Gluten-Free Sourdough Based on Quinoa and Sorghum: Characterization and Applications in Breadmaking
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proximate Composition
2.3. Sourdough Preparation
2.4. Sourdough Characterization
2.5. Lactic Acid Bacteria Enumeration
2.6. Thermo-Mechanical Properties of the Dough
2.7. Bread Preparation
2.8. Bread Characterization
2.9. Statistical Analysis
3. Results
3.1. Proximate Composition of the Gluten-Free Flours
3.2. Sourdough Properties
3.3. Thermo-Mechanical Behavior of the Gluten-Free Dough
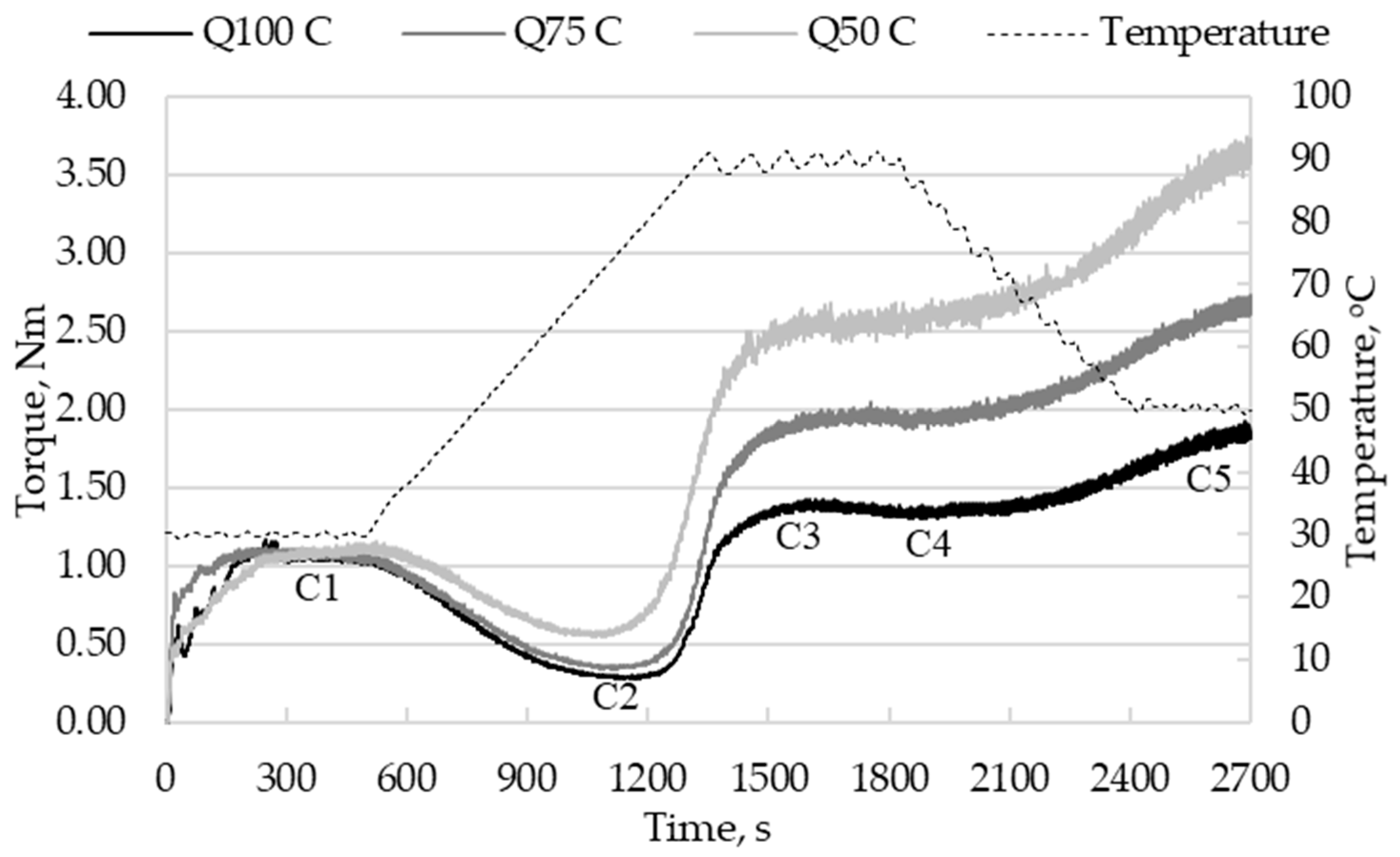
3.3.1. Effect of Sorghum Flour Addition on Thermo-Mechanical Properties of Quinoa Flour
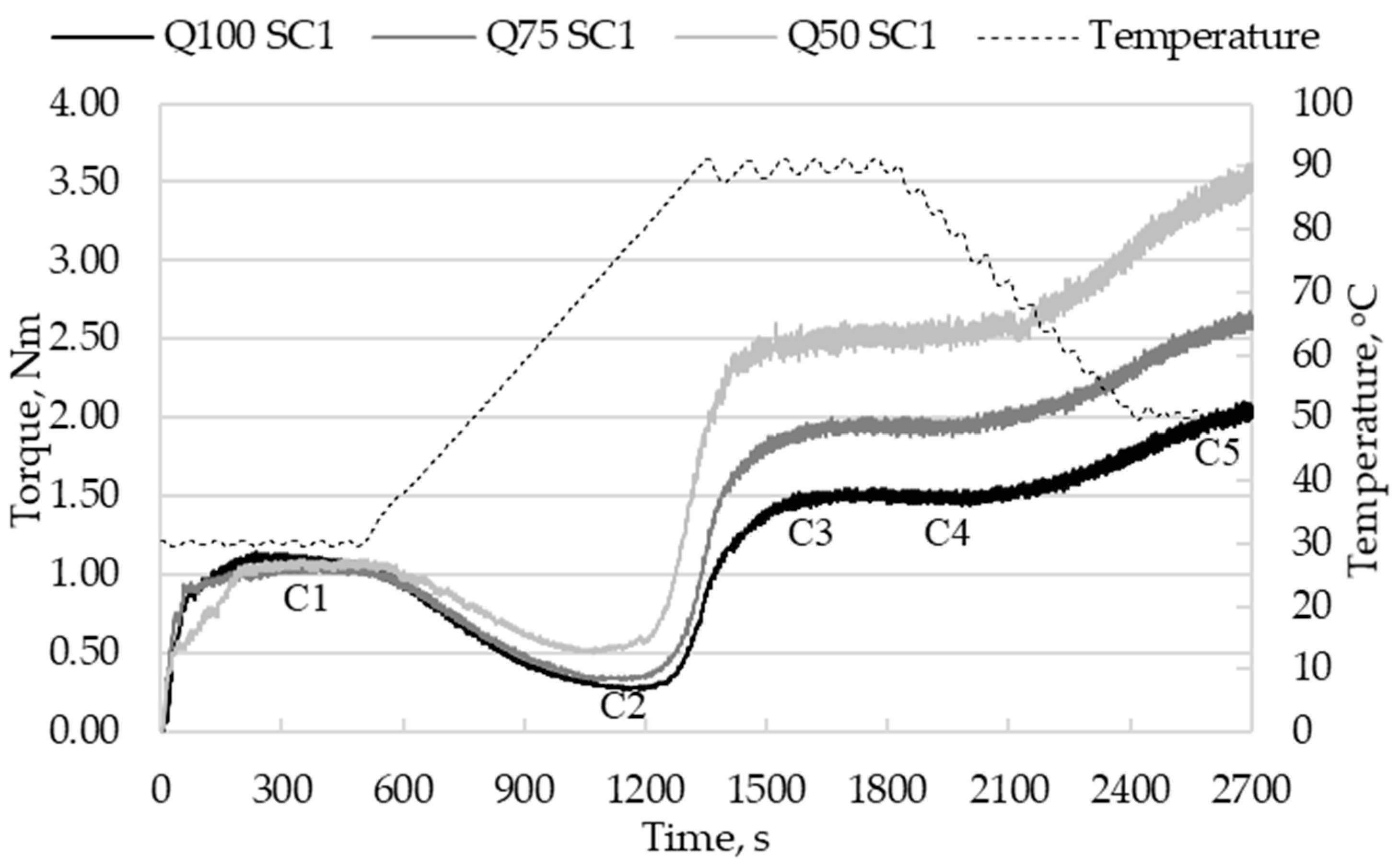
3.3.2. Effect of Sourdough Addition on Thermo-Mechanical Properties of Dough
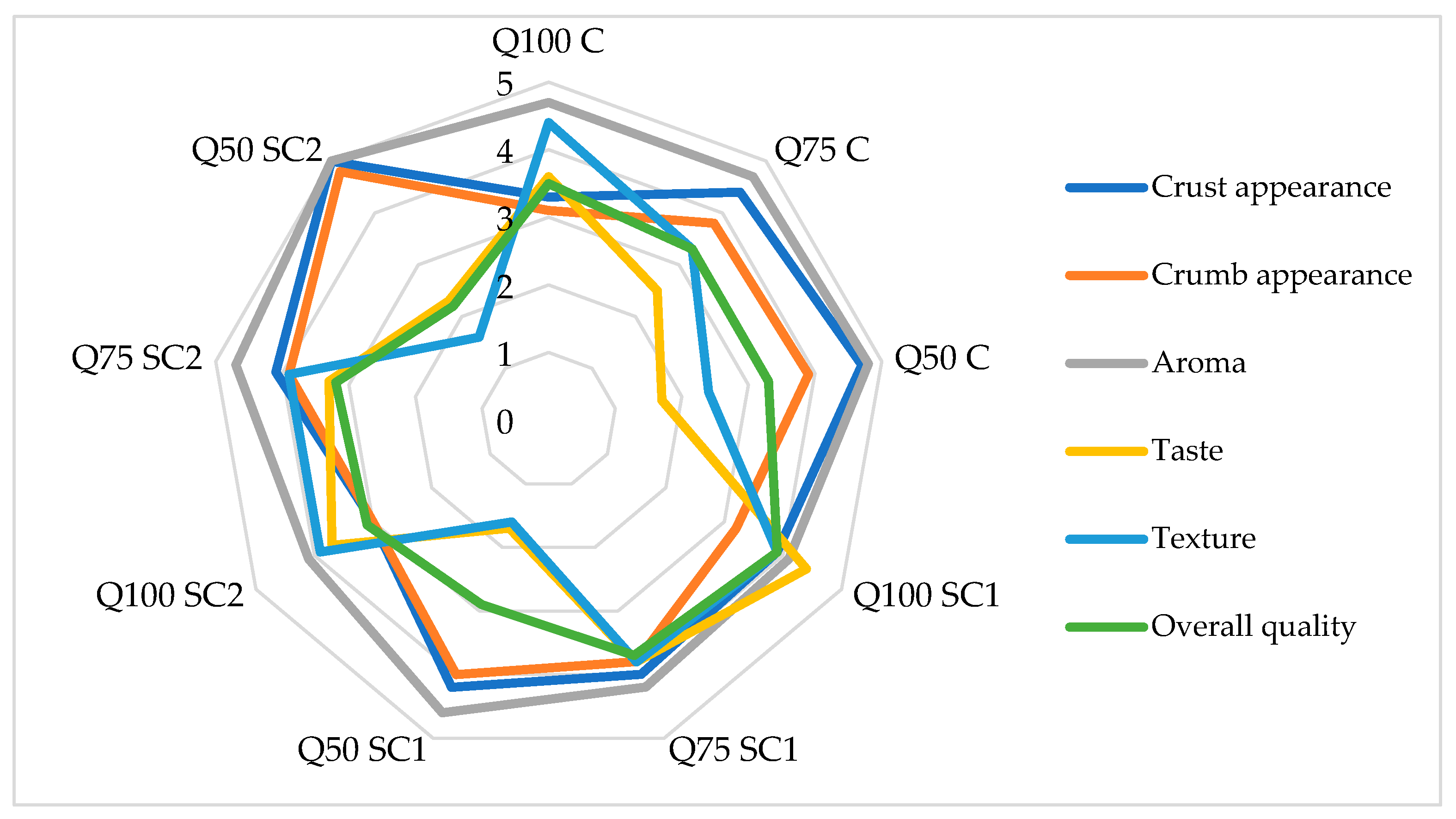
3.4. Bread Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matos, M.E.; Rosell, C.M. Understanding gluten-free dough for reaching breads with physical quality and nutritional balance. J. Sci. Food Agric. 2015, 95, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.S.; Farage, P.; Zandonadi, R.P.; Botelho, R.B.; de Oliveira, L.D.L.; Raposo, A.; Shakeel, F.; Alshehri, S.; Mahdi, W.A.; Araújo, W.M. A systematic review on gluten-free bread formulations using specific volume as a quality indicator. Foods 2021, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Demirkesen, I.; Kelkar, S.; Campanella, O.H.; Sumnu, G.; Sahin, S.; Okos, M. Characterization of structure of gluten-free breads by using X-ray microtomography. Food Hydrocoll. 2014, 36, 37–44. [Google Scholar] [CrossRef]
- Wang, K.; Lu, F.; Li, Z.; Zhao, L.; Han, C. Recent developments in gluten-free bread baking approaches: A review. Food Sci. Technol. 2017, 37 (Suppl. S1), 1–9. [Google Scholar] [CrossRef]
- Coronel, E.B.; Ixtaina, V.Y.; Capitani, M.I. Nutritional and Functional Characterization of Chia Expeller and Gluten-Free Flours as Ingredients for Premixes. Plant Foods Hum. Nutr. 2025, 80, 1–8. [Google Scholar]
- Khairuddin, M.A.N.; Lasekan, O. Gluten-free cereal products and beverages: A review of their health benefits in the last five years. Foods 2021, 10, 2523. [Google Scholar] [CrossRef]
