Conversion of Kitchen Waste into Sustainable Fertilizers: Comparative Effectiveness of Biological, Microbial, and Thermal Treatments in a Ryegrass Growth Trial
Abstract
1. Introduction
1.1. Kitchen Waste Generation
1.2. Methods for Processing Kitchen Waste
1.3. Usage of Effective Microorganisms
1.4. Focus of This Study—Decaying, EMs, Sterilization, Anaerobic Digestion, and Their Combinations
2. Materials and Methods
2.1. Fertilizers
2.2. Soil and Plants Preparation
2.3. Soil and Plant Analysis
3. Theory and Calculation
4. Results
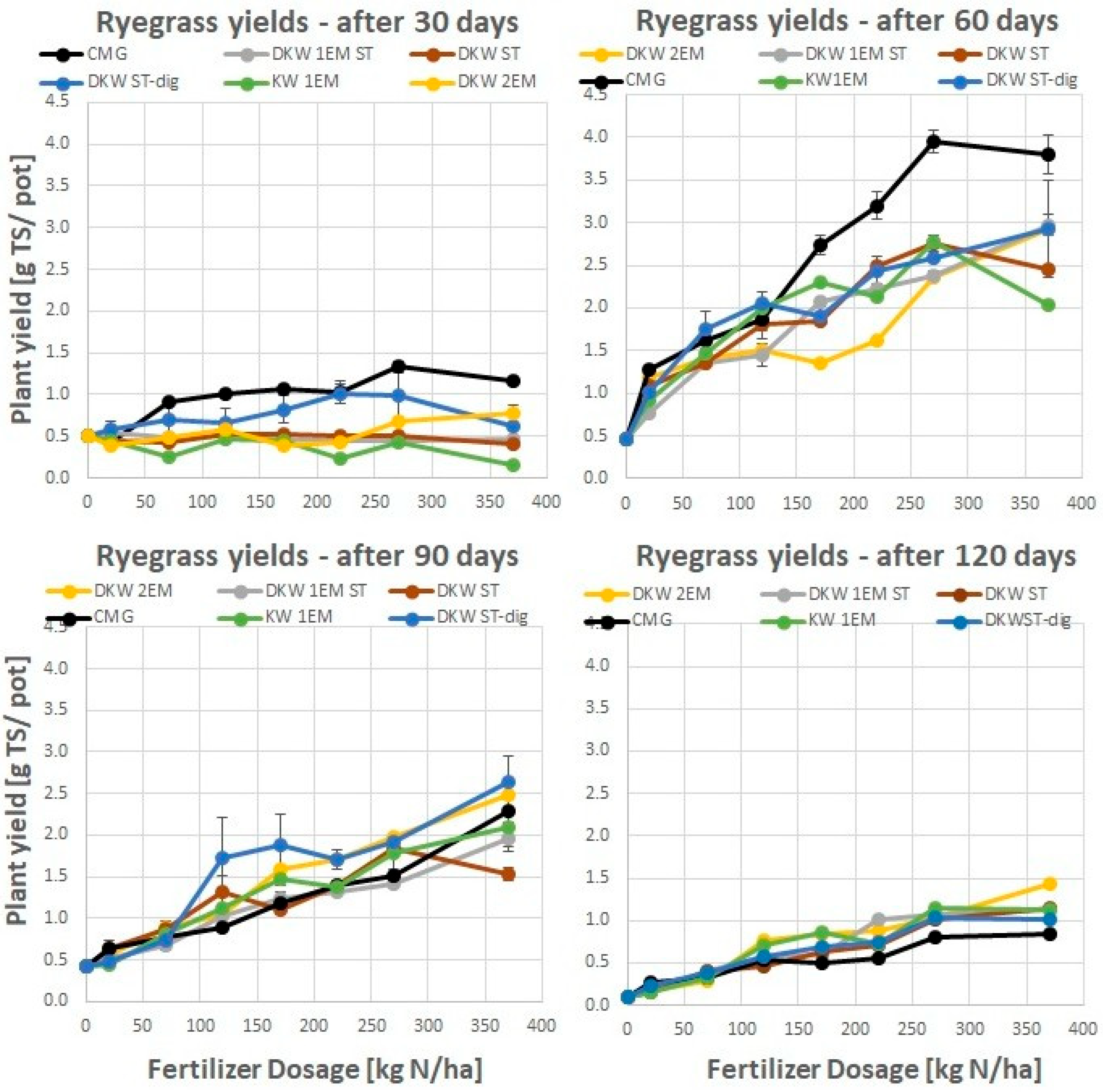
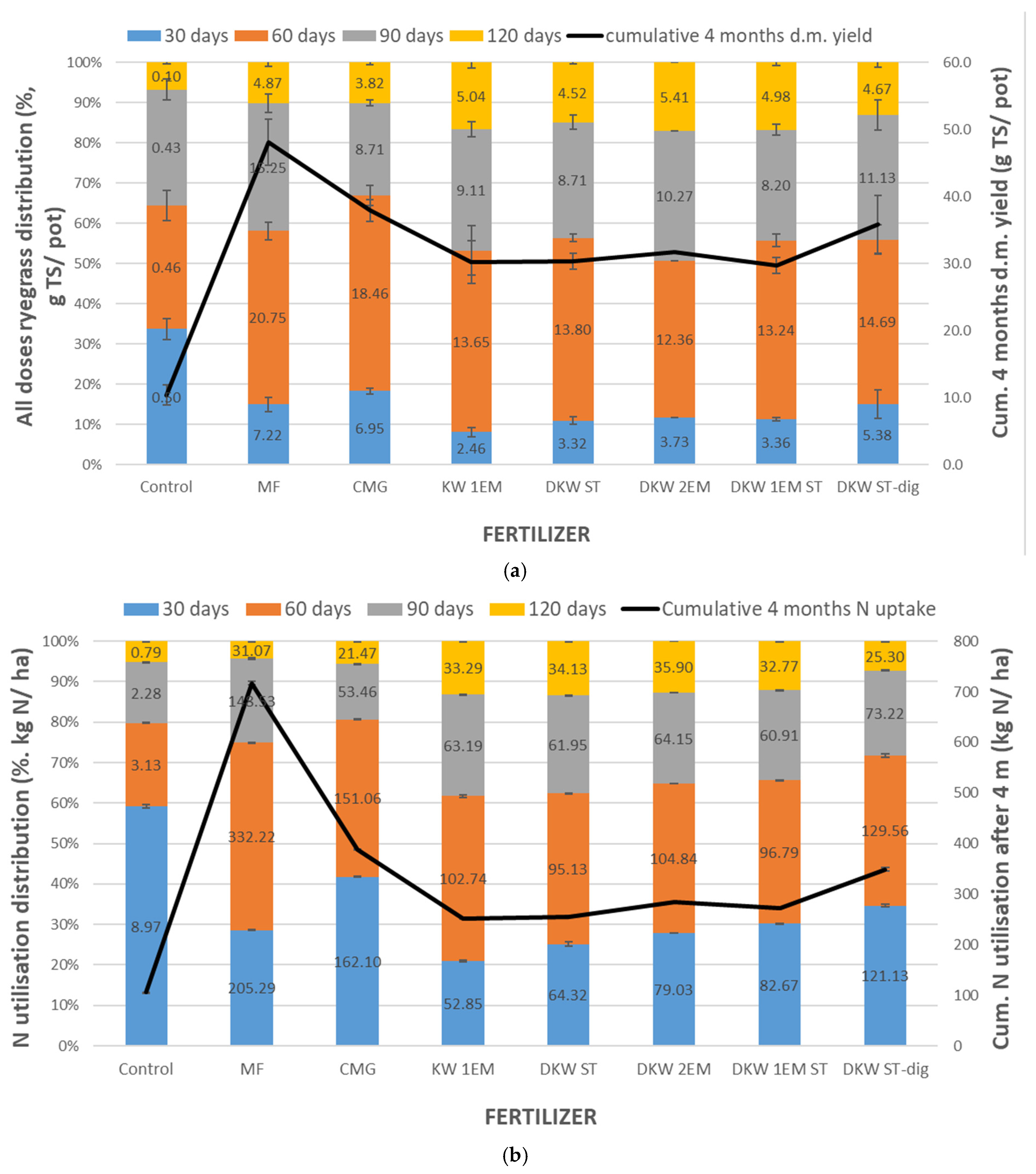
4.1. Response of the Ryegrass Biomass Yield to Kitchen Waste-Based Fertilizers
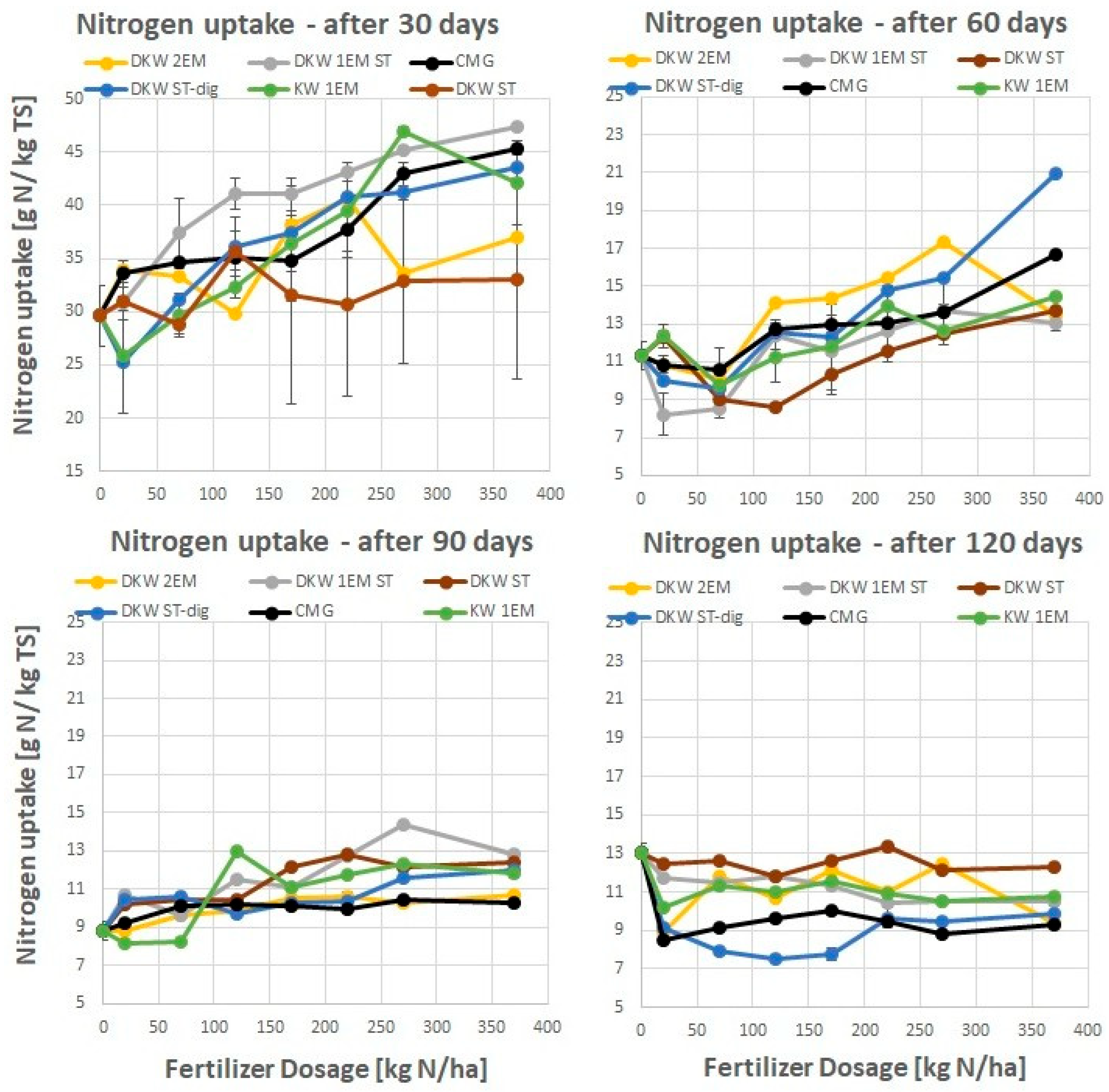
4.2. Nitrogen Uptake by Ryegrass Fertilised with Kitchen Waste-Based Fertilizers
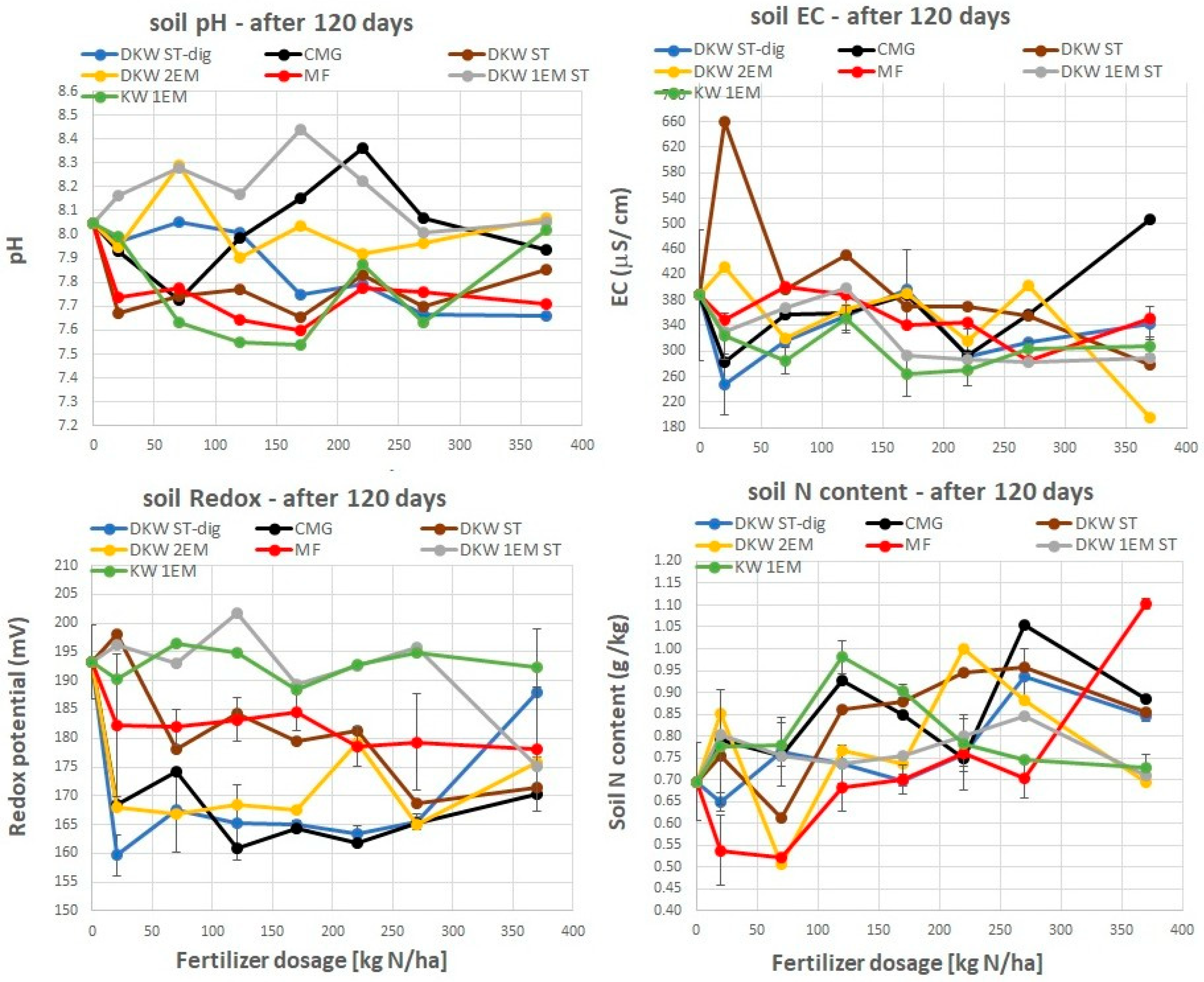
4.3. Residual Soil Properties After the Growth of Ryegrass
5. Discussion
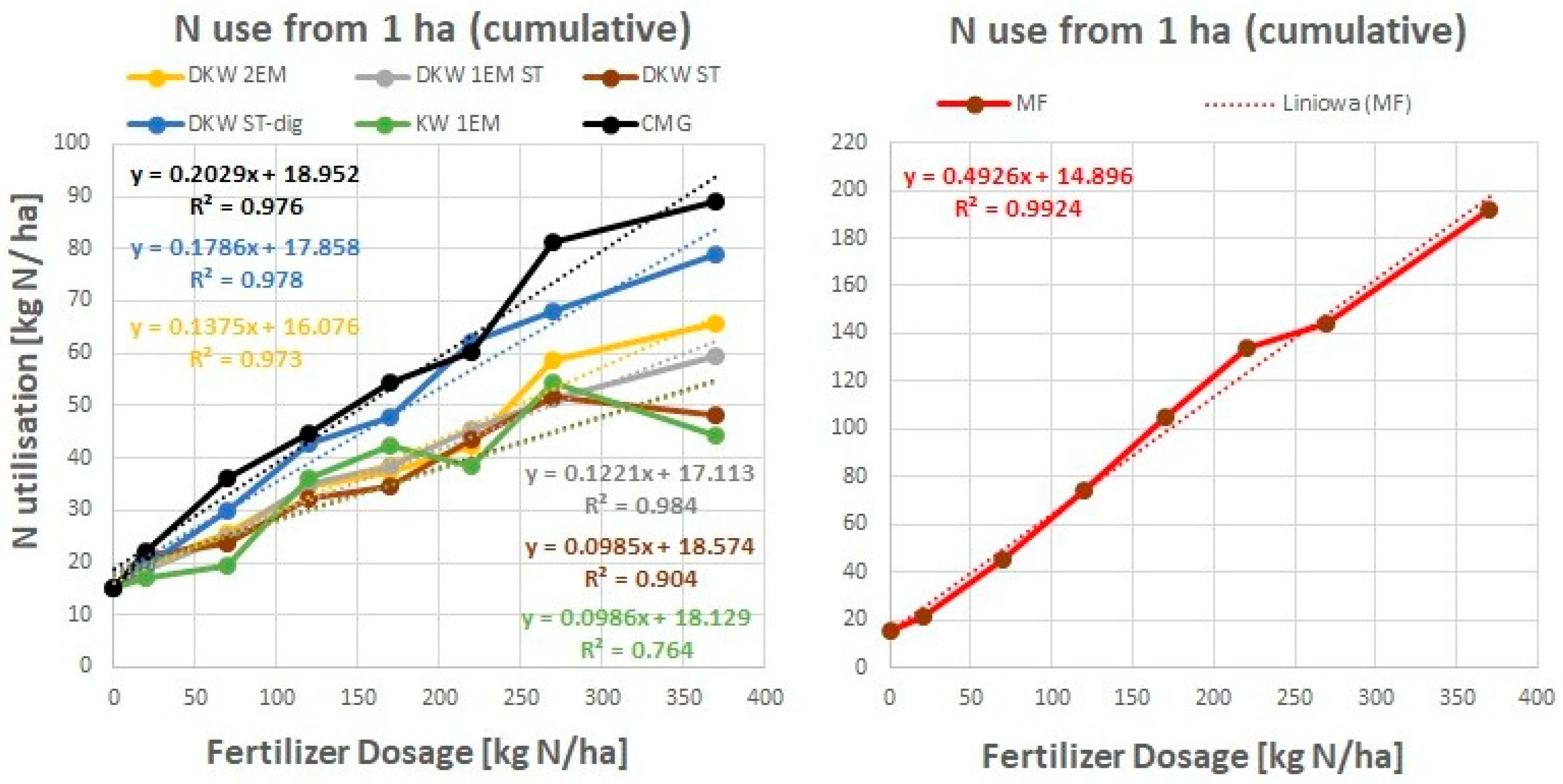
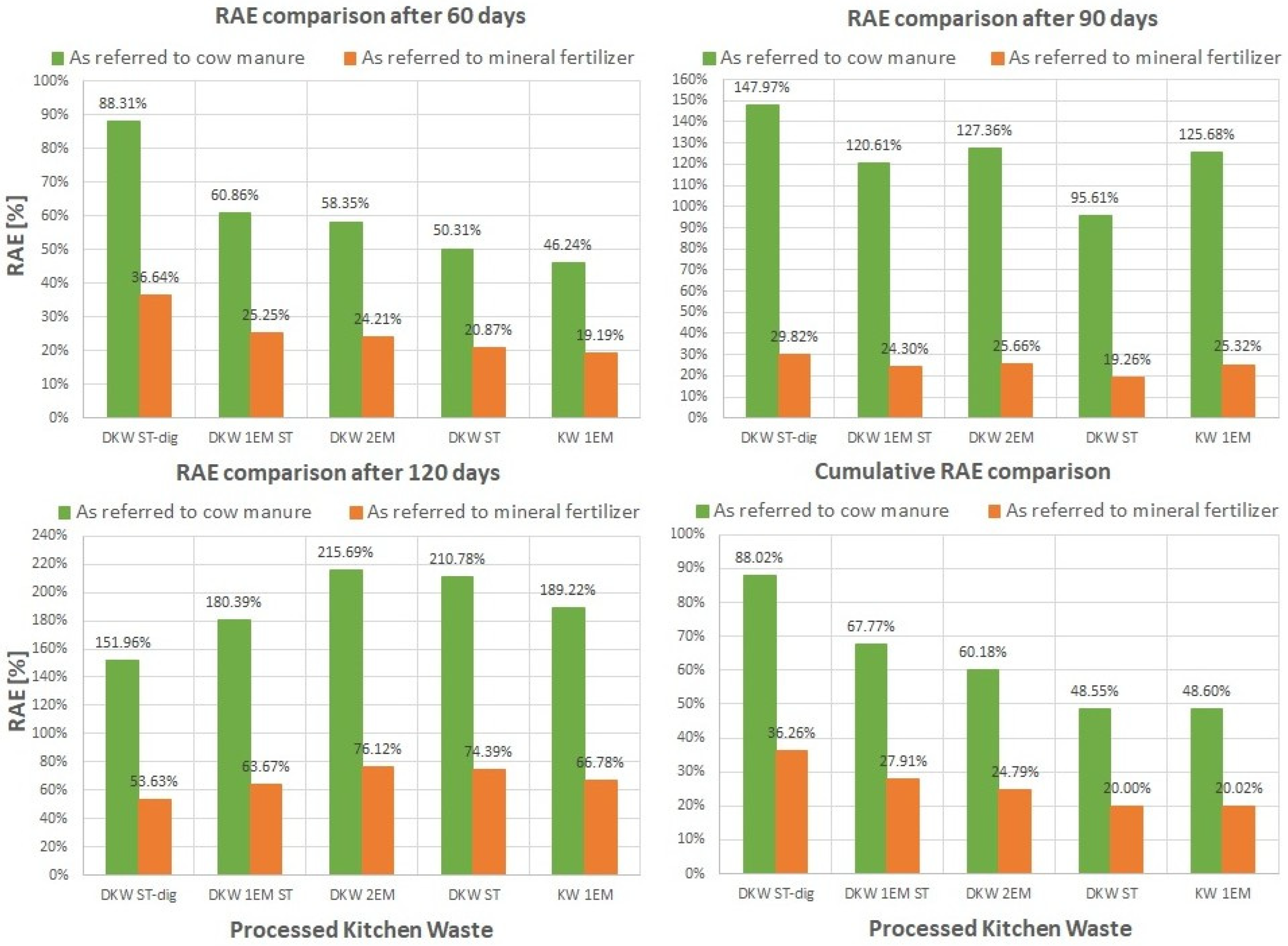
5.1. Absolute Agronomic Effectiveness
5.2. Relative Agronomic Effectiveness
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Sadhukhan, J.; Dugmore, T.I.J.; Matharu, A.; Martinez-Hernandez, E.; Aburto, J.; Rahman, P.K.S.M.; Lynch, J. Perspectives on “Game Changer” Global Challenges for Sustainable 21st Century: Plant-Based Diet, Unavoidable Food Waste Biorefining, and Circular Economy. Sustainability 2020, 12, 1976. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Sienkiewicz-Cholewa, U.; Baśladyńska, S.; Kocek, D.; Mironiuk, M.; Chojnacka, K. New Environmentally Friendly Bio-Based Micronutrient Fertilizer by Biosorption: From Laboratory Studies to the Field. Sci. Total Environ. 2020, 710, 136061. [Google Scholar] [CrossRef]
- Sun, X.; Dou, Z.; Shurson, G.C.; Hu, B. Bioprocessing to Upcycle Agro-Industrial and Food Wastes into High-Nutritional Value Animal Feed for Sustainable Food and Agriculture Systems. Resour. Conserv. Recycl. 2024, 201, 107325. [Google Scholar] [CrossRef]
- Zubair, M.A.; Esrafil, M.; Kona, F.T. Estimation of Nutritional Composition of Kitchen Wastes and Comparison of the Effect of Different Drying Methods on Bioactive Compounds in the Wastes. Food Humanit. 2023, 1, 1547–1558. [Google Scholar] [CrossRef]
