Abstract
Background: Physical activity is vital for promoting health and rehabilitation, and ensuring cardiovascular safety during such activities is paramount. Electrocardiography (ECG) and its longitudinal monitoring remain crucial for the early detection of cardiac diseases. Recent advancements in nonlinear RR analysis and machine learning offer promising approaches to identifying subtle precursors of cardiac pathologies in monitoring systems using simple heart rate (HR) wearable sensors. Therefore, using HR sensors in human activity recognition (HAR) is recommendable. After defining fatigue in a cardiological context, and focusing on an AI-based methods suite for HAR, the main research question of this scoping review is as follows: “Can RR time series be successfully used as physiological biomarkers for the early detection of cardiac fatigue?” The reported data on assessment of fatigue are focused on the last two decades. The aim of this scoping review was to collect, present and discuss the existing literature on the effectiveness of AI-based methods for processing RR time series as a predictive biomarker for cardiac fatigue compared to commonly used questionnaires for this outcome in adult populations. Methods: Queries were conducted in the PubMed, Scopus and Google Scholar databases for the time period 2005–2025. Only research articles and review papers were considered suitable candidates. Results: Data from 10 papers were considered, related to the information researched. Conclusions: Information on HRV-based objective measures is quite scarce and there is an urgent need to adopt a multidisciplinary approach and to improve advanced AI-based nonlinear analyses to differentiate cardiac physiological status from cardiac pathological status.
1. Introduction
Cardiac fatigue is a specific condition affecting the heart muscle’s ability to function efficiently, often seen in heart failure patients [1]. Cardiovascular fatigue is a broader term that includes both physical and mental fatigue related to the entire cardiovascular system, commonly seen in various cardiovascular diseases. Understanding these differences is crucial for accurate diagnosis and targeted treatment strategies. Several criteria, including age, training status and performance level, have been linked to the likelihood of electrocardiographic changes, and the diagnosis of cardiac fatigue remains a contentious topic [1].
Feelings of diminished physical and mental vitality are part of the vast, multifaceted concept of fatigue. In a broader context, although there are currently very few tools available to understand fatigue, it significantly impacts patients’ health-related quality of life across a wide range of diseases. Specifically, an objective measure of cardiac fatigue is missing. Early descriptors of cardiac fatigue, including molecular biomarkers and physiological indicators, may prove valuable for the early and objective identification of cardiovascular overload situations, such as those associated with intense physical exercise, chronic stress, or pathological conditions. A more general definition of fatigue describes it as “a state of excessive chronic tiredness characterized by a pervasive feeling of exhaustion and negative emotions such as anxiety and low mood” [2]. Numerous questionnaires are now available to assess how people subjectively perceive fatigue.
In Appendix A, some examples of questionnaires are reported.
1.1. ECG Features
Cardiac fatigue can prolong repolarization and increase electrical instability, reflected in the QT interval. Specifically, QTc prolongation (corrected QT interval) indicates repolarization abnormalities, and the QT Variability Index (QTVI) is sensitive to sympathetic stress and early cardiac dysfunction [3]. T-wave Alternans (TWA) reflects microvolt-level beat-to-beat variability in T-wave morphology, linked to ventricular repolarization instability; elevated TWA suggests metabolic fatigue, myocardial stress, or increased arrhythmic risk [4]. Fatigue and ischemic stress can cause transient ST depression or elevation, especially in endurance athletes or those under hemodynamic stress. Finally, a delayed return to baseline HR after exertion suggests autonomic fatigue and impaired vagal reactivation.
1.2. Gold Standard for Diagnosis
There is no universally accepted gold standard for diagnosing cardiac fatigue. However, it is typically assessed using a multimodal approach involving ECG (e.g., decreased heart rate variability—HRV; increased QT variability), echocardiography [5] and cardiac imaging (e.g., echocardiographic measures of ejection fraction and diastolic filling). Cardiopulmonary exercise testing, valuable even when performed by a portable measurement system (Cosmed™ K5, Rome, Italy) [6,7], is used to test the efficiency of the cardiopulmonary system and the metabolic thresholds. Because cardiac fatigue is reversible and dynamic, real-time or continuous monitoring (e.g., Holter ECG, wearable sensors) is often more informative than static assessments.
1.3. Wearable Devices
Recent advancements in wearable sensor technology [8,9], especially in the field of human–machine interaction, are increasingly aligned with the needs of clinical monitoring, including the early detection of cardiac fatigue [10]. While traditional wearable systems were limited by their low integration and measurement accuracy, emerging innovations have addressed these gaps through compact system designs, flexible biosensors and improved battery life. Notably, the integration of electrochemical sensors allows for the simultaneous detection of multiple biomarkers (such as sweat analysis) in non-invasive modes, offering a pain-free alternative to traditional methods. Coupled with modern machine learning (ML) algorithms, these systems now enable the indirect estimation of physiological states, including those relevant to cardiac performance [11]. These trends underscore a shift toward more smart, high-fidelity and clinically relevant wearable monitoring platforms that are highly compatible with the early detection of conditions like cardiac fatigue.
Some studies reported that strenuous endurance training may result in both skeletal and cardiac muscle fatigue [12,13]. This condition is called “exercise-induced cardiac fatigue” (EICF), and it manifests as a brief decrease in left (LV) or right (RV) ventricular function in otherwise healthy individuals [14,15]. While traditional echocardiographic metrics like LV ejection fraction (LVEF) can be used to evaluate EICF, myocardial deformation imaging—that is, global longitudinal strain measured by speckle tracking echocardiography—is becoming a more accurate and sensitive metric [16,17]. These studies tried to evaluate the occurrence of EICF in recreational ultramarathon runners and to ascertain potential predictive factors of myocardial dysfunction (as ventricular tachycardia and sudden cardiac death), but this type of activity is very far from those we found in the human activity recognition (HAR) context.
The detection of different human actions or gestures, as well as additional in-depth information about human activities (such as the quality of motions, emotions and gender) based on sensor data, are all parts of the large research topic known as HAR. The advent of wearable, inexpensive, low-power sensors and live data streaming has led to the application of numerous innovative data processing and analysis techniques to HAR in recent years.
Molecular biomarkers are substances detectable in the blood or other body fluids that indicate cardiovascular stress or damage. Burger et al. (2024) [18] observed that cardiovascular biomarkers can increase, and heart function may temporarily deteriorate, as a result of ultra-endurance event participation. For most molecular biomarkers, a blood sample is required.
The advantage of physiological indicators like heart rate (HR), is that they can be recorded and analyzed remotely. Moreover, they can be used for an initial screening to identify an at-risk subject, who can subsequently undergo advanced diagnostic tests and blood tests for molecular biomarkers [19]. Recently, there has been a significant proliferation of wearable devices such as smartwatches, rings and chest bands in cardiac care [20].
1.4. HR or HRV?
HR is considered as the simple count of heart beats per minute, whereas HRV refers to the variation in time intervals between consecutive R-peaks in the ECG signal. The body of evidence from physiological studies supports the validity of HR as a diagnostic and prognostic tool in cardiology. However, HR’s clinical utility may be influenced by factors such as age and comorbidities, time of day, measurement duration, physical activity and medications; thus, standardized protocols are essential in order to improve reproducibility and comparability.
