Integration and Validation of Soft Wearable Robotic Gloves for Sensorimotor Rehabilitation of Human Hand Function
Abstract
1. Introduction
2. Materials and Methods
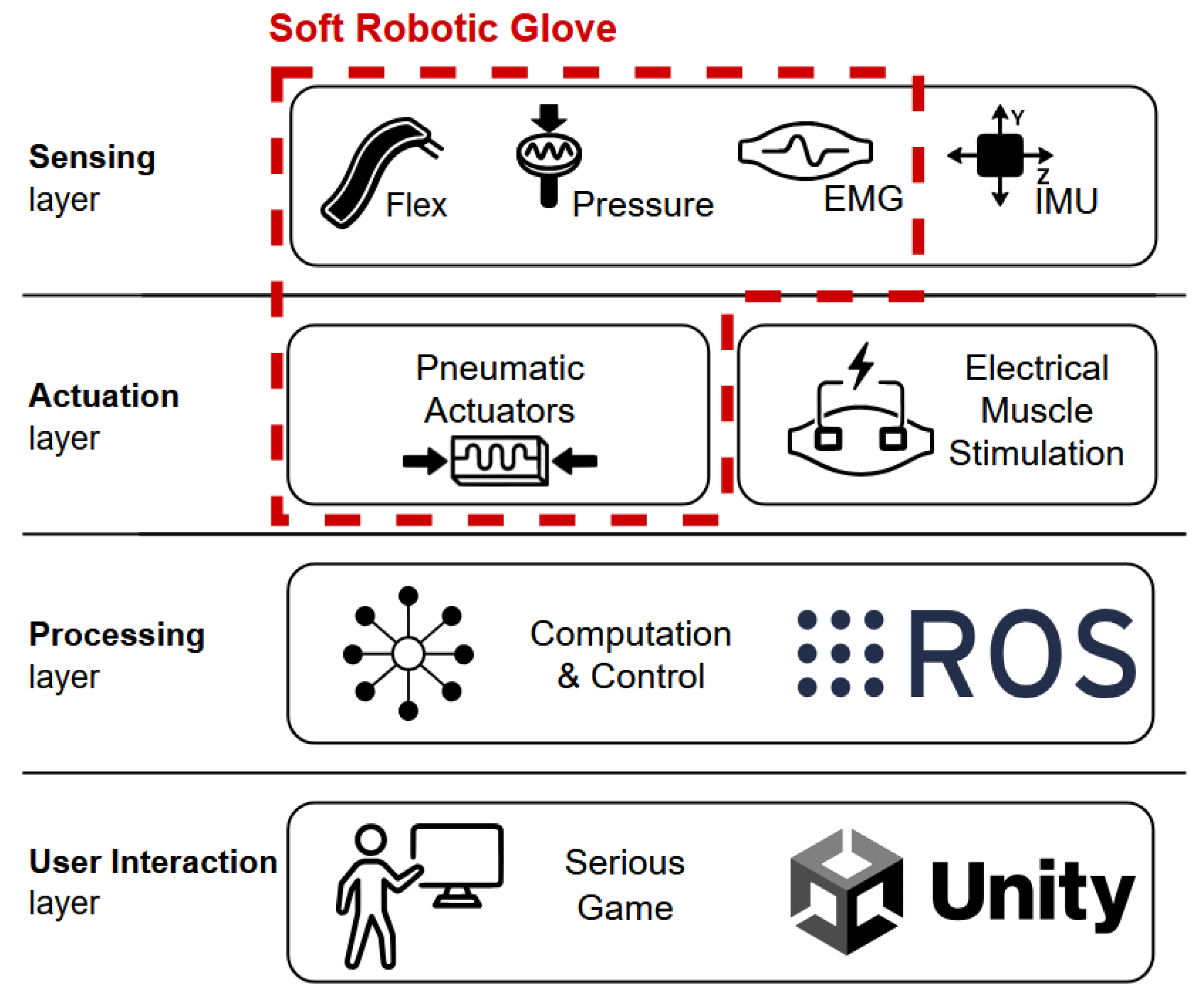
2.1. Body-Area-Distributed Sensor and Actuator System and the NeuroSuitUp Platform
The Wearable SRG
2.2. Design, Development, and Integration of SRGs
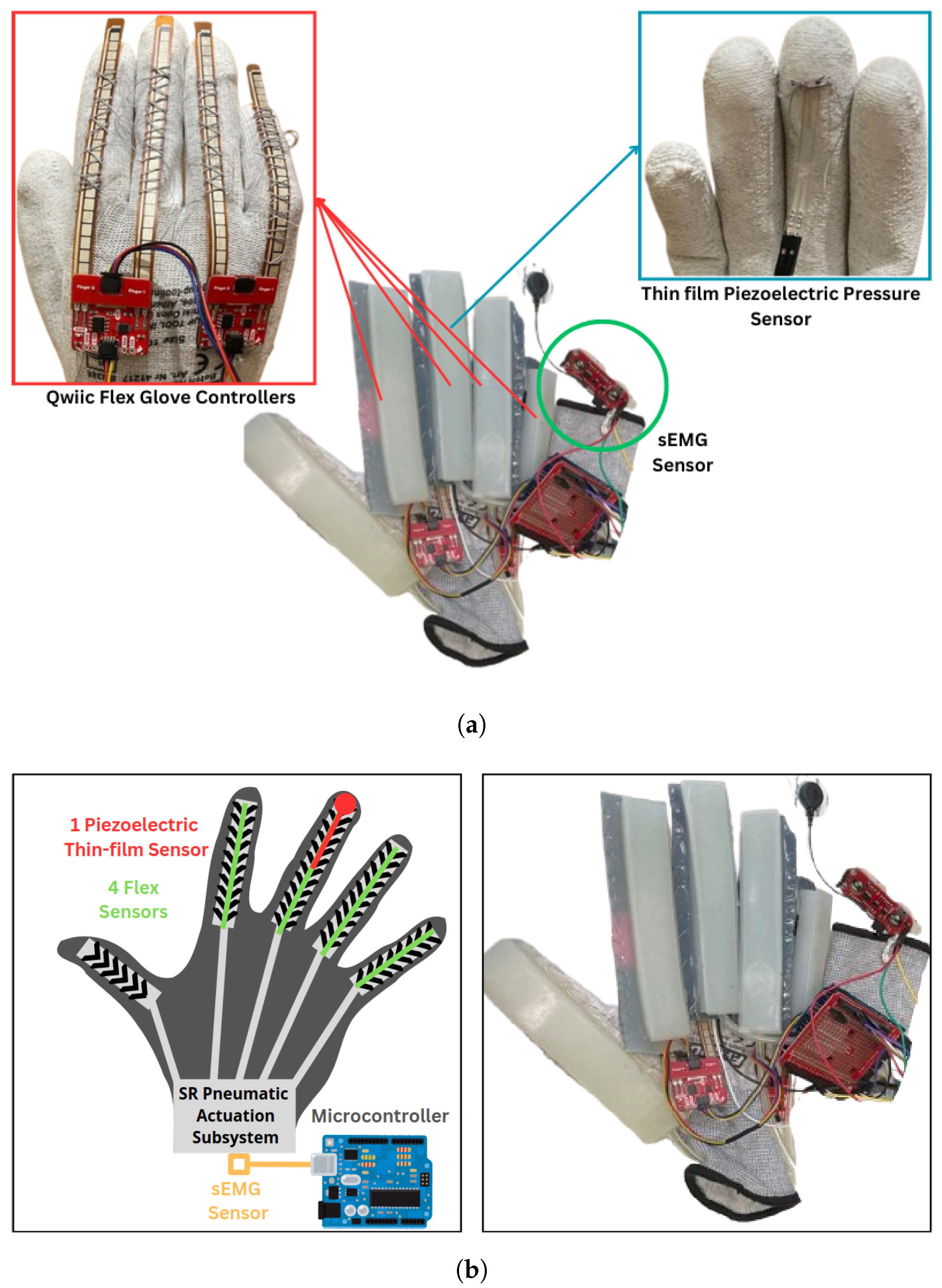
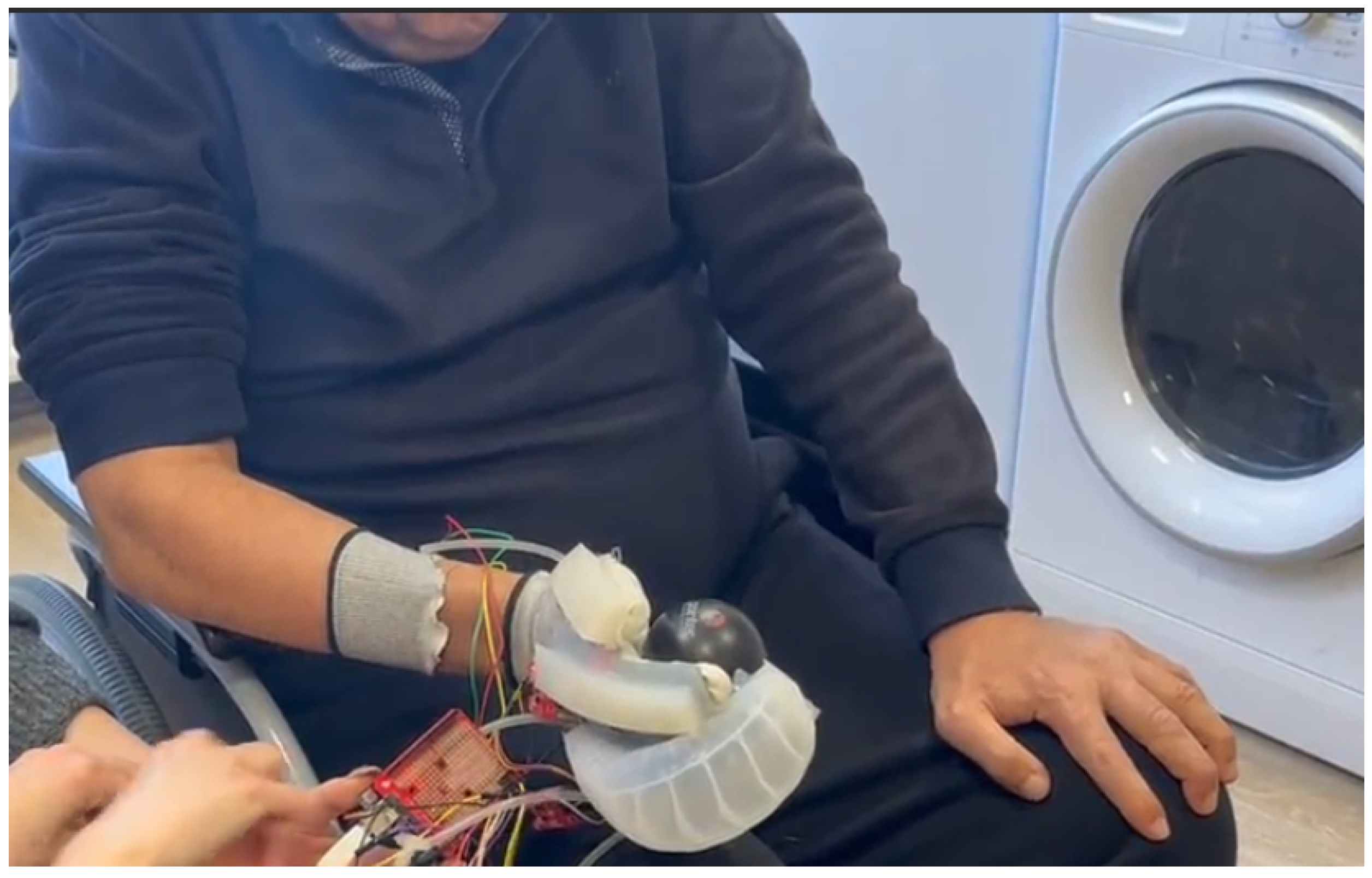
2.2.1. SRG Prototype for SCI Participants
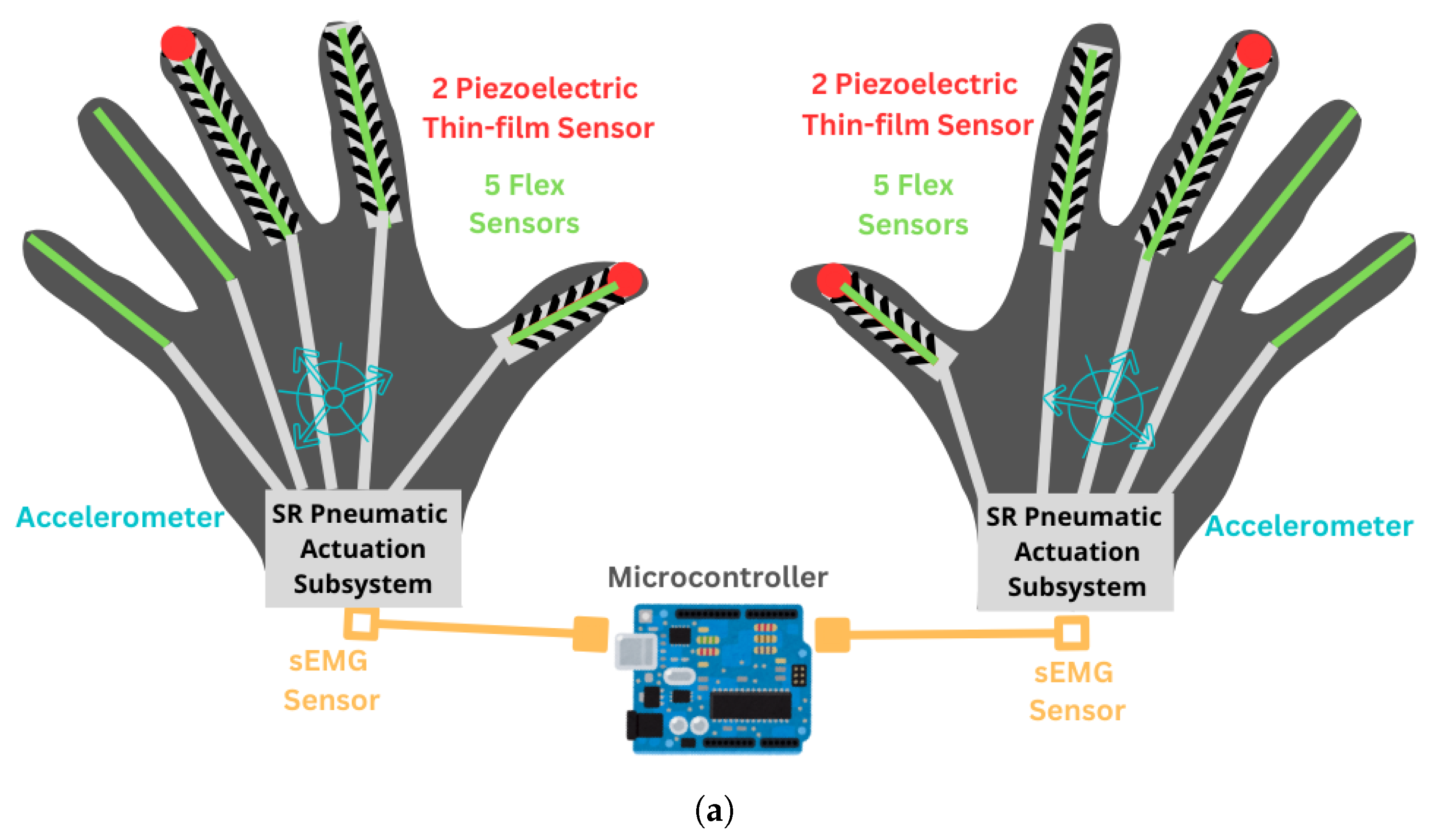
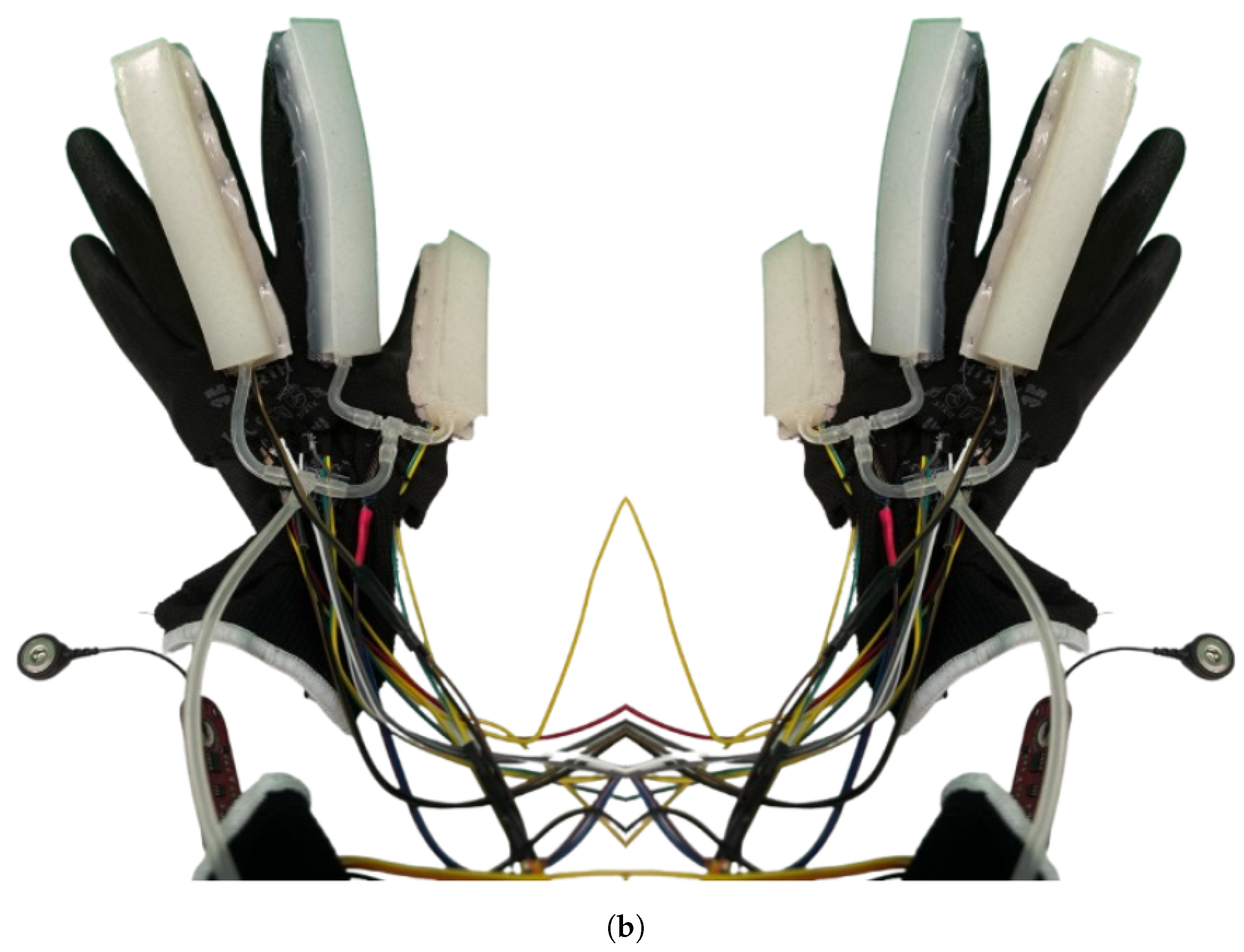
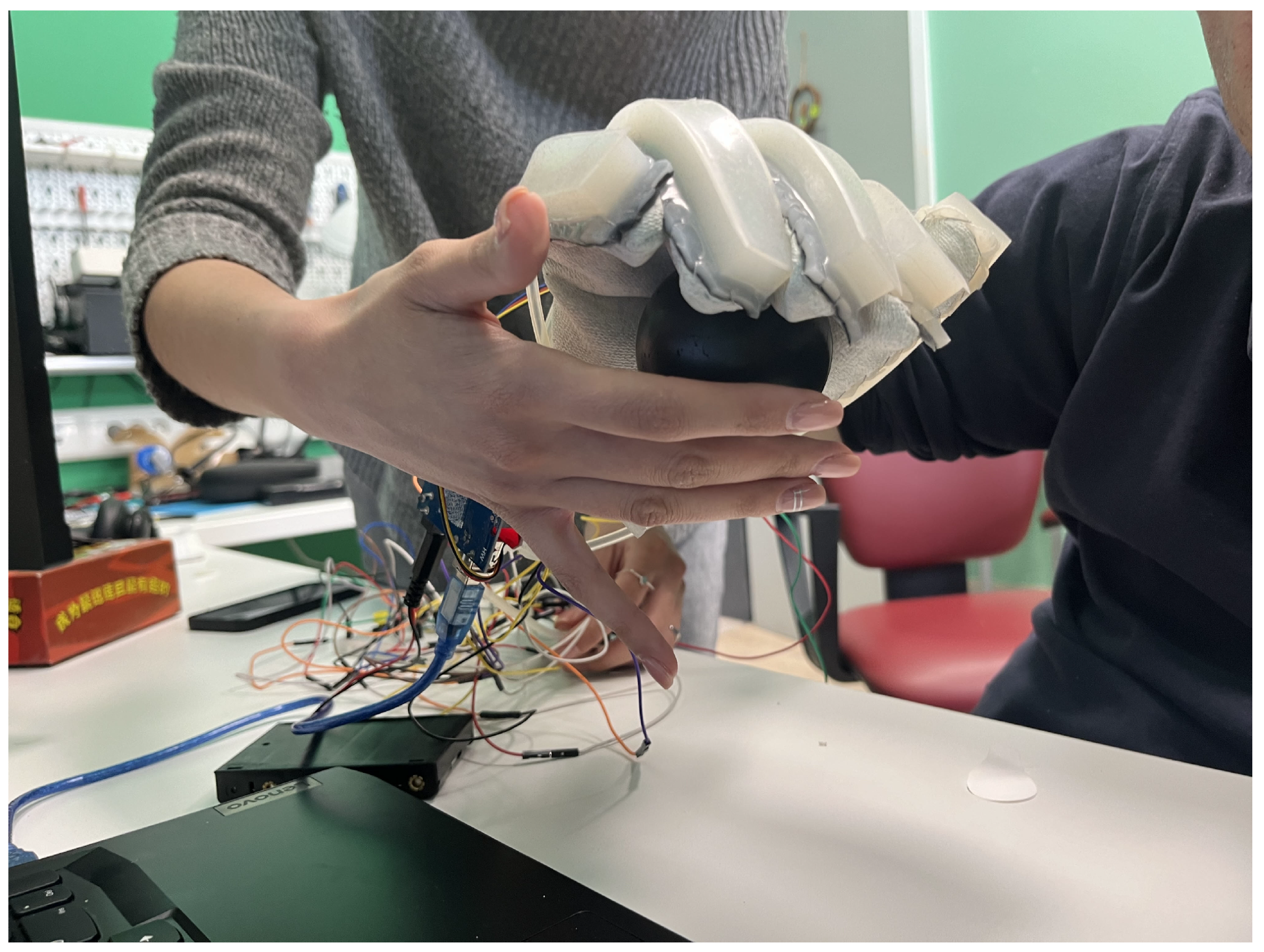
2.2.2. SRG Prototype for Post-Stroke Participants
2.3. Experimental Setup and Protocol
2.4. Data Acquisition and Analysis
2.4.1. Data Preprossesing
2.4.2. Data Analysis
3. Results
3.1. SCI Participants
3.2. Post-Stroke Participants
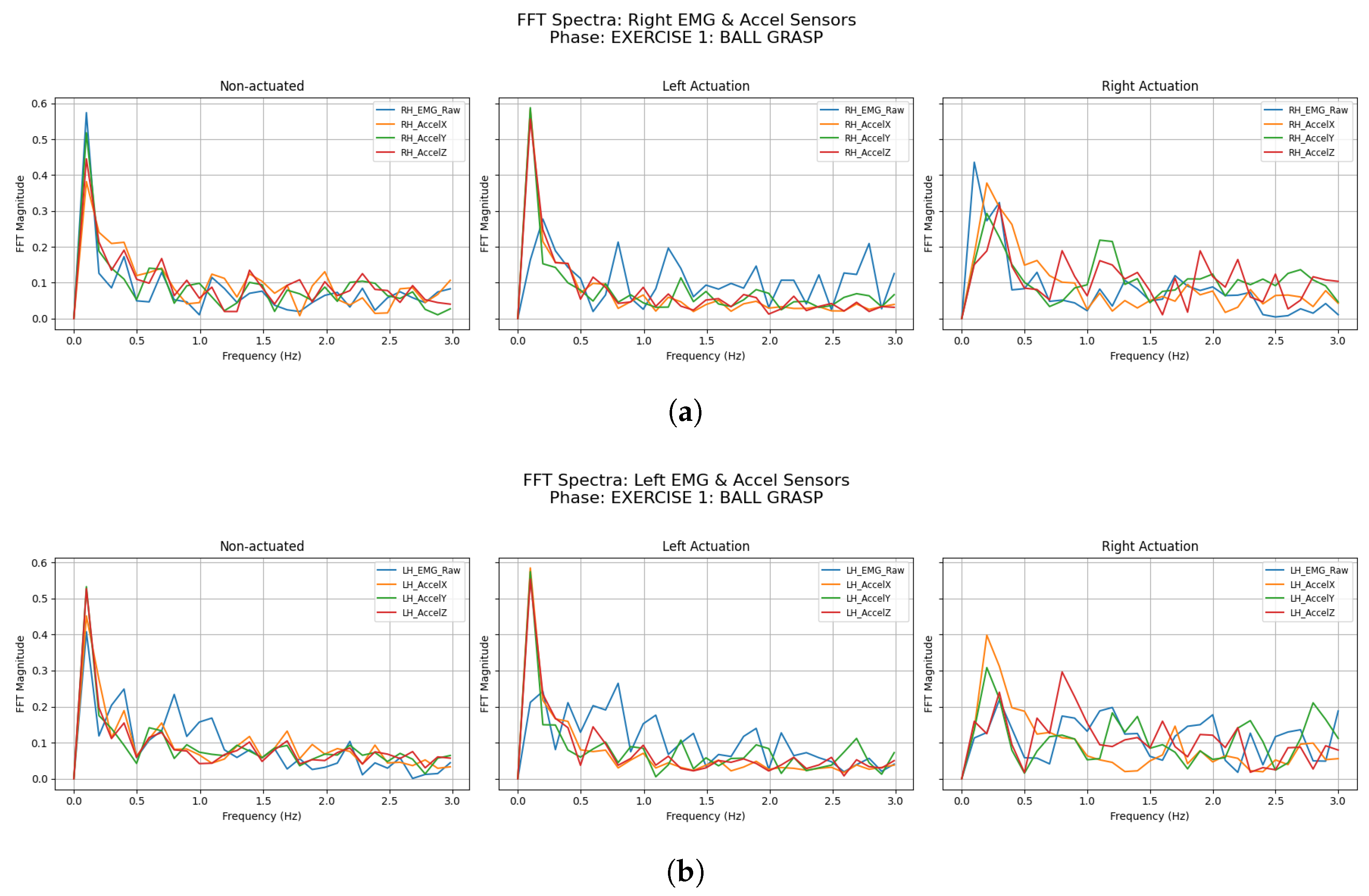
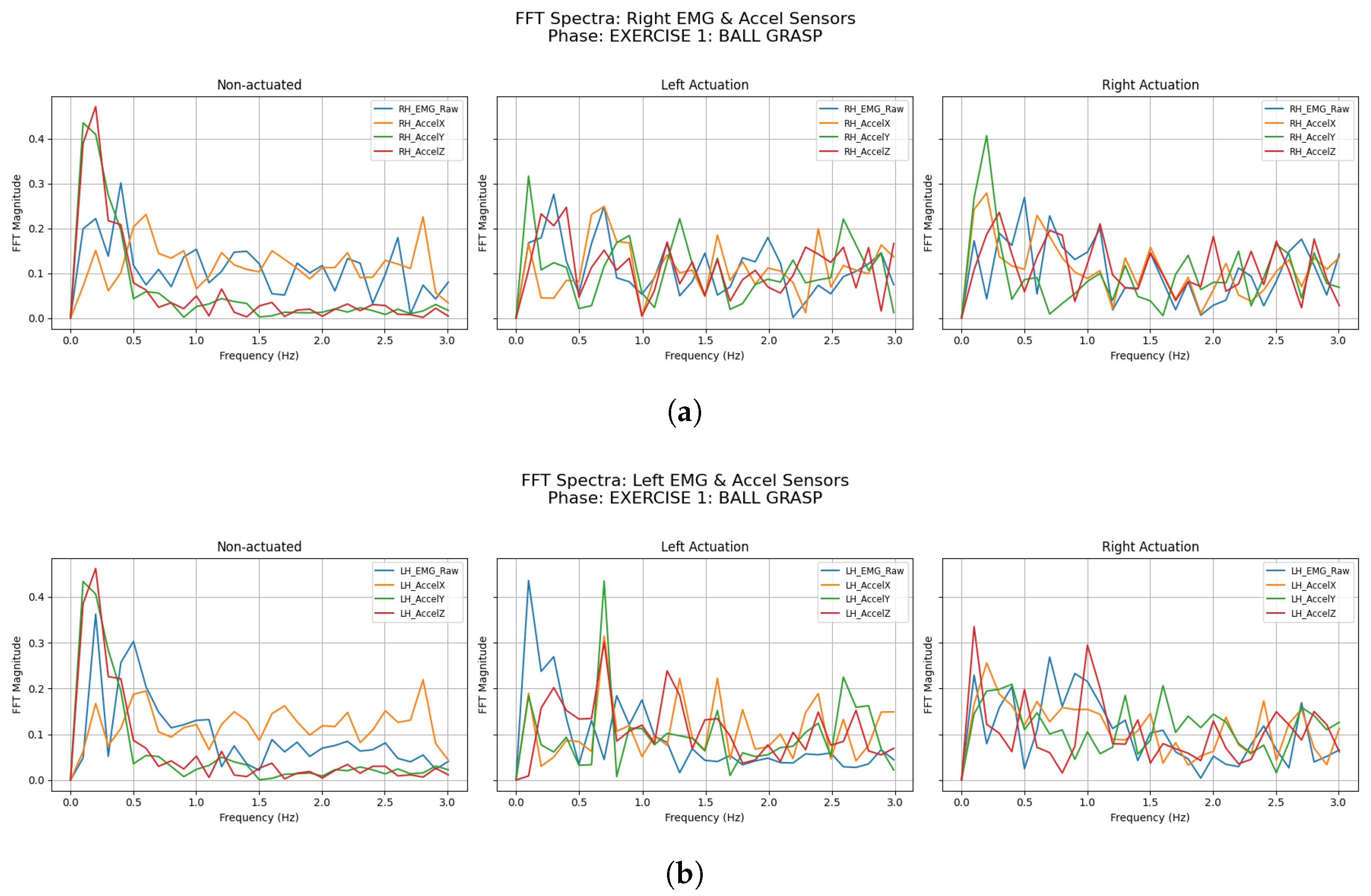
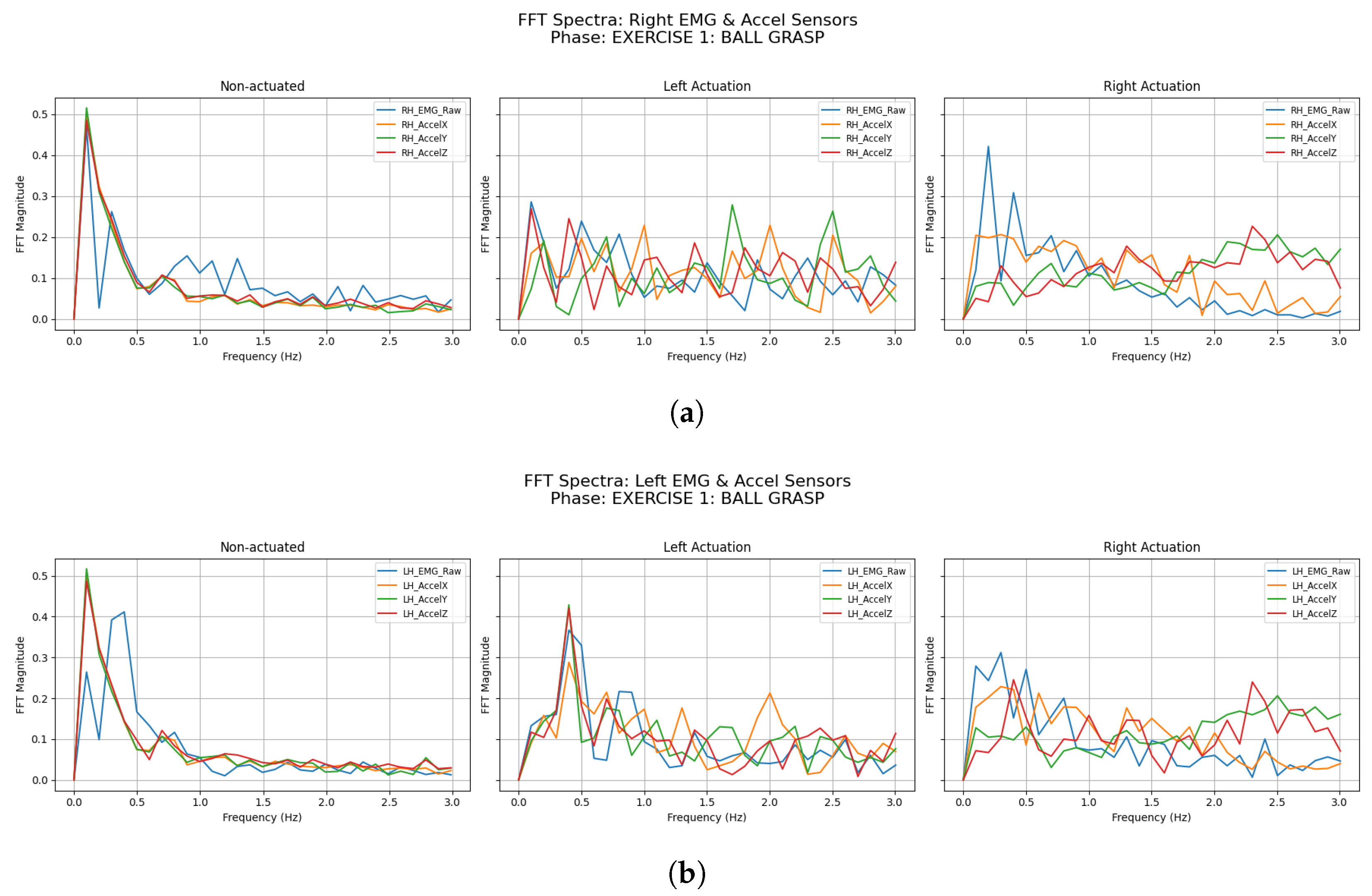
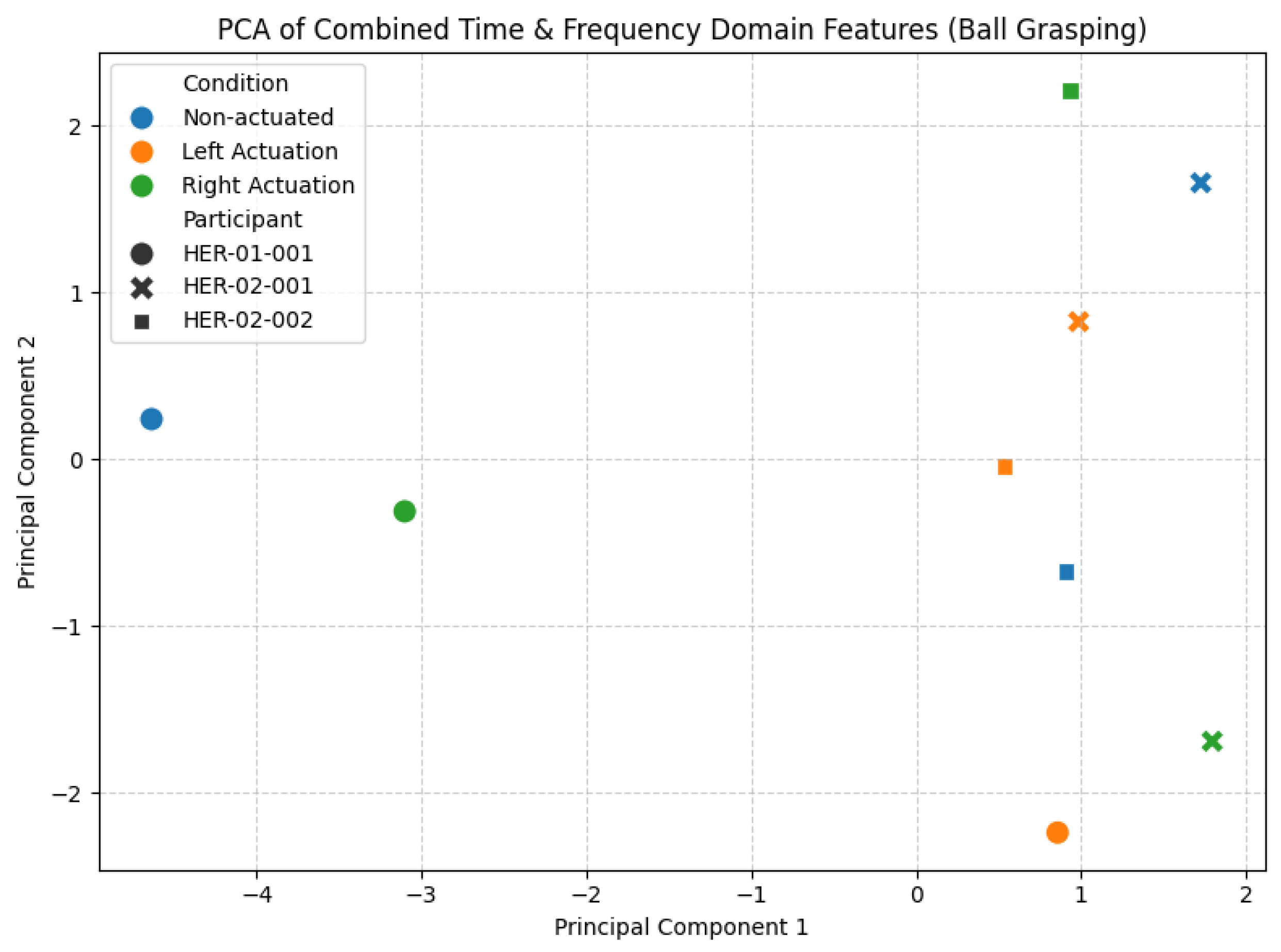
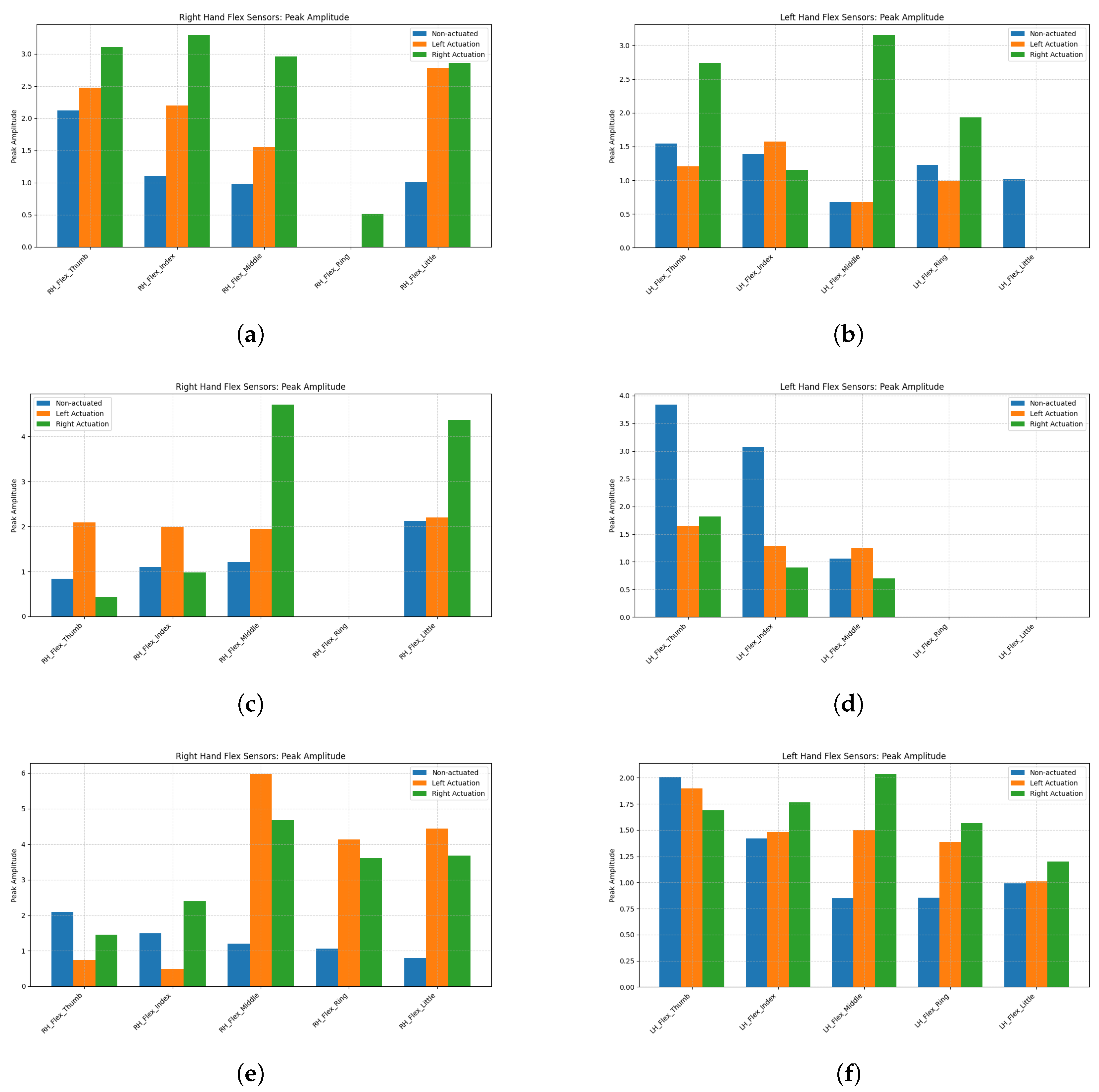
3.2.1. Ball Grasping
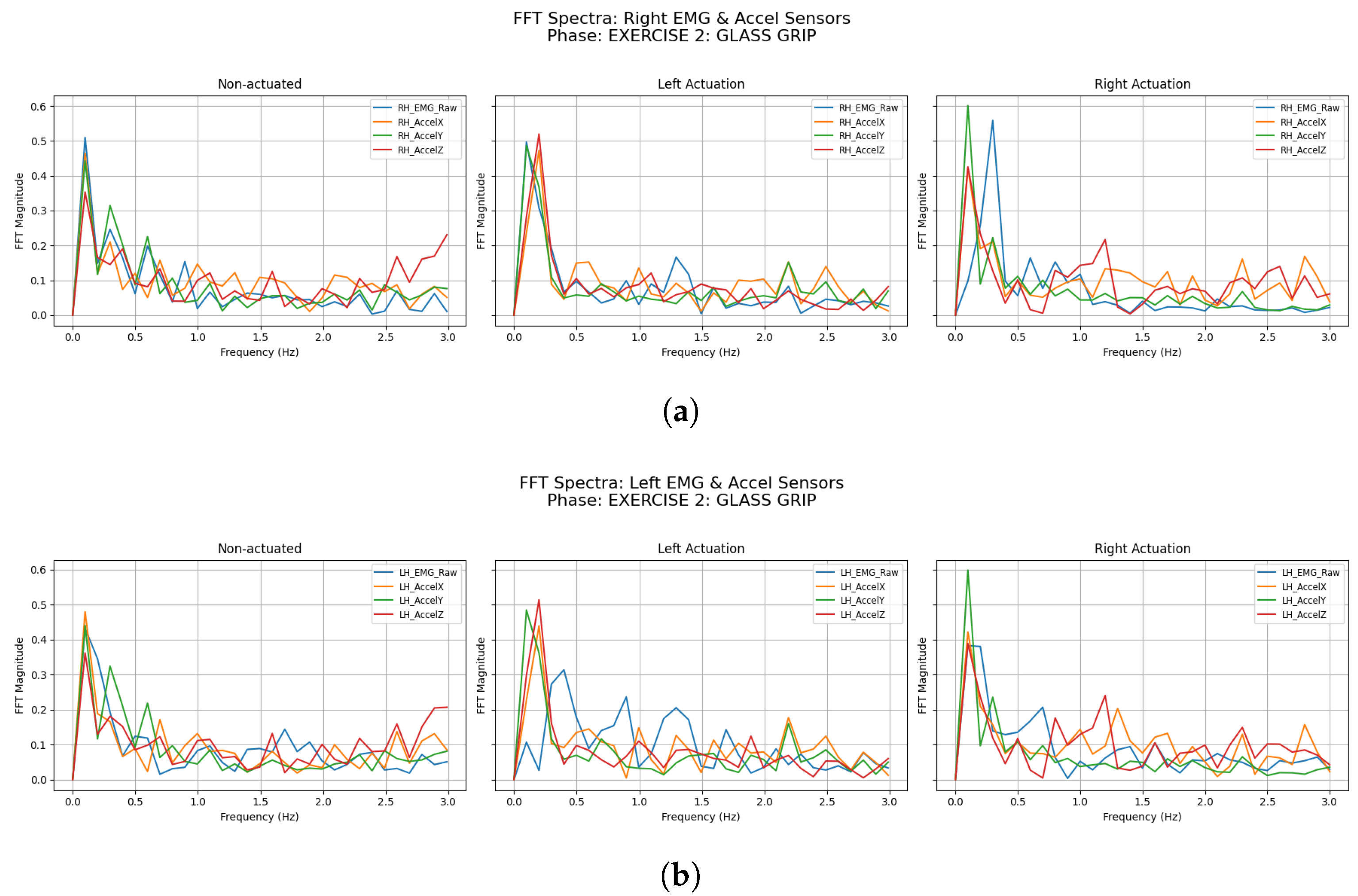
3.2.2. Glass Grip
