Abstract
Electronic medical records (EMRs) are fundamental to clinical decision support systems (CDSS). Conventional EMR structures still fail to capture the cyclical nature of treatment plans, leading to fragmented data representation and reduced decision accuracy. This study addresses this gap by proposing a cycle-based EMR framework that systematically integrates treatment cycles, enabling structured, sequential data organization. Treatment cycles categorize patient data into iterative phases, reflecting disease progression and repeated interventions, ensuring data continuity and analytical precision. A dataset inspired by MIMIC-III was developed to empirically evaluate this approach, incorporating treatment cycle fields to enhance data continuity and analytical precision. The results indicate that cycle-based structuring preserves critical variations in patient responses, improves treatment outcome predictions, and strengthens CDSS recommendations. While this approach offers substantial benefits, challenges such as workflow adaptation, usability, and interoperability must be addressed to facilitate seamless integration into clinical practice. Despite these challenges, this study establishes a scientifically validated foundation for cycle-based EMRs, aligning data structures with real-world clinical workflows. By rectifying data organization, this approach elevates diagnostic accuracy, optimizes treatment planning, and enhances patient outcomes, contributing to the future of precision medicine.
1. Introduction
The inherent complexity of medical settings has highlighted the importance of system design concerns. This sensitivity increases exponentially when medical decisions are made using data processed by clinical decision support systems (CDSS) [1]. This complexity has increased due to the growing volume and diversity of patient data, necessitating advanced tools like CDSS to manage and support decision-making in these complicated clinical environments [2]. Recent studies highlight the critical role of CDSS in improving clinical practice across diverse fields such as nursing, pediatrics, and oncology, emphasizing their practical usability and widespread applicability in addressing complex healthcare challenges globally [3]. The successful implementation of CDSS depends on their seamless integration into healthcare providers’ existing workflows and decision-making processes [4,5]. Usability experts have emphasized that effective system design significantly enhances usability and ensures physician adoption of CDSS [6]. Unlike interface design, which concentrates on a system’s visual and interactive aspects, system design encompasses the structural, functional, and operational frameworks of CDSS. It addresses how data is organized, retrieved, and presented to users, ensuring that the system is compatible with medical professionals’ real-world needs and workflows [7,8]. Poor system design hinders the fulfillment of physicians’ needs and may lead to overwhelm and dissatisfaction. It ultimately can negatively impact patient care [9].
One significant deficiency in the prevalent system design is incompatibility with current medical processes [10]. Among the deficiencies mentioned, the cyclical nature of the treatment plan can be firmly perceived as the prominent requirement. A treatment cycle often contains a sequence of distinct chain phases (diagnosis, treatment plan, and result evaluation), and the existing system designs within medical information systems still do not address the cyclical nature of these phases [11]. Electronic medical records (EMRs), as the principal platforms for medical data collection, are no exception to this. Since they are considered data providers for CDSS, this compatibility with real-world needs must be observed for EMR system design [12]. In contemporary system design, medical data are frequently presented in isolated fragments, which reduces physicians’ ability to comprehensively comprehend the patient’s medical history and current treatment needs. In other words, the treatment cycle to which the registered data belong is not explicit. This ambiguity reduces the necessary transparency for data analysis [13].
This problematic situation highlights a critical research gap: how can CDSS be designed to align with the inherent cyclical nature of treatment plans? Existing system designs often prioritize medical data entry and retrieval within EMRs, regardless of how data categorization and labeling affect the next-step data analysis and medical decision accuracy [14,15]. To the best of our knowledge, no prior research has explicitly investigated the impact of a cyclical treatment approach on the design of CDSS that integrate with EMR data. Addressing this gap is essential for several reasons. First, enhanced clinical decision-making hinges on our ability to understand and incorporate the cyclical nature of treatment plans into CDSS design. Second, concentrating on cyclical data tagging and organization within EMRs streamlines data retrieval and analysis, elevating the quality of patient care. Finally, this study aims to fill the gap in the existing studies regarding the relationship between the cyclical nature of treatment plans and CDSS design, establishing a foundation for future research and innovation in this critical area. To address this gap, the current study proposes a cycle-oriented approach to CDSS design, investigating different aspects of this approach. In this regard, the upcoming sections are defined as follows.
This study initially delves into a comprehensive review of accomplished studies on cyclical approaches within medical information systems (not limited to the treatment cycle concept with CDSS, but also encompassing medical outcome evaluation, the iterative nature of diagnostic processes, and the application of cyclical methodologies in calculating treatment costs). Separately, we review the articles investigating how the systematic organization of medical data within EMRs and the use of data tagging techniques contribute to more precise medical decision-making. To establish a theoretical foundation, we define the concept of treatment cycles and discuss their necessity in medical applications. A comparative analysis is then conducted to contrast traditional EMR system designs with the proposed cyclical approach, highlighting their advantages and limitations. The implementation of this model is detailed through both technical considerations and the implications for data retrieval and analysis. We further present an empirical evaluation demonstrating how the cyclical model improves the accuracy of medical decision-making. In addition, we discuss implementation feasibility, usability concerns, and ethical implications related to data privacy and decision transparency. Finally, we conclude that integrating cyclical methodologies into EMR structures can significantly enhance the accuracy and reliability of medical decision-making within CDSS.
2. Related Works
The recent and rapid growth in EMR usage due to today’s technological advancements has eliminated paper-based processes within healthcare systems [16]. In this realm, proper system design enhances systems’ user adoption and data quality, elevating the accuracy level of medical decisions [17]. In this regard, the success of system design strongly depends on its compatibility with ongoing medical processes [18]. From the physicians’ perspective, the treatment plan encompasses a series of medical functions that may result in the cure or non-cure of a patient’s condition [19]. Throughout the stages of system design, it is essential to consider all physicians’ needs, including those related to the treatment cycle, to improve system usability and medical decision-making. To comprehensively understand existing approaches, we review studies emphasizing the importance of cyclical methodologies across different facets of medical information systems. Then, we delve into studies investigating system design solutions that address the challenges of medical data arrangement and labeling within EMRs. Considering these factors, the accuracy and reliability of medical decisions supported by CDSS will be improved.
2.1. Literature Review on Cyclical Approaches in Medical Information Systems
CDSS have become central in enhancing clinical workflows by providing timely recommendations that integrate patient data with evidence-based guidelines. Several studies indicate that embedding iterative feedback loops into CDSS design can significantly improve performance. For instance, cyclic frameworks such as the Plan–Do–Study–Act (PDSA) system have been applied to continuously refine CDSS outputs, ensuring that the system adapts to evolving clinical needs and minimizes errors in real time [20]. The iterative nature of such cycles allows developers to test changes on a small scale, study the outcomes, and then act by modifying the system. On the other hand, the diagnostic process in clinical settings is intrinsically iterative. Diagnostic workflows often require repeated data collection cycles, hypothesis testing, and reinterpretation as new patient information becomes available. Studies in the field have demonstrated that the cyclical approach (in which clinicians and systems repeatedly “loop” through stages of assessment and re-evaluation) can significantly enhance the diagnostic accuracy [21]. Mark Smith et al. [22] emphasized that continuous learning processes in medical systems are essential in ensuring both clinical safety and cost-effectiveness. In this view, the cyclical approach is fundamental because it allows systems to respond dynamically to the complexities of real-world healthcare. Similarly, Loeb [23] found that incorporating iterative diagnostic cycles into CDSS improved the timeliness and accuracy of diagnoses, highlighting the inadequacy of a single, static implementation when patient data change rapidly.
Beyond diagnosis, cyclical approaches have proven valuable in outcome evaluation and cost management. Laka et al. [24] pointed out the limitations of static assessments, arguing that they fail to capture the ongoing benefits of system refinements over time. They recommended conducting repeated outcome assessments to accurately reflect the evolving nature of clinical practice and technological performance within the CDSS environment. White et al. [25] claimed that iterative cost recalibration enables more accurate resource utilization tracking. This guarantees that financial evaluations remain reflective of real-world operational conditions.
Furthermore, Cavour and Chui [26] demonstrated in their study that continuous monitoring provides essential feedback for effective care coordination across the diagnostic process. This aligns with the “continuum of care” concept, emphasizing seamless and integrated healthcare delivery across different stages and settings. This cyclical model supports continuous learning and the refinement of diagnostic processes by providing clinicians with consistent feedback on their diagnostic reasoning and outcomes [27]. The iterative nature of diagnostic processes, as described in the context of diagnostic paths, underscores the importance of cyclical approaches. By continuously gathering and integrating information, clinicians can refine their working diagnoses and improve the diagnostic accuracy over time [28,29].
2.2. Literature Review on System Design Solutions for Medical Data Arrangement and Labeling in EMRs
System design in medical settings, such as CDSS and EMRs, plays a vital role in managing the flow and quality of data that drives medical decision-making [30]. A crucial aspect of this process involves data tagging (or labeling), where raw data are organized and annotated to enhance their usability across systems. Proper data arrangement and labeling in EMRs are fundamental in enabling adequate clinical decision support [31]. While numerous studies explore system design and data flows, we attempt to collect different perspectives to articulate and compare them within the medical domain.