- Moonsamy, T.A.; Rajauria, G.; Priyadarshini, A.; Jansen, M.A.K. Food Waste: Analysis of the Complex and Variable Composition of a Promising Feedstock for Valorisation. Food Bioprod. Process. 2024, 148, 31–42. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, H.; Zhang, H.; Xu, S.; Lichtfouse, E.; Yun, Y. Anaerobic Digestion and Recycling of Kitchen Waste: A Review. Environ. Chem. Lett. 2022, 20, 1745–1762. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New Opportunities for Agricultural Digestate Valorization: Current Situation and Perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Shi, L.; Simplicio, W.S.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient Recovery from Digestate of Anaerobic Digestion of Livestock Manure: A Review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Menkveld, H.W.H.; Broeders, E. Recovery of Ammonium from Digestate as Fertilizer. Water Pract. Technol. 2017, 12, 514–519. [Google Scholar] [CrossRef]
- Tampio, E.; Salo, T.; Rintala, J. Agronomic Characteristics of Five Different Urban Waste Digestates. J. Environ. Manag. 2016, 169, 293–302. [Google Scholar] [CrossRef]
- Lu, J.; Xu, S. Post-Treatment of Food Waste Digestate towards Land Application: A Review. J. Clean. Prod. 2021, 303, 127033. [Google Scholar] [CrossRef]
- Ahn, K.; Lee, K.B.; Kim, Y.J.; Koo, Y.M. Quantitative Analysis of the Three Main Genera in Effective Microorganisms Using QPCR. Korean J. Chem. Eng. 2014, 31, 849–854. [Google Scholar] [CrossRef]
- Van Fan, Y.; Lee, C.T.; Klemeš, J.J.; Chua, L.S.; Sarmidi, M.R.; Leow, C.W. Evaluation of Effective Microorganisms on Home Scale Organic Waste Composting. J. Environ. Manag. 2018, 216, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Higa, T.; Parr, J.F. Beneficial and Effective Microorganisms for a Sustainable Agriculture and Environment; International Nature Farming Research Center: Atami, Japan, 1994. [Google Scholar]
- Kinati, C.; Ameha, N.; Girma, M.; Nurfeta, A. Effective Microorganisms, Turmeric (Curcuma Longa), and Their Combination on Performance and Economic Benefits in Broilers. Heliyon 2022, 8, e09568. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Mageed, T.A.; Rady, M.M.; Taha, R.S.; Abd El Azeam, S.; Simpson, C.R.; Semida, W.M. Effects of Integrated Use of Residual Sulfur-Enhanced Biochar with Effective Microorganisms on Soil Properties, Plant Growth and Short-Term Productivity of Capsicum annuum under Salt Stress. Sci. Hortic. 2020, 261, 108930. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.-Y.; Zhang, J.; Liu, Y.-H.; Tang, D.-Y.; Zhao, J.; Dai, C.-C. By Reconstructing a Multifunctional Intensive Microbiome, Effective Microorganisms (EM) Improve the Ecological Environment of Rice-Crayfish Cocropping. Agric. Ecosyst. Environ. 2023, 357, 108698. [Google Scholar] [CrossRef]
