Biomechanical Optimization of Lumbar Fusion Cages with a Porous Design: A Finite Element Analysis
Abstract
1. Introduction
2. Materials and Methods
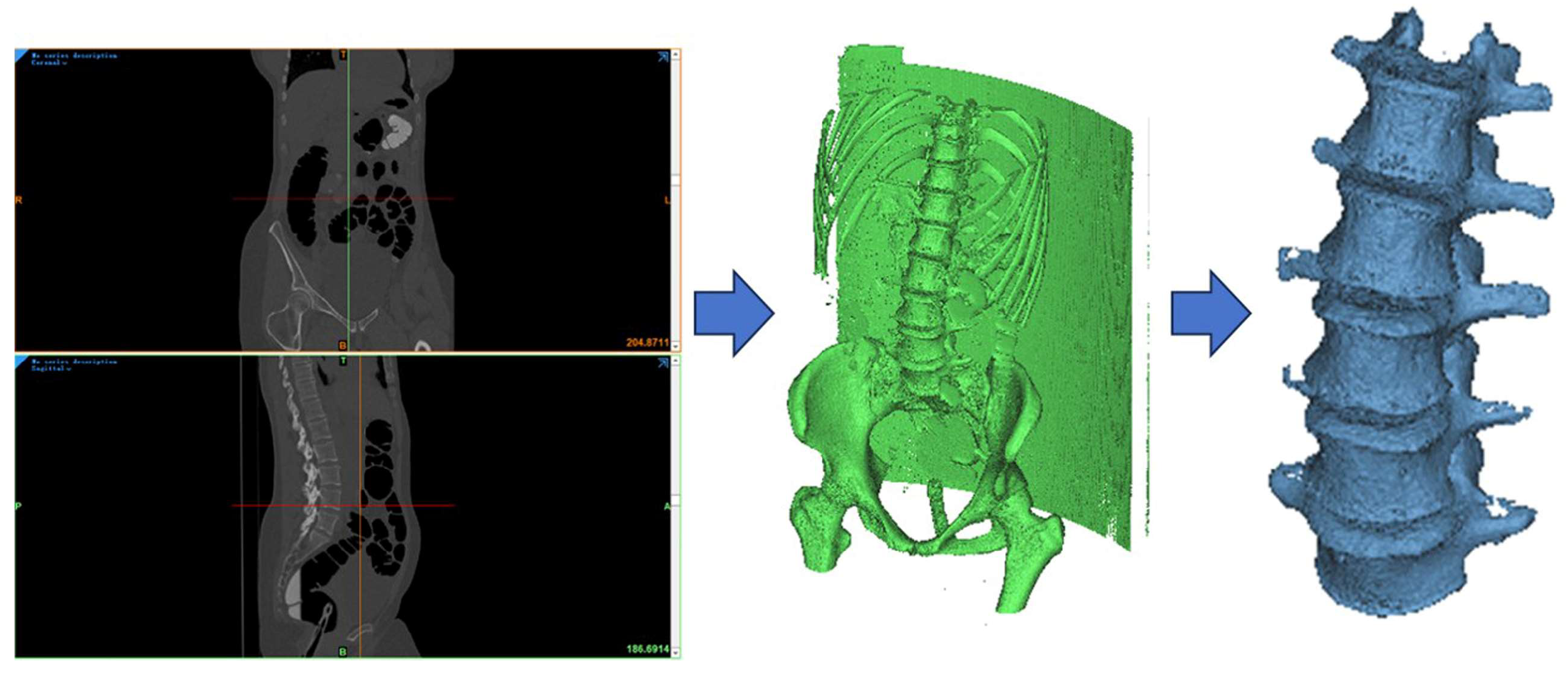
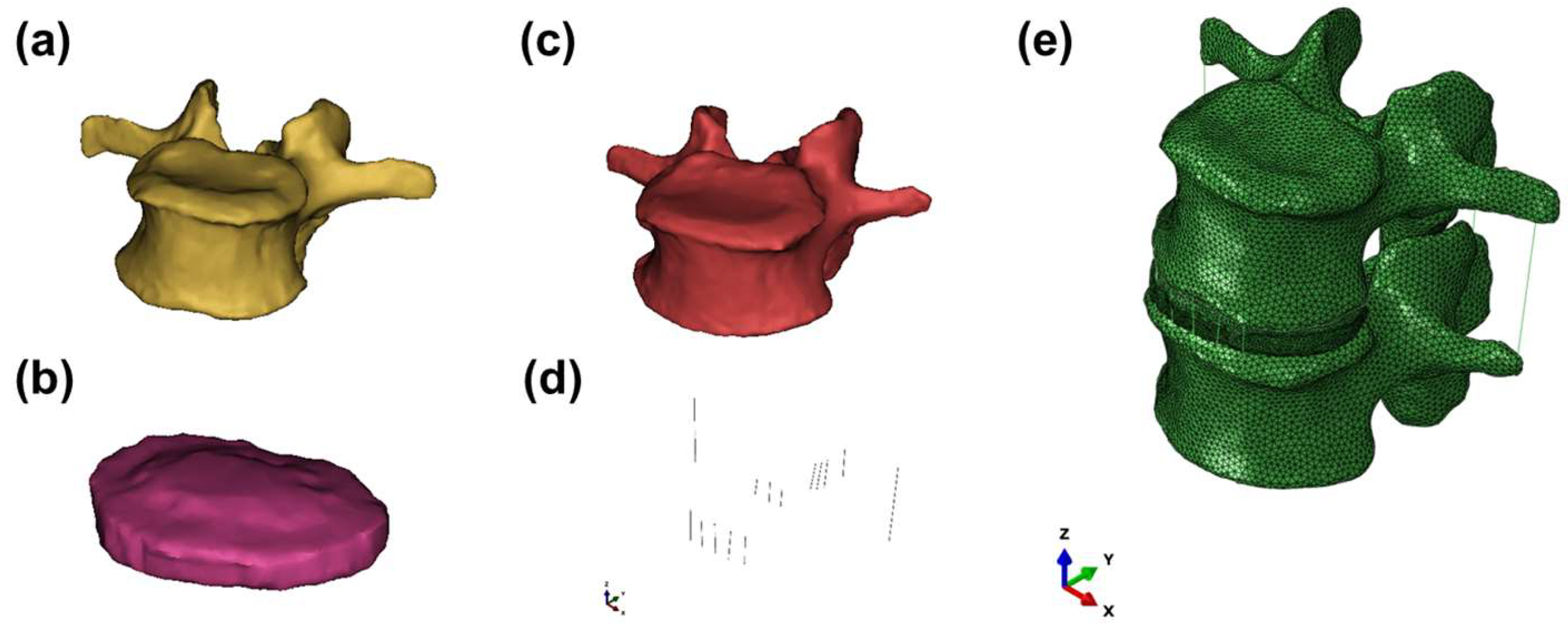
2.1. Development of the Lumbar Spine FE Model
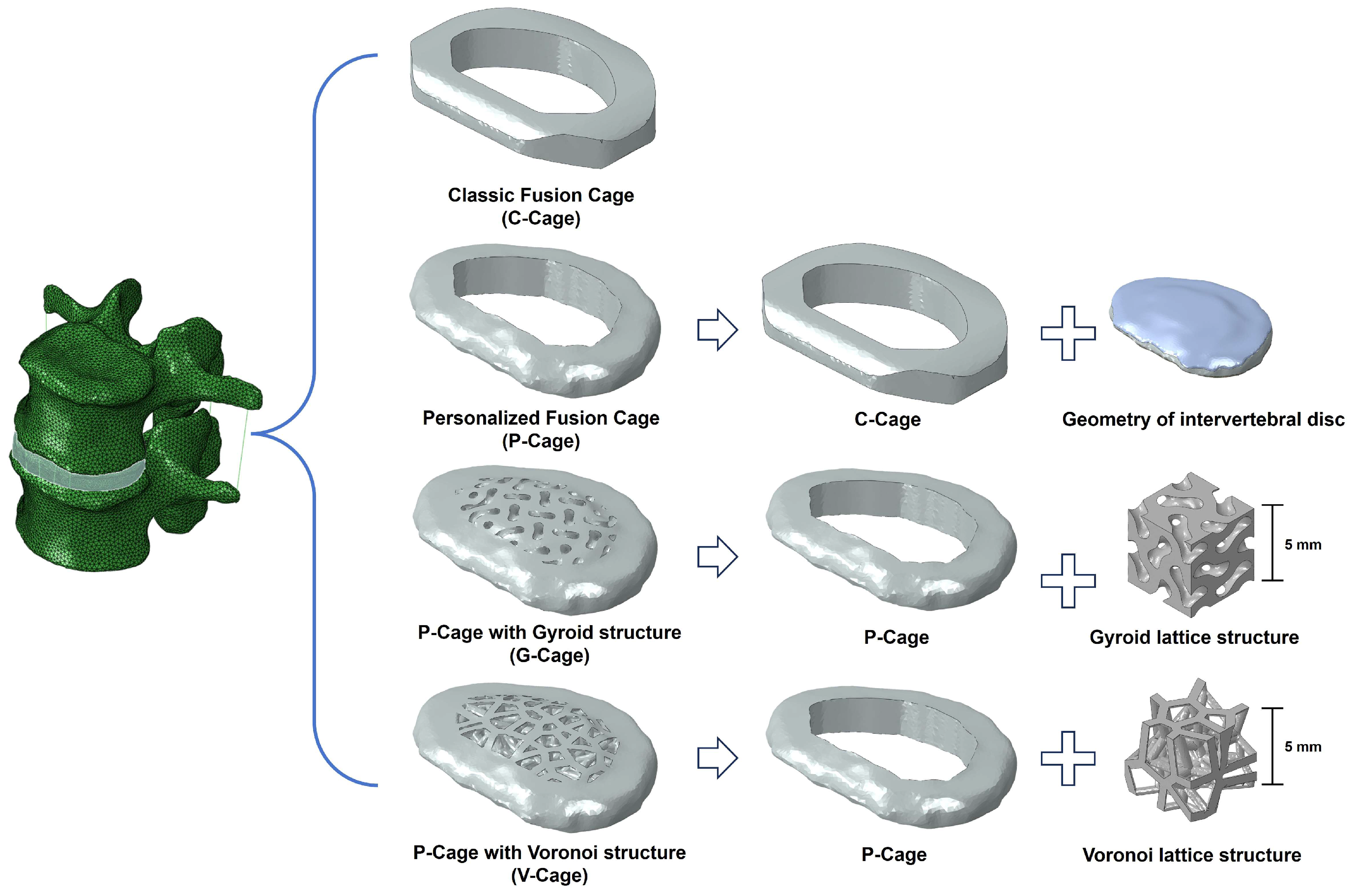
2.2. Design of Customized Lumbar Fusion Cage
2.3. Boundary and Loading Conditions of the FE Model
3. Results
3.1. Validation of the Intact Lumbar Spine Model
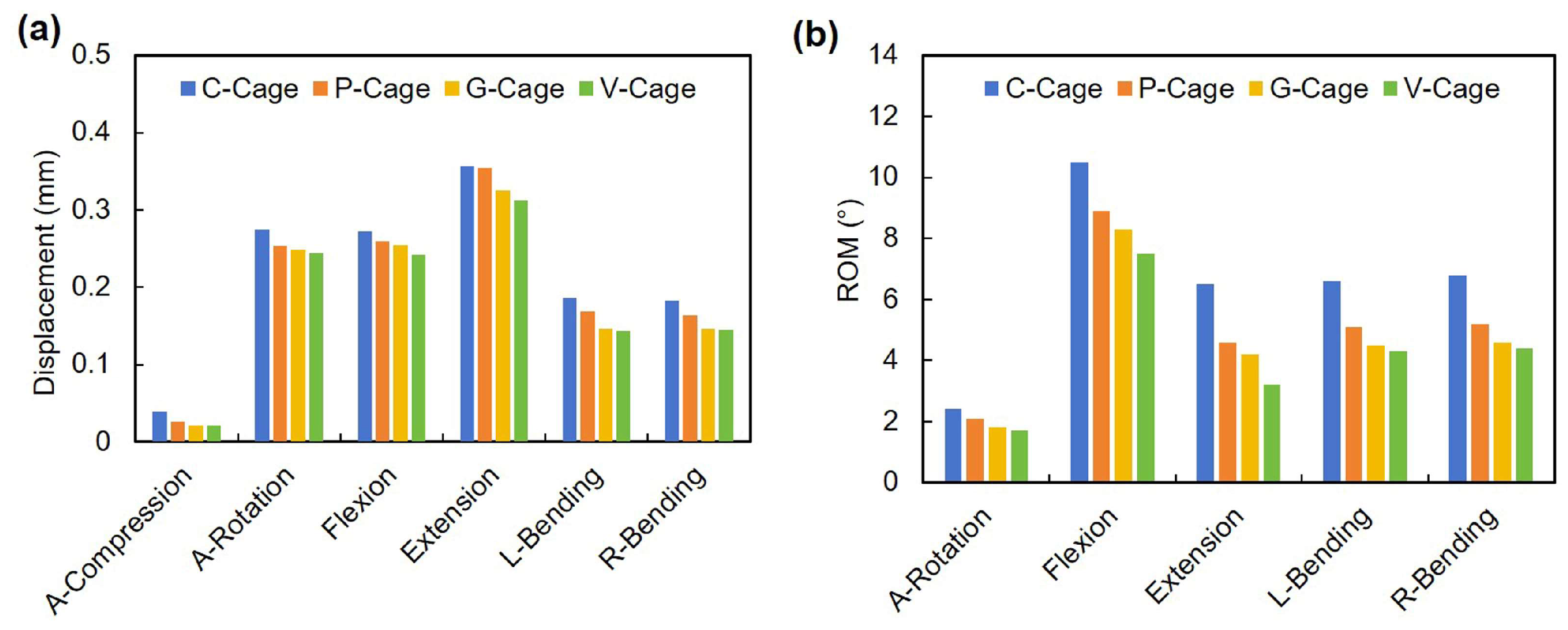
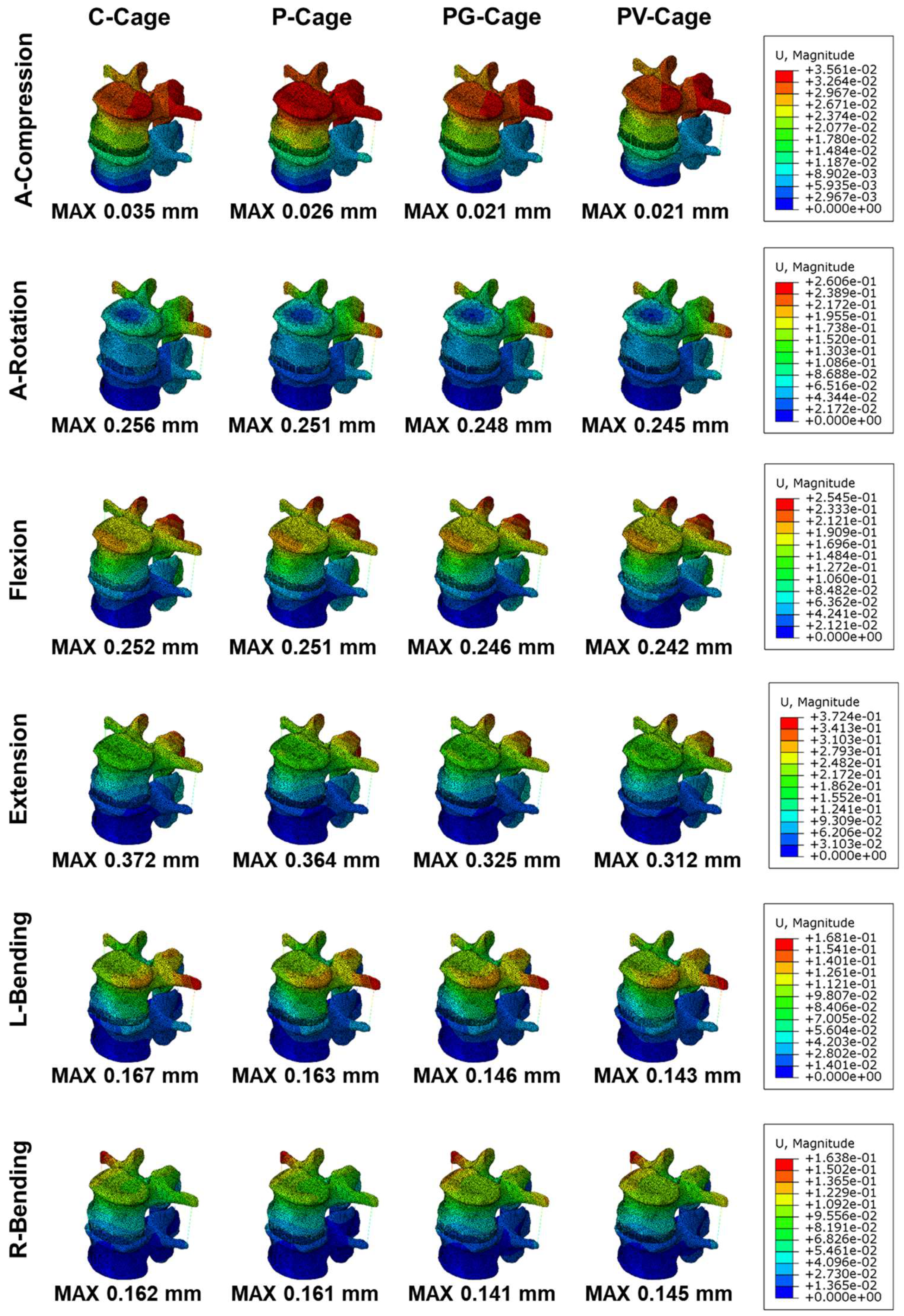
3.2. Stability of Endplates
3.3. Cage Stress
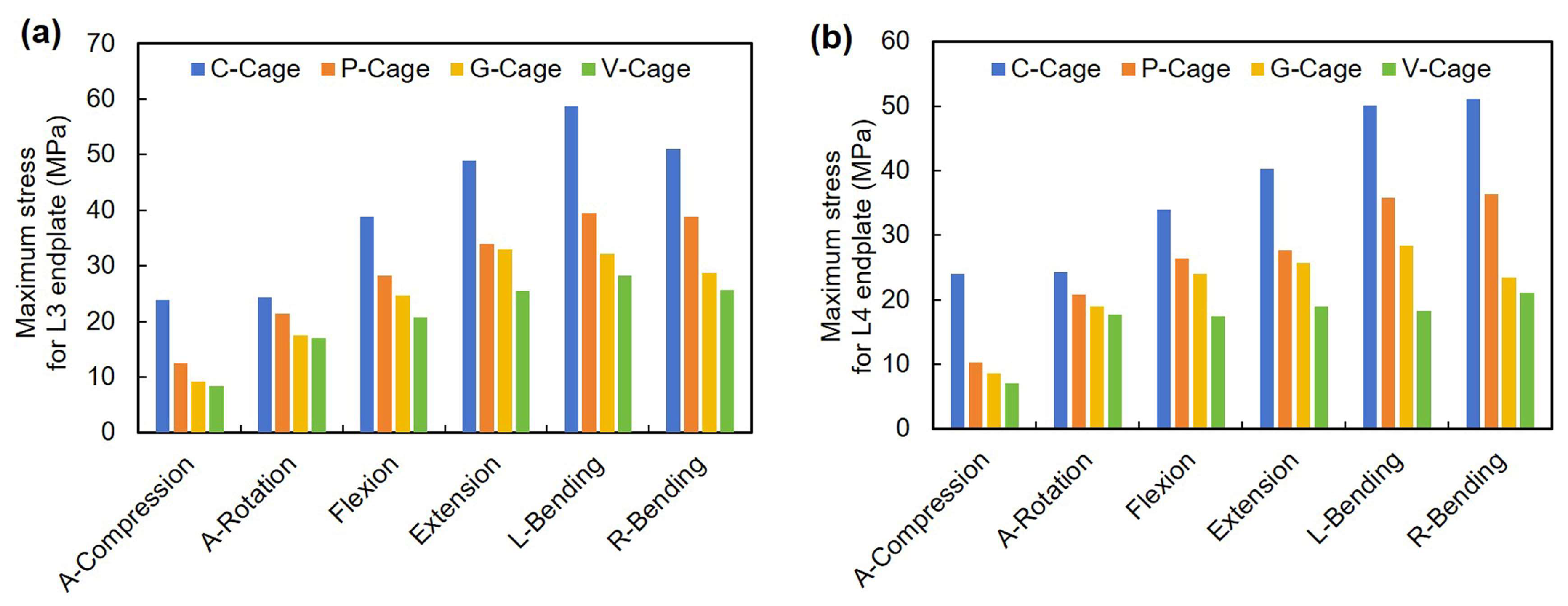
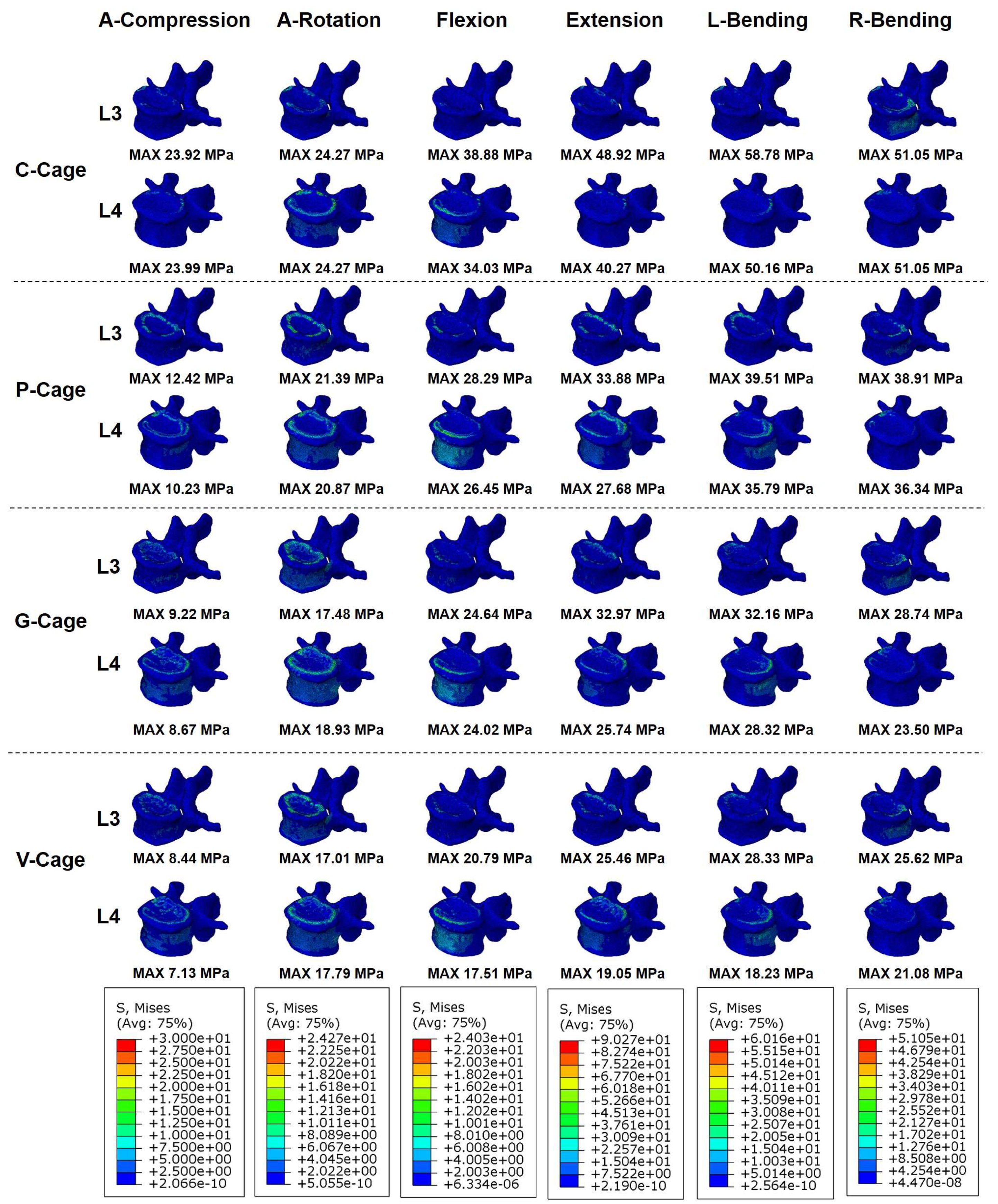
3.4. Endplate Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Kunder, S.L.; Rijkers, K.; Caelers, I.J.; de Bie, R.A.; Koehler, P.J.; van Santbrink, H. Lumbar interbody fusion: A historical overview and a future perspective. Spine 2018, 43, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Schnake, K.; Rappert, D.; Storzer, B.; Schreyer, S.; Hilber, F.; Mehren, C. Lumbale spondylodese–indikationen und techniken. Orthopade 2019, 48, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Taba, H.A.; Williams, S.K. Lateral lumbar interbody fusion. Neurosurg. Clin. 2020, 31, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Navarrete, R.; Gittens, R.A.; Schneider, J.M.; Hyzy, S.L.; Haithcock, D.A.; Ullrich, P.F.; Schwartz, Z.; Boyan, B.D. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012, 12, 265–272. [Google Scholar] [CrossRef]