Gustav and Jan [32] analyzed data flows within EMR systems, emphasizing the difficulties posed by unstructured data. They highlighted that the absence of robust tagging mechanisms makes retrieving specific patient information inefficient and prone to errors. On the other hand, Luis et al. [33] delved into the issue from a system integration perspective, emphasizing standardized data tags to streamline interoperability between EMRs and CDSS. These studies highlight the importance of tagging in two different aspects. Gustav and Jan investigated probable internal data flaws, while Luis et al. were concerned with external data compatibility during communication between two medical platforms.
Chih-Hsun et al. [34] explored tagging automation, introducing AI-powered tools that classify and annotate data during entry. Their study demonstrated that automated tagging significantly reduced the manual workload and enhanced the data consistency. Meanwhile, Mohamed et al. [35] investigated tagging from a user interface perspective, showing that user-friendly tagging systems improved the data entry accuracy and reduced clinician frustration. These findings underscore the interplay between technological advancements and user-centric design in optimizing tagging practices.
Structured tagging, such as labeling treatment cycles, enables the systematic organization of critical patient data, which is significant in generating actionable insights in clinical workflows [36]. In this respect, cyclical approaches (like the Plan–Do–Check–Act model) have successfully improved the timeliness and quality of EMR documentation by embedding iterative feedback loops into data entry processes, thereby aligning tags with evolving clinical phases [37]. Advanced frameworks, such as patient similarity-based EMR systems, leverage tagged diagnostic and demographic data to dynamically update treatment recommendations, illustrating how structured tagging supports adaptive decision-making [38]. Similarly, efforts to convert unstructured EMR text into standardized formats (like using BART-based models for entity extraction in lung cancer records) highlight the role of semantic tagging in harmonizing data for analytics [36].
However, challenges persist in psychiatric EMRs, where unstructured narrative data dominate. In such cases, hybrid tagging strategies will be needed to balance clinical nuance with computational usability [39]. These insights underscore that cyclical tagging and structured arrangement enhance data interoperability and drive predictive accuracy and clinical safety in CDSS applications [38,40].
3. Overview of the Treatment Cycle
Like many processes across diverse sectors, medical care follows a cyclical pattern. Treatment plans often involve repeating phases over time, like administrative, industrial, and financial operations that can be optimized for efficiency [41,42]. This section will delve especially into the context of “treatment cycles” within medical settings. A clear definition of this term and its significance in medical practice will be provided. By understanding the cyclical nature of treatment, we can identify opportunities to improve data organization and retrieval within EMR systems.
3.1. Definition of Treatment Cycle in Medical Settings
A treatment cycle is a structured process that consists of repeated sequences of therapy interspersed with rest periods, which may include either no treatment or alternative therapies. For example, one cycle may involve one week of active treatment and three weeks of rest. When this cycle is repeated consistently over time, it constitutes a comprehensive course of therapy, often referred to as a treatment plan [43].
This cyclical framework is integral to treatment plans, which outline the timing, dosage, and sequence of interventions tailored to individual patient needs. Clinical pathways (standardized, evidence-based protocols) guide these cycles by specifying diagnostic, therapeutic, and monitoring steps for specific patient populations, ensuring consistency while allowing adaptability to clinical progress [44].
The medical process comprises several sequential steps that must be executed. As illustrated in Figure 1, each stage has its priorities and specific position within the overall framework. The diagnosis and the chosen treatment plan will be assessed based on the preceding steps.
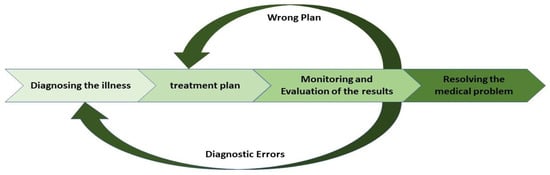
Figure 1.
Medical process overview with treatment cycle focus.
3.2. The Importance of a Cyclical Approach in Medical Processes
As indicated in Figure 1, a set of activities is typically performed for the diagnosis step and the treatment plan. Medical processes often require frequent review and reassessment [45]. Continuous evaluation and improvement during the treatment process are essential, leading to the development of a cyclical approach within the therapeutic framework. Clinical pathways are prescriptive models outlining the standard medical steps required to treat specific patient populations [46]. Clinical pathways represent a patient’s diagnostic and treatment journey [47]. This implication highlights the importance of a cyclical approach, facilitating continuous evaluation and improvement. This iterative process enhances patient outcomes and optimizes data analysis by allowing for the integration of feedback and adjustments at each stage of care [48].
Treatment plans typically consist of distinct phases that recur over time. The cyclical approach enables the linkage of data points to specific phases within a treatment cycle, enhancing data organization. This facilitates progress tracking, the identification of trends, and the comparison of data across different cycles [49].
4. Two Different Approaches Within EMRs for the Patient Treatment Process
EMR systems have reached a pivotal stage in their adoption and utilization. As healthcare professionals become more proficient in leveraging these systems, their potential to enhance clinical decision-making and patient outcomes grows consistently [50]. EMR system architectures must be designed with transparency and efficiency to maximize these benefits, ensuring that physicians can generate meaningful reports and make well-informed medical decisions. This section examines existing and proposed approaches to structuring medical processes within EMRs, evaluating their effectiveness and potential improvements.
4.1. Conventional Approach
Healthcare professionals increasingly leverage EMRs to streamline clinical workflows, with advancements such as smart forms enhancing functionality [51]. One of the primary advantages of EMRs is their ability to support clinical decision-making by providing software solutions tailored to individual patient needs [52]. Given the central role of electronic data forms in EMRs, optimizing their structure and functionality across multiple dimensions is crucial.
A key consideration is ensuring that EMR data entry processes align with real-world medical workflows while maintaining transparency in medical reports and, ultimately, in clinical decision-making. Physicians document relevant medical information within designated data forms when patients visit a hospital or medical center. As the treatment progresses, they continuously input updates into the data fields embedded within the EMR system. After treatment, the outcomes are recorded to facilitate a comprehensive evaluation.
For medical teams to assess both the initial diagnosis and the effectiveness of the chosen treatment path, a structured and accessible data framework is essential. Regardless of whether a treatment is successful, the recorded medical data and outcomes remain valuable for the individual patient and similar future cases. The critical limitation of the current EMR approach is the absence of a well-defined treatment cycle structure, leading to fragmented data entry where each data point is recorded in isolation, without considering its relationships with other relevant forms. This scattered approach impairs the ability to track and evaluate treatment progression effectively. The prevalent data entry workflow among physicians, which illustrates these challenges, is depicted in Figure 2.
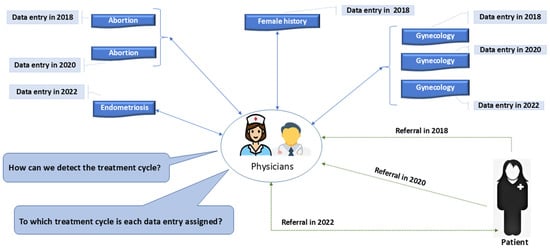
Figure 2.
Ambiguity in current EMR systems for assigning data entries to treatment cycles.
In the conventional EMR data entry approach, inconsistencies arise due to the lack of a structured treatment cycle framework. A crucial issue is that certain data forms may be filled out multiple times during a patient’s treatment course, while others (equally relevant to the case) may only be completed once. This irregularity creates ambiguity for physicians when tracking and analyzing a patient’s treatment route. Physicians struggle to determine whether a set of records represents a single continuous treatment plan or multiple fragmented encounters without a standardized cycle-based structure.
A well-defined treatment cycle should include a consistent set of core data forms for every patient referral, ensuring that each treatment iteration is comprehensively documented. However, in the current system, these essential forms are not systematically present across each patient’s visit. This inconsistency is exemplified in Figure 2, where the number of completed data forms does not align with the number of patient referrals. In the given case, a patient underwent three separate treatment attempts due to unsuccessful outcomes in prior visits. Despite this, some key data forms were only utilized once or twice rather than being completed systematically for each referral.
This fundamental flaw (the absence of a structured and clearly defined treatment cycle) leads to critical gaps in medical documentation, making it challenging to evaluate treatment progress, identify patterns, and ensure data completeness for clinical decision support. To address this issue, we propose a novel EMR model that systematically integrates treatment cycles, ensuring that each cycle is adequately defined, structured, and consistently documented across all patient encounters.