- Hermann-Bandera, A.A.; Uhlmann, L.O.; Bortoluzzi, E.C.; Petry, C. Do Effective Microorganisms and Supermagro Fertilizers Improve Gladiolus Quality? S. Afr. J. Bot. 2023, 161, 90–95. [Google Scholar] [CrossRef]
- Chaurasia, J.; Poudel, B.; Mandal, T.; Acharya, N.; Ghimirey, V. Effect of Micronutrients, Rhizobium, Salicylic Acid, and Effective Microorganisms in Plant Growth and Yield Characteristics of Green Gram [Vigna radiata (L.) Wilczek] in Rupandehi, Nepal. Heliyon 2024, 10, e26821. [Google Scholar] [CrossRef]
- Kannahi, M.; Dhivya, U. Production of Health Drink Using Effective Microorganisms and Medicinal Plant Extracts. J. Chem. Pharm. Res. 2014, 6, 496–500. [Google Scholar]
- Vijayanand, P.S.; Viswanathan, G.; Manjunath, N.V.; Balakrishnaraja, R.; Dharchana, R.; Ragashravanthi, R. Model Study on Bioremediation Process for the Treatment of Polluted River through Effective Microorganisms. Mater. Today Proc. 2022, 66, 1231–1234. [Google Scholar] [CrossRef]
- Tang, S.; Gong, J.; Song, B.; Cao, W.; Li, J. Remediation of Biochar-Supported Effective Microorganisms and Microplastics on Multiple Forms of Heavy Metals in Eutrophic Lake. J. Hazard. Mater. 2024, 465, 133098. [Google Scholar] [CrossRef]
- Kaur, B.; Choudhary, R.; Sharma, G.; Brar, L.K. Sustainable and Effective Microorganisms Method for Wastewater Treatment. Desalin. Water Treat. 2024, 319, 100419. [Google Scholar] [CrossRef]
- Boruszko, D. Research on the Influence of Anaerobic Stabilization of Various Dairy Sewage Sludge on Biodegradation of Polycyclic Aromatic Hydrocarbons PAHs with the Use of Effective Microorganisms. Environ. Res. 2017, 155, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Huseien, G.F.; Hussein Joudah, Z.; Hafizah, A.; Khalid, N.; Mohd Sam, A.R.; Tahir, M.; Abdul Shukor Lim, N.H.; Alyousef, R.; Mirza, J. Durability Performance of Modified Concrete Incorporating Fly Ash and Effective Microorganism. Constr. Build. Mater. 2021, 267, 120947. [Google Scholar] [CrossRef]