- Boukid, F.; Folloni, S.; Sforza, S.; Vittadini, E.; Prandi, B. Current trends in ancient grains-based foodstuffs: Insights into nutritional aspects and technological applications. Compr. Rev. Food Sci. F. 2018, 17, 123–136. [Google Scholar] [CrossRef]
- Păucean, A.; Man, S.M.; Chiş, M.S.; Mureşan, V.; Pop, C.R.; Socaci, S.A.; Muresan, C.C.; Muste, S. Use of pseudocereals preferment made with aromatic yeast strains for enhancing wheat bread quality. Foods 2019, 8, 443. [Google Scholar] [CrossRef]
- Gewehr, M.F.; Pagno, C.H.; Danelli, D.; Melo, L.M.D.; Flôres, S.H.; Jong, E.V.D. Evaluation of the functionality of bread loaves prepared with quinoa flakes through biological tests. Braz. J. Pharm. Sci. 2016, 52, 337–346. [Google Scholar] [CrossRef]
- El-Sohaimy, S.A.; Shehata, M.G.; Mehany, T.; Zeitoun, M.A. Nutritional, physicochemical, and sensorial evaluation of flat bread supplemented with quinoa flour. Int. J. Food Sci. 2019, 2019, 4686727. [Google Scholar] [CrossRef]
- Lamothe, L.M.; Srichuwong, S.; Reuhs, B.L.; Hamaker, B.R. Quinoa (Chenopodium quinoa W.) and amaranth (Amaranthus caudatus L.) provide dietary fibres high in pectic substances and xyloglucans. Food Chem. 2015, 167, 490–496. [Google Scholar] [CrossRef]
- Turkut, G.M.; Cakmak, H.; Kumcuoglu, S.; Tavman, S. Effect of quinoa flour on gluten-free bread batter rheology and bread quality. J. Cereal Sci. 2016, 69, 174–181. [Google Scholar] [CrossRef]
- Onyango, C.; Mutungi, C.; Unbehend, G.; Lindhauer, M.G. Modification of gluten-free sorghum batter and bread using maize, potato, cassava or rice starch. LWT-Food Sci. Technol. 2011, 44, 681–686. [Google Scholar] [CrossRef]
- Campo, E.; Del Arco, L.; Urtasun, L.; Oria, R.; Ferrer-Mairal, A. Impact of sourdough on sensory properties and consumers’ preference of gluten-free breads enriched with teff flour. J. Cereal Sci. 2016, 67, 75–82. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Weckx, S.; Van Kerrebroeck, S.; De Vuyst, L. Omics approaches to understand sourdough fermentation processes. Int. J. Food Microbiol. 2019, 302, 90–102. [Google Scholar] [CrossRef]