4.2. Treatment Cycle Approach Within EMRs
The effective utilization of EMRs is pivotal in promoting physician engagement with these systems, as it provides significant incentives for adoption while enforcing penalties for non-compliance [53,54]. However, to maximize the usability and clinical value of EMRs, it is essential to ensure that the data entered during the treatment process are structured in such a way that aligns with real-world medical workflows. Recognizing this necessity, we have introduced an innovative cycle-based approach that redefines how medical processes are documented and analyzed within EMRs. This proposed model systematically segments the entire treatment process into distinct cycles, allowing for a more structured and transparent representation of a patient’s progression. By explicitly defining treatment stages and outcomes within the EMR, this approach enhances the ability of physicians and medical decision support systems to track treatment effectiveness, recognize iterative patterns, and optimize patient care strategies. Ultimately, the cycle-based framework bridges the gap between digitalized medical data and practical clinical needs, offering a more intuitive and analytically powerful solution for the management of treatment histories within EMRs.
As illustrated in Figure 3, the proposed model establishes a structured sequence for data form arrangement, ensuring that, except for the initial form, each subsequent form has prerequisite entries before it can be accessed or completed. In this framework, the priority of data entry is a fundamental principle, with numbered data forms appearing sequentially based on their designated order of precedence. While each data form is associated with a specific department and utilized independently by its users, the model maintains interdependence and continuity, effectively linking them like a chain.
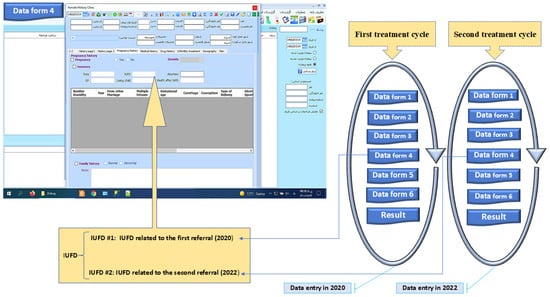
Figure 3.
Cyclical structure for organizing data forms in EMR treatment cycles.
This structured approach resolves the imbalance between the number of treatment cycles and the corresponding data forms used in the current system. By enforcing a logical sequence, the proposed model enhances data integrity and ensures a more coherent representation of the treatment process. To further illustrate the application of this method, Figure 3 presents a data field for intrauterine fetal demise (IUFD) as an example of a structured treatment cycle.
In this model, each IUFD data field per referral is assigned a unique cycle number, distinguishing treatment cycles. For instance, IUFD data from 2020 are separable from IUFD data from 2022 through the cycle number. This patient underwent two treatment cycles, each with a different path and outcome. The cycle number field in medical reports helps medical teams to identify the corresponding treatment cycle for each data entry, allowing for a more precise evaluation of treatment progress and effectiveness. Additionally, this model provides flexibility in defining treatment cycles, enabling medical personnel to include multiple data forms as needed. The cycle structure and its components can be modified based on medical requirements, ensuring adaptability to different treatment scenarios. An empirical evaluation is conducted to validate this approach, comparing it with the conventional EMR design and demonstrating its advantages in structuring and analyzing medical data.
5. Implementing the Proposed Model
Digitalization has become a fundamental aspect of modern healthcare, transforming traditional medical practices into digital formats. This transition involves utilizing electronic systems to collect, store, and analyze patient data, ultimately enhancing efficiency, accuracy, and accessibility in medical processes [55]. Moreover, AI-driven data collection, analysis, and assessment are increasingly integrated into healthcare, enabling more precise and reliable outcomes [49,50]. As discussed in the previous section, the cyclical approach is essential in structuring medical records. Therefore, integrating this approach into EMRs is a crucial consideration. Digitalization sometimes necessitates modifications to existing processes to ensure optimal implementation.
During EMR deployment, IT specialists frequently encounter a recurring request from physicians: “We need to determine which referral (treatment cycle) a specific data field (e.g., lab results, medical item) belongs to in the patient’s treatment”. Addressing this requirement is essential to prevent medical errors and ensure data integrity. To resolve this issue, our study proposes a structured model of sequential data forms that delineates treatment cycles within EMRs, enhancing clarity and decision-making.
5.1. Technical Considerations
EMR systems can enhance patient data retrieval, organization, and analysis by integrating treatment cycle-based data tagging. Employing unique cycle identifiers during data entry can significantly improve the data’s integrity, transparency, and traceability. This section discusses this framework’s technical aspects of data entry design, management, and retrieval.
5.1.1. Data Entry Design
The data entry design integrates cycle identifiers into form templates, automatically tagging each form with its respective treatment cycle. This ensures accurate and consistent data tagging while providing a user-friendly interface for healthcare providers to minimize errors and streamline the data entry process. These cycle-based form templates are implemented in the following way.
- Each data form (f1, f2, …, fn) within a treatment cycle is designed to include a field for a cycle identifier (flag).
- The form templates are pre-configured to automatically assign the appropriate treatment cycle flag according to the patient’s current treatment stage.
- For instance, during the first treatment cycle, forms will be tagged with “Cycle 1”. If the patient begins a new cycle, subsequent forms will be tagged with “Cycle 2”.
The system employs automated flagging to ensure that the cycle identifier is consistently applied to all relevant forms without manual input, reducing the risk of errors.
5.1.2. Data Management
Data management exploits a robust database schema that includes treatment cycle identifiers for each entry. This maintains data integrity and prevents the mixing of cycle information, with strict validation protocols and regular audits ensuring the accuracy and reliability of patient records. The data management concern has been considered in two axioms.
Database Schema: The database schema is designed to include a column for the treatment cycle identifier, ensuring that each data entry is associated with the correct cycle. An example of the mentioned schema is as follows.
As demonstrated in Table 1, the database schema includes columns for the patient ID, form ID, data, and treatment cycle. This structure ensures that each data entry is accurately linked to its treatment cycle. The patient with Patient ID 12004 has undergone two distinct treatment cycles in this example. In the first treatment cycle, forms F1 and F2 were used. Hence, both were tagged with “Cycle 1”. This tagging ensures that all data related to the first cycle are easily identifiable and can be retrieved or analyzed without confusion.

Table 1.
Database structure in the cycle model.
Subsequently, the same patient began a second treatment cycle, during which forms f1 and f3 were used, now tagged with the “Cycle 2” identifier. This demonstrates the model’s flexibility in reusing forms across different cycles while maintaining a clear separation between the data entries of each cycle.
Data Integration: The model is designed to integrate seamlessly with existing EMR systems, ensuring that the tagging process does not disrupt current workflows. In this regard, interoperability standards are adhered to, facilitating the exchange and management of tagged data across different healthcare information systems.
The flexibility of the cyclical model, allowing physicians to modify forms, directly impacts data integration. This adaptability necessitates dynamic data mapping and integration processes. When physicians can alter the structure or content of forms, it implies that the data structure is dynamic, meaning that the data model must be adaptable to accommodate changing form layouts and data elements.
5.2. Data Retrieval and Analysis
Effective data retrieval and analysis are essential in leveraging the benefits of the treatment cycle-based data tagging model. Advanced query functions enable healthcare providers to filter and access patient data based on treatment cycle identifiers, facilitating precise and relevant data retrieval. Clinicians can easily track a patient’s treatment history and analyze outcomes across different treatment cycles, with clear distinctions between different treatment cycles maintained by the system via the anticipated queries and analytical tools.
Query Functions: The EMR system incorporates advanced query functions that allow healthcare providers to filter and retrieve patient data based on treatment cycle identifiers. In this case, the system can provide the required data through various queries like the following:
SELECT ∗ FROM Patient Records WHERE Patient ID = 12004 AND Treatment Cycle = ‘Cycle 1’.
Reporting and Analytics Tools: Reporting tools are developed to generate insights and reports based on treatment cycle data, helping clinicians to analyze the treatment efficacy across different cycles [56]. Cyclical data tagging facilitates pattern recognition and trend analysis, enabling data-driven insights for improved healthcare outcomes and resource allocation. Tools like SQL 2019, Python 3.10 (with libraries like Pandas 1.5), or specialized healthcare analytics platforms (e.g., Tableau 2023.1 or Power BI) can extract insights from tagged data. For instance, analyzing treatment cycle data can reveal optimal treatment pathways or identify patient subgroups that respond better to specific therapies.
6. Empirical Evaluation of the Cycle Approach’s Utilization in EMRs
To investigate the efficacy of a cyclical approach within EMRs for data management and analysis, we constructed a synthetic dataset comprising 750 rows inspired by real-world clinical data and tailored to emphasize iterative treatment cycles and data tagging. This dataset, titled “CycleDiabetes750”, is available at https://bit.ly/MIMIC-Cycle750 (accessed on 4 March 2025), is rooted in the structure of the Medical Information Mart for Intensive Care III (MIMIC-III) database, a widely recognized public dataset sourced from Beth Israel Deaconess Medical Center and accessible via PhysioNet [57]. MIMIC-III was selected due to its comprehensive coverage of clinical variables pertinent to diabetes care, including patient demographics (e.g., subject_id, age, gender), laboratory measurements (e.g., glucose), medication administrations (e.g., treatment_type), diagnoses (e.g., diagnosis), and admission details (e.g., admission_type). These data fields align closely with the study’s requirements, providing a robust framework for the simulation of diabetes treatment scenarios.
