Featured Application
Natural products are an endless source of antimicrobial agents against many foodborne pathogenic bacteria. The present work demonstrates the potential of licorice root extract to efficiently inhibit the growth of a panel of Listeria strains and serotypes. Results support the utilization of the plant extract as a promising anti-Listeria agent for the food industry. Furthermore, it also demonstrates the combined use of advanced analytical and in silico methodologies to decode the active components of the extract, opening new horizons in the discovery of novel agents to control Listeria bacteria.
Abstract
Licorice roots are a rich source of bioactive compounds with multiple biological activities. The objective of this study was to evaluate the inhibitory effects of licorice root extract against a range of Listeria strains. In addition, the correlation of its phytochemical composition with antimicrobial properties was also investigated. Thus, the bacteriostatic and bactericidal effects of licorice root extract on seven Listeria monocytogenes strains, L. grayi, L. seeligeri, and L. ivanovii were determined. The minimum inhibitory and bactericidal concentrations ranged from 31.3 to 62.5 µg mL−1 and from 62.5 to 250 µg mL−1, respectively. The phytochemical composition of the extract was also analyzed using advanced LC-DAD- qTOF-MS; it is composed of fifty-one compounds belonging to different subgroups of flavonoids and triterpenoids. Subsequently, the anti-Listeria potency of the most abundant phytochemicals was determined using the AntiBac-Pred web tool. In silico calculation showed that liquiritin-apioside and licorice glycoside C1/C2 were strong growth inhibitors of L. monocytogenes, as their potency was comparable to well-known antibiotic substances. Overall, the present study demonstrates the potent antimicrobial effect of licorice root extract and reveals its active phytochemicals.
1. Introduction
Bacterial contamination is the primary cause of food poisoning and food-borne diseases, as well as a cause of losses due to food spoilage [1]. A range of Gram-positive and Gram-negative bacteria can cause foodborne infection; however, Listeria monocytogenes is recognized as a major risk by the food industry. It is a Gram-positive, rod-shaped, foodborne pathogen that is considered the major causative agent responsible for serious diseases in both humans and animals. L. monocytogenes can naturally contaminate a variety of refrigerated and ready-to-eat foods, such as milk and dairy products, vegetables, meat, poultry, and seafood products, and its growth is not prevented by low-temperature storage [2,3]. Infections of human or animal hosts result in clinical presentations that range from asymptomatic carriers to septicemia, encephalitis, or abortions. It is noteworthy that, in the EU, it is zoonosis that has the highest case fatality rate of 10% [4]. As an adaptive, environmental organism, L. monocytogenes is quite resistant to a range of standard physical and chemical food preservation methods; hence, there has been a growing interest in the application of synthetic and natural compounds to control L. monocytogenes in food [5]. In the last decade, numerous plant extracts and essential oils have been evaluated as potential inhibitors of L. monocytogenes bacteria, and the anti-listerial potential of the extracts is mostly correlated with their phenolic composition; various mechanisms of action involving membranes, cytoplasm, and genetic material have been proposed for these compounds [6,7].
Licorice is a native bush in Asia and the Mediterranean region that is used in traditional medicines and folk remedies to treat many diseases [8]. Currently, in vitro and in vivo studies have documented the diverse pharmacological properties of licorice, such as anti-inflammatory, anti-tussic and expectorant, anti-ulcerative, antioxidant, antiviral, anticarcinogenic and antimutagenic, hepato-protective, neuroprotective, anti-depressive, estrogenic and androgenic, and antimicrobial activities [9,10,11,12]. Regarding antimicrobial activity, polar and supercritical fluid extracts of licorice root have strong inhibitory effects on Gram-positive bacteria and Gram-negative bacteria, such as Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, and Bacillus subtilis. Based on the above inhibitory activities against bacteria, licorice root extracts may serve as an alternative therapy for treating diverse bacterial infections. Furthermore, possible mechanisms for the antimicrobial effects of licorice root phytochemicals have been proposed [13]. More specifically, 18β-glycyrrhetinic acid decreases the expression of key virulence genes of methicillin-resistant S. aureus [14]; licochalcone A inhibits biofilm formation [15]; licochalcone E reduces the production of α-toxin [16]; liquiritigenin lowers the production of α-hemolysin; and both licochalcone A and glabridin prevent yeast–hyphal transition [15,17]. Accumulating evidence additionally suggests that L. monocytogenes is significantly susceptible to licorice root extracts and fractions [18,19,20]. Furthermore, the aqueous extract of licorice root demonstrates synergistic effects on the growth of L. monocytogenes with aminoglycosides such as gentamicin, with the presence of licorice extract reducing the minimum inhibitory concentration of gentamicin by 32-fold [21]. Previous works mainly focused on the anti-Listeria potency of glabridin, a prenylated isoflavan with well-known inhibitory effects against L. monocytogenes, with its bactericidal activity defined at concentrations above 25 µg mL−1 [22,23].
