Does Pilates Breathing Affect the Biceps Brachii Neuromuscular Efficiency During Submaximal Contraction?
Abstract
1. Introduction
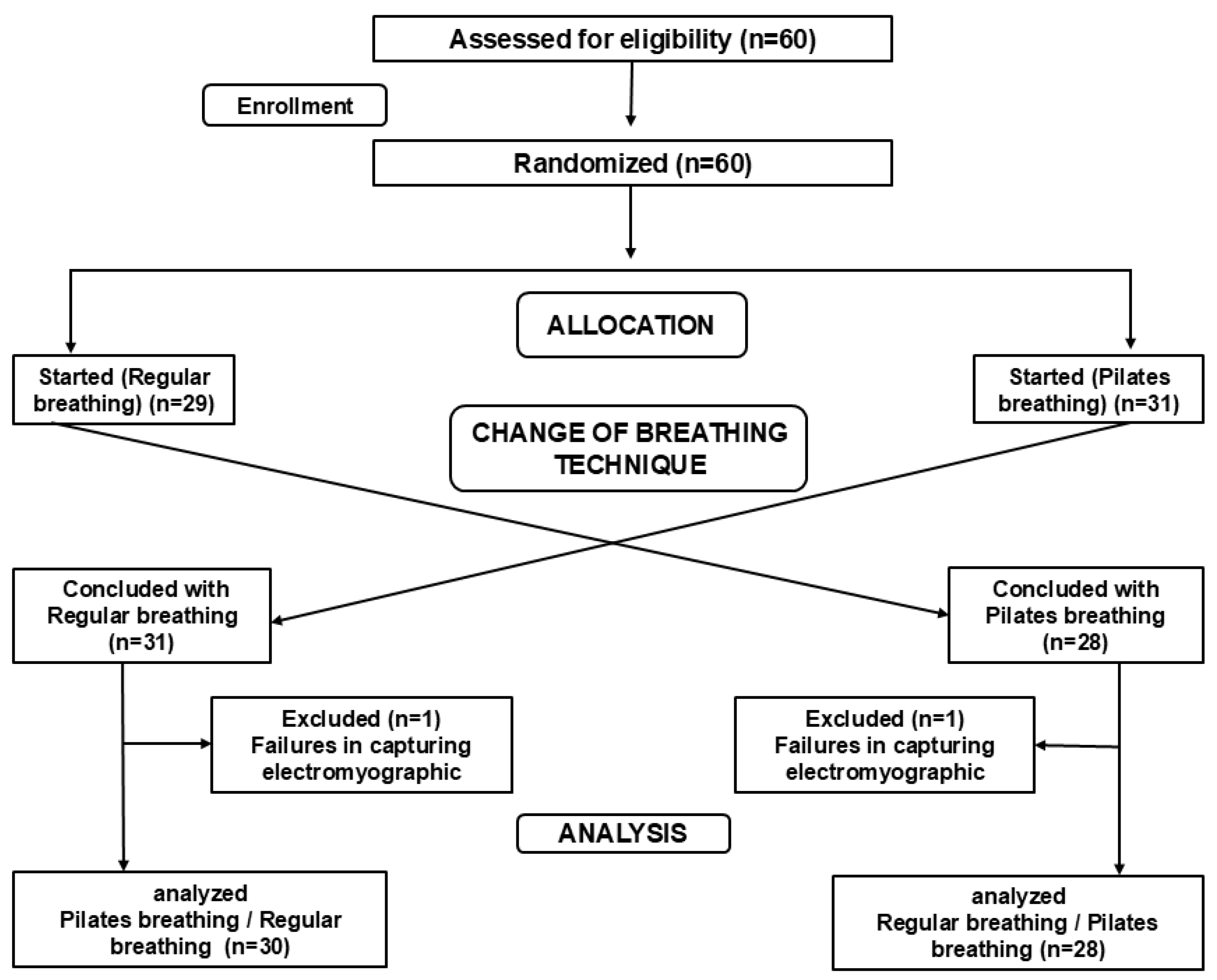
2. Materials and Methods
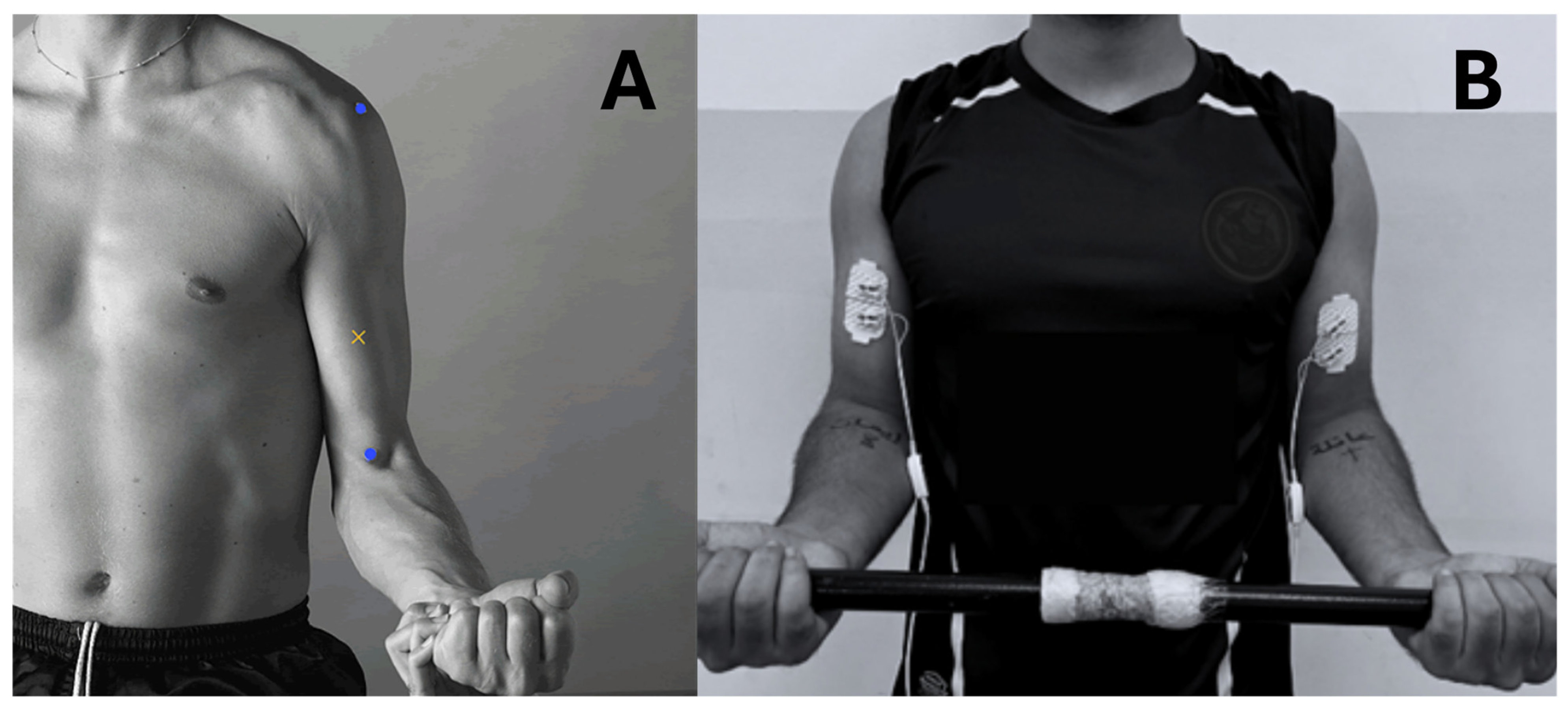
2.1. Data Recording
2.2. Exercise Procedures
2.3. Experimental Protocol
2.4. Data Extraction
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PME | Pilates Method of Exercise |
| MVIC | Maximal Voluntary Isometric Contraction |
| sEMG | Electromyographic Surface |
References
- Campos, J.L.; Vancini, R.L.; Rodrigues Zanoni, G.; Barbosa De Lira, C.A.; Santos Andrade, M.; Jacon Sarro, K. Effects of Mat Pilates Training and Habitual Physical Activity on Thoracoabdominal Expansion during Quiet and Vital Capacity Breathing in Healthy Women. J. Sports Med. Phys. Fit. 2018, 59, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.J.; Mendes, R.; Mendes, R.S.; Martins, F.; Gomes, R.; Gama, J.; Dias, G.; Castro, M.A. Benefits of Pilates in the Elderly Population: A Systematic Review and Meta-Analysis. Eur. J. Investig. Health Psychol. Educ. 2022, 12, 236–268. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.J.; André, A.; Monteiro, M.; Castro, M.A.; Mendes, R.; Martins, F.; Gomes, R.; Vaz, V.; Dias, G. Methodology and Experimental Protocol for Studying Learning and Motor Control in Neuromuscular Structures in Pilates. Healthcare 2024, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Pucci, G.C.M.F.; Neves, E.B.; Saavedra, F.J.F. Effect of pilates method on physical fitness related to health in the elderly: A systematic review. Rev. Bras. Med. Esporte 2019, 25, 76–87. [Google Scholar] [CrossRef]
- Byrnes, K.; Wu, P.-J.; Whillier, S. Is Pilates an Effective Rehabilitation Tool? A Systematic Review. J. Bodyw. Mov. Ther. 2018, 22, 192–202. [Google Scholar] [CrossRef]
- Barbosa, A.W.C.; Guedes, C.A.; Bonifácio, D.N.; De Fátima Silva, A.; Martins, F.L.M.; Almeida Barbosa, M.C.S. The Pilates Breathing Technique Increases the Electromyographic Amplitude Level of the Deep Abdominal Muscles in Untrained People. J. Bodyw. Mov. Ther. 2015, 19, 57–61. [Google Scholar] [CrossRef]