- Du, C.-F.; Cai, X.-Y.; Gui, W.; Sun, M.-S.; Liu, Z.-X.; Liu, C.-J.; Zhang, C.-Q.; Huang, Y.-P. Does oblique lumbar interbody fusion promote adjacent degeneration in degenerative disc disease: A finite element analysis. Comput. Biol. Med. 2021, 128, 104122. [Google Scholar] [CrossRef]
- Yuan, W.; Kaliya-Perumal, A.-K.; Chou, S.M.; Oh, J.Y.-L. Does lumbar interbody cage size influence subsidence? A biomechanical study. Spine 2020, 45, 88–95. [Google Scholar] [CrossRef]
- Suh, P.B.; Puttlitz, C.; Lewis, C.; Bal, B.S.; McGilvray, K. The effect of cervical interbody cage morphology, material composition, and substrate density on cage subsidence. JAAOS-J. Am. Acad. Orthop. Surg. 2017, 25, 160–168. [Google Scholar] [CrossRef]
- Li, S.; Huan, Y.; Zhu, B.; Chen, H.; Tang, M.; Yan, Y.; Wang, C.; Ouyang, Z.; Li, X.; Xue, J. Research progress on the biological modifications of implant materials in 3D printed intervertebral fusion cages. J. Mater. Sci. Mater. Med. 2022, 33, 2. [Google Scholar] [CrossRef]
- Yu, Y.; Robinson, D.L.; Ackland, D.C.; Yang, Y.; Lee, P.V.S. Influence of the geometric and material properties of lumbar endplate on lumbar interbody fusion failure: A systematic review. J. Orthop. Surg. Res. 2022, 17, 224. [Google Scholar] [CrossRef]
- Kang, J.; Dong, E.; Li, X.; Guo, Z.; Shi, L.; Li, D.; Wang, L. Topological design and biomechanical evaluation for 3D printed multi-segment artificial vertebral implants. Mater. Sci. Eng. C 2021, 127, 112250. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Mechanical performance of additively manufactured meta-biomaterials. Acta Biomater. 