Considering the growing interest in replacing synthetic food antimicrobials with natural ones, mainly because of the public’s conviction that natural antimicrobials are safer than synthetic analogs, the food industry is strongly inspired to discover safe and low-cost antimicrobial agents of natural origin [24]. Initial work has shown that licorice root extract could inhibit the growth of a range of Gram-positive bacteria, including L. monocytogenes [25]. Therefore, the objective of the present work was to explore for the first time the potential of licorice root extract (LRE) obtained by supercritical fluid extraction to control the growth of different Listeria species, since outstanding differences in susceptibility among the Listeria species were found for natural and synthetic substances. Subsequently, the major and minor phytochemicals in LRE were elucidated with the employment of LC-DAD-qTOF-MS in order to explain its antimicrobial effects. Finally, the AntiBac-Pred, a web application for predicting the antibacterial activity of chemical compounds, was used to pinpoint the active phytoconstituents of LRE.
2. Materials and Methods
2.1. Chemicals, Bacterial Strains and Extract
Listeria agar according to ISO 11,290 (Merck®, Darmstadt, Germany), brain–heart infusion (BHI) broth (Himedia®, Mumbai, India), and absolute ethanol (Scharlau Chemie, Barcelona, Spain) were used in microbiological experiments.
Quercetin and rutin standards were supplied by Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile of HPLC grade and acetic acid were also purchased from Sigma-Aldrich, and they were used for the phytochemical analysis of the extract.
L. monocytogenes EGD (serotype 1/2a), L. monocytogenes Scott A (serotype 4b), L. monocytogenes NCTC 4885 (serotype 4b), L. monocytogenes NCTC 4994 (serotype 4b), L. monocytogenes ATCC 23,074 (serotype 4b), L. monocytogenes NCTC 1792 (serotype 4b), L. monocytogenes NCTC 7973 (serotype 1/2a), L. grayi NCTC 10815, L. seeligeri NCTC 11856, and L. ivanovii NCTC 11,846 were used in the present study.
LRE was prepared by the CO2 extraction of dried Glycyrrhiza uralensis root and provided by Flavex Naturextrakte GmbH (Rehlingen, Germany). The extractor was filled with 70 kg of dried Glycyrrhiza uralensis root and was operated at 30 MPa and 40 °C using a CO2 mass flow rate of 50 kg per kg of plant material.
2.2. Determination of Antimicrobial Activity by Broth Microdilution Method
The broth microdilution method was performed using the Τecan Infinite 200 PRO plate reader (Tecan Group, Männedorf, Switzerland) to determine the minimum inhibitory concentrations (MIC) of the LRE. Briefly, 50 µL of serial dilutions of LRE, ranging from 4 to 500 µg mL−1, were transferred, in triplicate, into a 96-well plate. Then, the extract was diluted with 40 µL of BHI broth and 10 µL of microbial suspension of a final concentration of 106 cfu mL−1 in each well. The inoculated 96-well plates were then incubated for 16 h at 37 °C. Optical density was measured and recorded every 30 min after shaking for 20 s [26]. The MIC was defined as the lowest concentration of extract that reduced the growth to an OD of 0.2 or less at the time of measurement.
2.3. Determination of Bactericidal Activity
The bactericidal activity of LRE was also determined using the Miles and Misra technique. Briefly, 50 µL of serial dilutions of LRE, ranging from 4 to 500 µg mL−1, were transferred, in triplicate, into a 96-well plate. Then, the extract was diluted with 40 µL of BHI broth and 10 µL of microbial suspension of a final concentration of 106 cfu mL−1 in each well. After incubation for 24 h at 37 °C, an appropriate volume from each well was taken, and 10-fold serial dilutions were prepared for each concentration. After that, an aliquot of 10 µL was placed in a BHI petri dish, which was previously divided into 6 parts representing the number of serial dilutions. Every part of the petri dish had 6 spots, representing 6 replicates [27]. The MBC was deemed to be the minimum concentration of extract capable of inactivating more than 99.99% of the bacteria present, resulting in no increase in optical density.
2.4. Identification and Quantification of Individual Phytochemicals in LRE
The LRE was further analyzed using UPLC/ESI-QTOF-MS to elucidate its phytochemical composition. The chromatographic separation was performed on an ACQUITY Ultra Performance LC system (Waters Corporation, Milford, MA, United States) equipped with a vacuum degasser, an autosampler, a binary pump, and a DAD detector. A volume of 2 µL of diluted extract was loaded onto an RP18 column (1.7 µm, 2.1 mm × 100 mm; ACQUITY UPLC BEH Shield RP18) in the present chromatographic separation. The mobile phase was composed of acetic acid (1%, v/v) (Phase A) and acetonitrile (Phase B) and was pumped through the analytical column at a rate of 0.6 mL min−1. The gradient elution program was set as follows: 0 min, 1% B; 2.3 min, 1% B; 4.4 min, 7% B; 8.1 min, 14% B; 12.2 min, 24% B; 16 min, 40% B; 18.3 min, 100% B, 21 min, 100% B; 22.4 min, 1% B; and finally, a conditioning cycle of 3 min with the initial conditions.