To specifically address the cyclical nature of treatment plans and enable the detailed analysis of iterative interventions, we augmented the MIMIC-III-inspired dataset with custom fields not natively present in the original database. These included “treatment_cycle”, “cycle_tag”, “cycle_outcome”, “treatment_start_date”, “treatment_end_date”, and “total_cycles”. The “treatment_cycle” field distinguishes successive treatment phases (Cycle 1, 2, 3), while “cycle_tag” categorizes patient glucose states (Initial_VeryHigh, Followup_Moderate) to reflect the clinical severity and progression. The “cycle_outcome” field (Improved, Stable) evaluates the treatment efficacy related to each cycle, and the temporal fields (the treatment_start_date, the treatment_end_date) track intervention durations. Finally, “total_cycles” indicates the number of cycles per patient, facilitating longitudinal analysis. These modifications transform the static, single-event focus of traditional EMR datasets like MIMIC-III into a dynamic, cycle-based framework, enabling us to explore features and analytical capabilities unique to this design.
To ensure that incorporating treatment cycle fields does not introduce bias, the dataset’s construction followed rigorous validation protocols, aligning with established guidelines for synthetic dataset development in medical research [58,59]. The statistical distribution of patient characteristics was preserved to reflect real-world clinical settings, minimizing artificial distortions. Cycle assignments were structured based on predefined glucose thresholds rather than arbitrary labeling, preventing systematic misclassification. Additionally, confounding variables such as patient demographics and admission types were analyzed to confirm that cycle-based grouping did not disproportionately affect treatment cycle categorization [60]. Lastly, potential observer bias was mitigated by maintaining transparency in variable definitions and ensuring that treatment cycle classifications were based on objective glucose level thresholds rather than subjective clinical interpretations. These precautions collectively enhanced the study’s internal validity, ensuring that the findings were robust and not driven by dataset modifications.
The primary objective of this empirical analysis is to demonstrate enhanced analytical precision and multifaceted insights afforded by the cyclical approach in EMRs, which are inaccessible without such a design. The dataset comprises patient records with variables such as treatment dates, treatment cycles, post-treatment metrics, and success rates. To assess the effect of treatment cycles, we compared the results obtained from analyses considering cycle tagging with those obtained from studies that ignored cyclic patterns.
6.1. Data Preparation and Cleaning
The dataset was first in an Excel file and inspected for consistency, missing values, and outliers. Variables considered in this study (such as treatment_cycle, post_treatment_glucose, and treatment_success_rate) were identified and cleaned to ensure uniformity. Any erroneous entries were corrected, and the data were tagged based on the treatment cycle, creating a structured approach for subsequent analyses.
6.2. Cycling vs. Non-Cycling Approaches
In this regard, we conducted a detailed analysis of treatment outcomes by comparing metrics across treatment cycles, conducting statistical tests, and visualizing the results. The studies mentioned were performed within the Python environment, and the provided codes are available at the Zenodo repository (https://zenodo.org/records/15046756, accessed on 18 March 2025).
The cyclical and non-cyclical analyses were conducted using the same dataset, including identical patient records, variables, and outcome definitions. The only difference between the two approaches lies in the additional fields that we introduced to support treatment cycle identification (e.g., treatment_cycle, cycle_tag). These enhancements enabled data tagging that facilitated cycle-based analysis, without altering the underlying data.
Cyclical analysis: Rather than treating all patient records as part of a uniform dataset, entries were grouped based on their corresponding treatment cycles (e.g., Cycle 1, Cycle 2, Cycle 3), as defined by the treatment_cycle field. This cyclical approach enabled an evaluation of variations across individual cycles by calculating summary statistics (mean, standard deviation) and visualizing cycle-specific distributions. Such categorization allows for the detection of meaningful patterns and clinical insights that could be overlooked in aggregate-level analyses.
Table 2 shows that the data can be broken down by treatment cycle by exploiting the cyclical approach. For each cycle (for example, Cycle 1, Cycle 2, etc.), we calculated multiple metrics, such as the mean, standard deviation, median, minimum, maximum, and count for post-treatment glucose levels, alongside similar statistics for the treatment success rate. Each row corresponds to a treatment cycle, and each column shows a specific metric. For instance, regarding the cell in row [2, 1] of this table, it represents the value of the first metric (typically the post-treatment glucose mean) for the treatment cycle listed in the second row (which here corresponds to, e.g., Cycle 2). This cell gives a quick snapshot of this cycle’s average post-treatment glucose value, providing insights into how the treatment outcomes may vary from one cycle to another.

Table 2.
Cycle-based metrics.
Non-cyclical analysis: For comparison, an overall analysis was performed by treating the dataset as a single group, disregarding cycle information. This approach provides an aggregated view of the treatment outcomes, helping to highlight the potential loss of granularity when treatment cycles are not considered.
Table 3 demonstrates that the conventional approach aggregates all data without considering the treatment cycles. It computes the same metric types (mean, standard deviation, median, etc.) for the entire dataset. Essentially, it gives a single, overall view of the post-treatment glucose levels and treatment success rates for the whole patient population. This approach is practical when we require an aggregate metric. Still, as our further analysis shows, it hides any differences or variability across treatment cycles.

Table 3.
Overall metrics (non-cyclical approach).
In summary, the cycle-based table reveals nuances between individual treatment cycles (highlighted by values such as cell [2, 1] for Cycle 2’s average post-treatment glucose level). In contrast, the overall table provides a single, unified benchmark. This two-pronged approach illustrates that significant differences between information stemming from treatment cycles might be lost if one only looks at the global (non-cyclical) summary.
6.3. Statistical Analysis
To rigorously assess the impact of the cyclical approach on treatment outcomes, we conducted a statistical evaluation using analysis of variance (ANOVA) [61]. This method was chosen because it allows us to compare means across multiple treatment cycles and determine whether the observed differences are statistically significant. Unlike simple pairwise comparisons, which increase the risk of Type I errors [62], ANOVA efficiently tests for variations across all cycles simultaneously, ensuring a more reliable interpretation of the treatment effects.
According to the structure of our dataset (where each treatment cycle may involve a distinct treatment plan tailored to evolving clinical judgments), we considered each cycle an independent analysis unit. While some individual patients may have undergone multiple cycles, the treatment strategy in each cycle was often adjusted depending on patient-specific responses. Therefore, treatment cycles were not strictly repeated measures of a fixed protocol but potentially distinct interventions with unique clinical contexts.
Given the independent nature of the treatment cycles in our study, ANOVA was selected as the most suitable statistical method, as it effectively evaluates the variance across cycles without assuming systematic correlations within patients. The frequent adjustments in treatment plans reinforce this choice. These adjustments minimize the dependency between treatment cycles. As a result, the assumptions of a mixed-effects model become less applicable. At the same time, ANOVA ensures the delivery of robust and clinically relevant insights into cycle-specific outcomes [63].
ANOVA determines whether the means of multiple groups differ significantly by comparing the variance within groups to the variance between groups. The test statistics (F-statistic) are calculated as follows:
ANOVA works by comparing two types of variances.
Variance between groups (treatment cycles): This specifies how much the average values (means) of different treatment cycles differ from the overall mean of all cycles combined. If the treatment cycles are firmly different, their means should be spread apart. Conversely, if they are not, their means will be close together.
Variance within groups (individual patients in the same cycle): This specifies how much individual patient outcomes vary within each treatment cycle. If a treatment cycle is consistent, individual patients will have similar results, and this variance will be low. Otherwise, much variation within the same treatment cycle might indicate external factors affecting treatment.
The first ANOVA test examined post-treatment glucose levels across different cycles. The results are as follows:
F-statistic: 64.6814, p-value: 0.0.
It is interpreted that, since the p-value is zero, we reject the null hypothesis and conclude that glucose levels significantly differ across treatment cycles. This suggests that the treatment effectiveness is not uniform across cycles and that iterative interventions are critical in stabilizing glucose levels.
Similarly, an ANOVA test was conducted to analyze the treatment success rates across cycles. The results obtained are as follows:
F-statistic = 14.4841, p-value = 0.0.
In this case, the very small p-value indicates that the treatment success rates significantly differ between cycles. This confirms that the treatment success rates also vary between cycles, reinforcing the importance of tracking treatment progress over the mentioned cycles rather than evaluating overall outcomes.
Although some minor within-patient dependency may exist, the magnitude of the observed differences (evidenced by extremely small p-values) indicates that the main findings are robust. The strong statistical significance and clinical rationale for cycle independence support the use of ANOVA as a reliable and interpretable method in this context.
These findings highlight the necessity of incorporating a cyclical approach into EMRs, unlike traditional EMRs, which often treat patient visits as isolated events and miss critical trends in the treatment response. The cycle-based structure provides deeper insights into patient progression, enabling more personalized and adaptive treatment strategies.
6.4. Results
6.4.1. Success Rate Evaluation
The results from the statistical analysis highlight the significance of adopting a cyclical approach in EMRs for the evaluation of treatment effectiveness. To illustrate the impact of a cyclical approach in treatment analysis, we visualized the treatment success rates from two different perspectives. This visualization (Figure 4) compares the treatment success rates under cyclical and non-cyclical conditions.