- Zhang, G.; Tu, J.; Sadiq, F.A.; Zhang, W.; Wang, W. Prevalence, Genetic Diversity, and Technological Functions of the Lactobacillus sanfranciscensis in Sourdough: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1209–1226. [Google Scholar] [CrossRef]
- Gänzle, M.; Ripari, V. Composition and function of sourdough microbiota: From ecological theory to bread quality. Int. J. Food Microbiol. 2016, 239, 19–25. [Google Scholar] [CrossRef]
- De Vuyst, L.; Comasio, A.; Kerrebroeck, S.V. Sourdough production: Fermentation strategies, microbial ecology, and use of non-flour ingredients. Crit. Rev. Food Sci. Nutr. 2023, 63, 2447–2479. [Google Scholar] [CrossRef]
- Hansen, A. Sourdough Bread; Taylor and Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar]
- De Vuyst, L.; Vancanneyt, M. Biodiversity and identification of sourdough lactic acid bacteria. Food Microbiol. 2007, 24, 120–127. [Google Scholar] [CrossRef]
- Fernández-Peláez, J.; Paesani, C.; Gómez, M. Sourdough technology as a tool for the development of healthier grain-based products: An update. Agronomy 2020, 10, 1962. [Google Scholar] [CrossRef]
- Torrieri, E.; Pepe, O.; Ventorino, V.; Masi, P.; Cavella, S. Effect of sourdough at different concentrations on quality and shelf life of bread. LWT-Food Sci. Technol. 2014, 56, 508–516. [Google Scholar] [CrossRef]
- ASRO. Romanian Standards Catalog for Cereal and Milling Products Analysis; SR ISO 712:2005, SR 90:2007, SR 91:2007 and SR ISO 2171/2002 and SR EN ISO 20483:2007; ASRO: Bucharest, Romania, 2008. [Google Scholar]
- Banu, I.; Aprodu, I. Investigations on functional and thermo-mechanical properties of gluten free cereal and pseudocereal flours. Foods 2022, 11, 1857. [Google Scholar] [CrossRef] [PubMed]
- Banu, I.; Vasilean, I.; Aprodu, I. Effect of lactic fermentation on antioxidant capacity of rye sourdough and bread. Food Sci. Technol. Res. 2010, 16, 571–576. [Google Scholar] [CrossRef]
- ISO 15214:1998; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 Degrees C. International Organization for Standardization: Genève, Switzerland, 1998.