- De Luca, C.J.; Contessa, P. Biomechanical Benefits of the Onion-Skin Motor Unit Control Scheme. J. Biomech. 2015, 48, 195–203. [Google Scholar] [CrossRef]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to Endurance and Strength Training. Cold Spring Harb. Perspect. Med. 2018, 8, a029769. [Google Scholar] [CrossRef]
- Campos, D.B.; Ferreira, I.C.; Souza, M.A.; Amorim, M.; Intelangelo, L.; Silveira-Nunes, G.; Barbosa, A.C. Acceleration Profiles and the Isoinertial Squatting Exercise: Is There a Direct Effect on Concentric–Eccentric Force, Power, and Neuromuscular Efficiency? J. Sport Rehabil. 2021, 30, 646–652. [Google Scholar] [CrossRef]
- Panhan, A.C.; Gonçalves, M.; Cardozo, A.C. Electromyographic Activation and Co-Contraction of the Thigh Muscles During Pilates Exercises on the Wunda Chair. J. Chiropr. Med. 2023, 22, 322–327. [Google Scholar] [CrossRef]
- Cardoso, E.A.; Neto, F.R.; Martins, W.R.; Bottaro, M.; Carregaro, R.L. Neuromuscular Efficiency of the Knee Joint Muscles in the Early-Phase of Strength Training: Effects of Antagonist’s Muscles Pre-Activation. Motricidade 2018, 14, 24–32. [Google Scholar] [CrossRef]
- Alvarenga, G.M.D.; Charkovski, S.A.; Santos, L.K.D.; Silva, M.A.B.D.; Tomaz, G.O.; Gamba, H.R. The Influence of Inspiratory Muscle Training Combined with the Pilates Method on Lung Function in Elderly Women: A Randomized Controlled Trial. Clinics 2018, 73, e356. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Sawilowsky, S.S. New Effect Size Rules of Thumb. J. Mod. App. Stat. Meth. 2009, 8, 597–599. [Google Scholar] [CrossRef]
- Magalhães, I.; Bottaro, M.; Mezzarane, R.A.; Neto, F.R.; Rodrigues, B.A.; Ferreira-Júnior, J.B.; Carregaro, R.L. Kinesiotaping Enhances the Rate of Force Development but Not the Neuromuscular Efficiency of Physically Active Young Men. J. Electromyogr. Kinesiol. 2016, 28, 123–129. [Google Scholar] [CrossRef]