HR can be used in different subject conditions; for example, at rest, an elevated resting heart rate is associated with increased cardiac morbidity and mortality, independent of other risk factors. It is considered a marker of sympathetic dominance, low cardiorespiratory fitness, or systemic inflammation [21]. During physical exercise, an abnormal HR response (blunted rise or delayed recovery) is associated with autonomic dysfunction, poor prognosis in heart failure and an increased risk of sudden cardiac death [22]. Moreover, circadian HR patterns can be studied and their changes can serve as early indicators of autonomic imbalance, athletes’ overtraining, or the onset of sepsis or systemic inflammation in critical care patients [23]. Nevertheless, HR has some limitations, as its context-dependence is influenced by a wide range of non-pathological factors (e.g., temperature, emotional state, caffeine consumption, hydration) (low-specificity). A high HR may be pathological in one setting (e.g., resting tachycardia) and physiological in another (e.g., during exercise). In conclusion, HR provides a gross marker of cardiac workload and autonomic tone, but it is less sensitive to subtle regulatory changes than HRV, which captures beat-to-beat dynamics and offers a more detailed view of autonomic control mechanisms [24]. For these reasons, we chose HRV as crucial indicator of cardiac health and fatigue, since it refers to the variation in time intervals between consecutive heartbeats (RR time series) [25].
HRV parameters can be easily recorded using a huge variety of wearable devices [8,9,20]. Therefore, the main research focus was to collect, present and discuss the existing literature on the effectiveness of AI-based methods for processing RR time series as a predictive biomarker for cardiac fatigue compared to commonly used questionnaires for this outcome in an adult population.
1.5. Physiological Biomarkers in Complex Systems Theoretical Framework
The use of RR time series as physiological biomarkers for the early detection of cardiac fatigue is supported by several studies that highlight the potential of HRV analysis and related metrics. RR time series and HRV analysis are promising tools for the early detection of cardiac fatigue. The integration of nonlinear analysis techniques and advanced ML models can enhance their predictive accuracy and provide valuable insights for both clinical and athletic applications. The frequent monitoring and analysis of RR intervals can help in the timely intervention and management of cardiac health. Cardiac health, especially when monitoring older people, necessitates careful evaluation.
Since cardiac fatigue has been shown to affect the complexity of physiological signals, the fatigue-induced loss of complexity in RR time series may represent a key aspect of nonlinear analysis [26].
It has been suggested that the loss of complexity is a general characteristic of pathologic dynamics, since illness and aging seem to impair people’s ability to adapt [27]. HRV analysis reflects the autonomic nervous system’s influence on the heart, which can be indicative of cardiac stress and fatigue [28].
Despite their widespread use, standard linear techniques like time-domain and frequency-domain analysis may oversimplify the complex dynamics of HR regulation [29]. By taking into consideration the patterns seen in cardiovascular dynamics, nonlinear approaches provide a more comprehensive strategy.
To address this purpose, the nonlinear Recurrence Quantification Analysis (RQA) method was used to properly analyze RR time series. RQA is a statistical, graphical and analytical tool successfully used in various domains, including medicine, seismic geology and finance, to explore nonlinear dynamical systems [30,31]. Specifically, RQA is an accurate method for analyzing the stochastic and chaotic dynamics of physiological data (such as RR time series) [29] and describing physiological changes [6,31,32]. In terms of complex systems theory, fatigue was hypothesized as an order parameter [7].
Another approach is that proposed by using detrended fluctuation analysis via an index called the Cardiac Stress Index, derived from the RR time series, which has been shown to correlate with perceived exertion during exercise [33]. This index can quantify cardiac stress and potentially detect early signs of cardiac fatigue [30].
HRV can be evaluated using time-based measures or frequency-domain measures [29]. The basis of frequency-domain analysis is the mathematical transformation of signals from the time domain to the frequency domain (represented in cycles per beat with different amplitudes and frequencies) using Fast Fourier Transforms. Power spectral density analysis provides fundamental knowledge of how power (i.e., variance) is distributed as a function of frequency [34]. Entropy has been used as a measure of the complexity of physiological systems, and other nonlinear techniques provide more details regarding HRV based on the entropy measure [35]. Specifically, as in [36,37], entropy approaches can be effective in the diagnosis of heart diseases. The main ECG and HRV features are compared in Table 1.

Table 1.
ECG and HRV features related to cardiac fatigue.
1.6. AI and ML
The use of artificial intelligence (AI) in healthcare is expanding. In recent years, various tools and advanced statistical algorithms have been effectively used to support medical professionals. AI-based devices must undergo proper training to assess large amounts of data and make fast, accurate decisions [38]. Specifically, an ML model is trained by exposing it to examples and adjusting its internal parameters accordingly. Once training is complete, the model is given new data and applies what it has learned to analyze and interpret it. The more accurate the training, the better the model’s performance.
ML can be divided into supervised ML and unsupervised ML, depending on whether the algorithm is trained with labeled or unlabeled data. Examples of supervised learning include Logistic Regression [39], Support Vector Machines (SVMs) [40], Decision Trees (DTs) [41] and Neural Networks (NNs) [42,43], which are used for tasks such as image and pattern classification. In contrast, unsupervised learning techniques, such as clustering and dimensionality reduction, are used to identify patterns in data without predefined labels [44]. A third category of ML is Reinforcement Learning, in which a computer program interacts with a dynamic environment to accomplish a specific task by learning through rewards and penalties [45].
The early detection of cardiac fatigue using HR sensors requires a multi-stage pipeline that includes sensor technology, signal acquisition, preprocessing, feature extraction and clinical interpretation.
The block scheme shown in Figure 1 illustrates the procedure used to classify RR time series measured by ECG or by any different wearable device type. The dimensionality reduction step, which follows the parameter extraction procedure, implies the reduction in the number of extracted parameters in the set prior to the ECG data classification attempted in the final stage of the process. The key preprocessing steps include, for example, the following: (i) bandpass filtering (e.g., 0.5–40 Hz for ECG) to remove baseline wander and powerline interference; (ii) motion artifact reduction: adaptive filtering or signal decomposition (e.g., empirical mode decomposition [46]); (iii) peak detection: accurate R-peak detection (by ECG) or pulse wave peak detection (by PPG) using methods like the Pan–Tompkins algorithm or derivative-based algorithms [47].
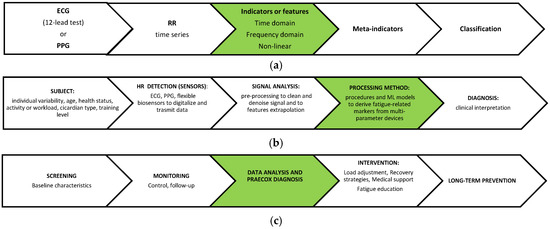
Figure 1.
Block schemes. (a) From ECG to classification for clinical interpretation in a theoretical framework; (b) the multi-stage pipeline with the main sources of variability and noise: limitations in the whole HRV data processing method can be found in these steps (subject, sensor, analysis, method, etc.); (c) the timeline of possible healthcare applicative procedures. The sections in green focus on the main research question.
ECG is by far the most popular cardiac monitoring technique in healthcare, and it has advantages over PPG in terms of stability and reliability.
1.7. Cardiovascular or Cardiac Fatigue?
It is important to specify that the general term “cardiovascular fatigue” was used in the literature search, although the focus of our search was specifically “cardiac fatigue”. The manuscript is structured as follows: Section 2 outlines the literature search process, with a focus on annual publication trends. In Section 3, the specific ML procedures used in the studies selected for this scoping review are described. Finally, Section 4 addresses the main research question—whether RR time series can be used as physiological biomarkers for the early detection of cardiac fatigue—focusing on AI-based methods, based on the findings from the selected papers.
2. Materials and Methods
We sourced scientific articles and reviews using the PubMed, Scopus and Google Scholar search engines. As in our previous review on NHS practices in Europe, we selected a search time-span window of 20 years. The literature search, conducted in January 2025, followed the PRISMA 2020 guidelines (the PRISMA website was visited in January 2025) and utilized the following keywords (mesh terms): “ECG”, “cardiovascular fatigue”, “cardiac pathologies”, “HRV” or “heart rate”, “wearable devices”, “AI” or “Machine Learning”. Only research and review articles were deemed appropriate. The standard English language filter was not used. Table 2 displays the results of the most notable queries. Figure 2 reports the flow diagram of the literature search. The PRISMA checklist is available in the Supplementary Materials.

