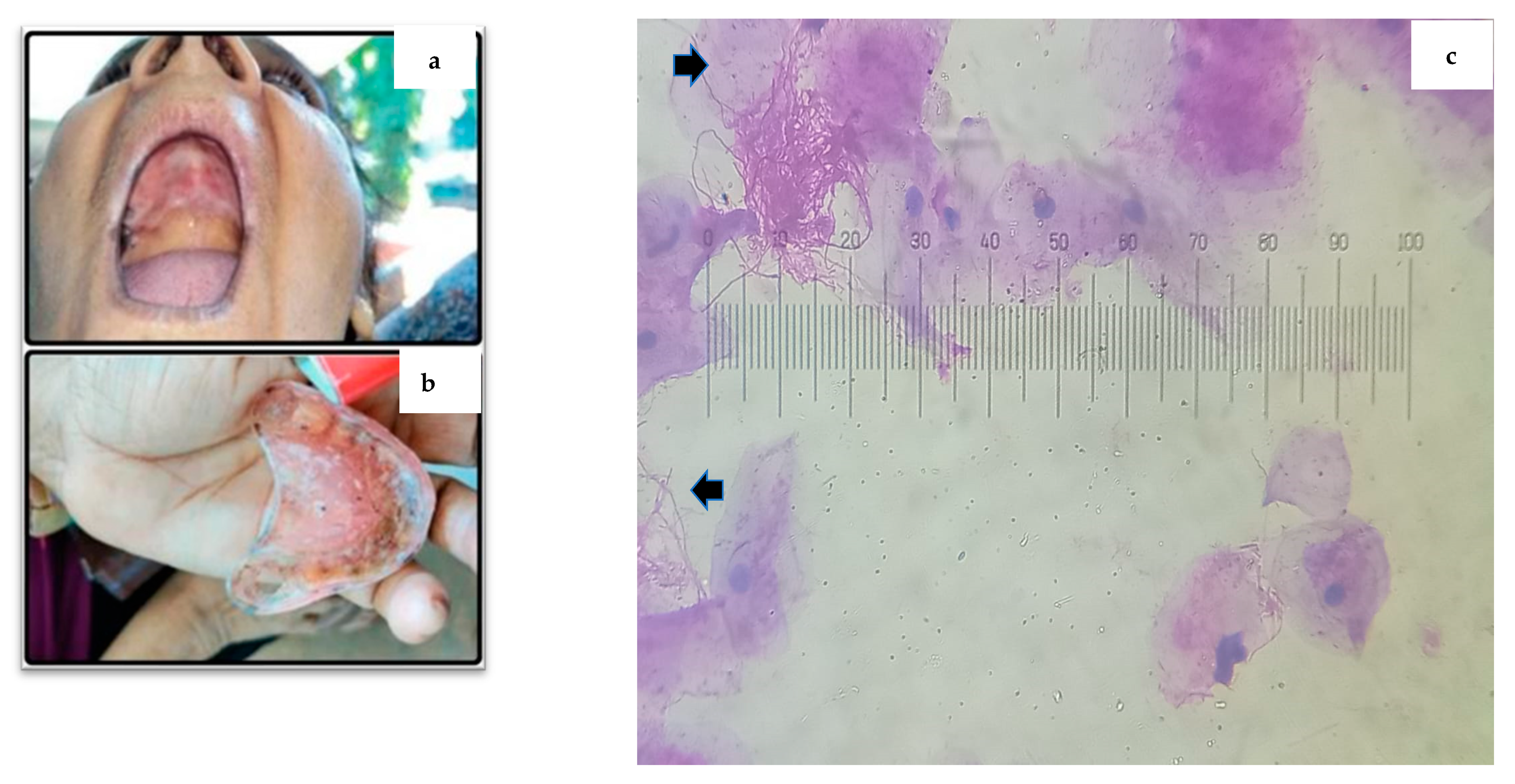
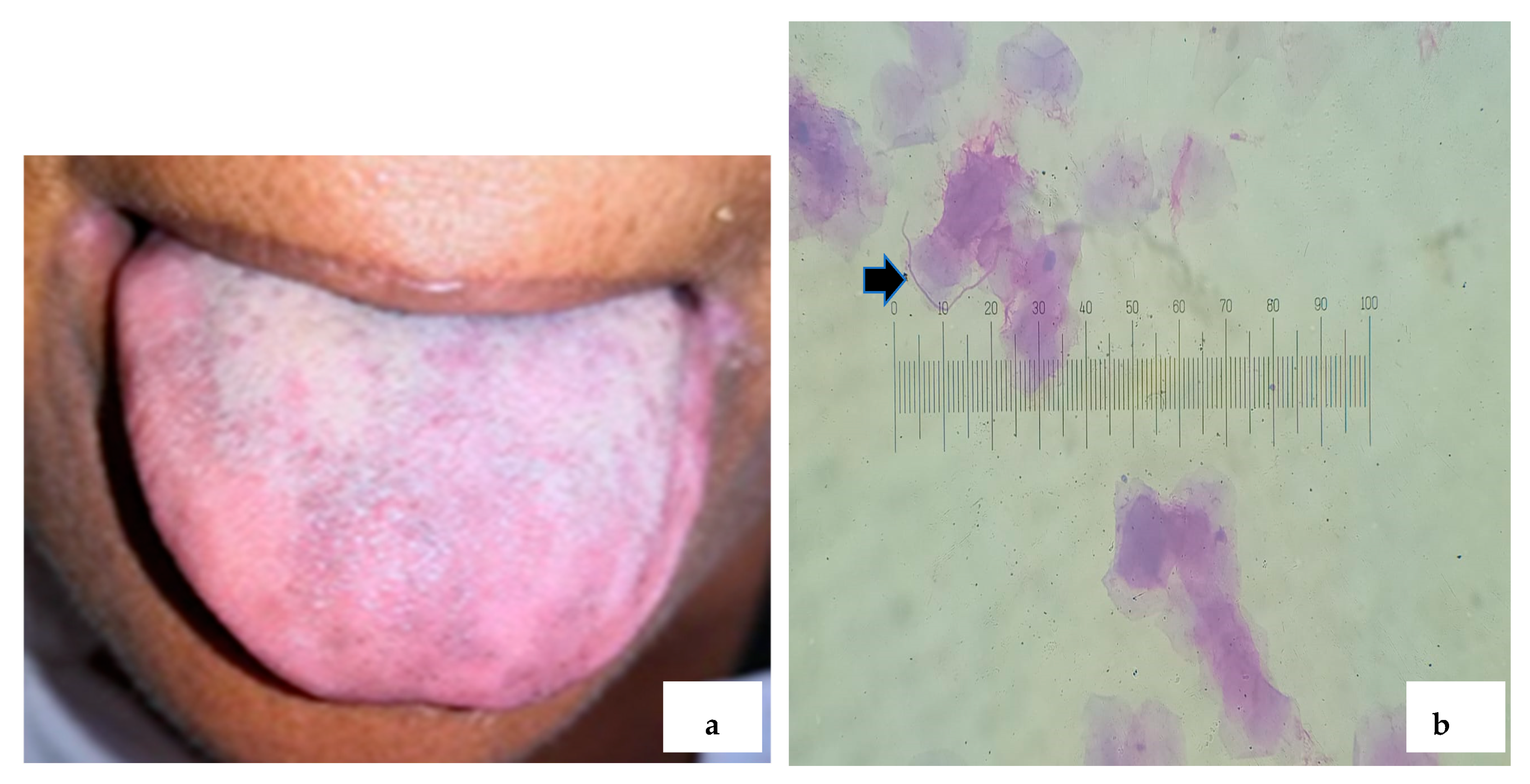
2.2. Cytological Sample Collection and Staining Protocol with PAS
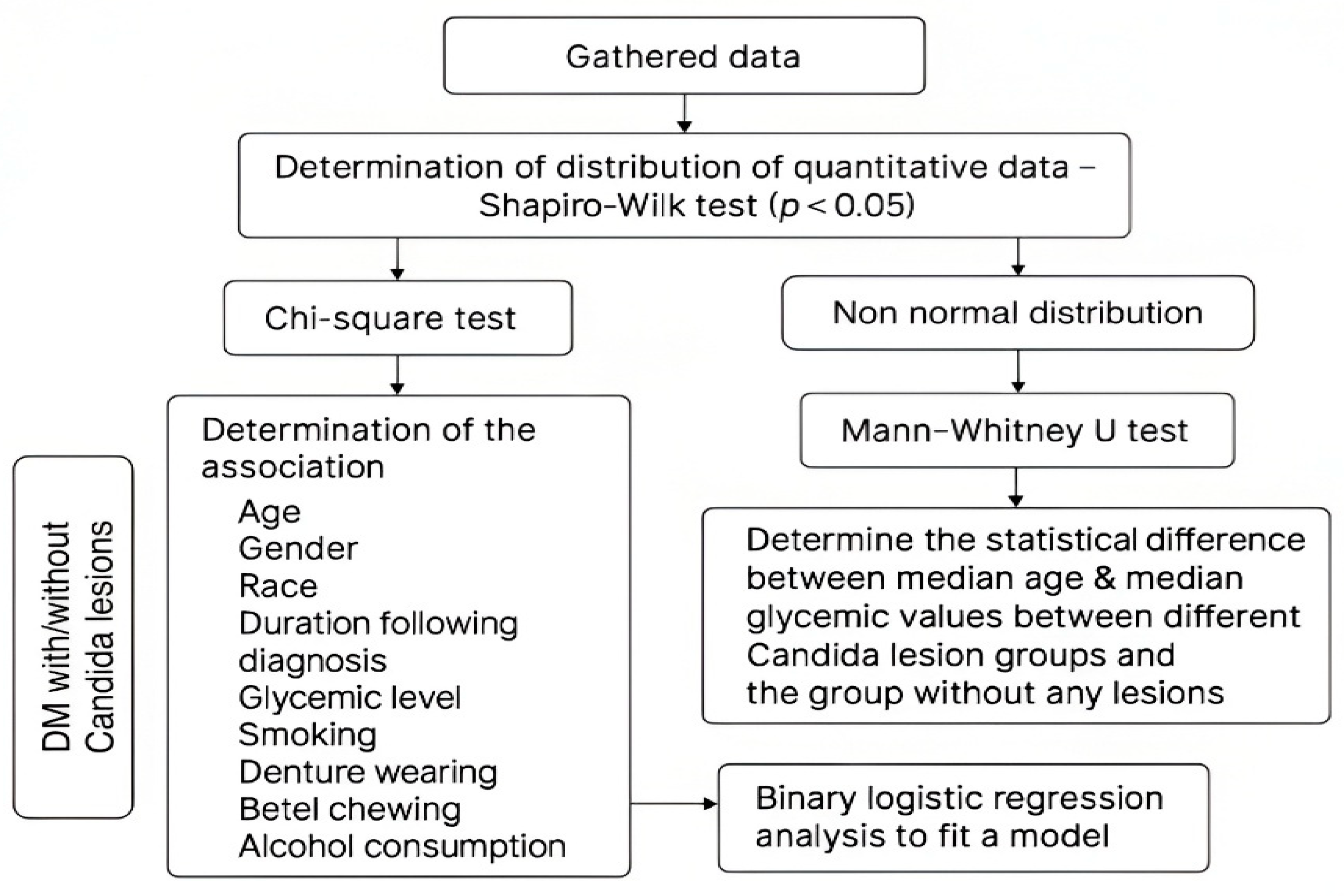
2.3. Statistical Analysis
2.4. Methodology Overview
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rannan-Eliya, R.P.; Wijemunige, N.; Perera, P.; Kapuge, Y.; Gunawardana, N.; Sigera, C.; Jayatissa, R.; Herath, H.M.M.; Gamage, A.; Weerawardena, N.; et al. Prevalence of diabetes and pre-diabetes in Sri Lanka: A new global hotspot–estimates from the Sri Lanka Health and Ageing Survey 2018/2019. BMJ Open Diabetes Res. Care 2023, 11, e003160. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, K.; Banach, M.; Bekiari, E.; Rizzo, M.; Edmonds, M. Complications of Diabetes 2017. J. Diabetes Res. 2018, 2018, 3086167. [Google Scholar] [CrossRef]
- Rohani, B. Oral manifestations in patients with diabetes mellitus. World J. Diabetes. 2019, 10, 485–489. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M. Candida sp. Infections in patients with diabetes mellitus. J. Clin. Med. 2019, 8, 76. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6352194/ (accessed on 27 September 2023). [CrossRef] [PubMed]
- Martinez, R.F.F.; Jaimes-Aveldañez, A.; Hernández-Pérez, F.; Arenas, R.; Miguel, G.F.S. Oral Candida spp carriers: Its prevalence in patients with type 2 diabetes mellitus. An. Bras. Dermatol. 2013, 88, 222–225. [Google Scholar] [CrossRef]
- Rodríguez-Archilla, A.; Piedra-Rosales, C. Candida species oral detection and infection in patients with diabetes mellitus: A meta-analysis. Iberoam. J. Med. 2021, 3, 115–121. [Google Scholar] [CrossRef]