- Savitri, M.; Kumar, V.; Kumari, A.; Angmo, K.; Bhalla, T.C. Isolation and characterization of lactic acid bacteria from traditional pickles of Himachal Pradesh, India. J. Food. Sci. Technol. 2017, 54, 1945–1952. [Google Scholar]
- Dubat, A.; Boinot, N. Mixolab Applications Handbook. Rheological and Enzymes Analyses; Chopin Technology: Villenueve, France, 2012; pp. 14–126. [Google Scholar]
- Martinez, O.D.M.; Toledo, R.C.L.; Queiroz, V.A.V.; Pirozi, M.R.; Martino, H.S.D.; de Barros, F.A.R. Mixed sorghum and quinoa flour improves protein quality and increases antioxidant capacity in vivo. LWT-Food Sci. Technol. 2020, 129, 109597. [Google Scholar] [CrossRef]
- Schoenlechner, R.; Bender, D.; D’Amico, S.; Kinner, M.; Tomoskozi, S.; Yamsaengsung, R. Dry Fractionation and Gluten-Free Sourdough Bread Baking from Quinoa and Sorghum. Foods 2023, 12, 3125. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Gobbetti, M. Use of sourdough made with quinoa (Chenopodium quinoa) flour and autochthonous selected lactic acid bacteria for enhancing the nutritional, textural and sensory features of white bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Gaide, I.; Basinskiene, L. Effect of lactic acid fermentation on quinoa characteristics and quality of quinoa-wheat composite bread. Foods 2021, 10, 171. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Rezaïki, L.; Cesselin, B.; Yamamoto, Y.; Vido, K.; Van West, E.; Gaudu, P.; Gruss, A. Respiration metabolism reduces oxidative and acid stress to improve long-term survival of Lactococcus lactis. Mol. Microbiol. 2004, 53, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Vera, A.; Rigobello, V.; Demarigny, Y. Comparative study of culture media used for sourdough lactobacilli. Food Microbiol. 2009, 26, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, R.; Panghal, A.; Chaudhary, G.; Kumari, A.; Chhikara, N. Nutritional, phytochemical and functional potential of sorghum: A review. Food Chem. Adv. 2023, 3, 100501. [Google Scholar] [CrossRef]
- Banu, I.; Vasilean, I.; Aprodu, I. Effect of select parameters of the sourdough rye fermentation on the activity of some mixed starter cultures. Food Biotechnol. 2011, 25, 275–291. [Google Scholar] [CrossRef]
- Aslankoohi, E.; Rezaei, M.N.; Vervoort, Y.; Courtin, C.M.; Verstrepen, K.J. Glycerol production by fermenting yeast cells is essential for optimal bread dough fermentation. PLoS ONE 2015, 10, 0119364. [Google Scholar] [CrossRef]
- Banu, I.; Aprodu, I. Studies concerning the use of Lactobacillus helveticus and Kluyveromyces marxianus for rye sourdough fermentation. Eur. Food Res. Technol. 2012, 234, 769–777. [Google Scholar] [CrossRef]
- Banu, I.; Aprodu, I. Assessing the performance of different grains in gluten-free bread applications. Appl. Sci. 2020, 10, 8772. [Google Scholar] [CrossRef]
- Apostol, L.; Belc, B.; Gaceu, L.; Oprea, O.B.; Popa, M.E. Sorghum Flour: A Valuable Ingredient for Bakery Industry? Appl. Sci. 2020, 10, 8597. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Bugusu, B.A. Overview: Sorghum Proteins and Food Quality. In Proceedings of the Workshop on the Proteins of Sorghum and Millets: Enhancing Nutritional and Functional Properties for Africa, Pretoria, South Africa, 2–4 April 2003. [Google Scholar]
- Yousif, A.; Nhepera, D.; Johnson, S. Influence of sorghum flour addition on flat bread in vitro starch digestibility, antioxidant capacity and consumer acceptability. Food Chem. 2012, 134, 880–887. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; O’Mahony, J.A. Composition, Protein Profile and Rheological Properties of Pseudocereal-Based Protein-Rich Ingredients. Foods 2018, 7, 73. [Google Scholar] [CrossRef]