- Silva, Y.; Melo, M.; Gomes, L.; Bonezi, A.; Loss, J. Análise da resistência externa e da atividade eletromiográfica do movimento de extensão de quadril realizado segundo o método Pilates. Braz. J. Phys. Ther. 2009, 13, 82–88. [Google Scholar] [CrossRef]
- Carvalho Barbosa, A.W.; Martins, F.L.M.; Vitorino, D.F.D.M.; Almeida Barbosa, M.C.S. Immediate Electromyographic Changes of the Biceps Brachii and Upper Rectus Abdominis Muscles Due to the Pilates Centring Technique. J. Bodyw. Mov. Ther. 2013, 17, 385–390. [Google Scholar] [CrossRef]
- Barbosa, A.C.; Martins, F.M.; Silva, A.F.; Coelho, A.C.; Intelangelo, L.; Vieira, E.R. Activity of Lower Limb Muscles During Squat With and Without Abdominal Drawing-in and Pilates Breathing. J. Strength. Cond. Res. 2017, 31, 3018–3023. [Google Scholar] [CrossRef]
- Panhan, A.C.; Gonçalves, M.; Eltz, G.D.; Villalba, M.M.; Cardozo, A.C.; Bérzin, F. Neuromuscular Efficiency of the Multifidus Muscle in Pilates Practitioners and Non-Practitioners. Complement. Ther. Med. 2018, 40, 61–63. [Google Scholar] [CrossRef]
- Marques, N.R.; Morcelli, M.H.; Hallal, C.Z.; Gonçalves, M. EMG Activity of Trunk Stabilizer Muscles during Centering Principle of Pilates Method. J. Bodyw. Mov. Ther. 2013, 17, 185–191. [Google Scholar] [CrossRef]
- Siedlecki, P.; Ivanova, T.D.; Shoemaker, J.K.; Garland, S.J. The Effects of Slow Breathing on Postural Muscles during Standing Perturbations in Young Adults. Exp. Brain Res. 2022, 240, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.A.; Santarelli, D.M.; O’Rourke, D. The Physiological Effects of Slow Breathing in the Healthy Human. Breathe 2017, 13, 298–309. [Google Scholar] [CrossRef]
- Carroll, T.J.; Riek, S.; Carson, R.G. Neural Adaptations to Resistance Training: Implications for Movement Control. Sports Med. 2001, 31, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-T.; Lee, J.-H. The Effects of Pilates Breathing Trainings on Trunk Muscle Activation in Healthy Female Subjects: A Prospective Study. J. Phys. Ther. Sci. 2017, 29, 194–197. [Google Scholar] [CrossRef]