2019, 85, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, A. 3D topology optimization and mesh dependency for redesigning locking compression plates aiming to reduce stress shielding. Int. J. Bioprinting 2021, 7, 339. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Tang, Q.; Feng, Q.; Song, J.; Han, X.; Guo, F. Mechanical behaviours and mass transport properties of bone-mimicking scaffolds consisted of gyroid structures manufactured using selective laser melting. J. Mech. Behav. Biomed. 2019, 93, 158–169. [Google Scholar] [CrossRef]
- Zhang, L.; Feih, S.; Daynes, S.; Chang, S.; Wang, M.Y.; Wei, J.; Lu, W.F. Energy absorption characteristics of metallic triply periodic minimal surface sheet structures under compressive loading. Addit. Manuf. 2018, 23, 505–515. [Google Scholar] [CrossRef]
- Sotomayor, O.E.; Tippur, H.V. Role of cell regularity and relative density on elastoplastic compression response of 3-D open-cell foam core sandwich structure generated using Voronoi diagrams. Acta Mater. 2014, 78, 301–313. [Google Scholar] [CrossRef]
- Jiao, C.; Xie, D.; He, Z.; Liang, H.; Shen, L.; Yang, Y.; Tian, Z.; Wu, G.; Wang, C. Additive manufacturing of Bio-inspired ceramic bone Scaffolds: Structural Design, mechanical properties and biocompatibility. Mater. Des. 2022, 217, 110610. [Google Scholar] [CrossRef]
- Luo, L.; Li, J.; Lin, Z.; Cheng, X.; Wang, J.; Wang, Y.; Yang, Y.; Li, S.; Ling, Q.; Dai, J.; et al. Anisotropic biomimetic trabecular porous three-dimensional-printed Ti-6Al-4V cage for lumbar interbody fusion. Mater. Des. 2023, 233, 112254. [Google Scholar] [CrossRef]
- Jain, P.; Rana, M.; Biswas, J.K.; Khan, M.R. Biomechanics of spinal implants—A review. Biomed. Phys. Eng. Express 2020, 6, 042002. [Google Scholar] [CrossRef]