- Sudbery, P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011, 9, 737–748. [Google Scholar] [CrossRef]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical appearance of oral Candida infection and therapeutic strategies. Front. Microbiol. 2015, 6, 1391. [Google Scholar] [CrossRef]
- Ghabanchi, J.; Andisheh Tadbir, A.; Darafshi, R.; Sadegholvad, M. The prevalence of median rhomboid glossitis in diabetic patients: A case-control study. Iran. Red Crescent. Med. J. 2011, 13, 503–506. [Google Scholar]
- Yao, Y.; Shi, L.; Zhang, C.; Sun, H.; Wu, L. Application of fungal fluorescent staining in oral candidiasis: Diagnostic analysis of 228 specimens. BMC Microbiol. 2019, 19, 96. [Google Scholar] [CrossRef]
- Sampath, A.; Weerasekera, M.; Dilhari, A.; Gunasekara, C.; Bulugahapitiya, U.; Fernando, N.; Samaranayake, L. Type 2 diabetes mellitus and oral Candida colonization: Analysis of risk factors in a Sri Lankan cohort. Acta Odontol. Scand. 2019, 77, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Anita Kumar, S.; Pandya, H. Speciation and antifungal susceptibility testing of Candida isolated from immunocompromised patients of a tertiary care centre in Gujarat, India. J. Pure Appl. Microbiol. 2023, 17, 427–433. [Google Scholar] [CrossRef]
- Alrashed, F.A.; Iqbal, M.; Alsubiheen, A.M.; Ahmad, T. Exploring determinants of sex and family history-based disparity in type 2 diabetes mellitus prevalence among clinical patients. BMC Public Health 2024, 24, 682. [Google Scholar] [CrossRef]
- Loster, J.E.; Wieczorek, A.; Loster, B.W. Correlation between age and gender in Candida species infections of complete denture wearers: A retrospective analysis. Clin. Interv. Aging 2016, 11, 1707. [Google Scholar] [CrossRef] [PubMed]