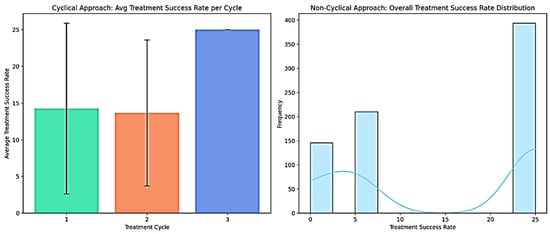
Figure 4.
Treatment success rate evaluation using different approaches.
The left panel in Figure 4 presents a bar plot illustrating the average treatment success rate per cycle, with the error bars indicating standard deviations. This visualization highlights a progressive improvement in the success rate as the treatment cycles advance. The observed trend suggests that treatment responses are not uniform across cycles, reinforcing the importance of capturing cyclical data structures in EMRs. Different colours are used for each bar (Cycle 1, Cycle 2, and Cycle 3) to visually distinguish the treatment cycles, enhancing the clarity of the progressive success rate trend. The third cycle exhibits the highest success rate, indicating that iterative treatments may yield cumulative benefits over time. Conversely, the right panel depicts a histogram with a kernel density estimate (KDE) overlay [64], showing the overall distribution of the treatment success rates across all patients, irrespective of cycle classification. Without the cyclical structure, the distribution appears heterogeneous, failing to differentiate the impacts of successive treatment phases. This limitation underscores the loss of critical insights when treatment cycles are not explicitly incorporated into the analysis.
These findings demonstrate that incorporating a cyclical approach enables a more nuanced treatment success analysis, distinguishing patterns hidden in traditional, non-cyclical datasets. The Python code used to generate these visualizations is available in the Zenodo repository at https://zenodo.org/records/15066593 (accessed on 21 March 2025), allowing further exploration of this approach.
6.4.2. Information Loss Analysis
To examine the impact of the cyclical approach, we analyzed the post-treatment glucose levels across different treatment cycles and compared them to a non-cyclical perspective. The left panel of Figure 5 presents a box plot illustrating the post-treatment glucose levels for each treatment cycle. In contrast, the right panel displays a histogram accompanied by a KDE representing the overall post-treatment glucose distribution without consideration of cycle distinctions.
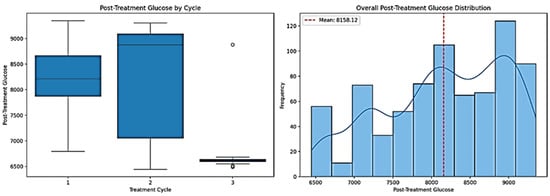
Figure 5.
Post-treatment glucose evaluation using different approaches.
As depicted in the left panel of Figure 5, the post-treatment glucose levels fluctuate significantly across treatment cycles. The box plot shows that Cycle 3 has substantially lower post-treatment glucose levels than Cycles 1 and 2, indicating a potential trend where the glucose levels decrease as the treatment progresses. This suggests that successive cycles contribute to more effective glucose regulation, an insight that would be overlooked without cycle-specific analysis. Additionally, the variation in glucose levels, as indicated by the interquartile ranges and whiskers, differs significantly among cycles, underscoring the importance of evaluating treatment phases separately.
The right panel of Figure 5, which aggregates all data without accounting for cycles, provides a significantly less detailed view. The histogram shows the overall distribution of the glucose levels but does not reveal whether specific cycles are responsible for higher or lower values. The red dashed line indicates the mean glucose level. Still, without distinguishing between treatment cycles, assessing whether this average is influenced by specific cycles performing better or worse is impossible.
A quantitative analysis further supports these findings. By incorporating treatment cycles, we can observe distinct differences between the cycles. These variations are lost without this distinction, as shown in the overall distribution, leading to a less precise understanding of glucose trends. Specifically, our analysis shows that 14.96% of the variance in the post-treatment glucose levels can be explained by the treatment cycles. This means that, by ignoring the cyclical nature of treatment, we lose about 15% of the relevant information, potentially overlooking significant patterns in the patient response.
A similar trend is observed in the treatment success rates. The grouping of the data by cycle explains 37.31% of the variance, meaning that over one-third of the variation in treatment outcomes is directly related to the treatment cycle structure. Without tracking the treatment cycles separately, the mentioned information will be lost, leading to an incomplete or potentially misleading interpretation of the treatment efficacy.
These percentages originate from our ANOVA test, which quantifies how much of the total variance in the dataset is explained by differences between treatment cycles. Specifically, the ANOVA partitions the variance into two components:
Variance between treatment cycles—how much the mean values differ across cycles.
Variance within cycles—how much individual values vary within each treatment cycle.
The proportion of the variance explained by the treatment cycles, known as the eta-squared (η²), is calculated as the ratio of the between-group variance to the total variance [65]. This metric quantifies the extent to which treatment cycles contribute to variations in the post-treatment glucose levels and treatment success rates. The obtained values suggest that incorporating treatment cycles is essential in accurately assessing glucose regulation and treatment efficacy, as it accounts for a significant portion of the observed variance.
These findings reinforce the importance of a cyclical EMR design, demonstrating that a non-cyclical system fails to capture crucial variations in patient outcomes. A complete set of Python codes for statistical analysis and visualization is available in the Zenodo repository at https://zenodo.org/records/15046756 (accessed on 18 March 2024) for further exploration and validation.
7. Discussion
This study highlights the importance of integrating a cyclical approach into EMRs to improve clinical data management and enhance the effectiveness of CDSS. Traditional EMRs primarily rely on independent, event-driven data entry, often neglecting the iterative nature of treatment plans. Our findings address this critical research gap by demonstrating how treatment cycles impact medical decision-making and the quality of predictive analytics within CDSS.
The current study’s findings align with existing studies investigating the role of structured data organization in EMRs. Hripcsak et al. [66] found that longitudinal data structuring in EMRs enhances predictive analytics for chronic diseases, particularly diabetes management. However, their approach focused on temporal event sequences rather than explicitly modeling treatment cycles. Similarly, Batavia et al. [67] examined the benefits of structured data entry in EMRs, finding that predefined templates improve consistency in medical documentation and facilitate better AI-driven decision-making. While their study reinforced the value of standardization, it did not address the need for a cyclical framework. Moreover, Gifford et al. [68] investigated the effectiveness of repeated intervention tracking in EMRs and found that tracking sequential medical interventions improved treatment predictions. However, their study primarily analyzed repeat hospital admissions rather than structured treatment cycles within a single disease management framework. Our research provides a more granular approach by demonstrating how each treatment cycle uniquely contributes to the overall effectiveness of medical interventions.
A quantitative analysis of the proposed cyclical framework confirms that incorporating treatment cycles significantly enhances the precision of clinical assessments. Specifically, we observed that 14.96% of the variance in post-treatment glucose levels and 37.31% of the variance in treatment success rates could be attributed to the cyclical structure of treatments. These findings suggest that ignoring treatment cycles leads to substantial information loss, making capturing patterns in patient responses challenging over time. Without a cycle-based framework, crucial insights regarding treatment efficacy and disease progression may be overlooked, ultimately reducing the accuracy of CDSS recommendations. Although the potential benefits are evident, implementing this model in real-world practice requires addressing several critical considerations. Key topics include practical implementation strategies, usability impacts, ethical concerns, and opportunities for future research.
7.1. Implementation Challenges in Real-World EMRs
The proposed cyclical framework is currently undergoing pilot implementation in two infertility centers. This domain is particularly well suited to a cycle-based structure, given the complex and multi-staged nature of infertility treatments, which involve different levels of intervention (from prescribing medication to suggesting surgical procedures). At the end of each treatment cycle, clinical outcomes are carefully evaluated based on all practical medical elements and the patient’s exclusive medical history, highlighting the critical importance of sequential data tracking.
The initial implementation phase leverages existing medical forms to mitigate workflow disruption and prevent system overload. Retaining their established structure, these forms are strategically grouped into treatment cycles through sequential sorting. Data form and field revisions will be introduced systematically only after stabilization at this stage. This gradual, structured approach facilitates a seamless transition, enabling the integration of the cyclical method into EMRs without overwhelming users.
The cyclical approach may be incompatible with existing EMR processes, which primarily support independent, event-based data entry rather than iterative, cycle-oriented documentation. To overcome these challenges, physicians and system designers must collaboratively define standardized treatment cycles, specifying structured data forms, mandatory fields, and sequence requirements to ensure alignment with clinical decision-making needs. Furthermore, flexibility in modifying treatment cycles is crucial, as protocols may evolve due to advancements in medical knowledge, patient-specific considerations, or administrative regulations. Adapting to these dynamic requirements will require continuous evaluation and refinement, ensuring that structured cycle-based data entry enhances workflow efficiency rather than impeding it.
