Antibacterial and Antibiofilm Effect of Lavandula dentata L. Essential Oil as Endodontic Irrigant Against Standard and Clinical Strains of Enterococcus spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oil Selection
2.2. Selection of Bacterial Strains
2.3. Antibacterial Evaluation of L. dentata Essential Oil Against the Selected Strains
2.4. Antibiofilm Evaluation of L. dentata Essential Oil Against the Selected Strains
2.5. Statistical Analysis
3. Results
3.1. Antibacterial Effect of L. dentata Essential Oil Against the Selected Strains
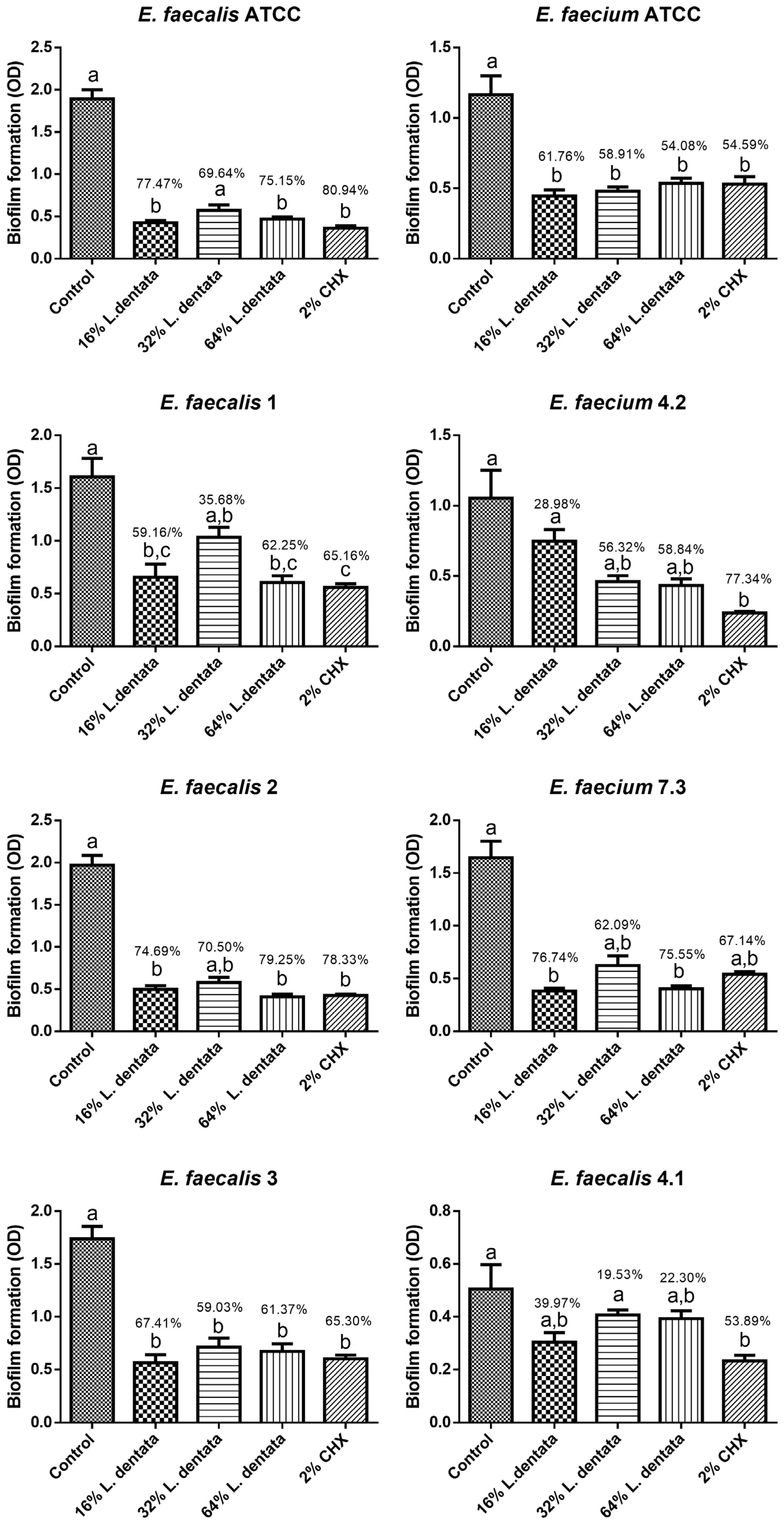
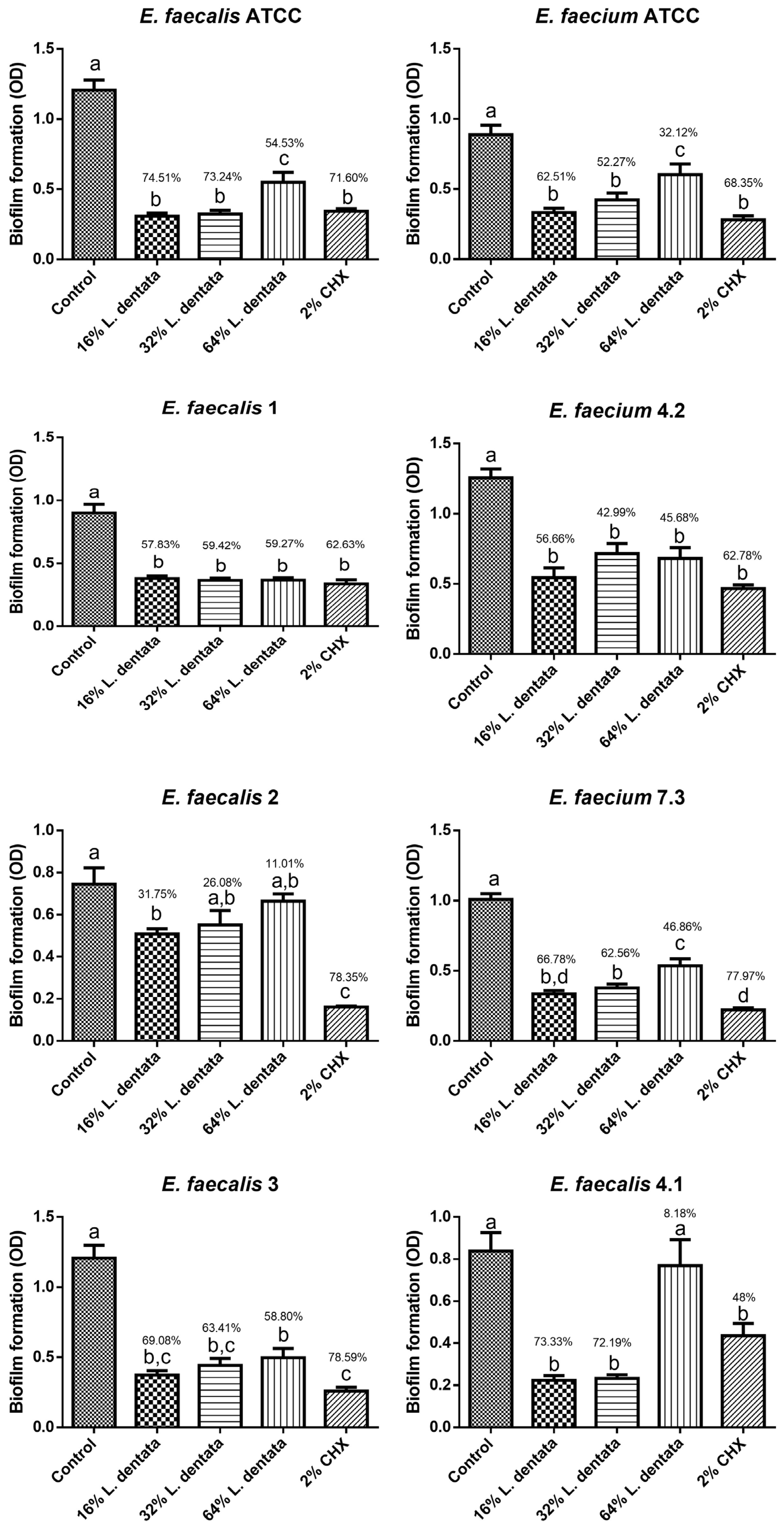
3.2. Antibiofilm Effect of L. dentata Essential Oil Against the Selected Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devriese, L.A.; Pot, B. The genus Enterococcus. In The Genera of Lactic Acid Bacteria; Wood, B.J.B., Holzapfel, W.H., Eds.; Springer: Boston, MA, USA, 1995; pp. 327–367. ISBN 978-1-4613-7666-8. [Google Scholar]
- Foulquié Moreno, M.R.; Sarantinopoulos, P.; Tsakalidou, E.; De Vuyst, L. The role and application of enterococci in food and health. Int. J. Food Microbiol. 2006, 106, 1–24. [Google Scholar] [CrossRef] [PubMed]
- García-Solache, M.; Rice, L.B. The enterococcus: A model of adaptability to its environment. Clin. Microbiol. Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef] [PubMed]