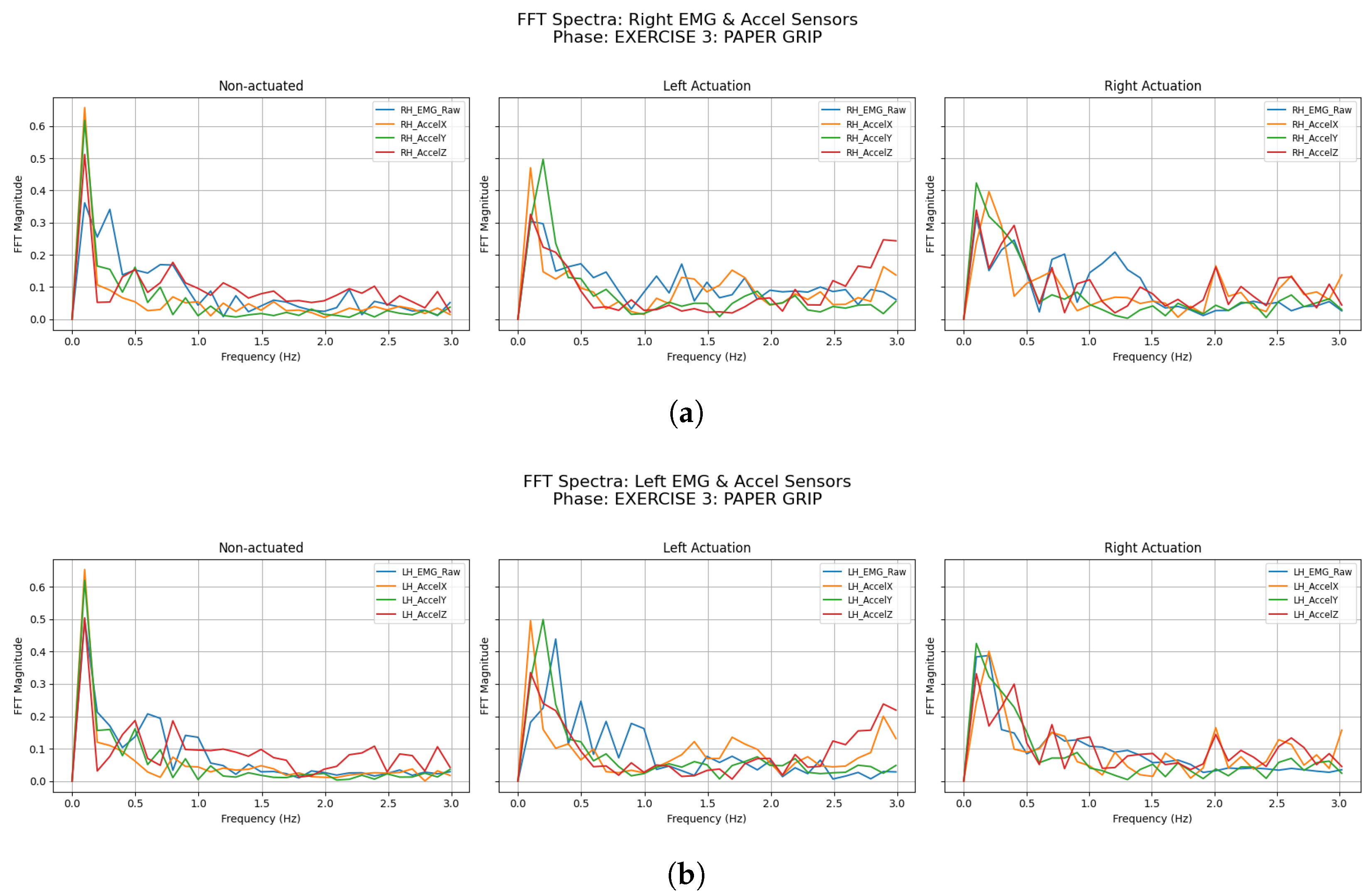
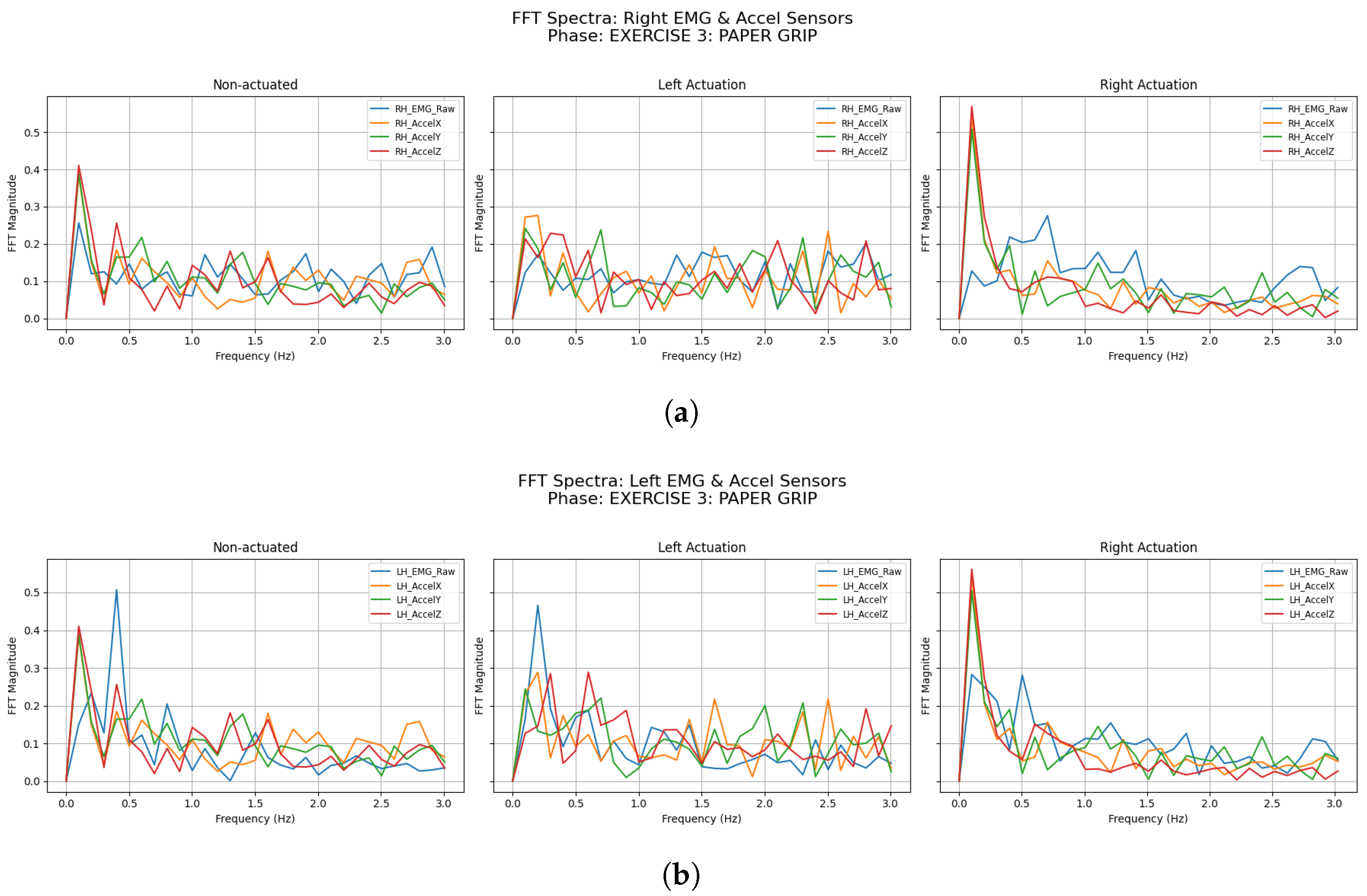
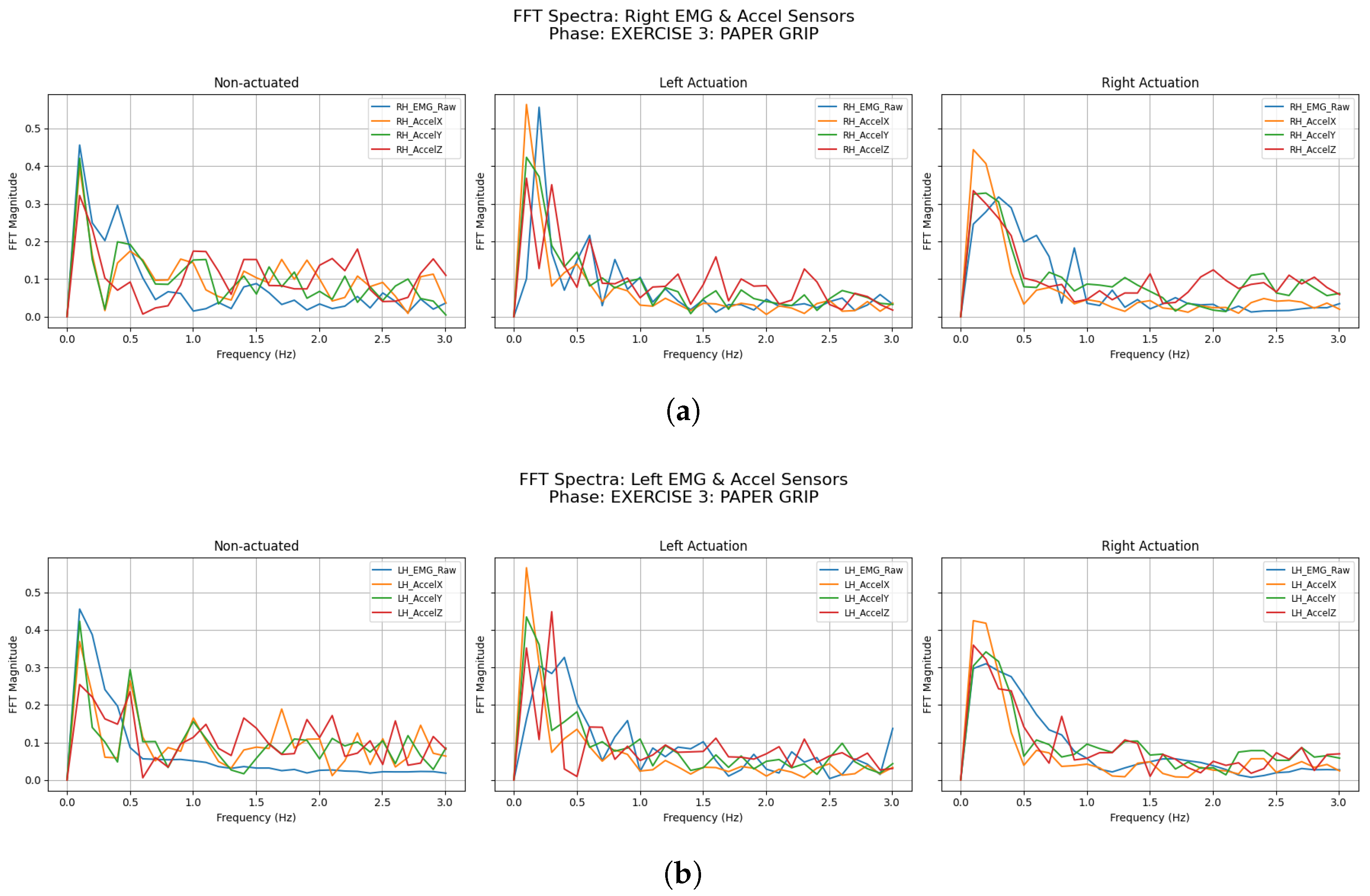
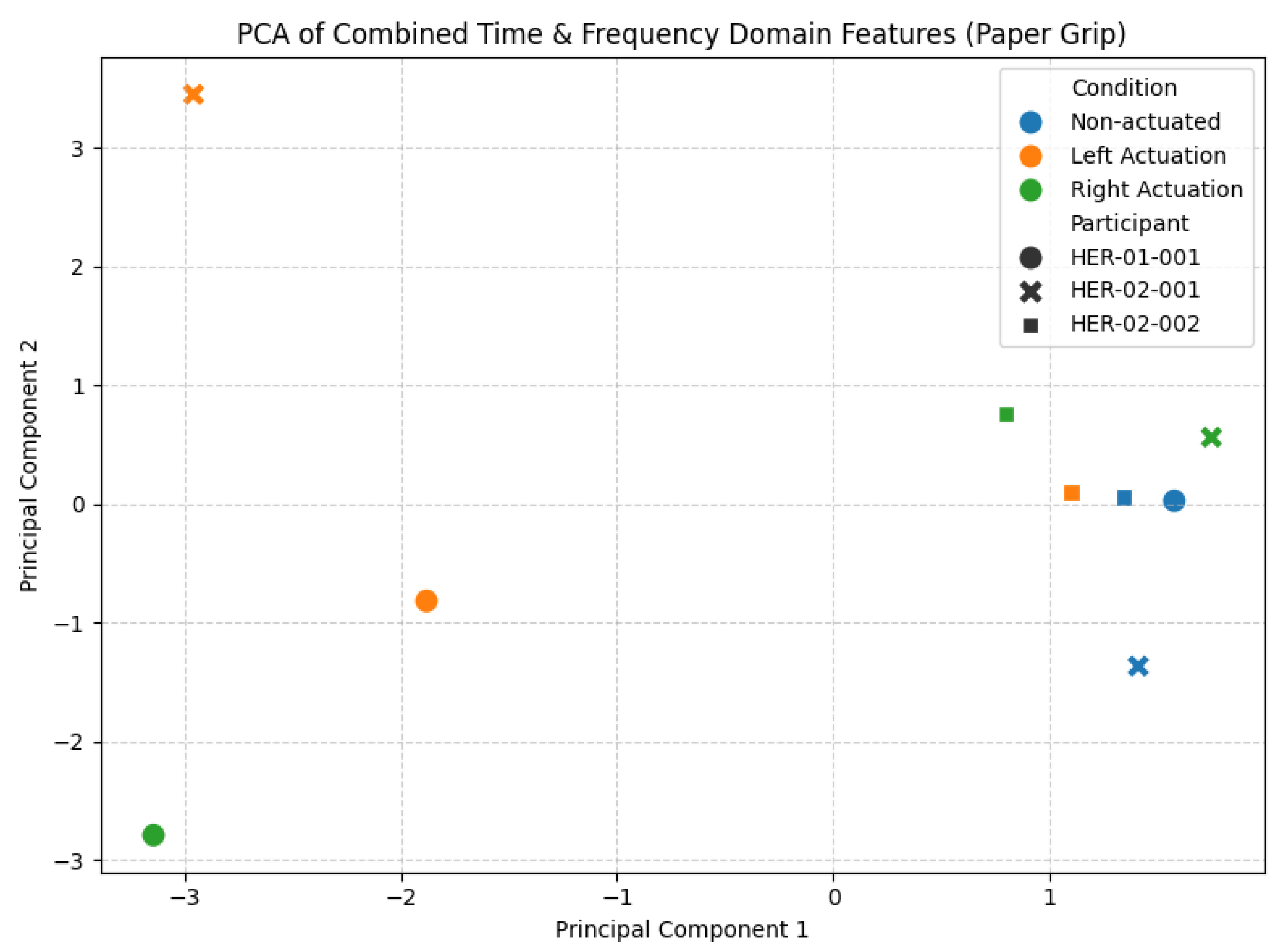
3.2.3. Paper Grip
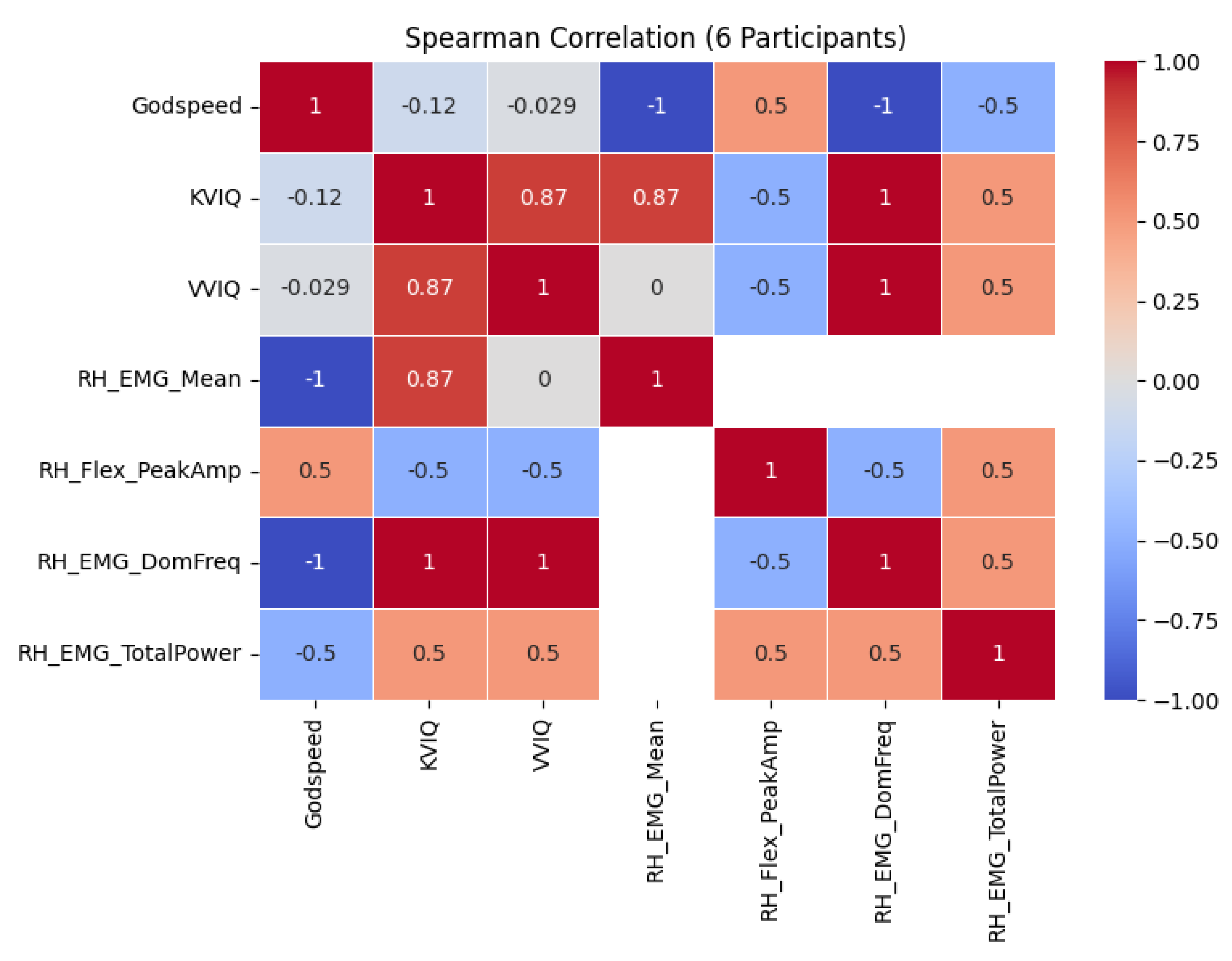
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADLs | Activities of Daily Living |
| AES | Apathy Evaluation Scale |
| BAI | Beck Anxiety Index |
| BDI | Beck’s Depression Inventory |
| ComSS | Communication Software Subsystem |
| DoF | Degrees of Freedom |
| EMS | Electrical Muscle Stimulation |
| g-SCIM-III | Spinal Cord Independence Measure |
| IMUs | Inertial Measurement Units |
| ISNCSCI | International Standard for Neurological Classification of Spinal Cord Injury |
| KVIQ | Kinesthetic and Visual Imagery Questionnaire |
| MAS | Modified Ashworth Scale |
| MSS | Multi-Sensor Subsystem |
| PCA | Principal Component Analysis |
| QoL | Quality of Life |
| ROS | Robot Operating System |
| RSE | Rosenberg Self-Esteem Scale |
| SCI | Spinal Cord Injury |
| sEMG | Surface Electromyography |
| SR | Soft Robotics |
| SRG(s) | Soft Robotic Glove(s) |
| SRPAS | Soft Robotic Pneumatic Actuation Subsystem |