MS analyses were performed with the employment of a Q/TOF micro mass spectrometer (Waters Corporation, Milford, MA, USA). The negative ionization mode was used, and the conditions were as follows: cone gas flow, 40 L/h; drying gas flow (N2), 11,000 L/h; nebulizer pressure, 50 psi; gas drying temperature, 360 °C; capillary voltage, 2500 V; fragmentor voltage and scan range, 3500 V and m/z 50–1500, respectively. The compounds were monitored at 280 nm. Integration and data elaboration were performed using MassLynx 4.1 software (Waters Corporation, Milford, MA, USA). The quantification was performed using the calibration curves that were prepared from the limit of quantification (LOQ) to 100 µg mL−1 obtained by MS. All calibration curves showed good linearity among different concentrations (r > 0.999).
2.5. In Silico Screening of the Antimicrobial Potency of LRE
The major phytoconstituents of LRE, identified by LC-DAD-qTOF-MS, were included in the computational analysis. The two-dimensional structures and canonical simplified molecular input line entry system (SMILES) of the compounds were obtained from the PubChem Compound page at https://pubchem.ncbi.nlm.nih.gov/, accessed on 10 September 2024. Subsequently, the chemical structures were subjected on the AntiBac-Pred web tool of the Way2Drug platform (https://www.way2drug.com/antibac/), accessed on 10 September 2024, in order to predict their inhibitory effect on the growth of Listeria monocytogenes bacteria.
3. Results & Discussion
3.1. Evaluation of the Anti-Listerial Potency of LRE
The anti-Listerial potency of LRE was investigated by the employment of the broth microdilution method. Initially, the MIC of the licorice root extract was determined for each Listeria species and strain, as this value reflects the concentration required to inhibit visible bacterial growth and serves as an indicator of the extract’s bacteriostatic effect against each test organism. LRE was obtained by supercritical carbon dioxide extraction; thus, water and ethanol were used for the solubilization of the extract for the assessment of its inhibitory effect against Listeria bacteria. Table 1 summarizes the MIC values of LRE dissolved in ethanol and water against Listeria strains. In particular, the MICs of LRE ranged from 31.3–65.5 µg mL−1 when dissolved in ethanol, whereas MICs were significantly higher when water was used for solubilization (125–250 µg mL−1). The significant differences in MIC values may be attributed to the better solubilization of LRE phytochemicals in ethanol than water. The superiority of alcohol for the solubilization of phytochemicals against water has been described thoroughly [28]. Findings also support the great potential of LRE as a growth inhibitor of Listeria bacteria, since its MIC values were similar to well-known classes of antibiotics such as cephalosporins and quinolones and comparable with streptogramins and lincosamides [29]. The MIC values of LRE are also substantially lower than those of over a hundred plant materials (entire plants, bulbs, fruits, flowers, roots, seeds, and by-products) published from 2017 to 2023 [6]. The potential of LRE to inhibit the growth of Listeria bacteria is equivalent to extracts of Hypericum perforatum L. leaves [30], Berberis libanotica Ehrenb. leaves [31], Pistacia lentiscus leaves [32], Origanum ehrenbergii Boiss aerial parts [33], and Alpinia galanga (Linn.) flowers [34]. In addition, LRE is more effective in controlling the growth of Listeria bacteria than extracts originating from the aerial parts and roots of Glycyrrhiza glabra. Its aerial parts and root extracts present MIC values of 620 µg mL−1 and 290 µg mL−1, respectively [18,19]. Since bacteriostatic effects alone are insufficient to confer antiseptic and disinfectant properties on an extract or antibacterial agent, the bactericidal activity of LRE was also determined. According to Table 1, the MBC values of the extract dissolved in ethanol ranged from 62.5 µg mL−1 to 250 µg mL−1. Interestingly, the variation of MBC values is greater than that of MICs. L. monocytogenes ATCC 23,074 and L. monocytogenes NCTC 7973 were the most susceptible strains among those studied. On the other hand, significantly higher amounts of LRE were required to kill the bacteria of L. monocytogenes EGD, L. grayi NCTC 10815, and L. seeligeri NCTC 11856. Results also highlighted that non-pathogenic Listeria species are more resistant to the effect of the extract in comparison with pathogenic Listeria. Results disclose remarkable differences in inherent susceptibility among the studied species and strains. The susceptibility diversity of Listeria species to antibiotics and natural products has also been described [29,35,36]. In addition, the MIC values reveal a great potential of LRE to act as a potent inhibitor of Listeria bacteria growth, since they meet stringent end point criteria for “activity” as a previous study described [37]. In particular, plant extracts should be considered efficacious if they exhibit IC50 values ≤ 100 µg mL−1; the present extract is notably promising, as its MBC values are lower than the described critical concentration. Therefore, the elucidation of phytochemical composition is of great importance in order to decipher the antimicrobial potential of LRE.

Table 1.
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of licorice root extract dissolved in ethanol and water against Listeria strains. MIC and MBC values are expressed as (µg mL−1).
