Insights into the Pyrolysis Properties of Environmentally Friendly PMVE/N2 Gas Mixtures: A Collaborative Analysis Based on Density Functional Theory and Reaction Kinetics
Abstract
1. Introduction
2. Calculation Methods
2.1. Reaction Dynamics Simulation
2.2. Density Functional Theory Simulation
3. Results
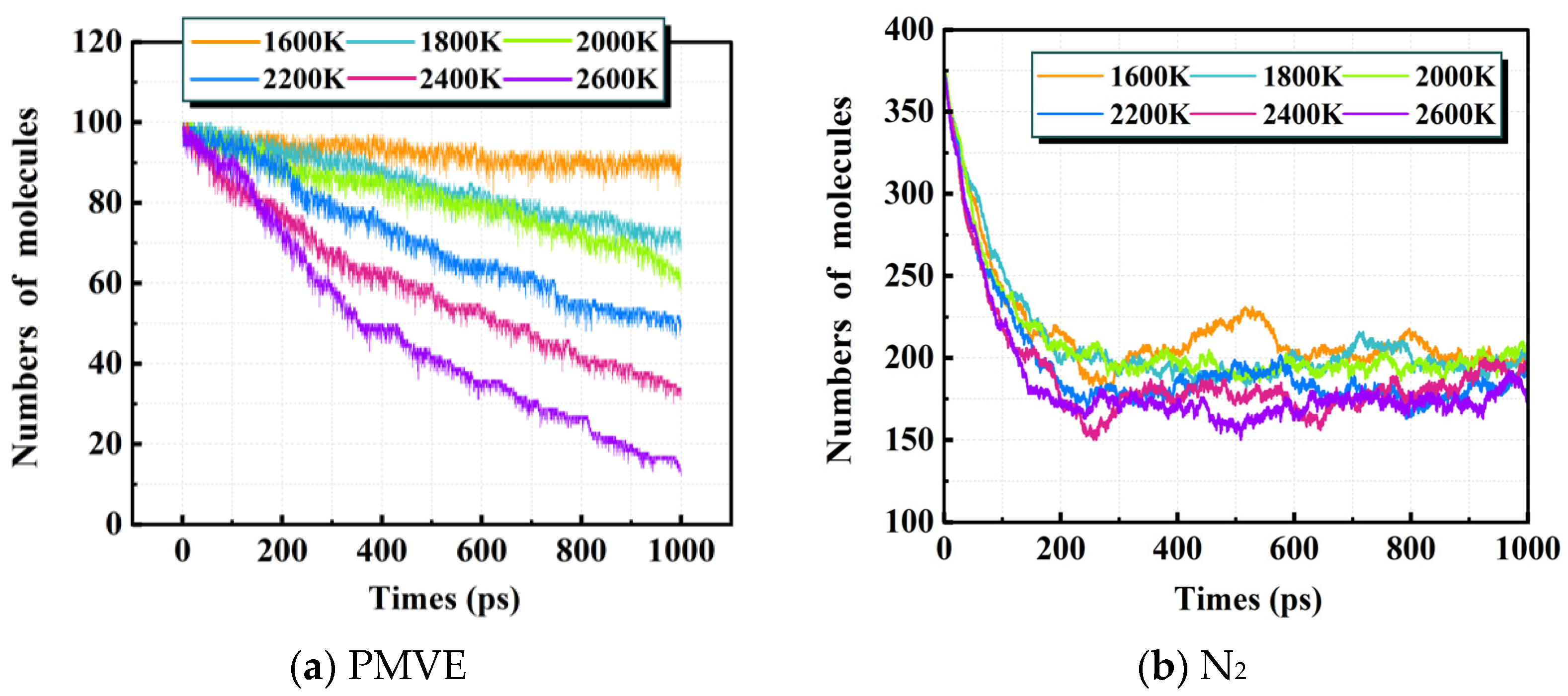
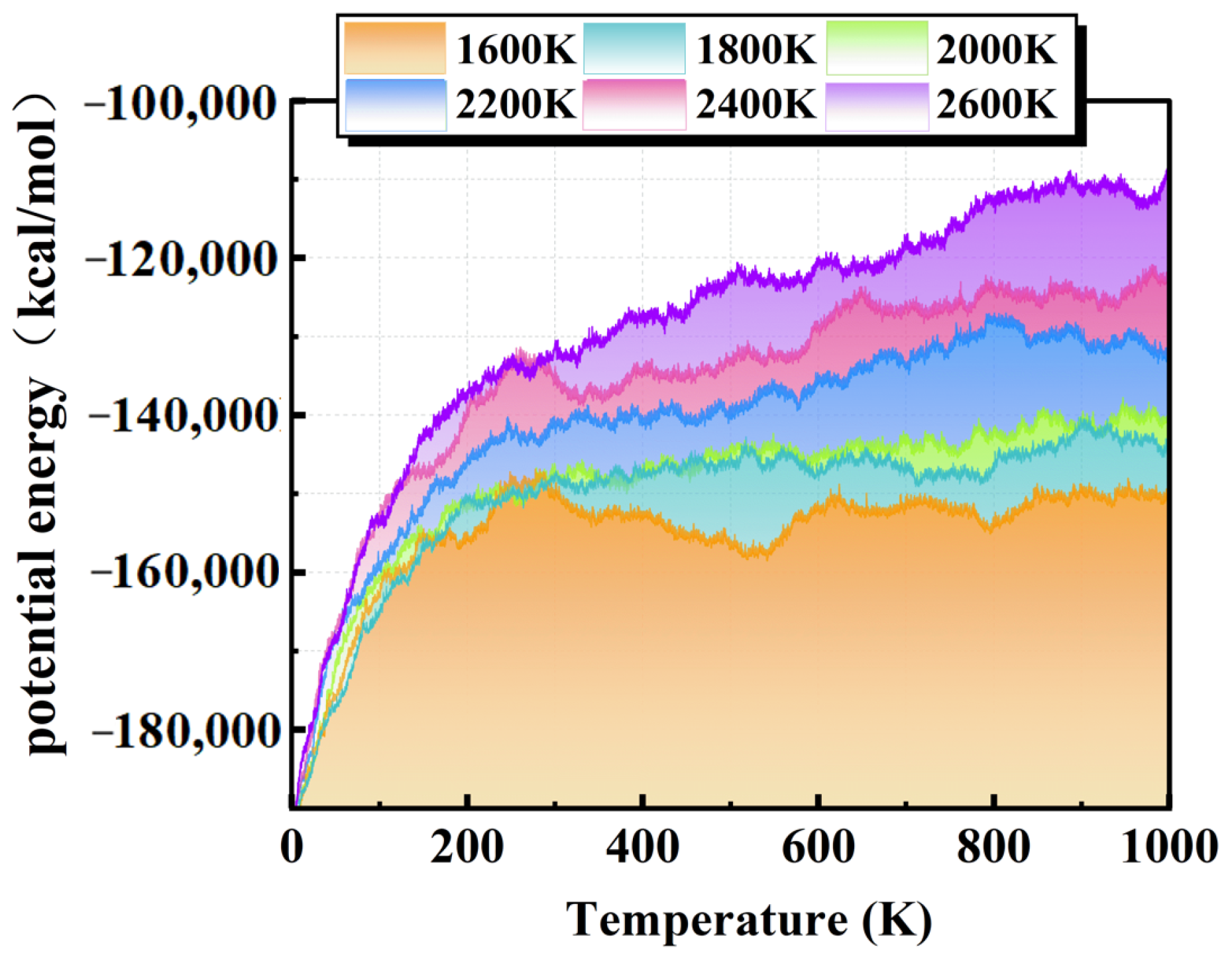