- Nathaniel, O.; Sam, A.R.M.; Lim, N.H.A.S.; Adebisi, O.; Abdulkareem, M. Biogenic Approach for Concrete Durability and Sustainability Using Effective Microorganisms: A Review. Constr. Build. Mater. 2020, 261, 119664. [Google Scholar] [CrossRef]
- Huseien, G.F.; Memon, R.P.; Baghban, M.H.; Faridmehr, I.; Wong, L.S. Effective Microorganism Solution-Imbued Sustainable Self-Curing Concrete: Evaluation of Sorptivity, Drying Shrinkage and Expansion. Case Stud. Constr. Mater. 2024, 20, e03255. [Google Scholar] [CrossRef]
- Cai, J.Z.; Yu, Y.L.; Yang, Z.B.; Xu, X.X.; Lv, G.C.; Xu, C.L.; Wang, G.Y.; Qi, X.; Li, T.; Man, Y.B.; et al. Synergistic Improvement of Humus Formation in Compost Residue by Fenton-like and Effective Microorganism Composite Agents. Bioresour. Technol. 2024, 400, 130703. [Google Scholar] [CrossRef]
- Boruszko, D. Impact of Effective Microorganisms on the Vermicomposting of Sewage Sludge. Desalin. Water Treat. 2023, 288, 273–282. [Google Scholar] [CrossRef]
- Onn, M.; Ahmad, Z.; Zainuddin, A.; Mohammad Iliyas, S.M. Morphology and Characterization Study on Effective Microorganism (EM) Water Based Epoxy Coatings. Mater. Today Proc. 2022, 66, 4026–4032. [Google Scholar] [CrossRef]
- Iranzo, M.; Cañizares, J.V.; Roca-Perez, L.; Sainz-Pardo, I.; Mormeneo, S.; Boluda, R. Characteristics of Rice Straw and Sewage Sludge as Composting Materials in Valencia (Spain). Bioresour. Technol. 2004, 95, 107–112. [Google Scholar] [CrossRef]
- Zhong, Z.; Bian, F.; Zhang, X. Testing Composted Bamboo Residues with and without Added Effective Microorganisms as a Renewable Alternative to Peat in Horticultural Production. Ind. Crops Prod. 2018, 112, 602–607. [Google Scholar] [CrossRef]
- Jusoh, M.L.C.; Manaf, L.A.; Latiff, P.A. Composting of Rice Straw with Effective Microorganisms (EM) and Its Influence on Compost Quality. J. Environ. Health Sci. Eng. 2013, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.B.; Maung, C.E.H.; Kim, K.Y. Metagenomic Analysis Reveals Enhanced Biodiversity and Composting Efficiency of Lignocellulosic Waste by Thermoacidophilic Effective Microorganism (TEM). J. Environ. Manag. 2020, 276, 111252. [Google Scholar] [CrossRef] [PubMed]
- Mupondi, L.T.; Mnkeni, P.N.S.; Brutsch, M.O. The Effects of Goat Manure, Sewage Sludge and Effective Microorganisms on the Composting of Pine Bark. Compost. Sci. Util. 2006, 14, 201–210. [Google Scholar] [CrossRef]