Implementing this model requires economic, temporal, and human resource investments during the transition phase. However, by providing structured data for the evaluation of outcomes per cycle, this approach ultimately supports cost-saving through better decision-making, more efficient resource allocation, and the precise calculation of treatment cycle expenses, which is not achievable with non-cyclical models.
7.2. Usability and Clinician Adoption Concerns
Despite the revealed advantages of this approach, implementing a cycle-based framework in EMRs presents several challenges that must be carefully addressed. One of the primary concerns in implementing a cycle-based framework within EMRs is usability, which, according to ISO 9241-11 [69], encompasses effectiveness, efficiency, and user satisfaction. While the cyclical approach enhances effectiveness by improving data accuracy and supporting better clinical decision-making, it may also introduce complexity in data entry workflows. The structured nature of cycle-based documentation imposes stricter data entry protocols, which could reduce efficiency and satisfaction among healthcare providers accustomed to more flexible data input methods.
Several features and technologies proposed in prior studies can be applied to mitigate the potential documentation burden introduced by the cyclical approach. These include automated text generation (e.g., automatically generating textual reports like sonography or radiology reports from structured data inputs), default data values, and data fetching from external medical sources [70,71]. These enhancements can improve efficiency and user satisfaction, aligning with the ISO 9241-11 standard.
Moreover, the usability of the cyclical model will be systematically evaluated using the think-aloud method [72]. During the pilot project, physicians will provide real-time feedback through this technique, facilitating the identification of usability challenges and iterative system refinements. By incorporating clinician insights early in the process, we aim to elevate the usability level of the cyclical approach and foster greater acceptance among end-users.
7.3. Ethical Implications of Cycle-Based EMRs
Implementing a cyclical data structure in EMRs introduces significant ethical considerations that must be carefully addressed. A primary concern is balancing the need for standardized cycle templates (which enable cross-institutional learning and enhance clinical decision-making) with the imperative to safeguard patient confidentiality and ensure data privacy. While some structured elements of treatment cycles (such as generalized data forms and standardized fields) can be shared across systems to enhance collective medical knowledge and improve decision support, it is crucial to ensure that only non-private, non-identifiable information is used for broader applications. Data anonymization and selective filtering must be employed to protect patient confidentiality before sharing data [73,74].
Furthermore, transparency in CDSS recommendations derived from cyclical data is essential. Physicians must explicitly understand how patient data across treatment cycles shapes AI-driven or system-generated recommendations, mitigating the risk of over-reliance on automated outputs. It is crucial to underscore that the ultimate responsibility for medical decisions rests with the physicians [75,76]. The proposed approach is intended to support clinicians by offering structured insights that improve the decision-making accuracy while preserving the primacy of professional judgment.
Finally, the growing structure and data-driven nature of EMR systems necessitate continuous evaluation to ensure that patient safety, data privacy, and data ownership rights are fully respected in all implementation and data utilization stages [77].
7.4. Future Research Directions
This study establishes a foundation for several vital directions in future research. Expanding upon our findings, subsequent investigations could focus on developing machine learning models trained on cyclical treatment data to refine personalized treatment recommendations by capturing the iterative nature of patient responses more effectively. Such models could significantly improve AI-driven care, particularly for rare diseases and atypical conditions, where conventional static data structures often fail to identify nuanced response patterns.
Future research should prioritize a thorough and systematic evaluation of the usability of cycle-based frameworks. Employing iterative user feedback throughout different stages of system design and implementation (guided by established scientific usability evaluation frameworks) will be essential. This approach will help to continuously refine the system based on real-world physician experiences, enhancing its effectiveness, efficiency, and user satisfaction.
Moreover, a critical research direction entails the development of standardized treatment cycles customized for specific medical domains. Academic efforts aimed at defining and validating cycle templates for fields such as infertility treatment, oncology, and chronic disease management would ensure that cyclical documentation aligns with the clinical practices of these specialties while preserving system interoperability and adaptability. These initiatives would significantly enhance the practical utility and adoption potential of cycle-based EMRs across diverse healthcare settings.
8. Conclusions
This study introduces a scientifically grounded definition of cyclical treatment structures and explores their implications for EMRs and CDSS. A comprehensive review of existing studies identified a critical gap in how treatment cycles are represented in EMRs, which often fail to capture the iterative nature of medical interventions. We proposed a structured, cycle-based approach to EMR design to address this issue, outlining its necessity in improving data organization and supporting more precise clinical decision-making.
Beyond the conceptual foundation, we examined the practical implementation of this approach, detailing how cyclical treatment structures can be integrated within EMRs to enhance the accuracy and relevance of stored medical data. We empirically demonstrated its advantages by analyzing structured treatment cycles, showing that this method preserves crucial variations in patient responses that would otherwise be overlooked. The findings indicate that incorporating treatment cycles accounts for a substantial portion of the variance in both post-treatment glucose levels and treatment success rates, reinforcing the value of this structured approach in medical decision-making.
By aligning EMR data structures more closely with real-world clinical workflows, this study provides a framework that meets physicians’ needs for structured, context-aware data analysis. The proposed approach ensures that patient records reflect the actual progression of treatment, aiding physicians in evaluating past interventions and optimizing future treatment strategies. This is particularly critical in complex and long-term conditions where treatment plans evolve.
Moreover, this research contributes to the ongoing evolution of medical data management by proposing a structured methodology that balances data granularity with usability. While workflow integration and user adoption must be addressed, this study establishes a foundation for future research into AI-driven analytics, predictive modeling, and personalized medicine.
In conclusion, the current study provides a theoretical and empirical basis for the integration of cyclical treatment structures into EMRs, offering a scientifically validated solution to a pressing challenge in CDSS design. As medical data systems evolve continuously, adopting this structured approach will improve diagnostic accuracy, refine treatment plans, and ultimately enhance patient care.
Author Contributions
Conceptualization, A.A.; Methodology, A.A. and F.J.G.-P.; Formal analysis, A.A. and F.J.G.-P.; Investigation, A.A.; Data curation, A.A. and F.J.G.-P.; Validation, A.A. and F.J.G.-P.; Visualization, A.A.; Writing—original draft, A.A.; Writing—review and editing, F.J.G.-P.; Supervision and project administration, F.J.G.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are publicly available on Zenodo repository at https://zenodo.org/records/14968776 (accessed on 4 March 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zikos, D.; Delellis, N. CDSS-RM: A Clinical Decision Support System Reference Model. BMC Med. Res. Methodol. 2018, 18, 137. [Google Scholar] [CrossRef] [PubMed]