- Cai, X.Y.; Sun, M.S.; Huang, Y.P.; Liu, Z.X.; Liu, C.J.; Du, C.F.; Yang, Q. Biomechanical effect of L4–L5 intervertebral disc degeneration on the lower lumbar spine: A finite element study. Orthop. Surg. 2020, 12, 917–930. [Google Scholar] [CrossRef]
- Welch-Phillips, A.; Gibbons, D.; Ahern, D.P.; Butler, J.S. What is finite element analysis? Clin. Spine Surg. 2020, 33, 323–324. [Google Scholar] [CrossRef]
- Cai, X.-Y.; Bian, H.-M.; Chen, C.; Ma, X.-L.; Yang, Q. Biomechanical study of oblique lumbar interbody fusion (OLIF) augmented with different types of instrumentation: A finite element analysis. J. Orthop. Surg. Res. 2022, 17, 269. [Google Scholar] [CrossRef] [PubMed]
- Du, C.-F.; Yang, N.; Guo, J.-C.; Huang, Y.-P.; Zhang, C. Biomechanical response of lumbar facet joints under follower preload: A finite element study. BMC Musculoskelet. Dis. 2016, 17, 126. [Google Scholar] [CrossRef] [PubMed]
- Andrea, B.-F.; Elena, N.; Bentivoglio, D.; Aprato, A.; Terzini, M.; Bignardi, C.; Giaretta, S.; Momoli, A. In Silico Evaluation of the Primary Stability of Acetabular Revision Cups: Standard Versus Locking Screws. ASME J. Biomech. Eng. 2025, 147, 051007. [Google Scholar]
- Schmidt, H.; Heuer, F.; Simon, U.; Kettler, A.; Rohlmann, A.; Claes, L.; Wilke, H.-J. Application of a new calibration method for a three-dimensional finite element model of a human lumbar annulus fibrosus. Clin. Biomech. 2006, 21, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-K.; Meng, Y.; Wang, B.-Y.; Rong, X.; Hong, Y.; Ding, C.; Chen, H.; Liu, H. Biomechanics following skip-level cervical disc arthroplasty versus skip-level cervical discectomy and fusion: A finite element-based study. BMC Musculoskelet. Dis. 2019, 20, 49. [Google Scholar] [CrossRef]