3.2. Elucidation of the Phytochemical Composition of LRE
The phytochemical composition of extracts is strongly linked to their antimicrobial potential. Previous works focused on glabridin, a prenylated flavonoid that could be extracted from licorice roots and has shown antimicrobial activity against Listeria bacteria and other foodborne pathogens and spoilage microorganisms [38,39]. Glabridin can reduce L. monocytogenes motility and hemolytic activity, but was not found to have antimicrobial activity [38,39]. Furthermore, prenylated (iso)flavonoids and chalcones from roots exert potent inhibitory effects on many bacteria and yeasts [40]. Thus, the phytochemical composition of LRE was elucidated using an advanced LC-DAD- qTOF-MS set-up to accurately identify its active phytoconstituents. Peak identification was based on retention times, UV–Vis spectra, mass spectra, and mass information from the literature. Chromatographic analysis revealed an extract rich in phytochemicals with great structural diversity. Table 2 summarizes the identified compounds; the presence of all phytochemicals has been previously described in plants belonging to the Glycyrrhiza genus and their derivatives [41,42,43,44,45]. The majority of LRE phytochemicals belong to the flavonoid class, as previous studies reported [46,47]. More specifically, a great variety of flavanones, as well as flavones and chalcones, were found in the extract. In addition, LRE contains triterpenoids typical of licorice extracts, while the presence of coumarins and phenolic acid was also confirmed. The presence of all these compounds has been reported in licorice extracts in previously published works. Table 2 also clearly demonstrates that the liquiritin-apioside isomers are the major constituents of LRE, as their total content reaches 133.1 mg g−1 of extract. The results show a concentrated content of these isomers in LRE compared to licorice root extracts of different geographical origins [48]. [(2Z)-3-(4-tert-Butyl-2-hydroxyphenyl)-3-hydroxyprop-2-enoyl]benzoic acid was also found at high concentrations in LRE (32.9 ± 0.8 mg g−1 extract). Licorice glycoside isomers are also prominent constituents in LRE. These are glycosylated flavonoids, which are typical compounds in Glycyrrhiza glabra roots. In addition, LRE contains significant amounts of flavanone-type compounds: namely, kanzonol C, kanzonol W, and kanzonol Y. Unfortunately, the triterpenoid content of LRE could not be determined. According to previous works, the glycyrrhizin contents in G. glabra roots vary from 11 to 80 mg g−1, depending on genetic and environmental factors, while glycyrrhetinic and oxo glycyrrhetinic acids, hydrolytic products of glycyrrhizin, are present in licorice root extracts at very low levels [49,50].

Table 2.
Identification and quantification of phytoconstituents in licorice root extract using LC-DAD-qTOF-MS.
3.3. In Silico Evaluation of the Antibacterial Potency of LRE Phytoconstituents
Computational tools are widely used for the prediction of the antimicrobial activity of diverse groups of antimicrobials. The most common target predictions are based on individual molecules. Ligand-based methods include methods that are based on the principle of chemical similarity, which states that similar chemical structures tend to present similar antimicrobial activities more often than not, although even structurally similar compounds may interact with a protein target in different ways [54,55]. In this study, 51 phytochemicals were identified in LRE. The computational screening was applied to phytochemicals with content above 0.5% w/w, as well as to triterpenoids, whose content was not calculated for technical reasons. The application of computation tools is expected to pinpoint the antimicrobial components of extracts, avoiding laborious and time-consuming fractionation and purification procedures. Thus, the antibacterial activities of LRE phytochemicals against Listeria monocytogenes were determined using the online tool AntiBac-Pred of the Way2Drug platform. Antibac-Pred analyzes growth inhibitors or non-inhibitors of L. monocytogenes based on antibacterial activity data available in ChEMBL, a database of bioactive molecules with drug-like properties (Figure 1). The score for each compound is expressed as confidence in its activity, which represents the difference between the probabilities that a chemical compound inhibits or does not inhibit the growth of a given bacteria. As confidence increases, the chances of the prediction being true are greater [56]. The calculated confidence scores (Table 3) show that the most promising inhibitor of L. monocytogenes is liquiritin-apioside, followed by licorice glycoside C1/C2. Interestingly, similar confidence scores were calculated for well-established antibiotics such as ampicillin and gentamicin. There is no data yet for the antibacterial potency of both pure phytochemicals. Isoliquiritin-apioside, glycyroside, and kanzonol W also appear to have the potential to act as possible anti-Listeria agents. Surprisingly, the typical triterpenoids of licorice roots, namely, glycyrrhizin and glycyrrhetinic acid, do not exert inhibitory effects against Listeria according to the confidence scores. However, previous works demonstrate their strong antimicrobial effects against bacteria such as Bacillus subtilis, Escherichia coli, Staphylococcus aureus, and Salmonella typhi [51,52,53,57,58]. The low confidence scores do not preclude the potent activity of compounds, since their structures are not typical of the “active” structures of the training set. Overall, the computational screening revealed the presence of many compounds with anti-Listerial potential that act in an additive or synergistic way. In addition, further studies are required to investigate the potency of LRE triterpenoids as inhibitors of Listeria bacteria.