- Martorano-Fernandes, L.; Dornelas-Figueira, L.M.; Marcello-Machado, R.M.; Silva, R.B.; Magno, M.B.; Maia, L.C.; del Bel Cury, A.A. Oral candidiasis and denture stomatitis in diabetic patients: Systematic review and meta-analysis. Braz. Oral Res. 2020, 34, e113. [Google Scholar] [CrossRef]
- Mathew, A.L.; Daniel, M.P.; Cherian, S.A.; Joseph, B.B. Prevalence of oral mucosal lesions among diabetic patients in south Kerala, India. J. Oral Med. Toxicol. 2017, 1, 20–22. [Google Scholar]
- Javed, F.; Klingspor, L.; Sundin, U.; Altamash, M.; Klinge, B.; Engström, P.E. Periodontal conditions, oral Candida albicans and salivary proteins in type 2 diabetic subjects with emphasis on gender. BMC Oral Health 2009, 9, 12. [Google Scholar] [CrossRef]
- Ganapathy, D.M.; Joseph, S.; Ariga, P.; Selvaraj, A. Evaluation of the influence of blood glucose level on oral candidal colonization in complete denture wearers with type-II diabetes mellitus: An in vivo study. Dent. Res. J. 2013, 10, 87–92. [Google Scholar]
- Dorocka-Bobkowska, B.; Zozulinska-Ziolkiewicz, D.; Wierusz-Wysocka, B.; Hedzelek, W.; Szumala-Kakol, A.; Budtz-Jörgensen, E. Candida-associated denture stomatitis in type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2010, 90, 81–86. [Google Scholar] [CrossRef]
- Perić, M.; Miličić, B.; Kuzmanović Pfićer, J.; Živković, R.; Arsić Arsenijević, V. A systematic review of denture stomatitis: Predisposing factors, clinical features, etiology, and global Candida spp. distribution. J. Fungi 2020, 6, 328. [Google Scholar] [CrossRef]