- Shirazi-Adl, S.A.; Shrivastava, S.C.; Ahmed, A.M. Stress analysis of the lumbar disc-body unit in compression a three-dimensional nonlinear finite element study. Spine 1984, 9, 120–134. [Google Scholar] [CrossRef]
- Goel, V.K.; Monroe, B.; Gilbertson, L.; Brinckmann, P. Interlaminar shear stresses and laminae separation in a disc: Finite element analysis of the L3-L4 motion segment subjected to axial compressive loads. Spine 1995, 20, 689–698. [Google Scholar] [CrossRef]
- Shirazi-Adl, A.; Ahmed, A.M.; Shrivastava, S.C. Mechanical response of a lumbar motion segment in axial torque alone and combined with compression. Spine 1986, 11, 914–927. [Google Scholar] [CrossRef]
- Ganbat, D.; Kim, Y.H.; Kim, K.; Jin, Y.J.; Park, W.M. Effect of mechanical loading on heterotopic ossification in cervical total disc replacement: A three-dimensional finite element analysis. Biomech. Model. Mechanobiol. 2016, 15, 1191–1199. [Google Scholar] [CrossRef]
- Lin, M.; Shapiro, S.Z.; Doulgeris, J.; Engeberg, E.D.; Tsai, C.-T.; Vrionis, F.D. Cage-screw and anterior plating combination reduces the risk of micromotion and subsidence in multilevel anterior cervical discectomy and fusion—A finite element study. Spine J. 2021, 21, 874–882. [Google Scholar] [CrossRef]