- Brunson, D.N.; Colomer-Winter, C.; Lam, L.N.; Lemos, J.A. Identification of Multiple Iron Uptake Mechanisms in Enterococcus faecalis and Their Relationship to Virulence. Infect. Immun. 2023, 91, e0049622. [Google Scholar] [CrossRef]
- Goh, H.M.S.; Yong, M.H.A.; Chong, K.K.L.; Kline, K.A. Model systems for the study of Enterococcal colonization and infection. Virulence 2017, 8, 1525–1562. [Google Scholar] [CrossRef]
- Chidambar, C.K.; Shankar, S.M.; Raghu, P.; Gururaj, S.B.; Bushan, K.S. Detection of Enterococcus faecalis in subgingival biofilms of healthy, gingivitis, and chronic periodontitis subjects. J. Indian Soc. Periodontol. 2019, 23, 416–418. [Google Scholar] [CrossRef]
- Bhardwaj, S.B.; Mehta, M.; Sood, S. Enterococci in the oral cavity of periodontitis patients from different urban socioeconomic groups. Dent. Res. J. 2020, 17, 147–151. [Google Scholar] [CrossRef]
- Kristich, C.J.; Rice, L.B.; Arias, C.A. Enterococcal Infection—Treatment and Antibiotic Resistance. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Boccella, M.; Santella, B.; Pagliano, P.; De Filippis, A.; Casolaro, V.; Galdiero, M.; Borrelli, A.; Capunzo, M.; Boccia, G.; Franci, G. Prevalence and antimicrobial resistance of enterococcus species: A retrospective cohort study in italy. Antibiotics 2021, 10, 1552. [Google Scholar] [CrossRef]
- Schilder, H. Cleaning and shaping the root canal. Dent. Clin. N. Am. 1974, 18, 269–296. [Google Scholar] [CrossRef]