- Cesare, M.; D’Agostino, F.; Sebastiani, E.; Group, N.A.P.H.; Damiani, G.; Cocchieri, A. Deciphering the Link Between Diagnosis-Related Group Weight and Nursing Care Complexity in Hospitalized Children: An Observational Study. Children 2025, 12, 103. [Google Scholar] [CrossRef]
- Cesare, M.; Zega, M. Clinical Nursing Information Systems Based on Standardized Nursing Terminologies: How Are We Doing? J. Nurs. Sch. 2024, 56, 625–627. [Google Scholar] [CrossRef]
- Sutton, R.T.; Pincock, D.; Baumgart, D.C.; Sadowski, D.C.; Fedorak, R.N.; Kroeker, K.I. An Overview of Clinical Decision Support Systems: Benefits, Risks, and Strategies for Success. NPJ Digit. Med. 2020, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.; Marsden, P.; Soden, P.; Naylor, E.; New, J.; Dornan, T. Involving Users in the Design and Usability Evaluation of a Clinical Decision Support System. Comput. Methods Programs Biomed. 2002, 69, 123–135. [Google Scholar] [CrossRef]
- Bakken, S. Innovative Informatics Interventions to Improve Health and Health Care. J. Am. Med. Inform. Assoc. 2023, 30, 409–410. [Google Scholar] [CrossRef]
- Miller, K.; Mosby, D.; Capan, M.; Kowalski, R.; Ratwani, R.; Noaiseh, Y.; Kraft, R.; Schwartz, S.; Weintraub, W.S.; Arnold, R. Interface, Information, Interaction: A Narrative Review of Design and Functional Requirements for Clinical Decision Support. J. Am. Med. Inform. Assoc. 2017, 25, 585–592. [Google Scholar] [CrossRef]
- Raghupathi, W. Designing Clinical Decision Support Systems in Health Care: A Systemic View. Int. J. Health Inf. Syst. Inform. 2007, 2, 44–53. [Google Scholar] [CrossRef]
- Casalino, L.; Crosson, F. Physician Satisfaction and Physician Well-Being: Should Anyone Care? Prof. Prof. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Bonacina, S.; Pozzi, G.; Pinciroli, F.; Marceglia, S.; Ferrante, S. A Design Methodology for Medical Processes. Appl. Clin. Inf. 2016, 7, 191–210. [Google Scholar] [CrossRef]
- Turkcan, A.; Zeng, B.; Lawley, M. Chemotherapy Operations Planning and Scheduling. IIE Trans. Health Syst. Eng. 2012, 2, 31–49. [Google Scholar] [CrossRef]
- Grechuta, K.; Shokouh, P.; Alhussein, A.; Müller-Wieland, D.; Meyerhoff, J.; Gilbert, J.; Purushotham, S.; Rolland, C. Benefits of Clinical Decision Support Systems for the Management of Noncommunicable Chronic Diseases: Targeted Literature Review. Interact. J. Med. Res. 2024, 13, e58036. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Tian, Y.; Lyu, K.; Zhou, T.; Zhang, P.; Chen, J.; Li, J. Electronic Health Record–Oriented Knowledge Graph System for Collaborative Clinical Decision Support Using Multicenter Fragmented Medical Data: Design and Application Study. J. Med. Internet Res. 2024, 26, e54263. [Google Scholar] [CrossRef] [PubMed]
- Goli, R.; Hubig, N.; Min, H.; Gong, Y.; Sittig, D.; Rennert, L.; Robinson, D.; Biondich, P.; Wright, A.; Nøhr, C.; et al. Keyphrase Identification Using Minimal Labeled Data with Hierarchical Context and Transfer Learning. medRxiv 2023. [Google Scholar] [CrossRef]
- Bae, H.; Park, S.-Y.; Kim, C.-E. A Practical Guide to Implementing Artificial Intelligence in Traditional East Asian Medicine Research. Integr. Med. Res. 2024, 13, 101067. [Google Scholar] [CrossRef]
- Aldhafiri, M.F.; Alrashidi, S.S.; Almutairi, A.S.; Almutrfi, J.S.; Alshammari, A.A.; Almutairi, L.M.; Albugami, M.M. Electronic Medical Records: Impacts, Outcomes, Challenges, and Opportunities. Med. J. Cairo Univ. 2024, 92. [Google Scholar] [CrossRef]
- Kumar, M.; Gotz, D.; Nutley, T.; Smith, J. Research Gaps in Routine Health Information System Design Barriers to Data Quality and Use in Low- and Middle-income Countries: A Literature Review. Int. J. Health Plann. Manag. 2018, 33, e1–e9. [Google Scholar] [CrossRef]
- Köpcke, F.; Prokosch, H. Employing Computers for the Recruitment into Clinical Trials: A Comprehensive Systematic Review. J. Med. Internet Res. 2014, 16, e161. [Google Scholar] [CrossRef]
- Hansen, C. Phased Correction of a Worn Dentition with a Severe Occlusal Cant Using a Systematic Management System. Compend. Contin. Educ. Dent. (15488578) 2024, 45, 44. [Google Scholar]
- Taylor, M.J.; McNicholas, C.; Nicolay, C.; Darzi, A.; Bell, D.; Reed, J.E. Systematic Review of the Application of the Plan-Do-Study-Act Method to Improve Quality in Healthcare. BMJ Qual. Saf. 2014, 23, 290–298. [Google Scholar] [CrossRef]
- Dixit, R.; Boxley, C.; Samuel, S.; Mohan, V.; Ratwani, R.; Gold, J. Electronic Health Record Use Issues and Diagnostic Error: A Scoping Review and Framework. J. Patient Saf. 2023, 19, e25–e30. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, J.M.; Stuckhardt, L.; Saunders, R.; Smith, M. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America; National Academies Press: Washington, DC, USA, 2013. [Google Scholar]
- Loeb, G.E. A New Approach to Medical Diagnostic Decision Support. J. Biomed. Inf. 2021, 116, 103723. [Google Scholar] [CrossRef] [PubMed]
- Laka, M.; Carter, D.; Merlin, T. Evaluating Clinical Decision Support Software (CDSS): Challenges for Robust Evidence Generation. Int. J. Technol. Assess. Health Care 2024, 40, e16. [Google Scholar] [CrossRef]
- White, N.M.; Carter, H.E.; Kularatna, S.; Borg, D.N.; Brain, D.C.; Tariq, A.; Abell, B.; Blythe, R.; McPhail, S.M. Evaluating the Costs and Consequences of Computerized Clinical Decision Support Systems in Hospitals: A Scoping Review and Recommendations for Future Practice. J. Am. Med. Inform. Assoc. 2023, 30, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Carvour, M.L.; Chiu, A. A Cyclical Approach to Continuum Modeling: A Conceptual Model of Diabetic Foot Care. Front. Public Health 2017, 5, 337. [Google Scholar] [CrossRef]
- Burstin, H.; Cosby, K. Measuring Performance of the Diagnostic Process. JAMA 2022, 328, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Epner, P.; Bauer, V.; Solomonides, A.; Newman-Toker, D.E. Identifying and Analyzing Diagnostic Paths: A New Approach for Studying Diagnostic Practices. Diagnosis 2017, 4, 67–72. [Google Scholar] [CrossRef]
- Ball, J.R.; Miller, B.T.; Balogh, E.P. Improving Diagnosis in Health Care; National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Ghassemi, M.; Pushkarna, M.; Wexler, J.; Johnson, J.; Varghese, P. Clinicalvis: Supporting Clinical Task-Focused Design Evaluation. arXiv 2018, arXiv:1810.05798. [Google Scholar]
- Huang, M.; Han, H.; Wang, H.; Li, L.; Zhang, Y.; Bhatti, U.A. A Clinical Decision Support Framework for Heterogeneous Data Sources. IEEE J. Biomed. Health Inf. 2018, 22, 1824–1833. [Google Scholar] [CrossRef]
- Mikkelsen, G.; Aasly, J. Manual Semantic Tagging to Improve Access to Information in Narrative Electronic Medical Records. Int. J. Med. Inf. 2002, 65, 17–29. [Google Scholar] [CrossRef]
- Marco-Ruiz, L.; Pedrinaci, C.; Maldonado, J.A.; Panziera, L.; Chen, R.; Bellika, J.G. Publication, Discovery and Interoperability of Clinical Decision Support Systems: A Linked Data Approach. J. Biomed. Inf. 2016, 62, 243–264. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-H.; Allot, A.; Lai, P.-T.; Leaman, R.; Tian, S.; Luo, L.; Jin, Q.; Wang, Z.; Chen, Q.; Lu, Z. PubTator 3.0: An AI-Powered Literature Resource for Unlocking Biomedical Knowledge. Nucleic Acids Res. 2024, 52, W540–W546. [Google Scholar] [CrossRef] [PubMed]
- Landolsi, M.Y.; Hlaoua, L.; Ben Romdhane, L. Information Extraction from Electronic Medical Documents: State of the Art and Future Research Directions. Knowl. Inf. Syst. 2023, 65, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, P.; Hu, C.; Zhang, K.; Dai, D.; Chang, H.; Zhu, C. Research on the Structuring of Electronic Medical Records Based on Joint Extraction Using BART. In China Health Information Processing Conference; Xu, H., Chen, Q., Lin, H., Wu, F., Liu, L., Tang, B., Hao, T., Huang, Z., Eds.; Springer Nature: Singapore, 2024; pp. 212–226. [Google Scholar]
- Chen, J.; Li, Z.; Ma, W.; Tang, Y.; Liu, C.; Ma, S.; Xu, M.; Zhang, Q. Enhancing the Timeliness of EMR Documentation in Resident Doctors: The Role of PDCA Cycle Management. BMC Med. Educ. 2024, 24, 1367. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Y.; Tian, L.-L.; Qian, Y.-M.; Li, J.-S. An Electronic Medical Record System with Treatment Recommendations Based on Patient Similarity. J. Med. Syst. 2015, 39, 55. [Google Scholar] [CrossRef]