- Hu, C.; Qi, Y. Long-Term Effective Microorganisms Application Promote Growth and Increase Yields and Nutrition of Wheat in China. Eur. J. Agron. 2013, 46, 63–67. [Google Scholar] [CrossRef]
- Li, Y.; Luo, W.; Wang, B.; Lin, T.; Li, C.; Liu, H.; Huang, Y.; Lin, C.; Tong, Y.; Lei, Z. Effects of Probiotic Fermented Kitchen Waste on the Growth and Propagation of Rotifer Brachionus calyciflorus. J. Biobased Mater. Bioenergy 2021, 15, 83–89. [Google Scholar] [CrossRef]
- Giri, B.S.; Sarowgi, A.; Kaushik, Y.; Pal, A.; Jaiswal, A.; Kumari, S.; Singh, H.; Sonwani, R.; Thivaharan, V.; Singh, R.S. Indoor Potted Plant Based Biofilter: Performance Evaluation and Kinetics Study. Indian J. Exp. Biol. 2019, 57, 879–886. [Google Scholar]
- Tang, J.; Riley, W.J. Finding Liebig’s Law of the Minimum. Ecol. Appl. 2021, 31, e02458. [Google Scholar] [CrossRef]
- Cordovil, C.M.d.S.; Cabral, F.; Coutinho, J. Potential Mineralization of Nitrogen from Organic Wastes to Ryegrass and Wheat Crops. Bioresour. Technol. 2007, 98, 3265–3268. [Google Scholar] [CrossRef]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of Green Waste Compost and Biochar Soil Amendments for Reducing Lead and Copper Mobility and Uptake to Ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef]
- Murillo, J.M.; Cabrera, F.; López, R.; Martín-Olmedo, P. Testing Low-Quality Urban Composts for Agriculture: Germination and Seedling Performance of Plants. Agric. Ecosyst. Environ. 1995, 54, 127–135. [Google Scholar] [CrossRef]
- Rayment, G.E.; Higginson, F.R. Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata Press: Melbourne, VIC, Australia, 1992. [Google Scholar]
- European Union. The European Comission Commission Regulation (EU) No 142/2011 of 25 February 2011 Implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council Laying down Health Rules as Regards Animal by-Products and Derived Products Not Intended for Human Consumption. Off. J. Eur. Union 2011, L 54, 1–254. [Google Scholar]
- Konkol, I.; Świerczek, L.; Cenian, A. Biogas Production from Bakery Wastes-Dynamics, Retention Time and Biogas Potential. J. Res. Appl. Agric. Eng. 2018, 63, 32–34. [Google Scholar]
- VDI 4630; Fermentation of Organic Materials, Characterization of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. Verein Deutscher Ingenieure: Düsseldorf, Germany, 2006.