- Virgile, A.; Bishop, C. A Narrative Review of Limb Dominance: Task Specificity and the Importance of Fitness Testing. J. Strength. Cond. Res. 2021, 35, 846–858. [Google Scholar] [CrossRef]
- Schaffer, J.E.; Sainburg, R.L. Interlimb Differences in Coordination of Unsupported Reaching Movements. Neuroscience 2017, 350, 54–64. [Google Scholar] [CrossRef]
- Alkhawaldeh, I.M.; Altarawneh, M. Difference in One-Repetition Maximum and Electromyography between the Dominant and Non-Dominant Arms and Their Relationship to Exercise Onset: An Experimental Study of the Biceps Brachii Muscle. Ann. Appl. Sport Sci. 2023, 11. [Google Scholar] [CrossRef]
- Campos, D. Does Pilates Breathing Affect the Biceps Brachii Neuromuscular Efficiency During Submaximal Contraction. Mendeley Data, V1. 2025. Available online: https://data.mendeley.com/datasets/brrd6g8grb/1 (accessed on 2 April 2025). [CrossRef]
| Male | Female | All | p Male vs. Female | |
|---|---|---|---|---|
| n (%) | 20 (32.1%) | 38 (67.8%) | 58 (100%) | 0.008 * |
| Age, y, mean ± SD | 23.20 ± 2.62 | 22.6 ± 2.64 | 22.8 ± 2.62 | 0.47 |
| Weight, mean ± SD | 81.0 ± 10.2 | 63.3 ± 10.3 | 69.0 ± 13.2 | 0.07 |
| Height, mean ± SD | 1.76 ± 0.11 | 1.63 ± 0.05 | 1.67 ± 0.09 | 0.04 * |
| BMI, mean ± SD | 26.4 ± 4.11 | 23.7 ± 3.47 | 24.6 ± 3.88 | 0.60 |
| Right | Left | Right vs. Left | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regular | Pilates | p | ES | Regular | Pilates | p | ES | Regular | ES | Pilates | ES | ||
| 20% of MVIC | conc | 2.9 (0.5–10.5) | 3.2 (0.5–19.4) | 0.031 * | 0.51 moderate | 4.3 (0.9–19.2) | 3.5 (0.9–13.2) | 0.003 * | −0.71 moderate | <0.001 * | 0.62 moderate | 0.938 | 0.16 very small |
| ecc | 2.6 (0.3–11.5) | 2.6 (0.3–7.8) | 0.03 * | −0.47 small | 2.9 (0.5–14.4) | 2.7 (0.6–14.2) | 0.036 * | −0.5 moderate | 0.638 | 0.16 very small | 0.66 | 0.15 very small | |