Table 2.
The results of the three search engine queries (623, 455 articles and 168 reviews). The star symbols “*” indicate that, for the Google Scholar database, the term “Scientific” does not distinguish between articles and reviews. The shaded cells refer to the total number of manuscripts in 2024–25, per database. (^ indicates that Google Scholar database results were not included in the sum).
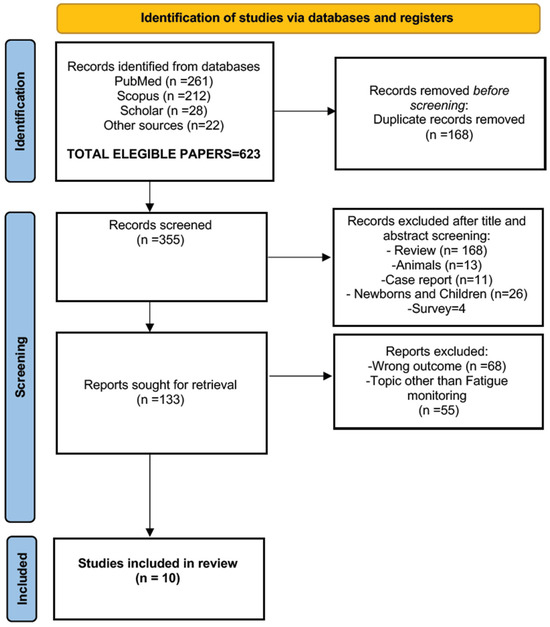
Figure 2.
A flow diagram of the literature search, according to the PRISMA criteria (http://www.prisma-statement.org/, accessed on 15 January 2025), with the steps followed in the manuscript selection procedure. After the application of the selection criteria, the initial 623 manuscripts were reduced to 10.
To create a comprehensive overview of the state of the art for this methodology, a manual search was also conducted to find analysis methods and connect them to pertinent research publications. Two reviewers (M.C. and G.Z.) extracted information about the studies’ features, equipment description and diagnostic usefulness metrics.
A preliminary literature search on “cardiovascular and fatigue and (ambient or assisted or living)” returned only 17 papers, revealing a lack of information on these topics (filters applied: clinical trial, meta-analysis, randomized controlled trial, humans, age: 65+ years).
Age-related specific research was carried out only on PubMed; of the 565 papers on “cardiovascular and fatigue” (filters applied: clinical trial, meta-analysis, randomized controlled trial, humans), 11% (62/565) focused on children and adolescents (age < 18 years) and 74% (417/565) on adults (age > 18 years). Among these, 34% (190/565) involved older adults (age > 65 years).
This first query outcome showed a linear increase in the number of publications mentioning “cardiovascular fatigue” (in the title or abstract) over the past 20 years. However, this trend was not observed in studies focusing on children and adolescents, as shown in Figure 3. The decrease in the number of publications in 2022–2023 could be influenced by the COVID-19 pandemic.
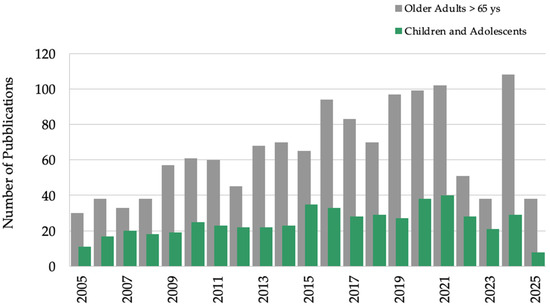
Figure 3.
Annual publications for “cardiovascular fatigue”. Older adults (>65 years) in gray, children and adolescents (birth-18 years) in green; other filters applied: humans, exclude preprints, PubMed from 2005–2025.
Searching “cardiac fatigue” returned only six results, all related to endurance exercise; the following filters were used: randomized controlled trials, meta-analyses, clinical trials, humans and any studies involving individuals aged 65 + years. This result led us to think that, up to now, “cardiac fatigue” has been rarely used and, in any case, is mainly applied in sports training contexts.
3. Results
From 623 total eligible papers, 355 publications were screened, and they were later sorted, first by title and abstract, and then by suitability for the review’s topic.
Studies examining whether RR time series could be used as physiological indicators for the early identification of cardiac fatigue in an adult population (see Figure 1’s green arrows) were selected.
In order to describe the most recent (in a 20-year span) literature information on cardiac fatigue in adults (>18 years of age), the data were classified according to the methods applied (and in order of year of publication) and to the healthy status of the participants; the obtained information is summarized in Table 3, and these selected papers are described in the following 10 paragraphs.
Regarding model performance (see the last column of Table 3), the most common evaluation metrics, accuracy, precision, recall and F1, are defined in Appendix B.
We report the full descriptive statistics: Seven of the ten papers (70%) selected were published in the last 5 years; the studies included from 8 to 139 subjects. All of these studies were conducted on a limited number of subjects, with the exception of one involving hypertensive patients. Of the 435 subjects included, 223 (51%) were healthy, 139 were hypertensive (32%) and 99 were spinal cord injury (SCI) (17%) subjects, respectively.
Of the 10 studies included, 40% used ECG-based RR interval acquisition, 40% used a chest belt sensor and 20% used PPG. In two studies, multi-sensor wearable devices were used. All studies reported using HRV features for fatigue detection, with nine studies applying ML methods and reporting accuracies > 80.5%. Specifically, four studies reported High Model Performance (≥90% accuracy or consistently strong across multiple metrics) and five studies reported Moderate to Good Model Performance (80–89% accuracy or F1/precision/recall in the ~0.80 range). Of the 10 studies included, 2 (20%) studies were conducted in Australia, 2 (20%) in China, 2 (20%) in USA and 4 (40%) in EU.
A word frequency analysis of the article titles revealed the key thematic focuses within the research field (Figure 4).
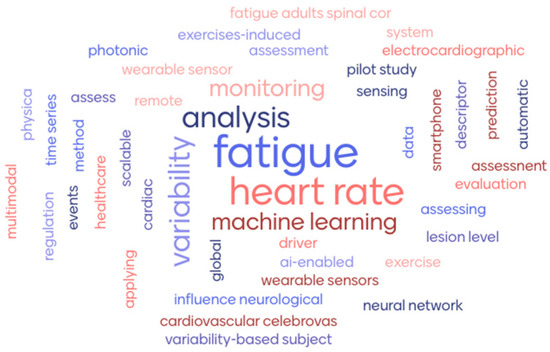
Figure 4.
Word cloud of 10 article titles by Mentimeter (www.mentimeter.com).
The most frequently occurring terms were “fatigue” (8/61), “heart rate”(6/61), “variability” (4/61) and “analysis” (3/61), highlighting the centrality of physiological monitoring, particularly HRV, in detecting fatigue. The repeated presence in the titles of terms like “wearable sensors,” “machine learning,” and “monitoring” confirm the strong interest in technological and AI-based approaches for real-time health assessment.
3.1. Multivariate Analysis and SCI
Rodrigues et al. (2016) [2] reported a comparison between a control group of able-bodied subjects and a group of subjects with SCI; this is particularly relevant because the SCI groups’ sympathetic nervous systems are damaged, and fatigue can significantly hinder the efficacy of their rehabilitation. Elevated fatigue was linked to higher sympathetic dysfunction, according to research based on HRV, Index Fatigue Scale (IFS) and eye blink duration [48]. Furthermore, fatigue was found to be associated with altered HRV, which may represent a potential cardiac risk.