- Abuhajar, E.; Ali, K.; Zulfiqar, G.; Al Ansari, K.; Raja, H.Z.; Bishti, S.; Anweigi, L. Management of chronic atrophic candidiasis (denture stomatitis)—A narrative review. J. Fungi 2023, 9, 3029. [Google Scholar] [CrossRef]
- Motta-Silva, A.C.; Aleva, N.A.; Chavasco, J.K.; Armond, M.C.; França, J.P.; Pereira, L.J. Erythematous oral candidiasis in patients with controlled type II diabetes mellitus and complete dentures. Mycopathologia 2010, 169, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, C.; Matsuse, H.; Tomari, S.; Obase, Y.; Miyazaki, Y.; Shimoda, T.; Kohno, S. Oral candidiasis associated with inhaled corticosteroid use: Comparison of fluticasone and beclomethasone. Ann. Allergy Asthma Immunol. 2003, 90, 646–651. [Google Scholar] [CrossRef]
- Berberi, A.; Noujeim, Z.; Aoun, G. Epidemiology of oropharyngeal candidiasis in human immunodeficiency virus/acquired immune deficiency syndrome patients and CD4+ counts. J. Int. Oral Health 2015, 7, 20–23. [Google Scholar] [PubMed]
- Lorenzo-Pouso, A.I.; Pérez-Jardón, A.; Caponio, V.C.A.; Spirito, F.; Chamorro-Petronacci, C.M.; Álvarez-Calderón-Iglesias, Ó.; Gándara-Vila, P.; Muzio, L.L.; Pérez-Sayáns, M. Oral chronic hyperplastic candidiasis and its potential risk of malignant transformation: A systematic review and prevalence meta-analysis. J. Fungi 2022, 8, 1093. [Google Scholar] [CrossRef] [PubMed]
- Federico, J.R.; Basehore, B.M.; Zito, P.M. Angular cheilitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK536929/ (accessed on 6 May 2024).
- Taylor, M.; Brizuela, M.; Raja, A. Oral candidiasis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK545282/ (accessed on 6 May 2024).