| VVIQ | Vividness of Visual Imagery Questionnaire |
References
- Fuglevand, A.J. Mechanical properties and neural control of human hand motor units. J. Physiol. 2011, 589, 5595–5602. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, C.D.; Irvine, T.A.; Sims, J.L.; Wang, Y.L.; Su, A.W.; Norris, D.O. Complex hand dexterity: A review of biomechanical methods for measuring musical performance. Front. Psychol. 2014, 5, 414. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Qi, Y.; Wu, X.; Zhu, J.; Zhang, J.; Wang, Y. Decoding Joint-Level Hand Movements With Intracortical Neural Signals in a Human Brain–Computer Interface. IEEE Trans. Cogn. Dev. Syst. 2024, 16, 2100–2109. [Google Scholar] [CrossRef]
- Vanderkolff, S.M.; Maibauer, I.; Amin, N. Beyond sight: Environmental interaction with the hands or feet? J. Neurophysiol. 2025, 133, 622–624. [Google Scholar] [CrossRef]
- Paraskevopoulos, E.; Herholz, S. Multisensory integration and neuroplasticity in the human cerebral cortex. Transl. Neurosci. 2013, 4, 337–348. [Google Scholar] [CrossRef]
- Dollar, A.M. Classifying human hand use and the activities of daily living. In The Human Hand as an Inspiration for Robot Hand Development; Springer International Publishing: Cham, Switzerland, 2014; pp. 201–216. [Google Scholar] [CrossRef]
- Wang, H.; Arceo, R.; Chen, S.; Ding, L.; Jia, J.; Yao, J. Effectiveness of interventions to improve hand motor function in individuals with moderate to severe stroke: A systematic review protocol. BMJ Open 2019, 9, e032413. [Google Scholar] [CrossRef]
- Nam, H.U.; Huh, J.S.; Yoo, J.N.; Hwang, J.M.; Lee, B.J.; Min, Y.S.; Kim, C.H.; Jung, T.D. Effect of dominant hand paralysis on quality of life in patients with subacute stroke. Ann. Rehabil. Med. 2014, 38, 450–457. [Google Scholar] [CrossRef]
- Rivers, C.S.; Fallah, N.; Noonan, V.K.; Whitehurst, D.G.; Schwartz, C.E.; Finkelstein, J.A.; Craven, B.C.; Ethans, K.; O’Connell, C.; Truchon, B.C.; et al. Health conditions: Effect on function, health-related quality of life, and life satisfaction after traumatic spinal cord injury. A prospective observational registry cohort study. Arch. Phys. Med. Rehabil. 2018, 99, 443–451. [Google Scholar] [CrossRef]
- Hmaied Assadi, S.; Barel, H.; Dudkiewicz, I.; Gross-Nevo, R.F.; Rand, D. Less-affected hand function is associated with independence in daily living: A longitudinal study poststroke. Stroke 2022, 53, 939–946. [Google Scholar] [CrossRef]
- Lili, L.; Sunnerhagen, K.S.; Rekand, T.; Alt Murphy, M. Independence and upper extremity functioning after spinal cord injury: A cross-sectional study. Sci. Rep. 2023, 13, 3148. [Google Scholar] [CrossRef]
- World Health Organization. Spinal Cord Injury: Fact Sheets. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury (accessed on 29 September 2024).
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; Grupper, M.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke 2025, 20, 132–144. [Google Scholar] [CrossRef] [PubMed]
- WHO EMRO | Stroke, Cerebrovascular Accident | Health Topics — emro.who.int. Available online: https://www.emro.who.int/health-topics/stroke-cerebrovascular-accident/index.html (accessed on 3 April 2025).
- CDC. Stroke Facts — cdc.gov. Available online: https://www.cdc.gov/stroke/data-research/facts-stats/index.html (accessed on 3 April 2025).