3.2. Entropy Analysis (Physical Fatigue)
Physical fatigue, understood as the inability to sustain performance over time, can be detected using the entropy analysis of HR signals, as proposed by Nasirzadeh et al. (2020) [37]. The authors used data collected by other authors who had measured the HR of eight workers wearing a chest strap. As previously observed, nonlinear entropy analysis can provide more details regarding the variability of HR [35,36]. The aim of the study was to develop a novel analysis method capable of predicting physical fatigue in real time using HR as the only indicator. For tiredness detection (the rating of perceived exertion (RPE) [49] was used to label the data), the suggested approach demonstrated accuracy, sensitivity and specificity rates of 90.36%, 82.26% and 96.2%, respectively, minimizing work accidents and injuries due to fatigue.
3.3. Meta-Learning and NNs
The performance of different HRV-based data mining approaches was tested in order to develop effective algorithms for predicting cardiovascular risk in a population of patients with hypertension. Melillo et al. (2015) [50] used a dataset of 24 h electrocardiograms from 139 hypertensive patients aged 72 ± 7 years who were followed for 12 months. The selected sample was divided into two groups: those at high risk who had suffered an event during follow-up (myocardial infarctions, strokes or syncope) and all others at a low risk. For each subject, a 5 min stationary segment was randomly selected and recorded during the day. The authors showed that HRV-based systems can be meaningful predictors of vascular events many weeks or months before their occurrence, and that they can outperform classification based on echographic evaluations.
3.4. Supervised ML (Physical Fatigue)
A novel approach for the objective and automatic classification of physical exhaustion based on HRV was presented by Ni et al. (2022) [51]. Their 80 healthy subjects were asked to perform a preset treadmill exercise, during which their ECG data were collected. From the ECG, 24 HRV features were computed. The 10 selected HRV features identified for physical fatigue detection are reported in Appendix C, as explained in [38]. The classification model adopted four supervised ML algorithms, namely DT, SVM, K-nearest neighbor (KNN) and a light gradient boosting machine (LightGBM). The LightGBM achieved the best performance and had an accuracy of 85.5% and an F1 score of 0.801. The results verify the feasibility of using HRV to evaluate physical fatigue. The development of psychosocial strategies that address mood, anxiety, pain and fatigue in SCI rehabilitation programs must consider these cumulative risks.
3.5. NNs and Fatigue at Work
Patel et al. (2011) [52] describe an AI-based system that uses HRV to identify drivers’ early signs of fatigue.
Each of the twelve participants drove a truck (mean age of 47 ± 11 years). This study examined the use of HRV and NNs to detect fatigue in drivers, suggesting a measurable physiological signature of fatigue.
The HRV spectrum analysis gives a direct relationship between fatigue and HRV. It was shown that, as weariness increased, the LF/HF ratio dropped. The accuracy provided by the NN was 90%. The results obtained suggest that it is possible to employ this HRV-based fatigue detection method as a fatigue countermeasure.
3.6. Photonic Sensing Smartphones and NNs (MobileNetV3)
Chen et al. (2024) [53] propose an innovative multi-sensor health monitoring system for human activity recognition and health monitoring based on smartphones; a breathing and HR monitoring device (BR&HRMD) and a gait monitoring device (GMD) were included in respiratory HAR by using an adapted MobileNetV3 NN structure. The suitability of a fiber optical sensor to evaluate HRV has been demonstrated in Chen’s previous studies [54]. The system’s potential as a telemedicine tool is demonstrated by its accuracy of 98.5% on the gait pattern identification task and 94% and 95.8% on the mental and muscle fatigue monitoring tasks, respectively. The limitation is that the method was only tested on eight subjects.
3.7. Unsupervised ML (Random Forest)
Luo et al. (2020) [55] examined the relationship between self-reported non-pathological fatigue and multimodal sensor data by comparing supervised and unsupervised ML techniques (after imputing missing time-series data) from a multi-sensor wearable device using a recurrent NN-based algorithm. The analysis was carried out on 405 recording days and 27 healthy participants. Continuous multimodal wearable sensor time series for vital signs, physical activity and other physiological parameters were recorded, as were three daily questionnaires on physical fatigue, mental fatigue and the visual analog scale (VAS).
Physical tiredness labels provided by the subjects were used to train a Random Forest classifier, and the causal convolutional NN model for the unsupervised representation learning of multivariate sensor data produced the best results (weighted precision of 0.70 ± 0.03 and recall of 0.73 ± 0.03). Physical activity (energy expenditure, intensity of motion and number of steps) and vital signs (HR, HRV and respiratory rate) were both crucial parameters for training the Random Forest (weighted precision of 0.70 ± 0.05 and recall of 0.72 ± 0.01).
3.8. Sensorized T-Shirt and Unsupervised ML
In order to create algorithms that could identify respondents’ levels of physical and cognitive weariness, the efficacy of several ML techniques based on multimodal data was evaluated by [42]. Using a sensorized t-shirt and a MUSE S headband, Jaiswal et al. (2022) [56] recorded various physiological signals (EEG; ECG; electromyography—EMG; EDA/GSR) from 32 healthy volunteers aged between 18 and 33 (average age: 24 years). While their data were being collected, participants were put through a series of standardized exercises designed to induce both physical and cognitive tiredness, such as running on a treadmill and performing N-Back tests. Following each task, participants used the VAS to rate their own level of weariness. In order to train the ML models, 113 time-domain and frequency-domain features—including HRV metrics—were finally extracted by using time segments of data recorded from every participant. For identifying physical weariness, the Random Forest classifier produced the best results (80.5% accuracy), whereas for cognitive fatigue, the Long Short-Term Memory (LSTM) NN produced the best results (84.1% accuracy).
3.9. SVM and Global Fatigue Descriptor (GFD)
According to its features, fatigue can be categorized as either objective or subjective [1]. In contrast to subjective fatigue, which can be caused directly by demanding or stressful mental tasks and indirectly by physical activities, objective fatigue is a physical phenomenon that manifests itself in both its causes and its effects (resulting in a decrease in the ability to exert the body in mechanical work). Ramos et al. (2020) [57] proposed a global descriptor, first by extracting individual fatigue descriptors from EMG and HRV measurements and then by using an SVM. Fourteen healthy men were monitored: fatigue was acquired at a constant rate, a condition reasonably ensured by using a constant work rate on a cyclo-ergometer test. Despite the authors reporting that “GFD corresponds to an exploratory approach that still needs a more profound validation”, it was very interesting that the participants showed a tendency over time toward 14 distinct fatigue descriptions, specifically 4 EMG (median frequency, from Fourier and Wavelet analysis; major frequency; major time) and 10 HRV parameters (maximum, minimum and average RR interval; SDNN; rmsSD; Triangular Index; SD2; power in LF and HF band; median frequency) (for more details see Kong [58] and Appendix C). A future modification of the system toward real-time analysis could be easy, because the implemented processing system uses a sliding window mechanism to extract all the information.
3.10. SVM and Exercise-Induced Fatigue (Physical Fatigue)
Studying exercise-induced fatigue can help to develop the best routines for improved training adaptation, assess athlete fatigue levels and improve injury prevention strategies. Gan et al. (2024) [59] proposed a novel nonlinear analysis method for analyzing the nonlinear dynamic indicator of HRV in subjects before and after exercise, with reference to RPE [49] as an objective judgment index and the selection of an SVM model for classification. Box-counting (the box-counting dimension, or Minkowski dimension, is a measure of the fractal dimension of a distance space), sandbox [60] (with a generalized fractal dimension) and relative distance entropy (reladisen) were used to quantify the degree of system chaos in HRV by performing a series of rotations and translations on one-dimensional time-series data. The accuracy, precision, recall and F1 score of these new measures proposed were compared with time–frequency, frequency-domain and other nonlinear metrics in classifying rested, slightly tired and tired states, with good performance.