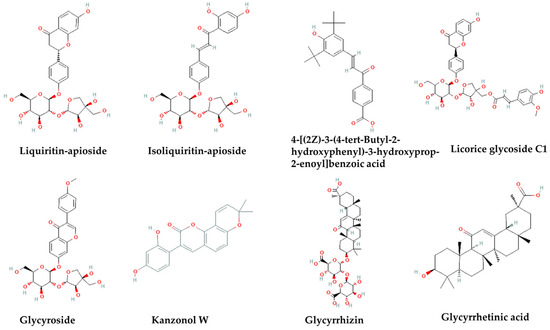
Figure 1.
Structures of phytochemicals tested as Listeria monocytogenes inhibitors via Antibac-Pred web tool.

Table 3.
Prediction of inhibitory effects of licorice root extract phytoconstituents against Listeria monocytogenes via Antibac-Pred web tool.
4. Conclusions
Overall, the results obtained herein suggest the ability of licorice root extract to control a panel of Listeria strains, a particularly important causative agent responsible for serious diseases in both humans and animals. In addition, the MIC and MBC values of this extract meet the endpoint criteria for “activity” as proposed in a previous critical review. LC-DAD-qTOF-MS analysis also demonstrated an extract rich in flavanones, flavones, chalcones, and triterpenoids, with their contribution to the antimicrobial effects of the extract having been studied. This work reveals the active components of the extract and offers new lead structures for controlling Listeria growth. Based on these findings, further fractionation and testing of the pure phytochemicals to assess their MIC and MBC values are strongly recommended. In conclusion, the licorice root can be included in the list of natural antimicrobial agents with potential uses in the food industry. However, more research is needed to fully understand the efficacy, safety, and regulatory aspects of using licorice in food preservation on a commercial scale.
Author Contributions
C.M. performed the microbiological experiments. A.C. carried out the in silico experiments. A.M.G.-C. performed the phytochemical analysis of the extract. C.E.D.R. and G.B. designed and interpreted the microbiological experiments. V.G. designed and interpreted the phytochemical and in silico experiments. V.G., G.B., and A.C. helped write and edit the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is unavailable due to privacy restrictions.
Acknowledgments
Licorice root extract was kindly provided by Alan Marson, New-Food Innovation Ltd., UK.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the existing affiliation information. This change does not affect the scientific content of the article.
References
- Farber, J.M.; Zwietering, M.; Wiedmann, M.; Schaffner, D.; Hedberg, C.W.; Harrison, M.A.; Hartnett, E.; Chapman, B.; Donnelly, C.W.; Goodburn, K.E.; et al. Alternative Approaches to the Risk Management of Listeria monocytogenes in Low Risk Foods. Food Control 2021, 123, 107601. [Google Scholar] [CrossRef]
- Lennox, J.A.; Patience, E.O.; Godwin, J.E.; Effiom, E.E. Prevalence of Listeria monocytogenes in Fresh and Raw Fish, Chicken and Beef. J. Adv. Microbiol. 2017, 3, 1–7. [Google Scholar]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A Review of Listeria onocytogenes: An Update on Outbreaks, Virulence, Dose-Response, Ecology, and Risk Assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Schoder, D.; Guldimann, C.; Märtlbauer, E. Asymptomatic Carriage of Listeria monocytogenes by Animals and Humans and Its Impact on the Food Chain. Foods 2022, 11, 3472. [Google Scholar] [CrossRef] [PubMed]
- Farid, N.; Waheed, A.; Motwani, S. Synthetic and Natural Antimicrobials as a Control against Food Borne Pathogens: A Review. Heliyon 2023, 9, e17021. [Google Scholar] [CrossRef]
- Ricci, A.; Lazzi, C.; Bernini, V. Natural Antimicrobials: A Reservoir to Contrast Listeria monocytogenes. Microorganisms 2023, 11, 2568. [Google Scholar] [CrossRef]
- Zamuz, S.; Munekata, P.E.S.; Dzuvor, C.K.O.; Zhang, W.; Sant’Ana, A.S.; Lorenzo, J.M. The Role of Phenolic Compounds against Listeria monocytogenes in Food. A Review. Trends Food Sci. Technol. 2021, 110, 385–392. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P.P. Liquorice (Glycyrrhiza glabra): A Phytochemical and Pharmacological Review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. Chemopreventive Effects of Licorice and Its Components. Curr. Pharmacol. Rep. 2015, 1, 60–71. [Google Scholar] [CrossRef]
- Karkanis, A.; Martins, N.; Petropoulos, S.A.; Ferreira, I.C.F.R. Phytochemical Composition, Health Effects, and Crop Management of Liquorice (Glycyrrhiza glabra L.): A Medicinal Plant. Food Rev. Int. 2018, 34, 182–203. [Google Scholar] [CrossRef]