- Zaremba, M.L.; Daniluk, T.; Rozkiewicz, D.; Cylwik-Rokicka, D.; Kierklo, A.; Tokajuk, G.; Dąbrowska, E.; Pawińska, M.; Klimiuk, A.; Stokowska, W.; et al. Incidence rate of Candida species in the oral cavity of middle-aged and elderly subjects. Adv. Med. Sci. 2006, 51 (Suppl. 1), 233–236. [Google Scholar]
- Kumar, B.V.; Padshetty, N.S.; Bai, K.Y.; Rao, M.S. Prevalence of Candida in the oral cavity of diabetic subjects. J. Assoc. Physicians India 2005, 53, 599–602. [Google Scholar]
- Dornelas Figueira, L.M.; Ricomini Filho, A.P.; da Silva, W.J.; Del Bel Cury, A.A.; Ruiz, K.G.S. Glucose effect on Candida albicans biofilm during tissue invasion. Arch. Oral Biol. 2020, 117, 104728. [Google Scholar] [CrossRef]
- Khalili, P.; Movagharipoor, A.; Sardari, F.; Movaghari Pour, F.; Jamali, Z. Oral candidiasis and cigarette, tobacco, alcohol, and opium consumption in Rafsanjan, a region in the southeast of Iran. BMC Oral Health 2023, 23, 262. [Google Scholar] [CrossRef]
- Deeiam, K.; Pankam, J.; Sresumatchai, V.; Visedketkan, P.; Jindavech, W.; Rungraungrayabkul, D.; Pimolbutr, K.; Klongnoi, B.; Khovidhunkit, S.-O.P. Presence of Candida and its associated factors in participants attending oral cancer screening in the lower northeastern area of Thailand. BMC Oral Health 2023, 23, 527. [Google Scholar] [CrossRef]
- Hasan, Z.; Islam, M.R.; Hasan, M.S.; Sakib, M.M.; Islam, M.S.; Chowdhury, M.B.; Sarker, S.; Islam, M.R.; Hasnat, M.A.; Nath, L.M.; et al. Risk analysis of chewing betel quid among diabetic patients from the northeastern part of Bangladesh. medRxiv. 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.01.15.20017731v1 (accessed on 10 June 2024).