Table 3.
The 10 eligible papers after the filtering process. NA implies that the data were not available.
Table 3.
The 10 eligible papers after the filtering process. NA implies that the data were not available.
| n | Healthy Status | Sample Size (F/M) | Reference Measure/Questionnaire | Method/Classifier Algoritms | Model Performance | Authors (First) | Publication Year |
|---|---|---|---|---|---|---|---|
| 1 | SCI | 99 (45 SCI) (38 M/7 F), 44 AB(37 M/7 F) | HRV from ECG, eye blink duration/IFS | Multivariate analysis and Pearson correl. | NA | Rodrigues [2] | 2016 |
| 2 | Healthy | 8 (3 F/5 M) | HRV from chest belt, Polar H3 senso/RPE | Entropy-based nonlinear features, ML | Accuracy (90.36%), sensitivity (82.26%), specificity (96.2%) | Nasirzadeh [37] * | 2020 |
| 3 | Hypertension | 139 (49 F/90 M) | HRV from ECG holter | Meta-learning and NN | Sensitivity, (71.4%) and specificity (87.8%) | Melillo [50] * | 2015 |
| 4 | Healthy | 80 (38 F/42 M) | HRV from ECG/RPE | DT, SVM, KNN and LightGBM | LightGBM: Accuracy (86%), F1score (0.801) | Ni [51] ^ | 2022 |
| 5 | Healthy drivers | 12 M | HRV from ECG | PSD of HRV, NN | Accuracy (90%) | Patel [52] * | 2011 |
| 6 | Healthy | 8 | HR from fiber optic sensor, RespR, GMD form fiber optic sensors/RPE | NN, MobileNetV3 | Mental task accuracy (94%); physical fatigue (95.8%) | Chen [53] * | 2024 |
| 7 | Healthy | 27 (13 F/14 M) | HRV from PPG, 14 parameters (HR, RespR…), PhF, MF, VAS (multi-sensor wearable device) | Unsupervised ML, Random Forest | Weighted precision 0.70 ± 0.03, recall 0.73 ± 0.03 | Luo [55] | 2020 |
| 8 | Healthy | 32 (9 F/23 M) | HRV from 3-lead ECG, EEG, EMG and EDA/GSR (sensor shirt) | LSTM, Random Forest | Cognitive fatigue: LSTM model, accuracy (84.1%), recall (0.90). Physical fatigue: Random Forest, accuracy (80.5%), recall (0.88) | Jaiswal [56] ^ | 2022 |
| 9 | Healthy | 14 M | HRV from chest belt, Polar RS 800 G3, EMG | Binary SVM | Accuracy (82%) | Ramos [57] ^ | 2020 |
| 10 | Healthy | 16 M | HRV from PPG | SVM | Accuracy (82.9%) | Gan [59] ^ | 2024 |
HR, heart rate; RespR, respiratory rate; ML, machine learning; PhF, physical fatigue; MF, mental fatigue; VAS, visual analog scale; SCI, spinal cord injury; AB, able-bodied (control group); NA, not applicable; IFS, Iowa fatigue scale; GMD, gait monitor device; PPG, photoplethysmography; RPE, rating of perceived exertion. * High Performance (≥90% accuracy or consistently strong across multiple metrics); ^ Moderate to Good Performance (80–89% accuracy or F1/precision/recall in ~0.80 range).
4. Discussion
When we started this study, we were surprised to find only six scientific articles when searching for “cardiac fatigue” on PubMed database and to receive the following alert: Quoted phrase not found in phrase index: “cardiovascular fatigue”. All the six papers regarded “exercise-induced cardiac fatigue”, and the review found by Oxborough et al. [5] was about echocardiographic literature. By adding terms such as HRV, “heart rate”, “AI” and “machine learning”, the number of papers increased tenfold, and the relation between nonlinear HRV features and ML models could be investigated.
A lot of research has been performed on HRV to find early indicators of cardiovascular diseases [3,24,59] but there is a lack of information regarding cardiac fatigue.
Since it is relatively inexpensive and non-invasive to collect, the automatic prediction of pathological events using cardiac activity data has been the subject of extensive research, particularly in the past five years, using ML techniques. The first two papers selected reinforce the importance of HR monitoring and the relationship between the cardiocirculatory system and fatigue; the others highlight the importance of considering additional physiological indices and integrated processing with AI.
Thus, to better understand the connection between self-reported non-pathological fatigue and multimodal sensor data, some researchers have employed supervised and unsupervised ML techniques. They first imputed missing time-series data from a multisensory wearable device using a recurrent NN-based algorithm.
The research on wearable sensors shows that they have rapidly improved in elasticity and weight; novel electrodes were proposed to effectively collect ECG signals, as in the case of the portable semi-dry electrodes proposed by Wang et al. [60], to both solve the issues of conventional dry electrodes’ high impedance and wet electrodes’ difficult conductive liquid replenishment. To increase the reliability of physical fatigue measurement, future research should involve a broader range of participants, including individuals with disorders such as hyperthyroidism, diabetes, hypertension and heart disease, as well as subjects of varying ages and levels of physical fitness. Additionally, further deep learning [61] and ML models, along with attributes derived from other physiological inputs, will be considered. In fact, especially in the cases of unhealthy individuals, the heartbeat alone is not sufficient to assess cardiac fatigue.
The answer to the research question regarding the use of HR time series as physiological biomarkers for the early detection of cardiac fatigue is positive, especially when a multidisciplinary approach is applied. Integrating cardiology, sports science, time-series nonlinear analysis and AI is essential for developing personalized, digitally enhanced cardiac monitoring aimed at the subjective assessment of cardiac fatigue. In the future, these objectives can be achieved by leveraging the potential of tele-exercise and real-time remote monitoring.
5. Conclusions
This scoping review supports the use of RR time series and HRV analysis as effective physiological biomarkers for the early detection of cardiac fatigue. The selected studies highlight that simple wearable sensors, when combined with advanced AI and nonlinear analysis techniques, can be reliable, non-invasive monitoring tools.
However, the current evidence is limited by small sample sizes, heterogeneous protocols and a lack of standardization. Future studies should adopt multidisciplinary approaches involving cardiology, sports science and AI to validate and optimize these methodologies.
Furthermore, integrating cardiac fatigue monitoring within HAR frameworks could open new perspectives for personalized healthcare and athletic performance assessment. Continuous RR monitoring via wearable technologies, combined with AI-based models, holds significant promise for advancing early diagnosis and intervention strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15105489/s1, Table S1: PRISMA 2020 Checklist [62].
Author Contributions
Conceptualization, L.G., G.Z., M.C.G.; methodology, L.G., G.Z., M.C.G. and M.R.; data curation, M.C. and M.A.; writing—original draft preparation, L.G., G.Z., M.C.G., M.C., M.A. and M.R.; writing—review and editing, L.G., G.Z., M.C.G. and M.R.; supervision, L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HR | heart rate |
| RespR | respiratory rate |
| ML | machine learning |
| PhF | physical fatigue |
| MF | mental fatigue |
| SCI | spinal cord injury |
| AB | able-bodied |
| NA | not applicable |
| IFS | Index Fatigue Scale |
| SVM | Support Vector Machine |
| LightGBM | light gradient boosting machine |
| DT | decision tree |
| VAS | visual analog scale |
| EMG | electromyography |
| IMU | inertial measuring units |
| PPG | photoplethysmography |
| RPE | rating of perceived exertion |
| LSTM | Long Short-Term Memory |
Appendix A
Scopus AI Results for the Query “Questionnaires to Assess the Subjective Experience of Fatigue”
Visually Induced Symptoms Questionnaire (VISQ): A 15-item questionnaire used to evaluate visually induced motion sickness (VIMS) and visual fatigue caused by stereoscopic 3D images. It was developed through factor analysis and is validated for assessing visual fatigue [63].