- DIN 38414 (S8); German Standard Methods for the Examination of Water, Waste Water and Sludge; Sludge and Sediments (Group S); Determination of the Amenability to Anaerobic Digestion (S 8). European Standard: Berlin, Germany, 2017.
- Act of 20 July 2017. Water Law. Article 105-Maximum Dose of Natural Fertilizers Used for Agricultural Purposes. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20170001566/U/D20171566Lj.pdf (accessed on 4 May 2025).
- Bolland, M.D.A.; Gilkes, R.J. The Poor Performance of Rock Phosphate Fertilizers in Western Australia: Part 1. The Crop and Pasture Response. Agric. Sci. 1990, 3, 8–43. [Google Scholar]
- Kuligowski, K.; Gilkes, R.J.; Poulsen, T.G.; Yusiharni, B.E. Ash from the Thermal Gasification of Pig Manure—Effects on Ryegrass Yield, Element Uptake, and Soil Properties. Soil Res. 2012, 50, 406–415. [Google Scholar] [CrossRef]
- Yusiharni, B.E.; Ziadi, H.; Gilkes, R.J. A Laboratory and Glasshouse Evaluation of Chicken Litter Ash, Wood Ash, and Iron Smelting Slag as Liming Agents and P Fertilisers. Aust. J. Soil Res. 2007, 45, 374–389. [Google Scholar] [CrossRef]
- Kuligowski, K.; Konkol, I.; Świerczek, L.; Chojnacka, K.; Cenian, A.; Szufa, S. Evaluation of Kitchen Waste Recycling as Organic N-Fertiliser for Sustainable Agriculture under Cool and Warm Seasons. Sustainability 2023, 15, 7997. [Google Scholar] [CrossRef]
- O’Connor, J.; Hoang, S.A.; Bradney, L.; Rinklebe, J.; Kirkham, M.B.; Bolan, N.S. Value of Dehydrated Food Waste Fertiliser Products in Increasing Soil Health and Crop Productivity. Environ. Res. 2022, 204, 111927. [Google Scholar] [CrossRef]
- Mandal, M.; Roy, A.; Das, S.; Rakwal, R.; Agrawal, G.K.; Singh, P.; Awasthi, A.; Sarkar, A. Food Waste-Based Bio-Fertilizers Production by Bio-Based Fermenters and Their Potential Impact on the Environment. Chemosphere 2024, 353, 141539. [Google Scholar] [CrossRef]
- Mahish, P.K.; Verma, D.K.; Ghritlahare, A.; Arora, C.; Otero, P. Microbial Bioconversion of Food Waste to Bio-Fertilizers. Sustain. Food Technol. 2024, 2, 689–708. [Google Scholar] [CrossRef]