- Newby, D.; Taylor, N.; Joyce, D.W.; Winchester, L.M. Optimising the Use of Electronic Medical Records for Large Scale Research in Psychiatry. Transl. Psychiatry 2024, 14, 232. [Google Scholar] [CrossRef]
- Johnson, R.; Chang, T.; Moineddin, R.; Upshaw, T.; Crampton, N.; Wallace, E.; Pinto, A.D. Using Primary Health Care Electronic Medical Records to Predict Hospitalizations, Emergency Department Visits, and Mortality: A Systematic Review. J. Am. Board Fam. Med. 2024, 37, 583–606. [Google Scholar] [CrossRef]
- Da Silva Mendes, V.; Nierer, L.; Li, M.; Corradini, S.; Reiner, M.; Kamp, F.; Niyazi, M.; Kurz, C.; Landry, G.; Belka, C. Dosimetric Comparison of MR-Linac-Based IMRT and Conventional VMAT Treatment Plans for Prostate Cancer. Radiat. Oncol. 2021, 16, 133. [Google Scholar] [CrossRef]
- Haekal, T.A. Inspection Program Effectiveness Key Performance Indicator for Pressurized Static Equipment Integrity at Offshore Platform. J. Mater. Explor. Find. 2004, 3, 4. [Google Scholar] [CrossRef]
- Yeh, Y.; Tsai, H.; Chen, Y.-C.; Su, W.; Chen, P.-J.; Chang, T.-K.; Li, C.-C.; Huang, C.-W.; Wang, J.-Y. Effects of the Number of Neoadjuvant Therapy Cycles on Clinical Outcomes, Safety, and Survival in Patients with Metastatic Colorectal Cancer Undergoing Metastasectomy. Oncol. Res. 2023, 30, 65–76. [Google Scholar] [CrossRef]
- Dickson, N.; Beauchamp, K.; Perry, T.; Roush, A.; Goldschmidt, D.; Edwards, M.L.; Blakely, L. Impact of Clinical Pathways on Treatment Patterns and Outcomes for Patients with Non-Small-Cell Lung Cancer: Real-World Evidence from a Community Oncology Practice. J. Comp. Eff. Res. 2022, 11, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Sultanovs, E.; Strebko, J.; Romanovs, A.; Lektauers, A. The Information Technologies in the Control Mechanism of Medical Processes. In Proceedings of the 2020 61st International Scientific Conference on Information Technology and Management Science of Riga Technical University (ITMS), Riga, Latvia, 15–16 October 2020; pp. 1–5. [Google Scholar]
- Moldovan, F.; Blaga, P. The Continuous Improvement Cycle Core Activities for the Sustainable Development of Healthcare Facilities. In International Conference Interdisciplinarity in Engineering; Springer: Cham, Switzerland, 2021; pp. 316–325. [Google Scholar]
- Caron, F.; Vanthienen, J.; Baesens, B. Healthcare Analytics: Examining the Diagnosis–Treatment Cycle. Procedia Technol. 2013, 9, 996–1004. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Tian, Y.; Zhang, K.; Hao, G.; Shen, L.; Du, Q. Application of the PDCA Cycle for Standardized Nursing Management in Sepsis Bundles. BMC Anesthesiol. 2022, 22, 39. [Google Scholar] [CrossRef]
- Castillo, C.M.; Harper, J.; Roberts, S.A.; O’Neill, H.C.; Johnstone, E.D.; Brison, D.R. The Impact of Selected Embryo Culture Conditions on ART Treatment Cycle Outcomes: A UK National Study. Hum. Reprod Open 2020, 2020, hoz031. [Google Scholar] [CrossRef] [PubMed]
- Manca, D.P. Do Electronic Medical Records Improve Quality of Care? Yes. Can. Fam. Physician 2015, 61, 846–847. [Google Scholar] [PubMed]
- Pace, K.B.; Sakulkoo, S.; Hoffart, N.; Cobb, A.K. Barriers to Successful Implementation of a Clinical Pathway for CHF. J. Health Qual. 2002, 24, 32–38. [Google Scholar] [CrossRef]
- Glauner, P.; Plugmann, P.; Lerzynski, G. Digitalization in Healthcare; Springer: Berlin/Heidelberg, Germany, 2021; ISBN 3030658953. [Google Scholar]
- García-Peñalvo, F.; Vázquez-Ingelmo, A. What Do We Mean by GenAI? A Systematic Mapping of the Evolution, Trends, and Techniques Involved in Generative AI. Int. J. Interact. Multimed. Artif. Intell. 2023, 4, 7–16. [Google Scholar] [CrossRef]
- Holmgren, A.J.; Downing, N.L.; Bates, D.W.; Shanafelt, T.D.; Milstein, A.; Sharp, C.D.; Cutler, D.M.; Huckman, R.S.; Schulman, K.A. Assessment of Electronic Health Record Use Between US and Non-US Health Systems. JAMA Intern. Med. 2021, 181, 251–259. [Google Scholar] [CrossRef]
- Raghupathi, W.; Raghupathi, V. Big Data Analytics in Healthcare: Promise and Potential. Health Inf. Sci. Syst. 2014, 2, 3. [Google Scholar] [CrossRef]
- Clarke, G.M.; Conti, S.; Wolters, A.T.; Steventon, A. Evaluating the Impact of Healthcare Interventions Using Routine Data. BMJ 2019, 365, l2239. [Google Scholar] [CrossRef]
- Johnson, A.E.W.; Pollard, T.J.; Shen, L.; Lehman, L.-W.H.; Feng, M.; Ghassemi, M.; Moody, B.; Szolovits, P.; Celi, L.A.; Mark, R.G. MIMIC-III, a Freely Accessible Critical Care Database. Sci. Data 2016, 3, 160035. [Google Scholar] [CrossRef]
- Liu, R.; Wei, J.; Liu, F.; Si, C.; Zhang, Y.; Rao, J.; Zheng, S.; Peng, D.; Yang, D.; Zhou, D. Best Practices and Lessons Learned on Synthetic Data. arXiv 2024, arXiv:2404.07503. [Google Scholar]
- Luo, W.; Phung, D.; Tran, T.; Gupta, S.; Rana, S.; Karmakar, C.; Shilton, A.; Yearwood, J.; Dimitrova, N.; Ho, T.B.; et al. Guidelines for Developing and Reporting Machine Learning Predictive Models in Biomedical Research: A Multidisciplinary View. J. Med. Internet Res. 2016, 18, e323. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y. Analysis of Variance (ANOVA) Comparing Means of More than Two Groups. Restor. Dent. Endod. 2014, 39, 74. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Lazar, N.A. The ASA Statement on P-Values: Context, Process, and Purpose. Am. Stat. 2016, 70, 129–133. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. arXiv 2014, arXiv:1406. [Google Scholar] [CrossRef]
- Wang, Z.; Su, Z.; Deng, Y.; Kurths, J.; Wu, J. Spatial Network Disintegration Based on Kernel Density Estimation. Reliab. Eng. Syst. Saf. 2024, 245, 110005. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and Reporting Effect Sizes to Facilitate Cumulative Science: A Practical Primer for t-Tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Hripcsak, G.; Albers, D.J.; Perotte, A. Exploiting Time in Electronic Health Record Correlations. J. Am. Med. Inf. Assoc. 2011, 18 (Suppl. 1), i109–i115. [Google Scholar] [CrossRef]
- Van Batavia, J.P.; Weiss, D.A.; Long, C.J.; Madison, J.; McCarthy, G.; Plachter, N.; Zderic, S.A. Using Structured Data Entry Systems in the Electronic Medical Record to Collect Clinical Data for Quality and Research: Can We Efficiently Serve Multiple Needs for Complex Patients with Spina Bifida? J. Pediatr. Rehabil. Med. 2018, 11, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Gifford, K.; Benson, J.; Kim, J. The Power of an Iterative Approach to Clinical Competence Assessment. Innov. Glob. Health Prof. Educ. 2018. [Google Scholar] [CrossRef]
- Bevan, N.; Carter, J.; Harker, S. ISO 9241-11 Revised: What Have We Learnt about Usability since 1998? In Human-Computer Interaction: Design and Evaluation: 17th International Conference, HCI International 2015, Los Angeles, CA, USA, 2–7 August 2015, Proceedings, Part I 17; Springer: Berlin/Heidelberg, Germany, 2015; pp. 143–151. [Google Scholar]
- Azadi, A.; García-Peñalvo, F.J. A Synergistic Bridge Between Human–Computer Interaction and Data Management Within CDSS. Data 2025, 10, 60. [Google Scholar] [CrossRef]
- Alley, A.; Cowles, S.; Rangan, P.; Gerkin, R.; Mahnert, N. The Effect of an Automated Order on Postpartum Opioid Use After Uncomplicated Vaginal Deliveries. J. Women’s Health 2022, 31, 842–847. [Google Scholar] [CrossRef]
- Vanicek, T.; Popelka, S. The Think-Aloud Method for Evaluating the Usability of a Regional Atlas. ISPRS Int. J. Geoinf. 2023, 12, 95. [Google Scholar] [CrossRef]
- Bonomi, L.; Gousheh, S.; Fan, L. Enabling Health Data Sharing with Fine-Grained Privacy. In Proceedings of the 32nd ACM International Conference on Information and Knowledge Management, Birmingham, UK, 21–25 October 2023. [Google Scholar] [CrossRef]
- Arora, D.; Sharma, O. Fog Computing in Healthcare. In Artificial Intelligence and Cybersecurity in Healthcare; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2025; pp. 57–84. ISBN 9781394229826. [Google Scholar]
- Contaldo, M.; Pasceri, G.; Vignati, G.; Bracchi, L.; Triggiani, S.; Carrafiello, G. AI in Radiology: Navigating Medical Responsibility. Diagnostics 2024, 14, 1506. [Google Scholar] [CrossRef]
- Wilson, S.; HadarSamuel, H.; Samuel; Norman, T.; Vaknin, S.; Saban, M. The Physician-AI Relationship: Partnering for Precision Medicine via Clinical Decision Support. Eur. J. Public Health 2024, 34, ckae144-1125. [Google Scholar] [CrossRef]
- Ademola, A.; George, C.; Mapp, G. Addressing the Interoperability of Electronic Health Records: The Technical and Semantic Interoperability, Preserving Privacy and Security Framework. Appl. Syst. Innov. 2024, 7, 116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).