Short Fatigue Questionnaire (pSFQ): Specifically designed for children, this four-item questionnaire assesses subjective fatigue. It has been validated for use in healthy children, children with chronic fatigue syndrome and children with chronic diseases, showing good psychometric properties [64].
Subjective Fatigue Scale: Used in a study to assess fatigue-related complaints and symptoms in young healthy males during head-down bed rest. This multidimensional questionnaire includes sections on drowsiness, difficulty in concentration and physical disintegration [65].
Fatigue Severity Scale (FSS): Commonly used in sleep disorder studies, this scale measures the severity of fatigue and its impact on daily functioning. It has been used alongside other scales to differentiate between fatigue and sleepiness [66].
Modified Fatigue Impact Scale (MFIS): This scale assesses the impact of fatigue on physical, cognitive and psychosocial functioning. It has been used in studies involving traumatic brain injury to compare self-reported fatigue with informant reports [67].
Chalder Fatigue Scale (CH): A unidimensional scale used to measure the severity of fatigue. It has been compared with the Fatigue Impact Scale (FI) in studies involving chronic fatigue syndrome and other conditions, showing strong correlations [66].
Multidimensional Fatigue Symptom Inventory—Short Form (MFSI-SF): This 30-item inventory assesses the physical, mental and emotional dimensions of fatigue. It has been widely used and evaluated for its psychometric properties [68].
Cancer-Related Fatigue (CRF) Questionnaires: Various questionnaires exist to measure fatigue in cancer patients, including both one-dimensional and multidimensional scales. These have been reviewed for their psychometric properties and user-friendliness [69].
Rating of perceived exertion (RPE) [49]. RPE is a tool used to measure how physically demanding an activity is perceived by an individual. The “Borg scale”, a psychophysical, category scale with rating ranges from 6 (no exertion at all) to 20 (maximal exertion), is the most popular RPE tool (ACSM 2010). While the OMNI-RPE has demonstrated higher reliability and validity with pediatric groups, the Borg and CR10 scales have demonstrated reliability and validity in healthy, clinical and athletic adult populations [49,70,71].
Appendix B
Accuracy, Precision, Recall and F1
The evaluation metrics considered for the models are accuracy (ACC), precision, recall and F1 score (F1). Their definitions are listed below:
where
- TP (true positives): The number of correctly classified samples belonging to a certain class.
- FP (false positives): The number of samples misclassified as a certain class when they actually belong to other classes.
- TN (true negatives): The number of correctly classified samples in other classes.
- FN (false negatives): The number of samples belonging to a certain class that were misclassified as other classes.
Accuracy measures the proportion of correct classifications relative to the total. Precision indicates the percentage of true positives out of all positive predictions. Recall measures the model’s ability to correctly identify samples of a specific class. The F1 score is the harmonic mean of precision and recall, providing a balanced measure of the model’s performance. The average of these metrics across different classes is calculated to obtain an overall evaluation of a model’s performance.
Appendix C
Appendix C.1. HRV Features Identified for Physical Fatigue by Ni et al. [51]
The mean of the HR sequence (meanHR), number of successive differences in NN interval sequences greater than 50 ms (NN50), standard deviation of the averages of the segmented chunks (SDANN), ratio of total number of all intervals to the height of the histogram (HRVTi), baseline width of the minimum square difference triangular interpolation of the highest peak of the histogram (TINN), absolute powers of the VLF band (aVLF), negative natural logarithm of the conditional probability that two sequences remain similar at the next point (sampan), standard deviations along the major (SD1) and minor (SD2) axis of the ellipse, SD1/SD2, the slope of a fitting line of the root mean square fluctuation of an integrated and detrended time series on a log–log scale (α) and α on the first linear region (α1).
Appendix C.2. HRV Features Identified for Physical Fatigue by Ramos et al. [57]
The mean of the maximum, minimum and average RR intervals (the time between consecutive heartbeats). The Triangular Index is a statistical measure that represents the overall distribution of RR intervals; a higher Triangular Index indicates a greater HRV, meaning a more adaptive and healthy autonomic nervous system. A lower Triangular Index suggests a reduced HRV, which may be associated with stress, fatigue or cardiovascular dysfunction.
The Triangular Index is useful in long-term HRV recordings and provides a global assessment of HRV by taking into account the overall shape of the RR interval distribution; the standard deviation of NN intervals (SDNN); the root mean square of successive differences (rmsSD); SD1 and SD2 in the Poincarè Standard Deviation/Dispersion of points perpendicular (SD1) or along (SD2) the axis of the line-of-identity (ellipse semiaxes 1 and 2) as defined in the previous paragraph and in [25]; low-frequency band ([0.040; 0.150] Hz); and high-frequency band ([0.150; 0.400] Hz).
For more details and definition of HRV features, see [3,24,59,72].
References
- Tran, P.; Banerjee, P. Myocardial fatigue at a glance. Curr. Heart Fail. Rep. 2023, 20, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Tran, Y.; Guest, R.; Middleton, J.; Craig, A. Influence of neurological lesion level on heart rate variability and fatigue in adults with spinal cord injury. Spinal Cord 2016, 54, 292–297. [Google Scholar] [CrossRef]
- Berger, R.D. QT interval variability: Is it a measure of autonomic activity? JACC 2009, 54, 851–852. [Google Scholar] [CrossRef][Green Version]
- Rosenbaum, D.S.; Jackson, L.E.; Smith, J.M.; Garan, H.; Ruskin, J.N.; Cohen, R.J. Electrical alternans and vulnerability to ventricular arrhythmias. N. Engl. J. Med. 1994, 330, 235–241. [Google Scholar] [CrossRef]
- Oxborough, D.; Birch, K.; Shave, R.; George, K. Exercise-induced cardiac fatigue—A review of the echocardiographic literature. Echocardiography 2010, 27, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, L.; Meucci, M.; Bolletta, F.; Emerenziani, G.P.; Gallotta, M.C.; Baldari, C. Validity, reliability and minimum detectable change of COSMED K5 portable gas exchange system in breath-by-breath mode. PLoS ONE 2018, 13, e0209925. [Google Scholar] [CrossRef]