- Noreen, S.; Mubarik, F.; Farooq, F.; Khan, M.; Khan, A.U.; Pane, Y.S. Medicinal Uses of Licorice (Glycyrrhiza glabra L.): A Comprehensive Review. Open Access Maced. J. Med. Sci. 2021, 9, 668–675. [Google Scholar]
- Husain, I.; Bala, K.; Khan, I.A.; Khan, S.I. A Review on Phytochemicals, Pharmacological Activities, Drug Interactions, and Associated Toxicities of Licorice (Glycyrrhiza Sp.). Food Front. 2021, 2, 449–485. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The Antiviral and Antimicrobial Activities of Licorice, a Widely-Used Chinese Herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef]
- Long, D.R.; Mead, J.; Hendricks, J.M.; Hardy, M.E.; Voyich, J.M. 18β-Glycyrrhetinic Acid Inhibits Methicillin-Resistant Staphylococcus Aureus Survival and Attenuates Virulence Gene Expression. Antimicrob. Agents Chemother. 2013, 57, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Messier, C.; Grenier, D. Effect of Licorice Compounds Licochalcone A, Glabridin and Glycyrrhizic Acid on Growth and Virulence Properties of Candida albicans. Mycoses 2011, 54, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Deng, X.; Qiu, J. Antimicrobial Activity of Licochalcone E against Staphylococcus aureus and Its Impact on the Production of Staphylococcal Alpha-Toxin. J. Microbiol. Biotechnol. 2012, 22, 800–805. [Google Scholar] [CrossRef]
- Dai, X.H.; Li, H.E.; Lu, C.J.; Wang, J.F.; Dong, J.; Wei, J.Y.; Zhang, Y.; Wang, X.; Tan, W.; Deng, X.M.; et al. Liquiritigenin Prevents Staphylococcus aureus-Mediated Lung Cell Injury via Inhibiting the Production of α-Hemolysin. J. Asian Nat. Prod. Res. 2013, 15, 390–399. [Google Scholar] [CrossRef]
- Gopal, S. In Vitro Antifungal and Antibacterial Activities of Root Extract of Glycyrrhiza glabra. J. of Appl. Sci. Res. 2009, 5, 1436–1439. [Google Scholar]
- Rahnama, M.; Fakheri, B.A.; Mashhady, M.A.; Saeidi, S. Anti-Bacterial and Anti-Biofilm Activity of Glycyrrhiza glabra, Rosmarinus officinalis and Saponaria officinalis Extracts on Important Food Pathogens. Gene Cell Tissue 2019, 6, e96326. [Google Scholar] [CrossRef]
- Sandasi, M.; Leonard, C.M.; Viljoen, A.M. The in Vitro Antibiofilm Activity of Selected Culinary Herbs and Medicinal Plants against Listeria Monocytogenes. Lett. Appl. Microbiol. 2010, 50, 30–35. [Google Scholar] [CrossRef]
- Park, M.; Horn, L.; Lappi, V.; Boxrud, D.; Hedberg, C.; Jeon, B. Antimicrobial Synergy between Aminoglycosides and Licorice Extract in Listeria Monocytogenes. Pathogens 2022, 11, 440. [Google Scholar] [CrossRef]
- Bombelli, A.; Araya-Cloutier, C.; Boeren, S.; Vincken, J.P.; Abee, T.; den Besten, H.M.W. Effects of the Antimicrobial Glabridin on Membrane Integrity and Stress Response Activation in Listeria monocytogenes. Food Res. Int. 2024, 175, 113687. [Google Scholar] [CrossRef] [PubMed]
- Bombelli, A.; Araya-Cloutier, C.; Abee, T.; den Besten, H.M.W. Disinfectant Efficacy of Glabridin against Dried and Biofilm Cells of Listeria monocytogenes and the Impact of Residual Organic Matter. Food Res. Int. 2024, 191, 114613. [Google Scholar] [CrossRef] [PubMed]
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Buelga, C.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Natural Food Colorants and Preservatives: A Review, a Demand, and a Challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef] [PubMed]
- El Awamie, M.; Reesm, C. Identification of the Antimicrobial Effect of Liquorice Extracts on Gram-Positive Bacteria: Determination of Minimum Inhibitory Concentration and Mechanism of Action Using a luxABCDE Reporter Strain. Int. J. Pharmacol. Pharm. Sci. 2017, 10, 325–335. [Google Scholar]
- Christou, A.; Stavrou, C.; Michael, C.; Botsaris, G.; Goulas, V. New Insights into the Potential Inhibitory Effects of Native Plants from Cyprus on Pathogenic Bacteria and Diabetes-Related Enzymes. Microbiol. Res. 2024, 15, 926–942. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Pereira, M.F.; Costa, M.R.; Pintado, M.E. Evaluation of the Antimicrobial Activity of Aqueous Extracts from Dry Vaccinium corymbosum Extracts upon Food Microorganism. Food Control 2013, 34, 645–650. [Google Scholar] [CrossRef]