- Ognean, C.-F. Technological and sensorial effects of sorghum addition at wheat bread. Agric. Food 2015, 3, 209–217. [Google Scholar]
- Torbica, A.; Hadnadev, M.; Dapcevic, T. Rheological, textural and sensory properties of gluten-free bread formulations based on rice and buckwheat flour. Food Hydrocol. 2010, 24, 626–632. [Google Scholar] [CrossRef]
- Nikolic, N.; Sakac, M.; Mastilovic, J. Effect of buckwheat flour addition to wheat flour on acylglycerols and fatty acids composition and rheology properties. LWT-Food Sci. Technol. 2011, 44, 650–655. [Google Scholar] [CrossRef]
- Collar, C.; Angioloni, A. Pseudocereals and teff in complex breadmaking matrices: Impact on lipid dynamics. J. Cereal Sci. 2014, 59, 145–154. [Google Scholar] [CrossRef]
- Przybylski, R.; Chauhan, G.S.; Eskin, N.A.M. Characterization of quinoa (Chenopodium quinoa) lipids. Food Chem. 1994, 51, 187–192. [Google Scholar] [CrossRef]
- Ragaee, S.; Abdel-Aal, E.-S.M.; Noaman, M. Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 2006, 98, 32–38. [Google Scholar] [CrossRef]
- Vargas-Solorzano, J.W.; Carvalho, C.W.P.; Takeiti, C.Y.; Ascheri, J.L.R.; Queiroz, V.A.V. Physicochemical properties of expanded extrudates from colored sorghum genotypes. Food Res. Int. 2014, 55, 37–44. [Google Scholar] [CrossRef]
- Nandini, C.D.; Salimath, P.V. Structural features of arabinoxylans from sorghum having good rotimaking quality. Food Chem. 2001, 74, 417–422. [Google Scholar] [CrossRef]
- Li, G.; Zhu, F. Physicochemical properties of quinoa flour as affected by starch interactions. Food Chem. 2017, 221, 1560–1568. [Google Scholar] [CrossRef]
- Ahmed, J.; Thomas, L.; Ali Arfat, Y. Functional, rheological, microstructural and antioxidant properties of quinoa flour in dispersions as influenced by particle size. Food Res. Int. 2019, 116, 302–311. [Google Scholar] [CrossRef]
- Taylor, J.R.N.; Schober, T.J.; Bean, S.R. Novel food and non-food uses for sorghum and millets. J. Cereal Sci. 2006, 44, 252–271. [Google Scholar] [CrossRef]
- Marston, K.; Khouryieh, H.; Aramouni, F. Effect of heat treatment of sorghum flour on the functional properties of gluten-free bread and cake. LWT-Food Sci. Technol. 2016, 65, 637–644. [Google Scholar] [CrossRef]
- Aprodu, I.; Banu, I. Effect of starch and dairy proteins on the gluten free bread formulation based on quinoa. J. Food Meas. Charact. 2021, 15, 2264–2274. [Google Scholar] [CrossRef]
- Rodriguez-Sandoval, E.; Sandoval, G.; Cortes-Rodriguez, M. Effect of quinoa and potato flours on the thermomechanical and breadmaking properties of wheat flour. Braz. J. Chem. Eng. 2012, 29, 503–510. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.; Wu, H.; Zhao, W.; Zhang, A.; Li, P.; Liu, J.; Yi, H. Insights into the starch and proteins molecular structure changes of foxtail millet sourdough: Effect of fermentation from grains of cereal to pre-meal. Int. J. Biol. Macromol. 2024, 272, 132729. [Google Scholar] [CrossRef]
- Valerio, F.; Bavaro, A.R.; Di Biase, M.; Lonigro, S.L.; Logrieco, A.F.; Lavermicocca, P. Effect of Amaranth and Quinoa Flours on Exopolysaccharide Production and Protein Profile of Liquid Sourdough Fermented by Weissella cibaria and Lactobacillus plantarum. Front. Microbiol. 2020, 11, 967. [Google Scholar] [CrossRef]
- Sekwati-Monang, B.; Valcheva, R.; Ganzle, M.G. Microbial ecology of sorghum sourdoughs: Effect of substrate supply and phenolic compounds on composition of fermentation microbiota. Int. J. Biol. Macromol. 2012, 159, 240–246. [Google Scholar] [CrossRef]
- Besbes, E.; Jury, V.; Monteau, J.Y.; Le Bail, A. Effect of baking conditions and storage with crust on the moisture profile, local textural properties and staling kinetics of pan bread. LWT-Food Sci. Technol. 2014, 58, 658–666. [Google Scholar] [CrossRef]