- Khoury, R.D.; de Carvalho, L.S.; do Nascimento, M.F.R.; Alhussain, F.; Abu Hasna, A. Endodontic irrigants from a comprehensive perspective. World J. Clin. Cases 2024, 12, 4460–4468. [Google Scholar] [CrossRef]
- Khoury, R.D.; Abu Hasna, A.; Gagliardi, C.F.; Marinho, R.M.d.M.; Carvalho, C.A.T.; Bresciani, E.; Valera, M.C. Antimicrobial and anti-endotoxin activity of N-acetylcysteine, calcium hydroxide and their combination against Enterococcus faecalis, Escherichia coli and lipopolysaccharides. PeerJ 2024, 12, e18331. [Google Scholar] [CrossRef]
- Yang, S.; Meng, X.; Zhen, Y.; Baima, Q.; Wang, Y.; Jiang, X.; Xu, Z. Strategies and mechanisms targeting Enterococcus faecalis biofilms associated with endodontic infections: A comprehensive review. Front. Cell. Infect. Microbiol. 2024, 14, 1433313. [Google Scholar] [CrossRef] [PubMed]
- Domingues, N.; Ramos, L.d.P.; Pereira, L.M.; do Rosário Estevam Dos Santos, P.B.; Scorzoni, L.; Pereira, T.C.; Abu Hasna, A.; Carvalho, C.A.T.; de Oliveira, L.D. Antimicrobial action of four herbal plants over mixed-species biofilms of Candida albicans with four different microorganisms. Aust. Endod. J. 2023, 49, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.d.S.A.; Meccatti, V.M.; Pereira, T.C.; Marcucci, M.C.; Hasna, A.A.; Valera, M.C.; de Oliveira, L.D.; Carvalho, C.A.T. Antibacterial Effect of Combinations of Salvia officinalis and Glycyrrhiza glabra Hydroalcoholic Extracts against Enterococcus spp. Coatings 2023, 13, 1579. [Google Scholar] [CrossRef]