- Kumar, A.; Nirmal, P.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; Sneha, V.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Troxler, R.; Von Graevenitz, A.; Funke, G.; Wiedemann, B.; Stock, I. Natural antibiotic susceptibility of Listeria species: L. grayi, L. innocua, L. ivanovii, L. monocytogenes, L. seeligeri and L. welshimeri strains. Clin. Microbiol. Inf. 2000, 6, 525–535. [Google Scholar] [CrossRef]
- Tsakni, A.; Chatzilazarou, A.; Tsakali, E.; Tsantes, A.G.; Van Impe, J.; Houhoula, D. Identification of Bioactive Compounds in Plant Extracts of Greek Flora and Their Antimicrobial and Antioxidant Activity. Separations 2023, 10, 373. [Google Scholar] [CrossRef]
- Dawra, M.; Nehme, N.; El Beyrouthy, M.; Abi Rizk, A.; Taillandier, P.; Bouajila, J.; El Rayess, Y. Comparative Study of Phytochemistry, Antioxidant and Biological Activities of Berberis libanotica Fruit and Leaf Extracts. Plants 2023, 12, 2001. [Google Scholar] [CrossRef] [PubMed]
- Nefzi, K.; Ben Jemaa, M.; Baraket, M.; Dakhlaoui, S.; Msaada, K.; Nasr, Z. In Vitro Antioxidant, Antibacterial and Mechanisms of Action of Ethanolic Extracts of Five Tunisian Plants against Bacteria. App. Sci. 2022, 12, 5038. [Google Scholar] [CrossRef]
- Dawra, M.; El Rayess, Y.; El Beyrouthy, M.; Nehme, N.; El Hage, R.; Taillandier, P.; Bouajila, J. Biological Activities and Chemical Characterization of the Lebanese Endemic Plant Origanum ehrenbergii Boiss. Flavour. Fragr. J. 2021, 36, 339–351. [Google Scholar] [CrossRef]
- Tang, X.; Xu, C.; Yagiz, Y.; Simonne, A.; Marshall, M.R. Phytochemical Profiles, and Antimicrobial and Antioxidant Activities of Greater Galangal [Alpinia galanga (Linn.) Swartz.] Flowers. Food Chem. 2018, 255, 300–308. [Google Scholar] [CrossRef]
- Rožman, T.; Jeršek, B. Antimicrobial Activity of Rosemary Extracts (Rosmarinus officinalis L.) against Different Species of Listeria. Acta Agric. Slov. 2009, 93, 51–58. [Google Scholar]
- De Niederhäusern, S.; Bondi, M.; Camellini, S.; Sabia, C.; Messi, P.; Iseppi, R. Plant Extracts for the Control of Listeria monocytogenes in Meat Products. Applied Sci. 2021, 11, 10820. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Vanden Berghe, D.; Maes, L. Anti-Infective Potential of Natural Products: How to Develop a Stronger In Vitro “Proof-of-Concept”. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Bombelli, A.; Araya-Cloutier, C.; Vincken, J.P.; Abee, T.; den Besten, H.M.W. Impact of food-relevant conditions and food matrix on the efficacy of prenylated isoflavonoids glabridin and 6,8-diprenylgenistein as potential natural preservatives against Listeria monocytogenes. Int. J. Food Microbiol. 2023, 390, 110109. [Google Scholar] [CrossRef]
- Liao, C.; Yu, C.; Guo, J.; Guan, M. Subinhibitory Concentrations of Glabridin from Glycyrrhiza glabra L. Reduce Listeria monocytogenes Motility and Hemolytic Activity but Do Not Exhibit Antimicrobial Activity. Front. Microbiol. 2024, 15, 1388388. [Google Scholar] [CrossRef]
- Van Dinteren, S.; Meijerink, J.; Witkamp, R.; van Ieperen, B.; Vincken, J.P.; Araya-Cloutier, C. Valorisation of Liquorice (Glycyrrhiza) Roots: Antimicrobial Activity and Cytotoxicity of Prenylated (Iso)Flavonoids and Chalcones from Liquorice Spent (G. glabra, G. inflata, and G. uralensis). Food Funct. 2022, 13, 12105–12120. [Google Scholar] [CrossRef]
- Celano, R.; Docimo, T.; Piccinelli, A.L.; Rizzo, S.; Campone, L.; Di Sanzo, R.; Carabetta, S.; Rastrelli, L.; Russo, M. Specialized Metabolite Profiling of Different Glycyrrhiza glabra Organs by Untargeted UHPLC-HRMS. Ind. Crops Prod. 2021, 170, 113688. [Google Scholar] [CrossRef]