- Gherman, B.; Zima, I.; Vaida, C.; Tucan, P.; Pisla, A.; Birlescu, I.; Machado, J.; Pisla, D. Robotic Systems for Hand Rehabilitation—Past, Present and Future. Technologies 2025, 13, 37. [Google Scholar] [CrossRef]
- Perera, O.; Liyanapathirana, R.; Gargiulo, G.; Gunawardana, U. A Review of Soft Robotic Actuators and Their Applications in Bioengineering, with an Emphasis on HASEL Actuators’ Future Potential. Actuators 2024, 13, 524. [Google Scholar] [CrossRef]
- Hong, R.; Li, B.; Bao, Y.; Liu, L.; Jin, L. Therapeutic robots for post-stroke rehabilitation. Med Rev. 2024, 4, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.; Sierro, T.; Niu, T.; Sarino, M.E.; Sarrafzadeh, M.; McArthur, D.; Edgerton, V.R.; Lu, D.C. Rehabilitation of hand function after spinal cord injury using a novel handgrip device: A pilot study. J. Neuroeng. Rehabil. 2017, 14, 22. [Google Scholar] [CrossRef]
- Marek, K.; Redlicka, J.; Miller, E.; Zubrycki, I. Objectivizing Measures of Post-Stroke Hand Rehabilitation through Multi-Disciplinary Scales. J. Clin. Med. 2023, 12, 7497. [Google Scholar] [CrossRef] [PubMed]
- Edemekong, P.F.; Bomgaars, D.L.; Sukumaran, S.; Schoo, C. Activities of Daily Living; StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ragnarsson, K.T. Functional electrical stimulation after spinal cord injury: Current use, therapeutic effects and future directions. Spinal Cord 2007, 46, 255–274. [Google Scholar] [CrossRef]
- Maggio, M.G.; Bonanno, M.; Manuli, A.; Calabrò, R.S. Improving Outcomes in People with Spinal Cord Injury: Encouraging Results from a Multidisciplinary Advanced Rehabilitation Pathway. Brain Sci. 2024, 14, 140. [Google Scholar] [CrossRef]
- Smith, S.N.; Crocker, A.F. Experiential learning in physical therapy education. Adv. Med. Educ. Pract. 2017, 8, 427–433. [Google Scholar] [CrossRef]
- Osuagwu, B.A.; Timms, S.; Peachment, R.; Dowie, S.; Thrussell, H.; Cross, S.; Shirley, R.; Segura-Fragoso, A.; Taylor, J. Home-based rehabilitation using a soft robotic hand glove device leads to improvement in hand function in people with chronic spinal cord injury:a pilot study. J. Neuroeng. Rehabil. 2020, 17, 40. [Google Scholar] [CrossRef]
- Polygerinos, P.; Wang, Z.; Galloway, K.C.; Wood, R.J.; Walsh, C.J. Soft robotic glove for combined assistance and at-home rehabilitation. Robot. Auton. Syst. 2015, 73, 135–143. [Google Scholar] [CrossRef]
- Proulx, C.E.; Beaulac, M.; David, M.; Deguire, C.; Haché, C.; Klug, F.; Kupnik, M.; Higgins, J.; Gagnon, D.H. Review of the effects of soft robotic gloves for activity-based rehabilitation in individuals with reduced hand function and manual dexterity following a neurological event. J. Rehabil. Assist. Technol. Eng. 2020, 7, 205566832091813. [Google Scholar] [CrossRef]
- Athanasiou, A.; Mitsopoulos, K.; Praftsiotis, A.; Astaras, A.; Antoniou, P.; Pandria, N.; Petronikolou, V.; Kasimis, K.; Lyssas, G.; Terzopoulos, N.; et al. Neurorehabilitation Through Synergistic Man–Machine Interfaces Promoting Dormant Neuroplasticity in Spinal Cord Injury: Protocol for a Nonrandomized Controlled Trial. JMIR Res. Protoc. 2022, 11, e41152. [Google Scholar] [CrossRef] [PubMed]
- Mitsopoulos, K.; Fiska, V.; Tagaras, K.; Papias, A.; Antoniou, P.; Nizamis, K.; Kasimis, K.; Sarra, P.D.; Mylopoulou, D.; Savvidis, T.; et al. NeuroSuitUp: System Architecture and Validation of a Motor Rehabilitation Wearable Robotics and Serious Game Platform. Sensors 2023, 23, 3281. [Google Scholar] [CrossRef]
- Fardipour, S.; Hadadi, M. Investigation of therapeutic effects of wearable robotic gloves on improving hand function in stroke patients: A systematic review. Curr. J. Neurol. 2022, 21, 125. [Google Scholar] [CrossRef]
- Fiska, V. Development of a Wearable Exoskeletal Device Based on Multi-Sensor Data Fusion & Soft Robotics for Neural Rehabilitation of the Human Hand. Master’s Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2022. [Google Scholar]
- Polygerinos, P.; Lyne, S.; Wang, Z.; Nicolini, L.F.; Mosadegh, B.; Whitesides, G.M.; Walsh, C.J. Towards a soft pneumatic glove for hand rehabilitation. In Proceedings of the 2013 IEEE/RSJ International Conference on Intelligent Robots and Systems, Tokyo, Japan, 3–7 November 2013; pp. 1512–1517. [Google Scholar] [CrossRef]
- Manns, M.; Morales, J.; Frohn, P. Additive manufacturing of silicon based PneuNets as soft robotic actuators. Procedia CIRP 2018, 72, 328–333. [Google Scholar] [CrossRef]
- SparkFun Electronics. SparkFun Qwiic Flex Glove Controller. Available online: https://www.sparkfun.com/products/14666 (accessed on 29 September 2024).
- FF Group. Antistatic PU Coated Gloves with Polyester Carbon Fiber Knitting. Available online: https://products.ffgroup-toolindustries.com/product/4888/antistatic-pu-coated-gloves-with-polyester-carbon-fiber-knitting (accessed on 29 September 2024).
- ClinicalTrials.gov, I.N. NeuroSuitUp: Neurorehabilitation through Synergistic Man–Machine Interfaces. 2022. Available online: https://clinicaltrials.gov/study/NCT05465486 (accessed on 3 April 2024).
- ClinicalTrials.gov, I.N. HEROES: Human Extremity Robotic Rehabilitation and Outcome Enhancement for Stroke (HEROES). 2024. Available online: https://clinicaltrials.gov/study/NCT06160453 (accessed on 3 April 2025).
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.; et al. International standards for neurological classification of spinal cord injury (revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef]
- Athanasiou, A.; Alexandrou, A.; Paraskevopoulos, E.; Foroglou, N.; Prassas, A.; Bamidis, P.D. Towards a Greek adaptation of the Spinal Cord Independence Measure (SCIM). In Proceedings of the 15th European Congress of Neurosurgery, Prague, Czech Republic, 12–17 October 2014; pp. 184–187. [Google Scholar] [CrossRef]
- Michailidou, C.; Marston, L.; De Souza, L.H. Translation into Greek and initial validity and reliability testing of a modified version of the SCIM III, in both English and Greek, for self-use. Disabil. Rehabil. 2016, 38, 180–188. [Google Scholar] [CrossRef]
- Duncan, P.W.; Bode, R.K.; Lai, S.M.; Perera, S.; Glycine Antagonist in Neuroprotection Americas Investigators. Rasch analysis of a new stroke-specific outcome scale: The Stroke Impact Scale. Arch. Phys. Med. Rehabil. 2003, 84, 950–963. [Google Scholar] [CrossRef]
- Patel, N.; Rao, V.A.; Heilman-Espinoza, E.R.; Lai, R.; Quesada, R.A.; Flint, A.C. Simple and reliable determination of the modified rankin scale score in neurosurgical and neurological patients: The mRS-9Q. Neurosurgery 2012, 71, 971–975. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [PubMed]
- Bohannon, R.W. Comfortable and maximum walking speed of adults aged 20—79 years: Reference values and determinants. Age Ageing 1997, 26, 15–19. [Google Scholar] [CrossRef]
- Meseguer-Henarejos, A.B.; Sánchez-Meca, J.; López-Pina, J.A.; Carles-Hernández, R. Inter-and intra-rater reliability of the Modified Ashworth Scale: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2017, 54, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893. [Google Scholar] [CrossRef]
- At, B. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Carbin, M.G. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 1988, 8, 77–100. [Google Scholar] [CrossRef]
- Rosenberg, M. Rosenberg self-esteem scale. In PsycTESTS Dataset; American Psychological Association: Washington, DC, USA, 1965. [Google Scholar] [CrossRef]
- Galanou, C.; Galanakis, M.; Alexopoulos, E.; Darviri, C. Rosenberg self-esteem scale Greek validation on student sample. Psychology 2014, 5, 819. [Google Scholar] [CrossRef]
- Marin, R.S.; Biedrzycki, R.C.; Firinciogullari, S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991, 38, 143–162. [Google Scholar] [CrossRef]
- Isaac, A.; Marks, D.F.; Russell, D.G. An instrument for assessing imagery of movement: The Vividness of Movement Imagery Questionnaire (VMIQ). J. Ment. Imag. 1986, 10, 23–30. [Google Scholar]
- Malouin, F.; Richards, C.L.; Jackson, P.L.; Lafleur, M.F.; Durand, A.; Doyon, J. The Kinesthetic and Visual Imagery Questionnaire (KVIQ) for assessing motor imagery in persons with physical disabilities: A reliability and construct validity study. J. Neurol. Phys. Ther. 2007, 31, 20–29. [Google Scholar] [CrossRef]
- Konstantinidis, E.I.; Billis, A.; Bratsas, C.; Siountas, A.; Bamidis, P.D. Thessaloniki Active and Healthy Ageing Living Lab: The roadmap from a specific project to a living lab towards openness. In Proceedings of the 9th ACM International Conference on PErvasive Technologies Related to Assistive Environments, Corfu Island, Greece, 29 June–1 July 2016; pp. 1–4. [Google Scholar] [CrossRef]
- Bartneck, C.; Kulić, D.; Croft, E.; Zoghbi, S. Measurement instruments for the anthropomorphism, animacy, likeability, perceived intelligence, and perceived safety of robots. Int. J. Soc. Robot. 2009, 1, 71–81. [Google Scholar] [CrossRef]
- Astaras, A.; Athanasiou, A.; Alexandrou, A.; Kartsidis, P.; Moustakas, N.; Bamidis, P. Double-blind greek translation and online implementation of the Godspeed robotics questionnaire. In Proceedings of the 6th Panhellenic Conference on Biomedical Technology Conference, Athens, Greece, 6–8 May 2015; p. 34. [Google Scholar]
- Zijlstra, F.; Van Doorn, L. The Construction of a Scale to Measure Perceived Effort; University of Technology: Delft, The Netherlands, 1985. [Google Scholar]