- de Lima, P.M.N.; Pereira, T.C.; de Carvalho, L.S.; Dos Santos, L.F.; Oliveira, C.E.R.; Ramos, L.d.P.; Marcucci, M.C.; Abu Hasna, A.; de Oliveira, L.D. Antimicrobial and synergistic effects of lemongrass and geranium essential oils against Streptococcus mutans, Staphylococcus aureus, and Candida spp. World J. Crit. Care Med. 2024, 13, 92531. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Vasudeva, A.; Jaiswal, N.; Garg, P.; Tyagi, S.; Singh, J. Antibacterial efficacy of Melaleuca alternifolia (Tea tree oil), Curcuma longa (Turmeric), 2% chlorhexidine, and 5% sodium hypochlorite against Enterococcus faecalis: An in vitro study. Saudi Endod. J. 2015, 5, 182. [Google Scholar] [CrossRef]
- Teja, K.V.; Janani, K.; Kaligotla, V.A.; Harini, K. Comparative antimicrobial efficacy of oregano oil, chlorhexidine, and sodium hypochlorite against Enterococcus faecalis. Endodontology 2021, 33, 97–101. [Google Scholar] [CrossRef]
- Naseri, N.; Kalantari Khandani, A.; Baherimoghadam, T.; Kalantari Khandani, A.; Hamedani, S.; Nouripour-Sisakht, S.; Safaeian, R. The effect of thymus vulgaris essential oil and chlorhexidine on candida albicans accumulated on removable orthodontic appliance: A clinical trial. J. Dent. 2022, 23, 190–197. [Google Scholar] [CrossRef]
- Lavandula dentata, L. |Plants of the World Online|Kew Science. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:449031-1 (accessed on 18 February 2025).