- Elgeti, D.; Nordlohne, S.D.; Föste, M.; Besl, M.; Linden, M.H.; Heinz, V.; Jekle, M.; Becker, T. Volume and texture improvement of gluten-free bread using quinoa white flour. J. Cereal Sci. 2014, 59, 41–47. [Google Scholar] [CrossRef]


| Quinoa Flour | Sorghum Flour | |
|---|---|---|
| Moisture, g/100 g | 11.19 ± 0.08 b | 11.71 ± 0.04 a |
| Protein, g/100 g d.w. | 15.83 ± 0.09 a | 9.50 ± 0.04 b |
| Fats, g/100 g d.w. | 6.58 ± 0.13 a | 3.38 ± 0.05 b |
| Fibers, g/100 g d.w. | 6.18 ± 0.08 b | 7.19 ± 0.28 a |
| Ash, g/100 g d.w. | 2.29 ± 0.08 a | 1.89 ± 0.10 b |
| Starter Culture | Q100 | Q75 | Q50 |
|---|---|---|---|
| <10 | <10 | <10 | |
| SC1 | (7.75 ± 0.35) × 108 a,B | (1.16 ± 0.04) × 109 a,A | (8.80 ± 0.14) × 108 a,B |
| SC2 | (1.78 ± 0.07) × 108 b,B | (2.51 ± 0.15) × 108 b,A | (1.45 ± 0.04) × 108 b,B |
| Sample | pH | Total Titratable Acidity | Lactic Acid, mg/100 g d.w | Acetic Acid, mg/100 g d.w | Glycerol, mg/100 g d.w | Ethanol, mg/100 g d.w |
|---|---|---|---|---|---|---|
| Q100 C | 5.898 ± 0.001 a,A | 6.41 ± 0.02 c,C | 1490.11 ± 38.31 b,C | 103.24 ± 4.05 a,A | 58.63 ± 1.65 b,B | 64.62 ± 3.06 a,A |
| Q75 C | 5.750 ± 0.006 b,A | 7.21 ± 0.02 b,C | 2330.04 ± 106.28 a,C | 114.25 ± 4.74 a,A | 95.54 ± 3.01 a,C | 52.87 ± 0.58 b,A |
| Q50 C | 5.756 ± 0.001 b,A | 7.41 ± 0.02 a,C | 1518.93 ± 26.21 b,C | 114.60 ± 4.52 a,A | 96.42 ± 3.15 a,B | 51.24 ± 1.88 b,A |
| Q100 SC1 | 4.454 ± 0.001 a,C | 9.74 ± 0.02 c,A | 2544.93 ± 37.74 b,B | 7.14 ± 0.04 c,C | 70.74 ± 1.80 b,A | 19.99 ± 1.02 a,B |
| Q75 SC1 | 4.439 ± 0.003 a,C | 10.99 ± 0.08 b,A | 3551.99 ± 26.01 a,B | 31.75 ± 1.43 b,C | 139.36 ± 1.13 a,A | 18.53 ± 0.66 a,B |
| Q50 SC1 | 4.408 ± 0.006 b,C | 11.57 ± 0.01 a,A | 2494.96 ± 23.81 b,A | 51.41 ± 2.59 a,C | 132.97 ± 3.90 a,A | 17.41 ± 0.57 a,B |
| Q100 SC2 | 4.507 ± 0.001 a,B | 8.94 ± 0.03 c,B | 3296.02 ± 51.16 b,A | 20.13 ± 1.39 b,B | 64.83 ± 2.73 c,A,B | 11.51 ± 0.47 b,C |
| Q75 SC2 | 4.514 ± 0.006 a,B | 9.23 ± 0.05 b,B | 4103.61 ± 60.39 a,A | 73.03 ± 2.87 a,B | 111.85 ± 2.80 b,B | 16.93 ± 0.85 a,B |
| Q50 SC2 | 4.510 ± 0.004 a,B | 9.65 ± 0.03 a,B | 2259.84 ± 11.98 c,B | 73.32 ± 3.36 a,B | 130.29 ± 3.13 a,A | 16.28 ± 0.13 a,B |
| Samples | Sourdough | Bread | ||||
|---|---|---|---|---|---|---|
| TPC, mg Ferulic acid/g d.w. | DPPH-RSA, µmols TE/g d.w. | ABTS-RSA, µmols TE/g d.w. | TPC, mg Ferulic acid/g d.w. | DPPH-RSA, µmols TE/ g d.w. | ABTS-RSA, µmols TE/g d.w. | |
| Q100 C | 3.83 ± 0.00 a,C | 247.24 ± 4.47 a,C | 320.93 ± 9.07 b,A | 2.61 ± 0.05 b,B | 212.97 ± 9.69 a,B | 165.79 ± 8.18 b,B |
| Q75 C | 3.79 ± 0.03 a,C | 254.95 ± 1.08 a,B | 338.36 ± 17.55 b,B | 2.83 ± 0.01 a,A | 241.69 ± 11.75 a,B | 216.75 ± 0.00 a,A |
| Q50 C | 3.02 ± 0.06 b,C | 233.20 ± 2.12 b,B | 402.53 ± 12.93 a,A | 2.60 ± 0.02 b,B | 244.49 ± 8.95 a,B | 226.57 ± 10.11 a,A |
| Q100 SC1 | 4.77 ± 0.01 a,B | 273.89 ± 1.08 a,A | 306.28 ± 17.49 c,A | 2.94 ± 0.05 a,b,A | 246.50 ± 12.65 b,A,B | 216.46 ± 8.98 b,A |
| Q75 SC1 | 4.35 ± 0.05 b,B | 267.39 ± 2.13 b,A | 359.87 ± 6.49 b,A,B | 3.05 ± 0.09 a,A | 267.83 ± 10.79 a,bA,B | 252.15 ± 2.04 a,A |
| Q50 SC1 | 3.72 ± 0.04 c,A | 254.94 ± 1.04 c,A | 411.07 ± 4.21 a,A | 2.78 ± 0.01 b,A | 312.37 ± 14.54 a,A | 250.74 ± 2.03 a,A |
| Q100 SC2 | 4.88 ± 0.02 a,A | 258.72 ± 2.11 a,B | 319.87 ± 6.43 b,A | 2.45 ± 0.02 c,B | 265.41 ± 10.17 a,A | 194.16 ± 8.89 b,A,B |
| Q75 SC2 | 4.53 ± 0.00 b,A | 267.62 ± 3.27 a,A | 422.13 ± 33.10 a,A | 2.87 ± 0.05 a,A | 301.40 ± 8.41 a,A | 230.84 ± 9.80 a,b,A |
| Q50 SC2 | 3.43 ± 0.01 c,B | 221.99 ± 4.29 b,B | 389.81 ± 10.89 a,A | 2.70 ± 0.03 b,A | 290.90 ± 8.65 a,A | 258.77 ± 12.10 a,A |
| Dough Sample | WA, % | C1, Nm | tC1, min | C8, Nm | A, Nm | S, min | S8, min |