- Sauro, J.; Dumas, J.S. Comparison of three one-question, post-task usability questionnaires. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Boston, MA, USA, 4–9 April 2009; pp. 1599–1608. [Google Scholar] [CrossRef]
- Song, X.; Van De Ven, S.S.; Liu, L.; Wouda, F.J.; Wang, H.; Shull, P.B. Activities of daily living-based rehabilitation system for arm and hand motor function retraining after stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Jarque-Bou, N.J.; Gracia-Ibáñez, V.; Vergara, M.; Sancho-Bru, J.L. The BE-UJI Hand Function Activity Set: A reduced set of activities for the evaluation of the healthy and pathological hand. J. Neuroeng. Rehabil. 2023, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Rudhe, C.; Van Hedel, H.J. Upper extremity function in persons with tetraplegia: Relationships between strength, capacity, and the spinal cord independence measure. Neurorehabilit. Neural Repair. 2009, 23, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Scivoletto, G.; Tamburella, F.; Laurenza, L.; Molinari, M. The spinal cord independence measure: How much change is clinically significant for spinal cord injury subjects. Disabil. Rehabil. 2013, 35, 1808–1813. [Google Scholar] [CrossRef]
- Kogan, E.; Twyman, K.; Heap, J.; Milentijevic, D.; Lin, J.H.; Alberts, M. Assessing stroke severity using electronic health record data: A machine learning approach. BMC Med. Inform. Decis. Mak. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Balasch i Bernat, M.; Balasch i Parisi, S.; Sebastián, E.N.; Moscardó, L.D.; Ferri Campos, J.; Lopez Bueno, L. Determining cut-off points in functional assessment scales in stroke. NeuroRehabilitation 2015, 37, 165–172. [Google Scholar] [CrossRef]
- Mills, P.B.; Holtz, K.A.; Szefer, E.; Noonan, V.K.; Kwon, B.K. Early predictors of developing problematic spasticity following traumatic spinal cord injury: A prospective cohort study. J. Spinal Cord Med. 2020, 43, 315–330. [Google Scholar] [CrossRef]
- Pundik, S.; Falchook, A.D.; McCabe, J.; Litinas, K.; Daly, J.J. Functional brain correlates of upper limb spasticity and its mitigation following rehabilitation in chronic stroke survivors. Stroke Res. Treat. 2014, 2014, 306325. [Google Scholar] [CrossRef]
- Dionne, A.; Mac-Thiong, J.M.; Alsofyani, M.A.; Richard-Denis, A. Are early onset spasms predictive of poor neurological recovery after traumatic spinal cord injury? J. Spinal Cord Med. 2024, 47, 566–572. [Google Scholar] [CrossRef]
- Sangari, S.; Chen, B.; Grover, F.; Salsabili, H.; Sheth, M.; Gohil, K.; Hobbs, S.; Olson, A.; Eisner-Janowicz, I.; Anschel, A.; et al. Spasticity predicts motor recovery for patients with subacute motor complete spinal cord injury. Ann. Neurol. 2024, 95, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Poveda-García, A.; Moret-Tatay, C.; Gómez-Martínez, M. The association between mental motor imagery and real movement in stroke. Healthcare 2021, 9, 1568. [Google Scholar] [CrossRef] [PubMed]
- Banyai, A.D.; Brișan, C. Robotics in physical rehabilitation: Systematic Review. Healthcare 2024, 12, 1720. [Google Scholar] [CrossRef] [PubMed]
- Beckerle, P.; Salvietti, G.; Unal, R.; Prattichizzo, D.; Rossi, S.; Castellini, C.; Hirche, S.; Endo, S.; Amor, H.B.; Ciocarlie, M.; et al. A human–robot interaction perspective on assistive and rehabilitation robotics. Front. Neurorobotics 2017, 11, 24. [Google Scholar] [CrossRef]
- Weiss, A.; Bartneck, C. Meta analysis of the usage of the godspeed questionnaire series. In Proceedings of the 2015 24th IEEE International Symposium on Robot and Human Interactive Communication (RO-MAN), Kobe, Japan, 31 August–September 2015; pp. 381–388. [Google Scholar]
- Arvanitidis, A.; Mitsopoulos, K.; Fiska, V.; Athanasiou, A.; Bamidis, P.D. Design of A Normative sEMG Database for Biometric Comparison in Rehabilitation Research. Glob. Clin. Eng. J. 2024, 6, 96–101. [Google Scholar] [CrossRef]
- Arsenidis, A.; Moraitopoulos, A.; Athanasiou, A.; Vildiridis, A.; Bamidis, P.; Stefaneas, P.; Astaras, A. Novel Functional Electrical Stimulation Parameter Optimization for Neurorehabilitation Using Both Conventional and AI Techniques. Glob. Clin. Eng. J. 2024, 6, 45–50. [Google Scholar] [CrossRef]








| Subsystem | Components | Function | Interaction |
|---|---|---|---|
| Multi-sensor subsystem (MSS) | Piezoelectric pressure sensor, finger flexion sensors (across fingers), sEMG sensor, accelerometer | Measures piezoelectric pressure, finger flexion, muscle activity, and hand 3D position. Collects data on hand movements and muscle responses | Sends sensory data to communication software subsystem (ComSS) for processing. Data used to evaluate motor performance and physiological responses. |
| Soft robotic pneumatic actuation subsystem (SRPAS) | PneuNet-based flexible soft robotic exoskeleton | Applies controlled forces to the hand for therapy and motor function improvement | Receives control signals from ComSS. |
| Communication software subsystem (ComSS) | Microcontroller-based, control algorithms. Data collection software | Processes real-time sensor data. Sends commands to the SRPAS | Orchestrates the interaction between MSS and SRPAS. Ensures real-time response to hand movements and therapy stimuli. |
| Participant Info | NSU-01-001 | NSU-02-001 | NSU-02-002 |
|---|---|---|---|
| Age, sex | 62, male | 21, male | 53, male |
| Time since injury | 18 months | 24 months | 37 years |
| Injury cause | MVA | MVA | Dive |
| Complete or incomplete | Complete | Incomplete | Incomplete |
| NLI | C6 | C4 | C7 |
| AIS | A | C | B |
| UEMS | 40 | 22/50 | 26 |
| LEMS | 0 | 2/50 | 0 |
| LT | 34 | 41/112 | 46 |
| PP | 32 | 39/112 | 84 |
| MAS level UE | 1 | 1+ | 2 |
| MAS level LE | 3 | 3 | 0 |
| SCIM total | 47/100 | 41/100 | 65/100 |
| self-care | 10/20 | 7/20 | 16/20 |
| respiration and sphincter | 26/40 | 26/40 | 34/40 |
| mobility | 11/40 | 8/40 | 15/40 |
| AES | 29 | 27 | 20 |
| BAI | 0 | 1 | 1 |
| BDI | 7 | 3 | 0 |
| RSE | 27 | 24 | 30 |
| KVIQ | 50 | 49 | 50 |
| VVIQ | 79 | 80 | 80 |
| Participant Info | HER-01-001 | HER-02-001 | HER-02-002 |
|---|---|---|---|
| Age, sex | 51, male | 54, male | 23, male |
| Time since stroke | 31 months | 39 months | 15 months |
| Type | Ischemic | Hemorrhagic | Ischemic |
| Lesion Side | Right | Left | Right |
| Affected Side | Left | Right | Left |
| Phenotype | Paraplegia | Paraparesis | Paraparesis |
| Oxford Arm | 0 | 3+ | 4 |
| Oxford Hand | 0 | 4+ | 2− |
| Oxford Leg | 3 | 4 | 5− |
| Oxford Foot | 0 | 3 | 4 |
| MAS level UE | 4 | 2 | 3 |
| MAS level LE | 2 | 4 | 2 |
| SIS-Strength | 6.25 | 75 | 75 |
| SIS-Hand | 0 | 70 | 40 |
| SIS-ADL | 37.5 | 82.5 | 57.5 |
| SIS-Mobility | 77.78 | 83.33 | 97.22 |
| SIS-Communication | 96.43 | 100 | 96.43 |
| SIS-Emotion | 58.33 | 41.67 | 55.56 |
| SIS-Memory and Thinking | 8.16 | 92.86 | 85.71 |
| SIS-Participation | 37.5 | 68.75 | 46.88 |
| SIS-Perceived Recovery | 60 | 60 | 55 |
| SIS Mean% | 42.44 | 74.90 | 67.70 |
| mRS-9Q | 3 | 3 | 2 |
| TUG 3 m (s) | 44.74 | 19.85 | 11.71 |
| Timed 10 m self (m/s) | 0.27 | 0.63 | 0.725 |
| Timed 10 m fast (m/s) | 0.37 | 0.94 | 1.37 |
| AES | 43 | 26 | 52 |
| BAI | 1 | 2 | 10 |
| BDI | 1 | 4 | 25 |
| RSE | 14 | 17 | 18 |
| KVIQ | 13 | 47 | 39 |
| KVIQL | 8 | 38 | 29 |
| KVIQR | 10 | 37 | 35 |
| VVIQ | 40 | 72 | 55 |
| Questionnaire | NSU -01-001 | NSU -02-001 | NSU -02-002 | NSU Mdn (IQR) | HER -01-001 | HER -02-001 | HER -02-002 | HER Mdn (IQR) | Total Mdn (IQR) |
|---|---|---|---|---|---|---|---|---|---|
| Godspeed-Total (/120) | 73 | 78 | 71 | 73 (3.5) | 90 | 58 | 70 | 70 (16) | 72 (6.5) |
| Anthropo-morphism (/25) | 12 | 9 | 8 | 9 (2) | 14 | 13 | 11 | 13 (1.5) | 11.5 (3.25) |
| Animosity (/30) | 14 | 9 | 13 | 13 (2.5) | 17 | 15 | 10 | 15 (3.5) | 13.5 (4) |
| Likeability (/25) | 17 | 23 | 20 | 20 (3) | 20 | 7 | 17 | 17 (6.5) | 18.5 (3) |
| Perceived Intelligence (/25) | 15 | 22 | 15 | 15 (3.5) | 24 | 8 | 17 | 17 (8) | 16 (5.75) |
| Perceived Safety (/15) | 15 | 15 | 15 | 15 (0) | 15 | 15 | 15 | 15 (0) | 15 (0) |
| LED | 0 | 0 | 0 | 0 (0) | 0 | 0 | 0 | 0 (0) | 0 (0) |
| SMEQ | 10 | 0 | 0 | 0 (5) | 0 | 10 | 70 | 10 (35) | 5 (10) |
| KVIQ | 50 | 49 | 50 | 50 (0) | 10 | 37 | 35 | 39 (17) | 48.5 (9) |
| VVIQ | 79 | 80 | 80 | 80 (0.5) | 40 | 72 | 55 | 55 (9) | 75.5 (20.5) |
| Timestamp | Event | Flex Index (V) | Flex Middle (V) | Flex Ring (V) | Flex Little (V) | Pressure Middle (V) | EMG (V) |
|---|---|---|---|---|---|---|---|
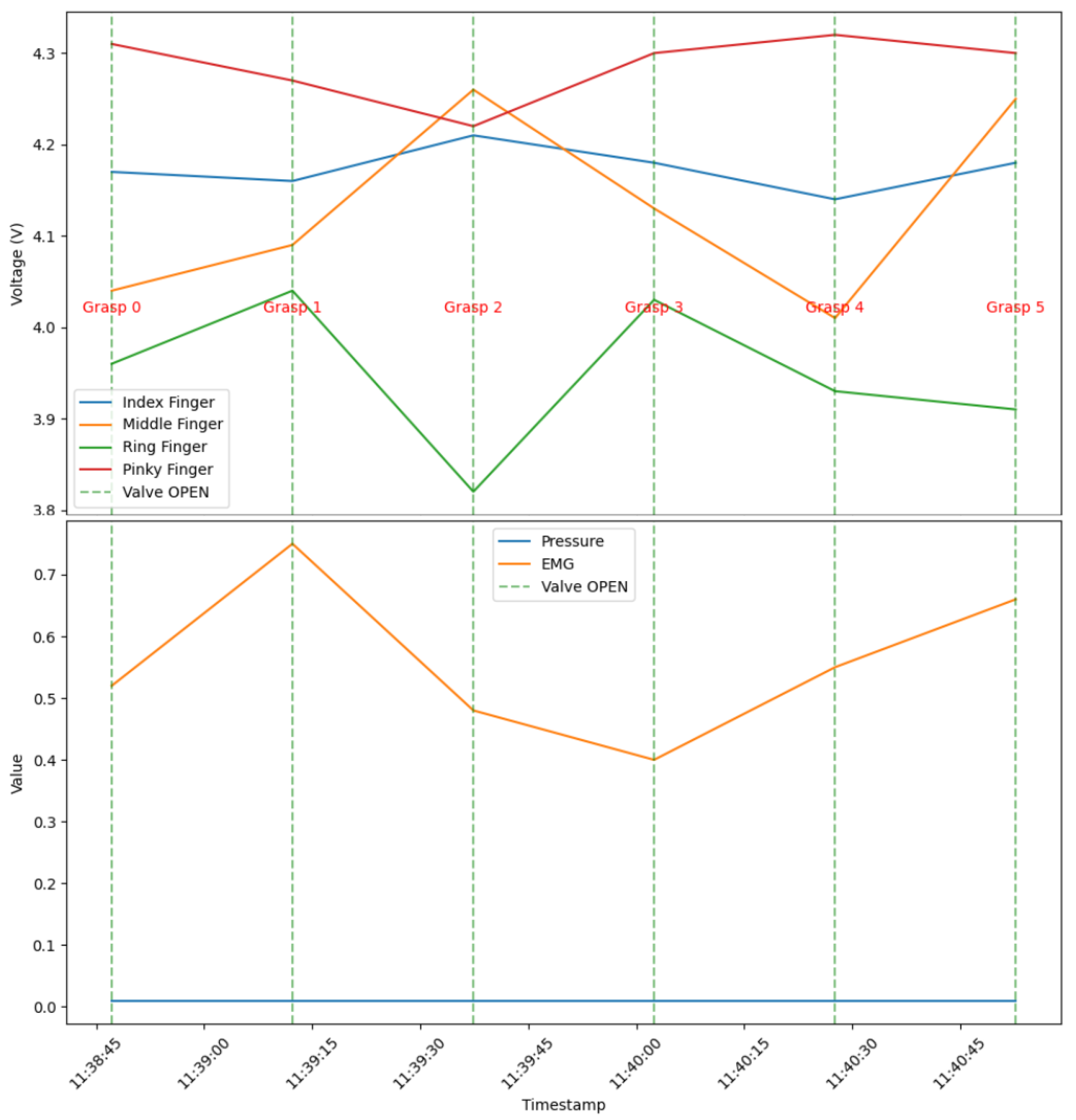
| 11:38:47 | 0 | 4.17 | 4.04 | 3.96 | 4.31 | 0.01 | 0.52 |
| 11:39:12 | 1 | 4.16 | 4.09 | 4.04 | 4.27 | 0.01 | 0.75 |
| 11:39:37 | 2 | 4.21 | 4.26 | 3.82 | 4.22 | 0.01 | 0.48 |
| 11:40:02 | 3 | 4.18 | 4.13 | 4.03 | 4.30 | 0.01 | 0.40 |
| 11:40:27 | 4 | 4.14 | 4.01 | 3.93 | 4.32 | 0.01 | 0.55 |
| 11:40:52 | 5 | 4.18 | 4.25 | 3.91 | 4.30 | 0.01 | 0.66 |
| Timestamp | Event | Flex Index (V) | Flex Middle (V) | Flex Ring (V) | Flex Pinky (V) | Pressure (V) | EMG (V) |
|---|---|---|---|---|---|---|---|
| 10:23:00 | 0 | 4.32 | 4.40 | 4.21 | 4.36 | 0.02 | 0.37 |
| 10:23:25 | 1 | 4.35 | 4.29 | 4.25 | 4.25 | 0.01 | 0.66 |
| 10:23:50 | 2 | 4.41 | 4.36 | 4.30 | 4.30 | 0.01 | 0.79 |
| 10:24:15 | 3 | 4.35 | 4.35 | 4.22 | 4.36 | 0.02 | 0.43 |