- Zimatore, G.; Serantoni, C.; Gallotta, M.C.; Guidetti, L.; Maulucci, G.; De Spirito, M. Automatic detection of aerobic threshold through recurrence quantification analysis of heart rate time series. Int. J. Environ. Res. Public Health 2023, 20, 1998. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, Z.; Al-Zaiti, S.S.; Bond, R.; Sejdić, E. Remote and Wearable ECG Devices with Diagnostic Abilities in Adults: A State-of-the-Science Scoping Review. Heart Rhythm 2022, 19, 1192–1201. [Google Scholar] [CrossRef]
- Veeramuthu, L.; Cho, C.J.; Liang, F.C.; Venkatesan, M.; Kumar, G.R.; Hsu, H.Y.; Kuo, C.C. Human skin-inspired electrospun patterned robust strain-insensitive pressure sensors and wearable flexible light-emitting diodes. ACS Appl. Mater. Interfaces 2022, 14, 30160–30173. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X. Perspectives in Wearable Systems in the Human-Robot Interaction (HRI) Field. Sensors 2023, 23, 8315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acharya, U.R.; Fujita, H.; Sudarshan, V.K.; Oh, S.L.; Adam, M.; Tan, J.H.; Koh, J.E.W. Application of deep convolutional neural network for automated detection of myocardial infarction using ECG signals. Inf. Sci. 2017, 415, 190–198. [Google Scholar] [CrossRef]
- Parry-Williams, G.; Sharma, S. The effects of endurance exercise on the heart: Panacea or poison? Nat. Rev. Cardiol. 2020, 17, 402–412. [Google Scholar] [CrossRef] [PubMed]
- George, K.; Whyte, G.P.; Green, D.J.; Oxborough, D.; Shave, R.E.; Gaze, D.; Somauroo, J. The endurance athletes heart: Acute stress and chronic adaptation. Br. J. Sports Med. 2012, 46 (Suppl. S1), 29–36. [Google Scholar] [CrossRef] [PubMed]
- La Gerche, A.; Burns, A.T.; Mooney, D.J.; Inder, W.J.; Taylor, A.J.; Bogaert, J.; MacIsaac, A.I.; Heidbüchel, H.; Prior, D.L. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur. Heart J. 2012, 33, 998–1006. [Google Scholar] [CrossRef]
- Neilan, T.G.; Yoerger, D.M.; Douglas, P.S.; Marshall, J.E.; Halpern, E.F.; Lawlor, D.; Picard, M.H.; Wood, M.J. Persistent and reversible cardiac dysfunction among amateur marathon runners. Eur. Heart J. 2006, 27, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.P.; Mahure, C.; Mungulmare, K.; Grewal, H.K.; Bansal, M. Myocardial fatigue in recreational marathon runners: A speckle-tracking echocardiography study. Indian Heart J. 2018, 70, S229–S234. [Google Scholar] [CrossRef]
- Wolff, S.; Picco, J.M.; Díaz-González, L.; Valenzuela, P.L.; Gonzalez-Dávila, E.; Santos-Lozano, A.; Matile, P.; Wolff, D.; Boraita, A.; Lucia, A. Exercise-induced cardiac fatigue in recreational ultramarathon runners at moderate altitude: Insights from myocardial deformation analysis. Front. Cardiovasc. Med. 2022, 8, 744393. [Google Scholar] [CrossRef]
- Burger, A.L.; Wegberger, C.; Tscharre, M.; Kaufmann, C.C.; Muthspiel, M.; Pogran, E.; Freynhofer, M.K.; Szalay, A.; Huber, K.; Jäger, B. Impact of an Ultra-Endurance Marathon on Cardiac Function in Association with Cardiovascular Biomarkers. Sports Med. Open 2024, 10, 67. [Google Scholar] [CrossRef]
- Palacios Le Blé, G.; Pedrero Chamizo, R.; Palacios Gil Antuñano, N.; Maroto Sanchez, B.; Aznar, S.; González Gross, M.M. Biomarkers of physical activity and exercise. Nutr. Hosp. 2015, 31, 237–244. [Google Scholar]
- Hughes, A.; Shandhi, M.M.H.; Master, H.; Dunn, J.; Brittain, E. Wearable devices in cardiovascular medicine. Circ. Res. 2023, 132, 652–670. [Google Scholar] [CrossRef]
- Fox, K.; Borer, J.S.; Camm, A.J.; Danchin, N.; Ferrari, R.; Lopez Sendon, J.L.; Steg, P.G.; Tardif, J.C.; Tavazzi, L.; Tendera, M.; et al. Resting heart rate in cardiovascular disease. J. Am. Coll. Cardiol. 2007, 50, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Amaral, L.A.N.; Hausdorff, J.M.; Ivanov, P.C.; Peng, C.K.; Stanley, H.E. Fractal dynamics in physiology: Alterations with disease and aging. Proc. Natl. Acad. Sci. USA 2002, 99 (Suppl. S1), 2466–2472. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Lai, Y.H.; Huang, P.H.; Hsiao, T.C. Use of sample entropy to assess sub-maximal physical load for avoiding exercise-induced cardiac fatigue. Appl. Sci. 2023, 13, 3813. [Google Scholar] [CrossRef]
- Pethick, J.; Winter, S.L.; Burnley, M. Fatigue reduces the complexity of knee extensor torque fluctuations during maximal and submaximal intermittent isometric contractions in man. J. Physiol. 2015, 593, 2085–2096. [Google Scholar] [CrossRef]
- Lewis, M.J.; Short, A.L. Exercise and cardiac regulation: What can electrocardiographic time series tell us? Scand. J. Med. Sci. Sports 2010, 20, 794–804. [Google Scholar] [CrossRef]
- Marwan, N.; Romano, M.C.; Thiel, M.; Kurths, J. Recurrence plots for the analysis of complex systems. Phys. Rep. 2007, 438, 237–329. [Google Scholar] [CrossRef]
- Marwan, N.; Wessel, N.; Meyerfeldt, U.; Schirdewan, A.; Kurths, J. Recurrence-plot-based measures of complexity and their application to heart-rate-variability data. Phys. Rev. E 2002, 66, 026702. [Google Scholar] [CrossRef]
- Zimatore, G.; Gallotta, M.C.; Campanella, M.; Skarzynski, P.H.; Maulucci, G.; Serantoni, C.; De Spirito, M.; Curzi, D.; Guidetti, L.; Baldari, C.; et al. Detecting metabolic thresholds from nonlinear analysis of heart rate time series: A review. Int. J. Environ. Res. Public Health 2022, 19, 12719. [Google Scholar] [CrossRef] [PubMed]
- Zimatore, G.; Serantoni, C.; Gallotta, M.C.; Meucci, M.; Mourot, L.; Ferrari, D.; Baldari, C.; De Spirito, M.; Maulucci, G.; Guidetti, L. Recurrence quantification analysis-based methodology in automatic aerobic threshold detection: Applicability and accuracy across age groups, exercise protocols and health conditions. Appl. Sci. 2024, 14, 9216. [Google Scholar] [CrossRef]
- Chen, S.W.; Liaw, J.W.; Chang, Y.J.; Chan, H.L.; Chiu, L.Y. A cycling movement based system for real-time muscle fatigue and cardiac stress monitoring and analysis. PLoS ONE 2015, 10, e0130798. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Rüdiger, H.; Ziemssen, T. Spectral analysis of heart rate variability: Time window matters. Front. Neurol. 2019, 10, 545. [Google Scholar] [CrossRef]
- Beckers, F.; Ramaekers, D.; Aubert, A.E. Approximate entropy of heart rate variability: Validation of methods and application in heart failure. Cardiovasc. Eng. 2001, 1, 177–182. [Google Scholar] [CrossRef]
- Srinivasa, M.; Pandian, P. Application of entropy techniques in analyzing heart rate variability using ECG signals. Int. J. Recent Innov. Trends Comput. Commun. 2019, 7, 9–16. [Google Scholar]
- Nasirzadeh, F.; Mir, M.; Hussain, S.; Tayarani Darbandy, M.; Khosravi, A.; Nahavandi, S.; Aisbett, B. Physical fatigue detection using entropy analysis of heart rate signals. Sustainability 2020, 12, 2714. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Nilanon, T.; Yao, J.; Hao, J.; Purushotham, S.; Liu, Y. Normal/abnormal heart sound recordings classification using convolutional neural network. In Proceedings of the 2016 Computing in Cardiology Conference (CinC), Vancouver, BC, Canada, 11–14 September 2016; pp. 585–588. [Google Scholar] [CrossRef]