- Srivastava, S.; Singh, P.; Barbora, L.; Baruah, D.; Saikia, R.; Mohanty, K.; Kalita, P. Performance Assessment of Innovative Waste Management System Developed for the Production of Bio-Fertilizer. Sustain. Chem. Environ. 2024, 7, 100148. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Mikulewicz, M. Valorisation of Agri-Food Waste to Fertilisers Is a Challenge in Implementing the Circular Economy Concept in Practice. Environ. Pollut. 2022, 312, 119906. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-Y.; Wang, S.-P.; Chu, X.-L.; Sun, Z.-Y.; Xia, Z.-Y.; Xie, C.-Y.; Gou, M.; Tang, Y.-Q. Valorizing Kitchen Waste to Produce Value-Added Fertilizer by Thermophilic Semi-Continuous Composting Followed by Static Stacking: Performance and Bacterial Community Succession Analysis. Bioresour. Technol. 2023, 373, 128732. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.-L.; Peng, X.-Y.; Sun, Z.-Y.; Xie, C.-Y.; Tang, Y.-Q. Converting Kitchen Waste into Value-Added Fertilizer Using Thermophilic Semi-Continuous Composting-Biofiltration Two-Stage Process with Minimized NH3 Emission. Bioresour. Technol. 2024, 406, 130955. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Li, Y.; Chi, B.; Huang, W.; Wu, J. Effects and Mechanisms of Kitchen Waste Organic Fertilizers Application on Soil Nitrogen Transformation, Plant Pathogenic Virulence Genes, and Metabolites. Chem. Eng. J. 2024, 496, 154125. [Google Scholar] [CrossRef]

| Material | Symbol | TS | VS | N-Total | P-Olsen | P-Total | K-Olsen | K-Total |
|---|---|---|---|---|---|---|---|---|
| Unit | % | % | g/kg | |||||
| Soil | - | 88.77 | 6.08 | 1.32 | 0.0190 | 0.186 | 0.0649 | 0.610 |
| S1 Kitchen waste 1 × dose EM-incubated | KW 1EM | 95 | 91.42 | 33.82 | 0.0486 | 1.511 | 5.715 | 8.482 |
| S2 Kitchen waste decayed, 2 × dose EM-incubated | DKW 2EM | 95 | 89.30 | 30.04 | NA | NA | NA | NA |
| S3 Kitchen waste decayed, 1 × dose, sterilised, EM-incubated | DKW 1EM ST | 95 | 89.30 | 32.18 | NA | NA | NA | NA |
| S4 Kitchen waste decayed, sterilized | DKW ST | 95 | 92.37 | 36.11 | ||||
| S5 Kitchen waste sterilized, digested | DKW ST-dig | 95 | 89.53 | 42.67 | NA | NA | NA | NA |
| Organic cow manure, granulated | CMG | 100 | NA | 30.00 | ||||
| Mineral fertilizer FLOROVIT NP. | MF | 100 | 0 | 190 | NA | NA | NA | NA |
| Scenario | Sample Weight [g] | Pre-Treatment | Fertilizer Symbol | ||||
|---|---|---|---|---|---|---|---|
| EM [ml] | Water [mL] | Decaying Time [Days] | Sterilization | Fermentation | |||
| S1 | 500 | 1 | 249 | - | - | - | KW 1EM |
| S2 | 2 | 248 | 12 | DKW 2EM | |||
| S3 | 1 | 249 | 1 h, 70 °C | DKW1 EM ST | |||
| S4 | - | - | DKW ST | ||||
| S5 | - | - | 21 days, 38 °C | DKW ST-dig | |||