|---|---|---|---|---|---|---|---|
| Q100 | 56.9 | 1.06 ± 0.01 b,C | 4.33 ± 0.04 c,D | 1.01 ± 0.02 b,B | 0.042 ± 0.003 b,B | 9.1 ± 0.02 a,A | 5.9 ± 0.02 c,A |
| Q75 | 52.0 | 1.02 ± 0.01 c,B | 4.52 ± 0.03 b,B | 0.96 ± 0.01 c,B | 0.047 ± 0.003 b,C | 9.1 ± 0.02 a,A | 6.2 ± 0.02 b,D |
| Q50 | 51.0 | 1.10 ± 0.01 a,B | 8.18 ± 0.05 a,A | 1.10 ± 0.02 a,A,B | 0.056 ± 0.002 a,B | 7.8 ± 0.03 b,B | 7.1 ± 0.02 a,C |
| Q100 C | 56.9 | 1.14 ± 0.02 a,A,B | 4.48 ± 0.03 b,C | 1.04 ± 0.02 b,A,B | 0.054 ± 0.003 c,A | 6.4 ± 0.02 c,D | 5.9 ± 0.02 c,A |
| Q75 C | 52.0 | 1.09 ± 0.02 b,A | 4.53 ± 0.04 b,B | 1.05 ± 0.01 b,A | 0.061 ± 0.002 b,A | 8.7 ± 0.02 b,C | 6.5 ± 0.02 b,B |
| Q50 C | 51.0 | 1.13 ± 0.01 a,A | 7.08 ± 0.03 a,C | 1.11 ± 0.02 a,A | 0.174 ± 0.002 a,A | 9.3 ± 0.03 a,A | 7.0 ± 0.02 a,D |
| Q100 SC1 | 56.9 | 1.11 ± 0.01 a,B | 4.87 ± 0.04 b,A | 1.06 ± 0.02 a,b,A | 0.039 ± 0.002 b,B | 7.5 ± 0.03 c,B | 5.9 ± 0.02 c,A |
| Q75 SC1 | 52.0 | 1.05 ± 0.01 b,B | 4.93 ± 0.04 b,A | 1.03 ± 0.02 b,A | 0.053 ± 0.002 a,B | 8.9 ± 0.02 b,B | 6.6 ± 0.02 b,A |
| Q50 SC1 | 51.0 | 1.08 ± 0.02 a,B | 8.08 ± 0.05 a,A | 1.07 ± 0.02 a,B | 0.041 ± 0.002 b,C | 9.3 ± 0.02 a,A | 8.2 ± 0.02 a,B |
| Q100 SC2 | 56.9 | 1.16 ± 0.02 a,A | 4.73 ± 0.04 c,B | 1.08 ± 0.02 a,A | 0.057 ± 0.002 a,A | 7.0 ± 0.02 c,C | 5.8 ± 0.02 c,B |
| Q75 SC2 | 52.0 | 1.11 ± 0.01 b,A | 4.98 ± 0.04 b,A | 1.05 ± 0.0 2a,A | 0.056 ± 0.002 a,B | 7.5 ± 0.02 b,D | 6.3 ± 0.02 b,C |
| Q50 SC2 | 51.0 | 1.08 ± 0.02 b,B | 7.68 ± 0.05 a,B | 1.07 ± 0.02 a,A,B | 0.043 ± 0.002 b,C | 9.3 ± 0.02 a,A | 8.7 ± 0.03 a,A |
| Dough Sample | C2, Nm | tC2, min | TC2, °C | C1-C2, Nm |
|---|---|---|---|---|
| Q100 | 0.28 ± 0.02 b,A | 18.85 ± 0.05 a,C | 58.4 ± 0.06 a,C | 0.78 ± 0.03 a,C |
| Q75 | 0.30 ± 0.02 b,B | 17.98 ± 0.04 b,C | 56.7 ± 0.06 b,C | 0.72 ± 0.03 b,B |
| Q50 | 0.52 ± 0.02 a,B | 16.87 ± 0.05 c,B | 57.2 ± 0.06 c,C | 0.58 ± 0.01 c,A |
| Q100 C | 0.29 ± 0.02 c,A | 18.92 ± 0.04 a,B,C | 59.8 ± 0.06 a,B | 0.84 ± 0.01 a,A,B |
| Q75 C | 0.35 ± 0.02 b,A | 18.62 ± 0.05 b,A | 59.3 ±0.06 b,A | 0.74 ± 0.03 b,B |
| Q50 C | 0.56 ± 0.02 a,A | 17.67 ± 0.04 c,A | 57.5 ± 0.06 c,B | 0.57 ± 0.01 c,A |
| Q100 SC1 | 0.28 ± 0.02 c,A | 19.32 ± 0.04 a,A | 61.5 ± 0.06 a,A | 0.83 ± 0.01 a,B |
| Q75 SC1 | 0.34 ± 0.02 b,A | 18.48 ± 0.04 b,B | 58.7 ± 0.06 b,B | 0.71 ± 0.01 b,B |
| Q50 SC1 | 0.52 ± 0.02 a,B | 17.70 ± 0.04 c,A | 57.6 ± 0.06 c,A | 0.57 ± 0.03 c,A |
| Q100 SC2 | 0.29 ± 0.02 c,A | 19.00 ± 0.04 a,B | 61.6 ± 0.06 a,A | 0.87 ± 0.00 a,A |
| Q75 SC2 | 0.35 ± 0.02 b,A | 18.43 ± 0.05 b,B | 59.3 ± 0.06 b,A | 0.76 ± 0.01 b,A |
| Q50 SC2 | 0.52 ± 0.02 a,A,B | 17.72 ± 0.04 c,A | 55.6 ± 0.06 c,D | 0.56 ± 0.04 c,A |
| Dough Sample | C3, Nm | tC3, min | TC3, °C | C3-C2, Nm |
|---|---|---|---|---|
| Q100 | 1.48 ± 0.02 c,B | 28.25 ± 0.04 a,A | 83.6 ± 0.06 b,A | 1.20 ± 0.04 c,A,B |
| Q75 | 1.87 ± 0.02 b,B | 27.53 ± 0.04 b,A | 84.2 ± 0.06 a,B | 1.56 ± 0.03 b,A |
| Q50 | 2.52 ± 0.02 a,B | 25.25 ± 0.04 c,C | 84.1 ± 0.06 a,C | 2.00 ± 0.00 a,B |
| Q100 C | 1.39 ± 0.01 c,C | 26.68 ± 0.05 b,D | 82.4 ± 0.06 c,C | 1.10 ± 0.03 c,C |
| Q75 C | 1.98 ± 0.01 b,A | 27.07 ± 0.05 a,B | 84.1 ± 0.06 b,B | 1.63 ± 0.03 b,A |
| Q50 C | 2.63 ± 0.03 a,A | 27.12 ± 0.05 a,A | 84.6 ± 0.06 a,B | 2.06 ± 0.01 a,A |
| Q100 SC1 | 1.47 ± 0.02 c,B | 27.15 ± 0.05 a,B | 83.1 ± 0.10 c,B | 1.19 ± 0.03 c,B |
| Q75 SC1 | 1.96 ± 0.02 b,A | 27.10 ± 0.05 a,B | 83.7 ± 0.06 b,C | 1.61 ± 0.01 b,A |
| Q50 SC1 | 2.57 ± 0.03 a,A | 27.13 ± 0.05 a,A | 84.8 ± 0.06 a,A | 2.06 ± 0.01 a,A |
| Q100 SC2 | 1.55 ± 0.02 c,A | 26.93 ± 0.04 b,C | 83.7 ± 0.06 c,A | 1.27 ± 0.01 c,A |
| Q75 SC2 | 1.94 ± 0.02 b,A | 27.12 ± 0.04 a,B | 84.4 ± 0.06 a,A | 1.60 ± 0.03 b,A |
| Q50 SC2 | 2.44 ± 0.02 a,C | 26.98 ± 0.04 b,B | 84.1 ± 0.04 b,C | 1.92 ± 0.01 a,C |