- Müller-Sepúlveda, A.; Chevecich, C.C.; Jara, J.A.; Belmar, C.; Sandoval, P.; Meyer, R.S.; Quijada, R.; Moura, S.; López-Muñoz, R.; Díaz-Dosque, M.; et al. Chemical Characterization of Lavandula dentata Essential Oil Cultivated in Chile and Its Antibiofilm Effect against Candida albicans. Planta Med. 2020, 86, 1225–1234. [Google Scholar] [CrossRef]
- Justus, B.; Almeida, V.P.d.; Gonçalves, M.M.; Assunção, D.P.d.S.F.d.; Borsato, D.M.; Arana, A.F.M.; Maia, B.H.L.N.S.; Paula, J.d.F.P.d.; Budel, J.M.; Farago, P.V. Chemical composition and biological activities of the essential oil and anatomical markers of lavandula dentata L. cultivated in brazil. Braz. Arch. Biol. Technol. 2018, 61, e18180111. [Google Scholar] [CrossRef]
- Bouazama, S.; Harhar, H.; Costa, J.; Desjobert, J.; Talbaoui, A.; Tabyaoui, M. Chemical composition and antibacterial activity of the essential oils of Lavandula pedunculata and Lavandula dentata. J. Mater. Environ. Sci. 2017, 8, 2154–2160. [Google Scholar]
- El Abdali, Y.; Beniaich, G.; Mahraz, A.M.; El Moussaoui, A.; Bin Jardan, Y.A.; Akhazzane, M.; Chebaibi, M.; Nafidi, H.-A.; Eloutassi, N.; Bourhia, M.; et al. Antibacterial, Antioxidant, and in silico NADPH Oxidase Inhibition Studies of Essential Oils of Lavandula dentata against Foodborne Pathogens. Evid. Based Complement. Alternat. Med. 2023, 2023, 9766002. [Google Scholar] [CrossRef] [PubMed]
- Benbelaïd, F.; Khadir, A.; Abdoune, M.A.; Bendahou, M.; Muselli, A.; Costa, J. Antimicrobial activity of some essential oils against oral multidrug-resistant Enterococcus faecalis in both planktonic and biofilm state. Asian Pac. J. Trop. Biomed. 2014, 4, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Saeloh, D.; Visutthi, M. Efficacy of Thai Plant Extracts for Antibacterial and Anti-Biofilm Activities against Pathogenic Bacteria. Antibiotics 2021, 10, 1470. [Google Scholar] [CrossRef] [PubMed]
- Teanpaisan, R.; Kawsud, P.; Pahumunto, N.; Puripattanavong, J. Screening for antibacterial and antibiofilm activity in Thai medicinal plant extracts against oral microorganisms. J. Tradit. Complement. Med. 2017, 7, 172–177. [Google Scholar] [CrossRef]
- dos Santos, A.C.C. Prevalência, Virulência e Sensibilidade às Terapias Antimicrobianas das cepas de Enterecoccus Faecalis e Enterococcus Faecium Isoladas de Infecções Endodônticas. Master’s Thesis, Institute of Science and Technology-Universidade Estadual Paulista, São José dos Campos, Brazil, 2014. [Google Scholar]
- Gomes, B.P.F.d.A.; Herrera, D.R. Etiologic role of root canal infection in apical periodontitis and its relationship with clinical symptomatology. Braz. Oral Res. 2018, 32, e69. [Google Scholar] [CrossRef]
- Wong, J.; Manoil, D.; Näsman, P.; Belibasakis, G.N.; Neelakantan, P. Microbiological aspects of root canal infections and disinfection strategies: An update review on the current knowledge and challenges. Front. Oral Health 2021, 2, 672887. [Google Scholar] [CrossRef]
- Gomes, B.P.F.A.; Francisco, P.A.; Godoi, E.P.; Endo, M.S.; Barbosa-Ribeiro, M.; Delboni, M.G.; Pecorari, V.G.A. Identification of culturable and nonculturable microorganisms, lipopolysaccharides, and lipoteichoic acids from root canals of teeth with endodontic failure. J. Endod. 2021, 47, 1075–1086. [Google Scholar] [CrossRef]
- Murad, C.F.; Sassone, L.M.; Faveri, M.; Hirata, R.; Figueiredo, L.; Feres, M. Microbial diversity in persistent root canal infections investigated by checkerboard DNA-DNA hybridization. J. Endod. 2014, 40, 899–906. [Google Scholar] [CrossRef]