- Javed, F.; Yakob, M.; Ahmed, H.B.; Al-Hezaimi, K.; Samaranayake, L.P. Oral Candida carriage among individuals chewing betel-quid with and without tobacco. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 427–432. [Google Scholar] [CrossRef] [PubMed]

| Lesions | N (%) [Out of 63 Lesions] | Site | * Median Age in Years | Gender | * Median Glycemic Level (mg/dL) | ||
|---|---|---|---|---|---|---|---|
| Male | Female | ||||||
| PMC | 11 17.5% | Dorsum of the tongue Buccal mucosa | 8 3 | 62 | 5 | 6 | 137 |
| EC | 12 19% | Dorsum of the tongue Buccal mucosa Hard palate Soft palate | 7 3 1 1 | 67 | 7 | 5 | 160 |
| CHC | 10 15.9% | Buccal mucosa Alveolar mucosa Lip | 8 1 1 | 61.5 | 8 | 2 | 152 |
| DS | 14 22.2% | Hard palate | 14 | 60.5 | 3 | 11 | 148 |
| Feature | DM with Candidal Lesions (n %) | DM Without Candidal Lesions (n %) | X2 Test p Value |
|---|---|---|---|
| 1. Age in years ≤60 ≥61 | 26 (7.38) 37 (10.51) | 148 (42.04) 141 (40.05) | 0.153 |
| 2. Gender Male Female | 31 (8.80) 32 (9.09) | 83 (23.57) 206 (58.52) | 0.002 |
| 3. Race Sinhala Tamil Muslim | 54 (15.34) 5 (1.42) 4 (1.13) | 242 (68.75) 20 (5.68) 27 (7.67) | 0.73 |
| 4. Duration following diagnosis in years ≤15 ≥16 | 44 (12.5) 19 (5.39) | 227 (64.48) 62 (17.61) | 0.173 |
| 5. Relationship with other NCDs DL + HTN Other NCDs present No other NCDs | 36 (10.22) 12 (3.40) 15 (4.26) | 157 (44.60) 53 (15.05) 79 (22.44) | 0.848 |
| 6. Glycemic level (mg/dL) ≤110 ≥111 | 16 (4.54) 47 (13.35) | 89 (25.28) 200 (56.81) | 0.396 |
| Risk Factors | DM with Candidal Lesions (n%) | DM Without Candidal Lesions (n%) | X2 | df | p |
|---|---|---|---|---|---|
| 1. Denture Denture wearer Non-denture wearer | 21 (33.33) 42 (66.7) | 73 (25.3) 216 (74.7) | 1.723 | 1 | 0.189 |
| 2. Smoking Present Absent | 14 (33.3) 28 (66.7) | 49 (15.8) 261 (84.2) | 7.733 | 1 | 0.005 |
| 3. Betel chewing Present Absent | 21 (28.4) 53 (71.6) | 42 (15.1) 236 (84.9) | 7.004 | 1 | 0.008 |
| 4. Alcohol misuse Present Absent | 13 (23.6) 42 (76.4) | 50 (16.8) 247 (83.2) | 1.461 | 1 | 0.227 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weerasinghe, J.; Weerasinghe, L.; Thirugnanasampanthar, V.; Jayasooriya, P.; Lombardi, T. Cross-Sectional Study on Proportions of Type 2 Diabetic Patients Presenting with Oral Candidal Lesions. Appl. Sci. 2025, 15, 5539. https://doi.org/10.3390/app15105539
Weerasinghe J, Weerasinghe L, Thirugnanasampanthar V, Jayasooriya P, Lombardi T. Cross-Sectional Study on Proportions of Type 2 Diabetic Patients Presenting with Oral Candidal Lesions. Applied Sciences. 2025; 15(10):5539. https://doi.org/10.3390/app15105539
Chicago/Turabian StyleWeerasinghe, Janitha, Lahiru Weerasinghe, Vinusika Thirugnanasampanthar, Primali Jayasooriya, and Tommaso Lombardi. 2025. "Cross-Sectional Study on Proportions of Type 2 Diabetic Patients Presenting with Oral Candidal Lesions" Applied Sciences 15, no. 10: 5539. https://doi.org/10.3390/app15105539
APA StyleWeerasinghe, J., Weerasinghe, L., Thirugnanasampanthar, V., Jayasooriya, P., & Lombardi, T. (2025). Cross-Sectional Study on Proportions of Type 2 Diabetic Patients Presenting with Oral Candidal Lesions. Applied Sciences, 15(10), 5539. https://doi.org/10.3390/app15105539