| 10:24:40 | 4 | 4.36 | 4.41 | 4.20 | 4.33 | 0.02 | 0.43 |
| 10:25:05 | 5 | 4.28 | 4.18 | 3.72 | 3.74 | 0.01 | 0.63 |
| Timestamp | Event | Flex Index (V) | Flex Middle (V) | Flex Ring (V) | Flex Pinky (V) | Pressure (V) | EMG (V) |
|---|---|---|---|---|---|---|---|
| 16:52:12 | 0 | 4.14 | 4.25 | 4.06 | 4.26 | 0.03 | 0.43 |
| 16:52:37 | 1 | 4.27 | 4.33 | 4.14 | 4.27 | 3.59 | 4.79 |
| 16:53:02 | 2 | 4.12 | 4.31 | 4.17 | 4.28 | 3.70 | 4.57 |
| 16:53:27 | 3 | 4.13 | 4.17 | 4.06 | 4.26 | 0.02 | 0.83 |
| 16:53:52 | 4 | 4.10 | 4.15 | 4.03 | 4.24 | 0.02 | 0.53 |
| 16:54:17 | 5 | 4.21 | 4.27 | 4.10 | 4.19 | 0.02 | 0.46 |
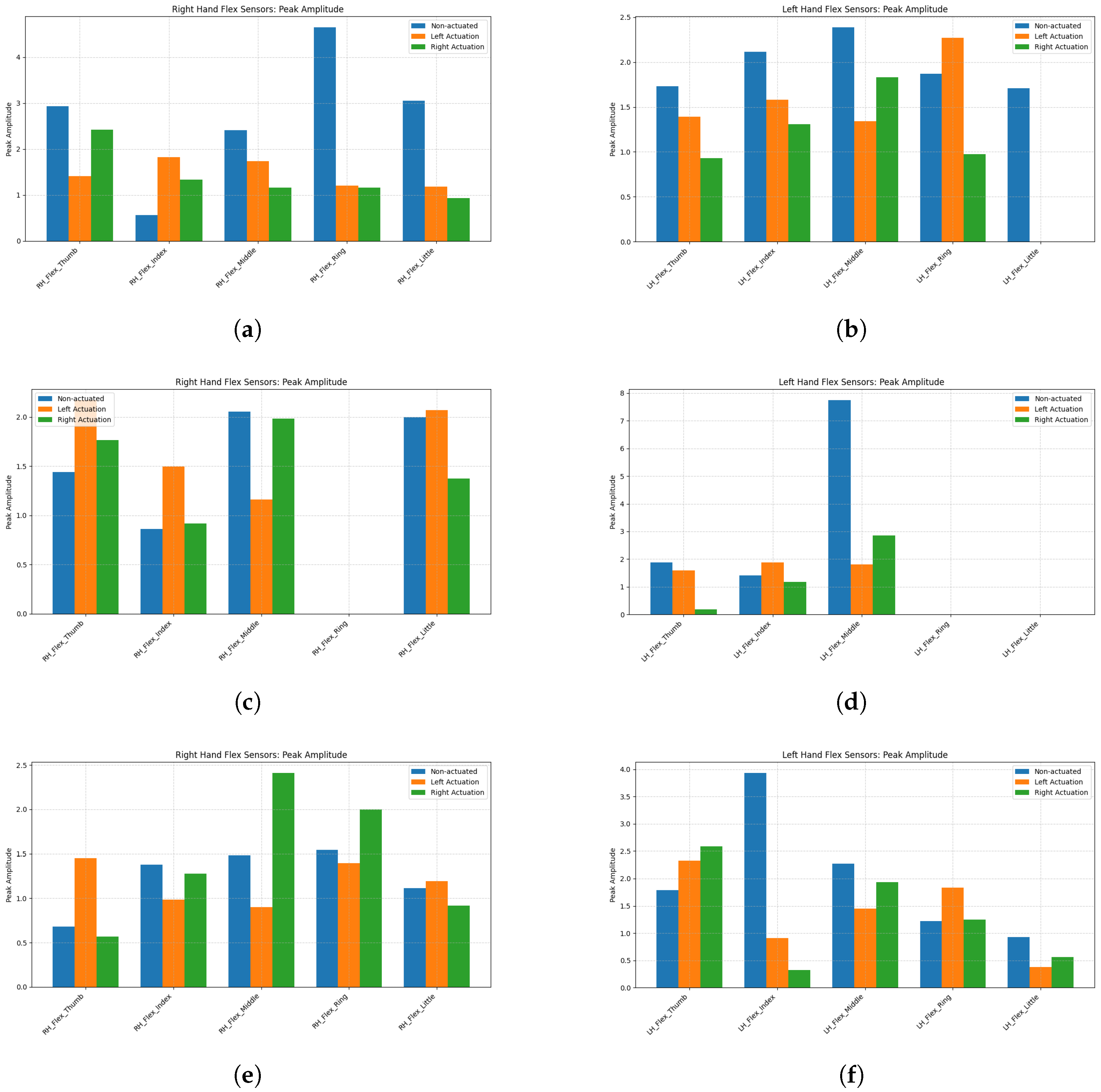
| Participant | Condition | LH Flex PeakAmp (V) | RH Flex PeakAmp (V) |
|---|---|---|---|
| HER-01-001 | Non-actuated | 1.962385 | 2.720541 |
| HER-01-001 | Left Actuation | 1.315986 | 1.473494 |
| HER-01-001 | Right Actuation | 1.009055 | 1.402194 |
| HER-02-001 | Non-actuated | 2.209620 | 1.271211 |
| HER-02-001 | Left Actuation | 1.057016 | 1.379960 |
| HER-02-001 | Right Actuation | 0.844384 | 1.207886 |
| HER-02-002 | Non-actuated | 2.028898 | 1.241410 |
| HER-02-002 | Left Actuation | 1.380525 | 1.186795 |
| HER-02-002 | Right Actuation | 1.331171 | 1.435131 |
| Participant | Condition | LH Accel Dominant Freq (Hz) | LH Accel Total Power () | RH Accel Dominant Freq (Hz) | RH Accel Total Power () |
|---|---|---|---|---|---|
| HER-01-001 | Non-actuated | 0.099354 | 1830.000000 | 0.099354 | 1830.000000 |
| HER-01-001 | Left Actuation | 0.099562 | 1888.322165 | 0.099562 | 1888.413307 |
| HER-01-001 | Right Actuation | 0.399840 | 1830.000000 | 0.233240 | 1830.000000 |
| HER-02-001 | Non-actuated | 1.035197 | 1889.605088 | 0.300541 | 1890.473058 |
| HER-02-001 | Left Actuation | 0.696933 | 1880.280903 | 0.398248 | 1884.340688 |
| HER-02-001 | Right Actuation | 0.233754 | 1875.938184 | 0.233754 | 1881.659661 |
| HER-02-002 | Non-actuated | 0.099562 | 1889.261171 | 0.099562 | 1888.783603 |
| HER-02-002 | Left Actuation | 0.400721 | 1889.190340 | 1.268951 | 1885.319101 |
| HER-02-002 | Right Actuation | 1.068590 | 1867.917990 | 1.703066 | 1871.429125 |
| Participant | Condition | LH EMG Dominant Freq (Hz) | LH EMG Total Power () | RH EMG Dominant Freq (Hz) | RH EMG Total Power () |
|---|---|---|---|---|---|
| HER-01-001 | Non-actuated | 0.099354 | 1830.000000 | 0.099354 | 1830.000000 |
| HER-01-001 | Left Actuation | 0.796495 | 1890.282550 | 0.199124 | 1854.152767 |
| HER-01-001 | Right Actuation | 0.299880 | 1830.000000 | 0.099960 | 1830.000000 |
| HER-02-001 | Non-actuated | 0.200361 | 1886.492856 | 0.400721 | 1889.190431 |
| HER-02-001 | Left Actuation | 0.099562 | 1887.675208 | 0.298686 | 1883.218107 |
| HER-02-001 | Right Actuation | 0.701262 | 1890.172429 | 0.500902 | 1888.627353 |
| HER-02-002 | Non-actuated | 0.398248 | 1890.866749 | 0.099562 | 1890.583968 |
| HER-02-002 | Left Actuation | 0.400721 | 1890.911656 | 0.100180 | 1823.081392 |
| HER-02-002 | Right Actuation | 0.300541 | 1886.715516 | 0.200361 | 1890.946518 |
| Participant | Condition | LH Flex PeakAmp (V) | RH Flex PeakAmp (V) |
|---|---|---|---|
| HER-01-001 | Non-actuated | 1.171659 | 1.042512 |
| HER-01-001 | Left Actuation | 0.889562 | 1.801664 |
| HER-01-001 | Right Actuation | 1.796198 | 2.546574 |
| HER-02-001 | Non-actuated | 1.593946 | 1.052788 |
| HER-02-001 | Left Actuation | 0.835917 | 1.644414 |
| HER-02-001 | Right Actuation | 0.682261 | 2.097046 |
| HER-02-002 | Non-actuated | 1.223903 | 1.329954 |
| HER-02-002 | Left Actuation | 1.455255 | 3.152663 |
| HER-02-002 | Right Actuation | 1.651295 | 3.163606 |
| Participant | Condition | LH Accel Dominant Freq (Hz) | LH Accel Total Power () | RH Accel Dominant Freq (Hz) | RH Accel Total Power () |
|---|---|---|---|---|---|
| HER-01-001 | Non-actuated | 0.099562 | 1878.102515 | 0.099562 | 1888.174548 |
| HER-01-001 | Left Actuation | 0.165937 | 1890.988507 | 0.099562 | 1887.806243 |
| HER-01-001 | Right Actuation | 0.099960 | 1830.000000 | 0.099960 | 1830.000000 |
| HER-02-001 | Non-actuated | 0.100180 | 1890.451842 | 0.100180 | 1890.495245 |
| HER-02-001 | Left Actuation | 0.268817 | 1889.718931 | 0.100806 | 1874.682196 |
| HER-02-001 | Right Actuation | 0.298686 | 1890.608011 | 0.099562 | 1832.358876 |
| HER-02-002 | Non-actuated | 0.133574 | 1889.471475 | 0.100180 | 1890.800619 |
| HER-02-002 | Left Actuation | 0.862870 | 1890.057772 | 0.365060 | 1878.191281 |
| HER-02-002 | Right Actuation | 0.333934 | 1890.839838 | 0.233754 | 1889.583496 |
| Participant | Condition | LH EMG Dominant Freq (Hz) | LH EMG Total Power () | RH EMG Dominant Freq (Hz) | RH EMG Total Power () |
|---|---|---|---|---|---|
| HER-01-001 | Non-actuated | 0.099562 | 1878.102515 | 0.099562 | 1888.174548 |
| HER-01-001 | Left Actuation | 0.398248 | 1890.988507 | 0.099562 | 1887.806243 |
| HER-01-001 | Right Actuation | 0.099960 | 1830.000000 | 0.299880 | 1830.000000 |
| HER-02-001 | Non-actuated | 0.100180 | 1890.451842 | 0.400721 | 1890.495245 |
| HER-02-001 | Left Actuation | 0.302419 | 1889.718931 | 0.302419 | 1862.682196 |
| HER-02-001 | Right Actuation | 0.199124 | 1890.608011 | 0.497810 | 1887.605027 |
| HER-02-002 | Non-actuated | 0.100180 | 1889.471475 | 0.500902 | 1890.800619 |
| HER-02-002 | Left Actuation | 0.099562 | 1890.057772 | 0.099562 | 1890.756482 |
| HER-02-002 | Right Actuation | 0.200361 | 1890.839838 | 0.500902 | 1890.997388 |
| Participant | Condition | LH Flex PeakAmp (V) | RH Flex PeakAmp (V) |
|---|---|---|---|
| HER-01-001 | Non-actuated | 1.962385 | 2.720541 |
| HER-01-001 | Left Actuation | 1.315986 | 1.473494 |
| HER-01-001 | Right Actuation | 1.009055 | 1.402194 |
| HER-02-001 | Non-actuated | 2.209620 | 1.271211 |
| HER-02-001 | Left Actuation | 1.057016 | 1.379960 |
| HER-02-001 | Right Actuation | 0.844384 | 1.207886 |
| HER-02-002 | Non-actuated | 2.028898 | 1.241410 |
| HER-02-002 | Left Actuation | 1.380525 | 1.186795 |
| HER-02-002 | Right Actuation | 1.331171 | 1.435131 |
| Participant | Condition | LH Accel Dominant Freq (Hz) | LH Accel Total Power () | RH Accel Dominant Freq (Hz) | RH Accel Total Power () |
|---|---|---|---|---|---|
| HER-01-001 | Non-actuated | 0.099354 | 1830.000000 | 0.099354 | 1830.000000 |
| HER-01-001 | Left Actuation | 0.099562 | 1888.322165 | 0.099562 | 1888.413307 |
| HER-01-001 | Right Actuation | 0.399840 | 1830.000000 | 0.233240 | 1830.000000 |
| HER-02-001 | Non-actuated | 1.035197 | 1889.605088 | 0.300541 | 1890.473058 |
| HER-02-001 | Left Actuation | 0.696933 | 1880.280903 | 0.398248 | 1884.340688 |
| HER-02-001 | Right Actuation | 0.233754 | 1875.938184 | 0.233754 | 1881.659661 |
| HER-02-002 | Non-actuated | 0.099562 | 1889.261171 | 0.099562 | 1888.783603 |
| HER-02-002 | Left Actuation | 0.400721 | 1890.911656 | 0.100180 | 1823.081392 |
| HER-02-002 | Right Actuation | 1.068590 | 1867.917990 | 1.703066 | 1871.429125 |
| Participant | Condition | LH EMG Dominant Freq (Hz) | LH EMG Total Power () | RH EMG Dominant Freq (Hz) | RH EMG Total Power () |
|---|---|---|---|---|---|
| HER-01-001 | Non-actuated | 0.099354 | 1830.000000 | 0.099354 | 1830.000000 |
| HER-01-001 | Left Actuation | 0.796495 | 1890.282550 | 0.199124 | 1854.152767 |
| HER-01-001 | Right Actuation | 0.299880 | 1830.000000 | 0.099960 | 1830.000000 |
| HER-02-001 | Non-actuated | 0.200361 | 1886.492856 | 0.400721 | 1889.190431 |
| HER-02-001 | Left Actuation | 0.099562 | 1887.675208 | 0.298686 | 1883.218107 |
| HER-02-001 | Right Actuation | 0.701262 | 1890.172429 | 0.500902 | 1888.627353 |
| HER-02-002 | Non-actuated | 0.398248 | 1890.866749 | 0.099562 | 1890.583968 |
| HER-02-002 | Left Actuation | 0.400721 | 1890.911656 | 0.100180 | 1823.081392 |
| HER-02-002 | Right Actuation | 0.300541 | 1886.715516 | 0.200361 | 1890.946518 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiska, V.; Mitsopoulos, K.; Mantiou, V.; Petronikolou, V.; Antoniou, P.; Tagaras, K.; Kasimis, K.; Nizamis, K.; Tsipouras, M.G.; Astaras, A.; et al. Integration and Validation of Soft Wearable Robotic Gloves for Sensorimotor Rehabilitation of Human Hand Function. Appl. Sci. 2025, 15, 5299. https://doi.org/10.3390/app15105299
Fiska V, Mitsopoulos K, Mantiou V, Petronikolou V, Antoniou P, Tagaras K, Kasimis K, Nizamis K, Tsipouras MG, Astaras A, et al. Integration and Validation of Soft Wearable Robotic Gloves for Sensorimotor Rehabilitation of Human Hand Function. Applied Sciences. 2025; 15(10):5299. https://doi.org/10.3390/app15105299
Chicago/Turabian StyleFiska, Vasiliki, Konstantinos Mitsopoulos, Vasiliki Mantiou, Vasileia Petronikolou, Panagiotis Antoniou, Konstantinos Tagaras, Konstantinos Kasimis, Konstantinos Nizamis, Markos G. Tsipouras, Alexander Astaras, and et al. 2025. "Integration and Validation of Soft Wearable Robotic Gloves for Sensorimotor Rehabilitation of Human Hand Function" Applied Sciences 15, no. 10: 5299. https://doi.org/10.3390/app15105299
APA StyleFiska, V., Mitsopoulos, K., Mantiou, V., Petronikolou, V., Antoniou, P., Tagaras, K., Kasimis, K., Nizamis, K., Tsipouras, M. G., Astaras, A., Bamidis, P. D., & Athanasiou , A. (2025). Integration and Validation of Soft Wearable Robotic Gloves for Sensorimotor Rehabilitation of Human Hand Function. Applied Sciences, 15(10), 5299. https://doi.org/10.3390/app15105299