| 40% of MVIC | conc | 2.6 (0.3–13.6) | 2.4 (0.8–10) | 0.008 * | 0.92 large | 4 (1.0–13.7) | 3.4 (0.8–17.9) | 0.002 * | 0.91 large | <0.001 * | 0.67 moderate | < 0.001 * | 0.62 moderate |
| ecc | 2.5 (0.2–10.8) | 2.3 (0.7–8.2) | 0.07 * | 0.95 large | 2.7 (0.4–11.5) | 2.4 (0.7–13.1) | 0.03 * | 0.94 large | <0.001 * | 0.13 very small | 0.579 | 0.08 very small | |
| 60% of MVIC | conc | 2.7 (0.7–8.7) | 2.5 (0.5–10) | <0.001 * | −0.7 moderate | 3.8 (1.0–22.4) | 3.4 (0.9–15.3) | 0.023 * | −0.59 moderate | <0.001 * | 0.68 moderate | <0.001 * | 0.63 moderate |
| ecc | 2.6 (0.5–7.8) | 2.2 (0.4–11.3) | 0.019 * | −0.45 small | 2.4 (0.9–17.3) | 2.2 (0.6–16.1) | 0.03 * | −0.65 moderate | 0.787 | 0.14 very small | 0.66 | 0.13 very small | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, D.B.; de Cassia Gomes Souza Macedo, M.; do Rosário Ferreira, K.R.; Esquirio, A.F.; Leal, A.C.; Gama, G.L.; Barbosa, A.C. Does Pilates Breathing Affect the Biceps Brachii Neuromuscular Efficiency During Submaximal Contraction? Appl. Sci. 2025, 15, 5528. https://doi.org/10.3390/app15105528
Campos DB, de Cassia Gomes Souza Macedo M, do Rosário Ferreira KR, Esquirio AF, Leal AC, Gama GL, Barbosa AC. Does Pilates Breathing Affect the Biceps Brachii Neuromuscular Efficiency During Submaximal Contraction? Applied Sciences. 2025; 15(10):5528. https://doi.org/10.3390/app15105528
Chicago/Turabian StyleCampos, Denys Batista, Maria de Cassia Gomes Souza Macedo, Kariny Realino do Rosário Ferreira, Arthur Ferreira Esquirio, Ana Clara Leal, Gabriela Lopes Gama, and Alexandre Carvalho Barbosa. 2025. "Does Pilates Breathing Affect the Biceps Brachii Neuromuscular Efficiency During Submaximal Contraction?" Applied Sciences 15, no. 10: 5528. https://doi.org/10.3390/app15105528
APA StyleCampos, D. B., de Cassia Gomes Souza Macedo, M., do Rosário Ferreira, K. R., Esquirio, A. F., Leal, A. C., Gama, G. L., & Barbosa, A. C. (2025). Does Pilates Breathing Affect the Biceps Brachii Neuromuscular Efficiency During Submaximal Contraction? Applied Sciences, 15(10), 5528. https://doi.org/10.3390/app15105528