- Montero, L.; Ibáñez, E.; Russo, M.; di Sanzo, R.; Rastrelli, L.; Piccinelli, A.L.; Celano, R.; Cifuentes, A.; Herrero, M. Metabolite Profiling of Licorice (Glycyrrhiza glabra) from Different Locations Using Comprehensive Two-Dimensional Liquid Chromatography Coupled to Diode Array and Tandem Mass Spectrometry Detection. Anal. Chim. Acta 2016, 913, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Rizzato, G.; Scalabrin, E.; Radaelli, M.; Capodaglio, G.; Piccolo, O. A New Exploration of Licorice Metabolome. Food Chem. 2017, 221, 959–968. [Google Scholar] [CrossRef]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Miyoshi, S.; Murakami, T.; Suzuki, R. Differentiating Between Three Licorice Species Using SFC-TOF/MS Analysis With Principal Component Analysis. Nat. Prod. Commun. 2023, 18, 1–10. [Google Scholar] [CrossRef]
- Liao, W.C.; Lin, Y.H.; Chang, T.M.; Huang, W.Y. Identification of Two Licorice Species, Glycyrrhiza uralensis and Glycyrrhiza glabra, Based on Separation and Identification of Their Bioactive Components. Food Chem. 2012, 132, 2188–2193. [Google Scholar]
- Avula, B.; Bae, J.Y.; Chittiboyina, A.G.; Wang, Y.H.; Wang, M.; Zhao, J.; Ali, Z.; Brinckmann, J.A.; Li, J.; Wu, C.; et al. Chemometric Analysis and Chemical Characterization for the Botanical Identification of Glycyrrhiza Species (G. glabra, G. uralensis, G. inflata, G. echinata and G. lepidota) Using Liquid Chromatography-Quadrupole Time of Flight Mass Spectrometry (LC-QToF). J. Food Comp. Anal. 2022, 112, 104679. [Google Scholar] [CrossRef]
- Montoro, P.; Maldini, M.; Russo, M.; Postorino, S.; Piacente, S.; Pizza, C. Metabolic Profiling of Roots of Liquorice (Glycyrrhiza glabra) from Different Geographical Areas by ESI/MS/MS and Determination of Major Metabolites by LC-ESI/MS and LC-ESI/MS/MS. J. Pharm. Biomed. Anal. 2011, 54, 535–544. [Google Scholar] [CrossRef]
- Basar, N.; Talukdar, A.D.; Nahar, L.; Stafford, A.; Kushiev, H.; Kan, A.; Sarker, S.D. A Simple Semi-Preparative Reversed-Phase HPLC/PDA Method for Separation and Quantification of Glycyrrhizin in Nine Samples of Glycyrrhiza glabra Root Collected from Different Geographical Origins. Phytochem. Anal. 2014, 25, 399–404. [Google Scholar] [CrossRef]
- Wang, Y.C.; Yang, Y.S. Simultaneous Quantification of Flavonoids and Triterpenoids in Licorice Using HPLC. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2007, 850, 392–399. [Google Scholar] [CrossRef]
- Kim, H.K.; Park, Y.; Kim, H.N.; Choi, B.H.; Jeong, H.G.; Lee, D.G.; Hahm, K.-S. Antimicrobial Mechanism of β-Glycyrrhetinic Acid Isolated from Licorice, Glycyrrhiza glabra. Biotechnol. Let. 2002, 24, 1899–1902. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Ovando-Martínez, M.; Velderrain-Rodríguez, G.R.; González-Aguilar, G.A.; Ayala-Zavala, J.F. Licorice (Glycyrrhiza glabra Linn.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2015; pp. 523–530. [Google Scholar]
- Wu, S.C.; Yang, Z.Q.; Liu, F.; Peng, W.J.; Qu, S.Q.; Li, Q.; Song, X.B.; Zhu, K.; Shen, J.Z. Antibacterial Effect and Mode of Action of Flavonoids From Licorice Against Methicillin-Resistant Staphylococcus Aureus. Front. Microbiol. 2019, 10, 2489. [Google Scholar] [CrossRef] [PubMed]
- Koutsoukas, A.; Simms, B.; Kirchmair, J.; Bond, P.J.; Whitmore, A.V.; Zimmer, S.; Young, M.P.; Jenkins, J.L.; Glick, M.; Glen, R.C.; et al. From in Silico Target Prediction to Multi-Target Drug Design: Current Databases, Methods and Applications. J. Proteomics 2011, 74, 2554–2574. [Google Scholar] [CrossRef] [PubMed]
- Torrent, M.; Victoria Nogues, M.; Boix, E. Discovering New In Silico Tools for Antimicrobial Peptide Prediction. Curr. Drug Targets 2012, 13, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.P.S.; Lima, L.R.; Silva, L.B.; Cruz, J.N.; Ramos, R.S.; Lima, L.S.; Cardoso, F.M.N.; Silva, A.V.; Rodrigues, D.P.; Rodrigues, G.S.; et al. Hierarchical Virtual Screening of Potential New Antibiotics from Polyoxygenated Dibenzofurans against Staphylococcus Aureus Strains. Pharmaceuticals 2023, 16, 1430. [Google Scholar] [CrossRef]
- Thakur, D.; Jain, A.; Ghoshal, G. Evaluation of Phytochemical, Antioxidant and Antimicrobial Properties of Glycyrrhizin Extracted from Roots of Glycyrrhiza glabra. J. Scientif Ind. Res. 2016, 75, 487–494. [Google Scholar]
- Fu, Y.; Chen, J.; Li, Y.J.; Zheng, Y.F.; Li, P. Antioxidant and Anti-Inflammatory Activities of Six Flavonoids Separated from Licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).