| Scenario | S1 | S2 | S3 | S4 | S5 | REF CM | |||
|---|---|---|---|---|---|---|---|---|---|
| Dosage Nr | kg N/ha | g N/pot | mg N/kg TS Soil | g Fertilizer/pot | |||||
| 1 (normal) | 20 | 0.033 | 0.023 | 1.027 | 1.157 | 1.080 | 0.962 | 0.814 | 1.100 |
| 2 | 70 | 0.116 | 0.079 | 3.596 | 4.048 | 3.779 | 3.368 | 2.850 | 3.851 |
| 3 | 120 | 0.198 | 0.135 | 6.164 | 6.940 | 6.479 | 5.773 | 4.886 | 6.602 |
| 4 (max in PL) | 170 | 0.281 | 0.192 | 8.733 | 9.832 | 9.178 | 8.179 | 6.922 | 9.353 |
| 5 | 220 | 0.363 | 0.248 | 11.301 | 12.723 | 11.877 | 10.585 | 8.957 | 12.103 |
| 6 | 270 | 0.446 | 0.304 | 13.870 | 15.615 | 14.577 | 12.990 | 10.993 | 14.854 |
| 7 | 370 | 0.611 | 0.417 | 19.007 | 21.399 | 19.976 | 17.801 | 15.065 | 20.356 |
| Logistic Step | Recommendations |
|---|---|
| 1. Source identification |
|
| 2. Waste segregation at source |
|
| 3. Collection system |
|
| 4. Transportation |
|
| 5. Initial processing and screening |
|
| 6. Storage and treatment |
|
| 7. Partnerships and incentives |
|
| 8. Monitoring and quality control |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuligowski, K.; Konkol, I.; Świerczek, L.; Woźniak, A.; Cenian, A. Conversion of Kitchen Waste into Sustainable Fertilizers: Comparative Effectiveness of Biological, Microbial, and Thermal Treatments in a Ryegrass Growth Trial. Appl. Sci. 2025, 15, 5281. https://doi.org/10.3390/app15105281
Kuligowski K, Konkol I, Świerczek L, Woźniak A, Cenian A. Conversion of Kitchen Waste into Sustainable Fertilizers: Comparative Effectiveness of Biological, Microbial, and Thermal Treatments in a Ryegrass Growth Trial. Applied Sciences. 2025; 15(10):5281. https://doi.org/10.3390/app15105281
Chicago/Turabian StyleKuligowski, Ksawery, Izabela Konkol, Lesław Świerczek, Adrian Woźniak, and Adam Cenian. 2025. "Conversion of Kitchen Waste into Sustainable Fertilizers: Comparative Effectiveness of Biological, Microbial, and Thermal Treatments in a Ryegrass Growth Trial" Applied Sciences 15, no. 10: 5281. https://doi.org/10.3390/app15105281
APA StyleKuligowski, K., Konkol, I., Świerczek, L., Woźniak, A., & Cenian, A. (2025). Conversion of Kitchen Waste into Sustainable Fertilizers: Comparative Effectiveness of Biological, Microbial, and Thermal Treatments in a Ryegrass Growth Trial. Applied Sciences, 15(10), 5281. https://doi.org/10.3390/app15105281