| Dough Sample | C4, Nm | tC4, min | C5, Nm | C5-C4, Nm |
|---|---|---|---|---|
| Q100 | 1.43 ± 0.02 c,B | 33.52± 0.05 a,A | 1.98 ± 0.02 c,B | 0.55 ± 0.00 b,A |
| Q75 | 1.79 ± 0.02 b,C | 32.38 ± 0.05 b,A | 2.38 ± 0.02 b,C | 0.59 ± 0.00 b,D |
| Q50 | 2.48 ± 0.03 a,A | 26.62 ± 0.04 c,C | 3.70 ± 0.02 a,B | 1.21 ± 0.05 a,A,B |
| Q100 C | 1.33 ± 0.02 c,C | 30.72 ± 0.04 a,C | 1.86 ± 0.02 c,C | 0.54 ± 0.02 c,A,B |
| Q75 C | 1.91 ± 0.02 b,A | 30.03 ± 0.04 c,C | 2.68 ± 0.02 b,A | 0.77 ± 0.01 b,A |
| Q50 C | 2.48 ± 0.03 a,A | 30.22 ± 0.04 b,A | 3.77 ± 0.03 a,A | 1.30 ± 0.05 a,A |
| Q100 SC1 | 1.53 ± 0.02 c,A | 29.98 ± 0.05 b,D | 2.04 ± 0.02 c,A | 0.51 ± 0.01 c,C |
| Q75 SC1 | 1.90 ± 0.02 b,A | 30.12 ± 0.04 a,C | 2.62 ± 0.02 b,B | 0.72 ± 0.00 b,C |
| Q50 SC1 | 2.42 ± 0.03 a,B | 30.20 ± 0.04 a,A | 3.66 ± 0.03 a,B | 1.24 ± 0.01 a,A |
| Q100 SC2 | 1.51 ± 0.02 c,A | 32.88 ± 0.05 a,B | 2.03 ± 0.02 c,A | 0.52 ± 0.01 c,B,C |
| Q75 SC2 | 1.84 ± 0.02 b,B | 30.22 ± 0.04 b,B | 2.57 ± 0.02 b,B | 0.73 ± 0.01 b,B |
| Q50 SC2 | 2.36 ± 0.02 a,B | 30.03 ± 0.04 c,B | 3.49 ± 0.03 a,C | 1.13 ± 0.01 a,B |
| Crumb Moisture (g/100 g) on 1st Day | Crumb Moisture (g/100 g) on 3rd Day | |||||
|---|---|---|---|---|---|---|
| Q100 | Q75 | Q50 | Q100 | Q75 | Q50 | |
| C | 43.22 ± 0.24 a,A | 38.93 ± 0.13 b,A | 36.30 ± 0.08 c,A | 41.38 ± 0.60 a,A | 37.77 ± 0.41 b,A | 35.18 ± 0.55 c,A |
| SC1 | 41.19 ± 0.04 a,B | 36.14 ± 0.05 b,B | 36.29 ± 0.05 b,A | 39.50 ± 0.36 a,A,B | 34.48 ± 0.06 b,B | 34.74 ± 0.30 b,A |
| SC2 | 41.58 ± 0.05 a,B | 37.60 ± 0.06 b,C | 35.80 ± 0.50 c,A | 39.81 ± 0.30 a,B | 35.98 ± 0.06 b,C | 34.76 ± 0.40 c,A |
| Sample | Specific Volume, cm3/g | L* | a* | b* | Firmness, N |
|---|---|---|---|---|---|
| Q100 C | 133.9 ± 6.3 a,A | 43.93 ± 0.30 c,B | 3.41 ± 0.02 a,B | 16.63 ± 0.10 a,B | 26.14 ± 1.40 a,A |
| Q75 C | 109.2 ± 6.4 a,A,B | 47.18 ± 0.26 b,A | 3.25 ± 0.01 b,A | 16.48 ± 0.11 a,A | 27.09 ± 0.93 a,A |
| Q50 C | 110.8 ± 6.5 a,A | 50.05 ± 0.37 a,A | 2.79 ± 0.02 c,A | 15.76 ± 0.08 b,B | 29.11 ± 5.12 a,A |
| Q100 SC1 | 124.4 ± 6.3 a,A | 45.14 ± 0.20 c,A | 3.31 ± 0.00 a,C | 17.15 ± 0.09 a,A | 27.20 ± 1.53 a,A |
| Q75 SC1 | 131.6 ± 6.6 a,A | 45.96 ± 0.09 b,B | 3.19 ± 0.01 a,A | 16.29 ± 0.39 a,A | 25.37 ± 2.38 a,A |
| Q50 SC1 | 110.3 ± 6.5 a,A | 47.42 ± 0.21 a,B | 2.94 ± 0.10 b,A | 16.50 ± 0.01 a,A | 29.14 ± 7.17 a,A |
| Q100 SC2 | 97.1 ± 6.9 a,B | 43.07± 0.07 c,B | 3.96 ± 0.01 a,A | 17.31 ± 0.16 a,A | 23.19 ± 2.65 b,A |
| Q75 SC2 | 100.0 ± 6.4 a,B | 46.61 ± 0.14 b,A,B | 3.32 ± 0.30 a,b,A | 16.45 ± 0.19 b,A | 25.20 ± 0.51 b,A |
| Q50 SC2 | 117.0 ± 6.4 a,A | 49.36 ± 0.13 a,A | 2.92 ± 0.01 b,A | 16.42 ± 0.07 b,A | 32.79 ± 3.34 a,A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupu, A.; Banu, I.; Grigore-Gurgu, L.; Vasilean, I.; Aprodu, I. Gluten-Free Sourdough Based on Quinoa and Sorghum: Characterization and Applications in Breadmaking. Appl. Sci. 2025, 15, 5468. https://doi.org/10.3390/app15105468
Lupu A, Banu I, Grigore-Gurgu L, Vasilean I, Aprodu I. Gluten-Free Sourdough Based on Quinoa and Sorghum: Characterization and Applications in Breadmaking. Applied Sciences. 2025; 15(10):5468. https://doi.org/10.3390/app15105468
Chicago/Turabian StyleLupu, Anca, Iuliana Banu, Leontina Grigore-Gurgu, Ina Vasilean, and Iuliana Aprodu. 2025. "Gluten-Free Sourdough Based on Quinoa and Sorghum: Characterization and Applications in Breadmaking" Applied Sciences 15, no. 10: 5468. https://doi.org/10.3390/app15105468
APA StyleLupu, A., Banu, I., Grigore-Gurgu, L., Vasilean, I., & Aprodu, I. (2025). Gluten-Free Sourdough Based on Quinoa and Sorghum: Characterization and Applications in Breadmaking. Applied Sciences, 15(10), 5468. https://doi.org/10.3390/app15105468