- Yamamoto, I.; Panjabi, M.M.; Crisco, T.; Oxland, T. Three-dimensional movements of the whole lumbar spine and lumbosacral joint. Spine 1989, 14, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zheng, Q.; Zhang, L.; Chen, R.; Li, Z.; Xu, W. Biomechanical evaluation of different oblique lumbar interbody fusion constructs: A finite element analysis. BMC Musculoskelet. Dis. 2024, 25, 97. [Google Scholar] [CrossRef] [PubMed]
- Nan, C.; Ma, Z.; Liu, Y.; Ma, L.; Li, J.; Zhang, W. Impact of cage position on biomechanical performance of stand-alone lateral lumbar interbody fusion: A finite element analysis. BMC Musculoskelet. Dis. 2022, 23, 920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, R.; Zhu, X. Multi-scale topology optimisation design and mechanical property analysis of porous interbody fusion cage. Bio-Med. Mater. Eng. 2024, 36, 110–123. [Google Scholar] [CrossRef]
- Talpeanu, G.; Awaja, F. Optimizing spinal fusion implants: Advanced biomaterials and technologies for improved outcomes. Biomed. Mater. Devices 2024, 1–33. [Google Scholar] [CrossRef]
- Han, C.; Wang, Y.; Wang, Z.; Dong, Z.; Li, K.; Song, C.; Cai, C.; Yan, X.; Yang, Y.; Wang, D. Enhancing mechanical properties of additively manufactured voronoi-based architected metamaterials via a lattice-inspired design strategy. Int. J. Mach. Tools Manuf. 2024, 202, 104199. [Google Scholar] [CrossRef]
- Kiselevskiy, M.V.; Anisimova, N.Y.; Kapustin, A.V.; Ryzhkin, A.A.; Kuznetsova, D.N.; Polyakova, V.V.; Enikeev, N.A. Development of bioactive scaffolds for orthopedic applications by designing additively manufactured titanium porous structures: A critical review. Biomimetics 2023, 8, 546. [Google Scholar] [CrossRef]