- Alarcón-Sánchez, M.A.; Rodríguez-Montaño, R.; Becerra-Ruiz, J.S.; Lomelí-Martínez, S.M.; Mosaddad, S.A.; Heboyan, A. Detection of Enterococcus faecalis and the red complex bacteria analyzed by the Checkerboard technique for DNA-DNA hybridization in endodontic infections: A systematic review and meta-analysis. Diagn. Microbiol. Infect. Dis. 2025, 111, 116654. [Google Scholar] [CrossRef]
- Masetto, M.A.M.; Deschamps, C.; Mógor, A.F.; Bizzo, H.R. Teor e composição do óleo essencial de inflorescências e folhas de Lavandula dentata L. em diferentes estádios de desenvolvimento floral e épocas de colheita. Rev. Bras. Plantas Med. 2011, 13, 413–421. [Google Scholar] [CrossRef]
- Martins, R.d.P.; Gomes, R.A.d.S.; Malpass, A.C.G.; Okura, M.H. Chemical characterization of Lavandula dentata L. essential oils grown in Uberaba-MG. Cienc. Rural 2019, 49, e20180964. [Google Scholar] [CrossRef]
- Giuliani, C.; Bottoni, M.; Ascrizzi, R.; Milani, F.; Papini, A.; Flamini, G.; Fico, G. Lavandula dentata from Italy: Analysis of Trichomes and Volatiles. Chem. Biodivers. 2020, 17, e2000532. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, K.; Manigandan, V.; Jeyapragash, D.; Bharathidasan, V.; Anandharaj, B.; Sathya, M. Eucalyptol inhibits biofilm formation of Streptococcus pyogenes and its mediated virulence factors. J. Med. Microbiol. 2020, 69, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; Rôças, I.N. Clinical implications and microbiology of bacterial persistence after treatment procedures. J. Endod. 2008, 34, 1291–1301.e3. [Google Scholar] [CrossRef]
| Bacterial Strain | AC | A + C | EM | AM | TC | CF | VM |
|---|---|---|---|---|---|---|---|
| E. faecalis 1 | S | S | S | S | S | S | S |
| E. faecalis 2 | S | S | S | S | S | I | S |
| E. faecalis 3 | S | S | I | S | R | I | I |
| E. faecalis 4.1 | S | S | S | S | R | S | I |
| E. faecium 4.2 | S | S | I | I | S | S | S |
| E. faecium 7.3 | S | S | S | S | S | S | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, C.T.; de Lima, P.M.N.; Pereira, T.C.; de Carvalho, L.S.; Diamantino, M.G.G.; Abu Hasna, A.; da Rocha, J.C.; de Oliveira, L.D.; Carvalho, C.A.T. Antibacterial and Antibiofilm Effect of Lavandula dentata L. Essential Oil as Endodontic Irrigant Against Standard and Clinical Strains of Enterococcus spp. Appl. Sci. 2025, 15, 5534. https://doi.org/10.3390/app15105534
Rocha CT, de Lima PMN, Pereira TC, de Carvalho LS, Diamantino MGG, Abu Hasna A, da Rocha JC, de Oliveira LD, Carvalho CAT. Antibacterial and Antibiofilm Effect of Lavandula dentata L. Essential Oil as Endodontic Irrigant Against Standard and Clinical Strains of Enterococcus spp. Applied Sciences. 2025; 15(10):5534. https://doi.org/10.3390/app15105534
Chicago/Turabian StyleRocha, Caroline Trefiglio, Patrícia Michelle Nagai de Lima, Thaís Cristine Pereira, Lara Steffany de Carvalho, Mariana Gadelho Gimenez Diamantino, Amjad Abu Hasna, João Carlos da Rocha, Luciane Dias de Oliveira, and Cláudio Antonio Talge Carvalho. 2025. "Antibacterial and Antibiofilm Effect of Lavandula dentata L. Essential Oil as Endodontic Irrigant Against Standard and Clinical Strains of Enterococcus spp." Applied Sciences 15, no. 10: 5534. https://doi.org/10.3390/app15105534
APA StyleRocha, C. T., de Lima, P. M. N., Pereira, T. C., de Carvalho, L. S., Diamantino, M. G. G., Abu Hasna, A., da Rocha, J. C., de Oliveira, L. D., & Carvalho, C. A. T. (2025). Antibacterial and Antibiofilm Effect of Lavandula dentata L. Essential Oil as Endodontic Irrigant Against Standard and Clinical Strains of Enterococcus spp. Applied Sciences, 15(10), 5534. https://doi.org/10.3390/app15105534