- Sansone, M.; Fusco, R.; Pepino, A.; Sansone, C. Electrocardiogram pattern recognition and analysis based on artificial neural networks and support vector machines: A review. J. Healthc. Eng. 2013, 4, 465–504. [Google Scholar] [CrossRef]
- Mert, A.; Kilic, N.; Akan, A. ECG signal classification using ensemble decision tree. J. Trends Dev. Mach. Assoc. Technol. 2012, 16, 179–182. [Google Scholar]
- Beritelli, F.; Capizzi, G.; Lo Sciuto, G.; Napoli, C.; Scaglione, F. Automatic heart activity diagnosis based on Gram polynomials and probabilistic neural networks. Biomed. Eng. Lett. 2018, 8, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Beritelli, F.; Capizzi, G.; Sciuto, G.L.; Napoli, C.; Woźniak, M. A novel training method to preserve generalization of RBPNN classifiers applied to ECG signals diagnosis. Neural Netw. 2018, 108, 331–338. [Google Scholar] [CrossRef]
- Domingos, P. A few useful things to know about machine learning. Commun. ACM 2012, 55, 78–87. [Google Scholar] [CrossRef]
- Mousavi, S.S.; Schukat, M.; Howley, E. Deep reinforcement learning: An overview. In Proceedings of the SAI Intelligent Systems Conference (IntelliSys) 2016; Bi, Y., Kapoor, S., Bhatia, R., Eds.; Springer: Cham, Switzerland, 2018; pp. 535–544. [Google Scholar] [CrossRef]
- Echeverria, J.C.; Crowe, J.A.; Woolfson, M.S.; Hayes-Gill, B.R. Application of empirical mode decomposition to heart rate variability analysis. Med. Biol. Eng. Comput. 2001, 39, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, W.J. Biomedical Digital Signal Processing; PTR Prentice-Hall: Upper Saddle River, NJ, USA, 1993. [Google Scholar]
- Caffier, P.P.; Erdmann, U.; Ullsperger, P. Experimental evaluation of eye-blink parameters as a drowsiness measure. Eur. J. Appl. Physiol. 2003, 89, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C. Rating of perceived exertion (RPE). J. Physiother. 2012, 58, 62. [Google Scholar] [CrossRef]
- Melillo, P.; Izzo, R.; De Luca, N.; Pecchia, L. Automatic prediction of cardiovascular and cerebrovascular events using heart rate variability analysis. PLoS ONE 2015, 10, e0128031. [Google Scholar] [CrossRef]
- Ni, Z.; Sun, F.; Li, Y. Heart rate variability-based subjective physical fatigue assessment. Sensors 2022, 22, 3199. [Google Scholar] [CrossRef]
- Patel, M.; Lal, S.K.L.; Kavanagh, D.; Rossiter, P. Applying neural network analysis on heart rate variability data to assess driver fatigue. Expert Syst. Appl. 2011, 38, 7235–7242. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Xiao, K.; Ferraro, M.; Ushakov, N.; Kumar, S.; Ge, F.; Li, X.; Min, R. AI-enabled scalable smartphone photonic sensing system for remote healthcare monitoring. IEEE Internet Things J. 2024, 12, 4510–4524. [Google Scholar] [CrossRef]
- Zhao, T.; Fu, X.; Zhou, Y.; Zhan, J.; Chen, K.; Li, Z. Noncontact Monitoring of Heart Rate Variability Using a Fiber Optic Sensor. IEEE Internet Things J. 2023, 10, 14988–14994. [Google Scholar] [CrossRef]
- Luo, H.; Lee, P.A.; Clay, I.; Jaggi, M.; De Luca, V. Assessment of fatigue using wearable sensors: A pilot study. Digit. Biomark. 2020, 4 (Suppl. S1), 59–72. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Zadeh, M.Z.; Hebri, A.; Makedon, F. Assessing fatigue with multimodal wearable sensors and machine learning. arXiv 2022, arXiv:2205.00287. [Google Scholar]
- Ramos, G.; Vaz, J.R.; Mendonça, G.V.; Pezarat-Correia, P.; Rodrigues, J.; Alfaras, M.; Gamboa, H. Fatigue evaluation through machine learning and a global fatigue descriptor. J. Healthc. Eng. 2020, 2020, 6484129. [Google Scholar] [CrossRef]
- Kong, X.; Chen, Z.; Liu, W.; Ning, K.; Zhang, L.; Marier, S.M.; Liu, Y.; Chen, Y.; Xia, F. Deep learning for time series forecasting: A survey. Int. J. Mach. Learn. Cybern. 2025; in press. [Google Scholar] [CrossRef]
- Gan, L.; Yang, Z.; Shen, Y.; Cao, R.; Xia, Y.; Shi, Y.; Cao, B. Heart rate variability analysis method for exercise-induced fatigue monitoring. Biomed. Signal Process. Control 2024, 92, 105966. [Google Scholar] [CrossRef]
- Wang, F.; Chen, D.; Zhang, X. Real-time Driving Fatigue Detection of ECG Signals Acquired Based on Novel Electrodes Using Wavelet Scattering Networks. Measurement 2025, 243, 116438. [Google Scholar] [CrossRef]
- Tél, T.; Fülöp, Á.; Vicsek, T. Determination of fractal dimensions for geometrical multifractals. Phys. A 1989, 159, 155–166. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Watanabe, H.; Wang, T.-Y.; Ando, H.; Mizushina, H.; Morita, T.; Emoto, M.; Hatada, T.; Bando, T.; Ujike, H. Visually induced symptoms questionnaire (VISQ): A subjective evaluation method for biomedical effects induced by stereoscopic 3D video. Appl. Ergon. 2024, 117, 104238. [Google Scholar] [CrossRef]
- Nap-van der Vlist, M.M.; Vroegindeweij, A.; Hoefnagels, J.W.; van der Ent, C.K.; Swart, J.F.; van de Putte, E.M.; Nijhof, S.L. Paediatric short fatigue questionnaire, a 4-item fatigue questionnaire for children. J. Psychosom. Res. 2023, 165, 111130. [Google Scholar] [CrossRef] [PubMed]
- Hossain, J.L.; Ahmad, P.; Reinish, L.W.; Kayumov, L.; Hossain, N.K.; Shapiro, C.M. Subjective fatigue and subjective sleepiness: Two independent consequences of sleep disorders? J. Sleep Res. 2005, 14, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Naschitz, J.E.; Rozenbaum, M.; Shaviv, N.; Fields, M.C.; Enis, S.; Babich, J.P.; Manor, H.; Yeshurun, D.; Sabo, E. The feeling of fatigue—Fatigue severity by unidimensional versus composite questionnaires. Behav. Med. 2004, 29, 167–174. [Google Scholar] [CrossRef]
- Chiou, K.S.; Chiaravalloti, N.D.; Wylie, G.R.; DeLuca, J.; Genova, H.M. Awareness of subjective fatigue after moderate to severe traumatic brain injury. J. Head Trauma Rehabil. 2016, 31, E60–E68. [Google Scholar] [CrossRef]
- Donovan, K.A.; Stein, K.D.; Lee, M.; Leach, C.R.; Ilozumba, O.; Jacobsen, P.B. Systematic review of the multidimensional fatigue symptom inventory-short form. Support. Care Cancer 2015, 23, 191–212. [Google Scholar] [CrossRef]
- Agasi-Idenburg, C.; Velthuis, M.; Wittink, H. Quality criteria and user-friendliness in self-reported questionnaires on cancer-related fatigue: A review. J. Clin. Epidemiol. 2010, 63, 705–711. [Google Scholar] [CrossRef]
- Roemmich, J.N.; Barkley, J.E.; Epstein, L.H.; Lobarinas, C.L.; White, T.M.; Foster, J.H. Validity of PCERT and OMNI walk/run ratings of perceived exertion. Med. Sci. Sports Exerc. 2006, 38, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, M.C.; Bonavolontà, V.; Zimatore, G.; Iazzoni, S.; Guidetti, L.; Baldari, C. Effects of open (racket) and closed (running) skill sports practice on children’s attentional performance. Open Sports Sci. J. 2020, 13, 105–113. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).