- Cheloni, J.P.M.; Zluhan, B.; Silveira, M.E.; Fonseca, E.B.; Valim, D.B.; Lopes, E.S. Mechanical behavior and failure mode of body-centered cubic, gyroid, diamond, and Voronoi functionally graded additively manufactured biomedical lattice structures. J. Mech. Behav. Biomed. 2025, 163, 106796. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Fogel, G.R.; Xiang, D.; Liao, Z.; Liu, W. Finite element model predicts the biomechanical performance of transforaminal lumbar interbody fusion with various porous additive manufactured cages. Comput. Biol. Med. 2018, 95, 167–174. [Google Scholar] [CrossRef]
- Logroscino, G.; Proietti, L.; Pola, E. Spine fusion: Cages, plates and bone substitutes. In Biomaterials for Spinal Surgery; Elsevier: Amsterdam, The Netherlands, 2012; pp. 265–294. [Google Scholar]
- Cheers, G.M.; Weimer, L.P.; Neuerburg, C.; Arnholdt, J.; Gilbert, F.; Thorwächter, C.; Holzapfel, B.M.; Mayer-Wagner, S.; Laubach, M. Advances in implants and bone graft types for lumbar spinal fusion surgery. Biomater. Sci.-UK 2024, 12, 4875–4902. [Google Scholar] [CrossRef]
- Reisener, M.-J.; Pumberger, M.; Shue, J.; Girardi, F.P.; Hughes, A.P. Trends in lumbar spinal fusion—A literature review. J. Spine Surg. 2020, 6, 752–761. [Google Scholar] [CrossRef]
- Shah, A.A.; Devana, S.K.; Lee, C.; Bugarin, A.; Lord, E.L.; Shamie, A.N.; Park, D.Y.; van der Schaar, M.; SooHoo, N.F. Prediction of major complications and readmission after lumbar spinal fusion: A machine learning–driven approach. World Neurosurg. 2021, 152, e227–e234. [Google Scholar] [CrossRef]

| Components | Young’s Modulus (MPa) | Poisson’s Ratio | Cross-Section Area (mm2) |
|---|---|---|---|
| Cortical bone | 12,000 | 0.3 | / |
| Cancellous bone | 100 | 0.2 | / |
| Bone endplate | 500 | 0.25 | / |
| Nucleus pulposus | 1 | 0.499 | / |
| Annulus fibrosus | 4 | 0.45 | / |
| Anterior longitudinal ligament (ALL) | 8 | 0.35 | 49.1 |
| Posterior longitudinal ligament (PLL) | 10 | 0.35 | 22.2 |
| Intertransverse ligaments (ITL) | 5 | 0.35 | 4 |
| Interspinous ligament (ISL) | 5 | 0.35 | 49.2 |
| Supraspinal ligament (SSL) | 5 | 0.35 | 70.3 |
| Cage Design | Cell Size | Seed Distance | Porosity Detail | Personalized Design |
|---|---|---|---|---|
| C-Cage | / | / | / | N |
| P-Cage | / | / | / | Y |
| G-Cage | 2.5 mm | / | 73.3% | Y |
| V-Cage | / | 3 mm | 73.5% | Y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Deng, K.; Shao, Z.; Wang, Y. Biomechanical Optimization of Lumbar Fusion Cages with a Porous Design: A Finite Element Analysis. Appl. Sci. 2025, 15, 5384. https://doi.org/10.3390/app15105384
Zhu C, Deng K, Shao Z, Wang Y. Biomechanical Optimization of Lumbar Fusion Cages with a Porous Design: A Finite Element Analysis. Applied Sciences. 2025; 15(10):5384. https://doi.org/10.3390/app15105384
Chicago/Turabian StyleZhu, Chenkai, Kan Deng, Zhenzong Shao, and Yong Wang. 2025. "Biomechanical Optimization of Lumbar Fusion Cages with a Porous Design: A Finite Element Analysis" Applied Sciences 15, no. 10: 5384. https://doi.org/10.3390/app15105384
APA StyleZhu, C., Deng, K., Shao, Z., & Wang, Y. (2025). Biomechanical Optimization of Lumbar Fusion Cages with a Porous Design: A Finite Element Analysis. Applied Sciences, 15(10), 5384. https://doi.org/10.3390/app15105384