3.1. Effect of Temperature on the Decomposition Process
3.1.1. Decomposition Rate of PMVE/N2 Gas Mixture
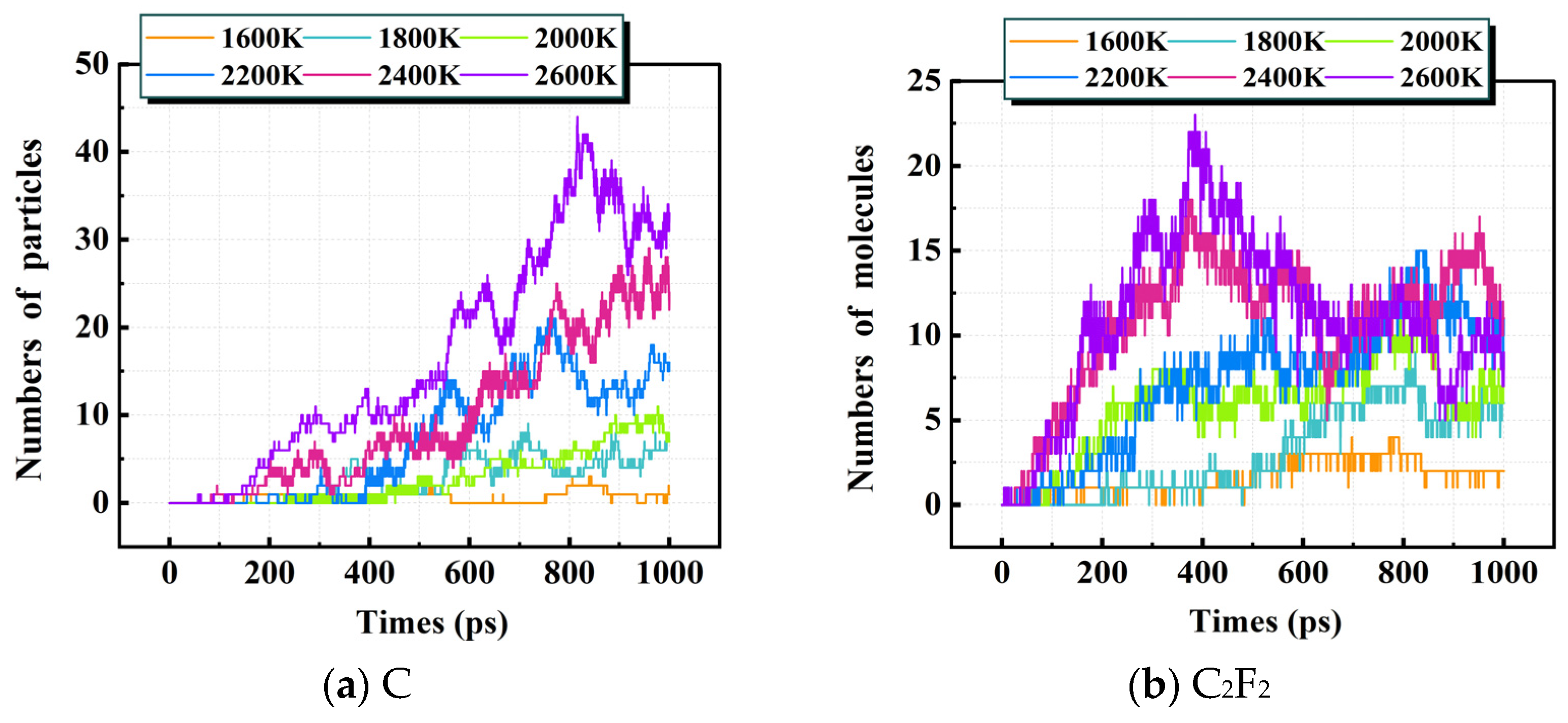
3.1.2. Distribution of Decomposition Products
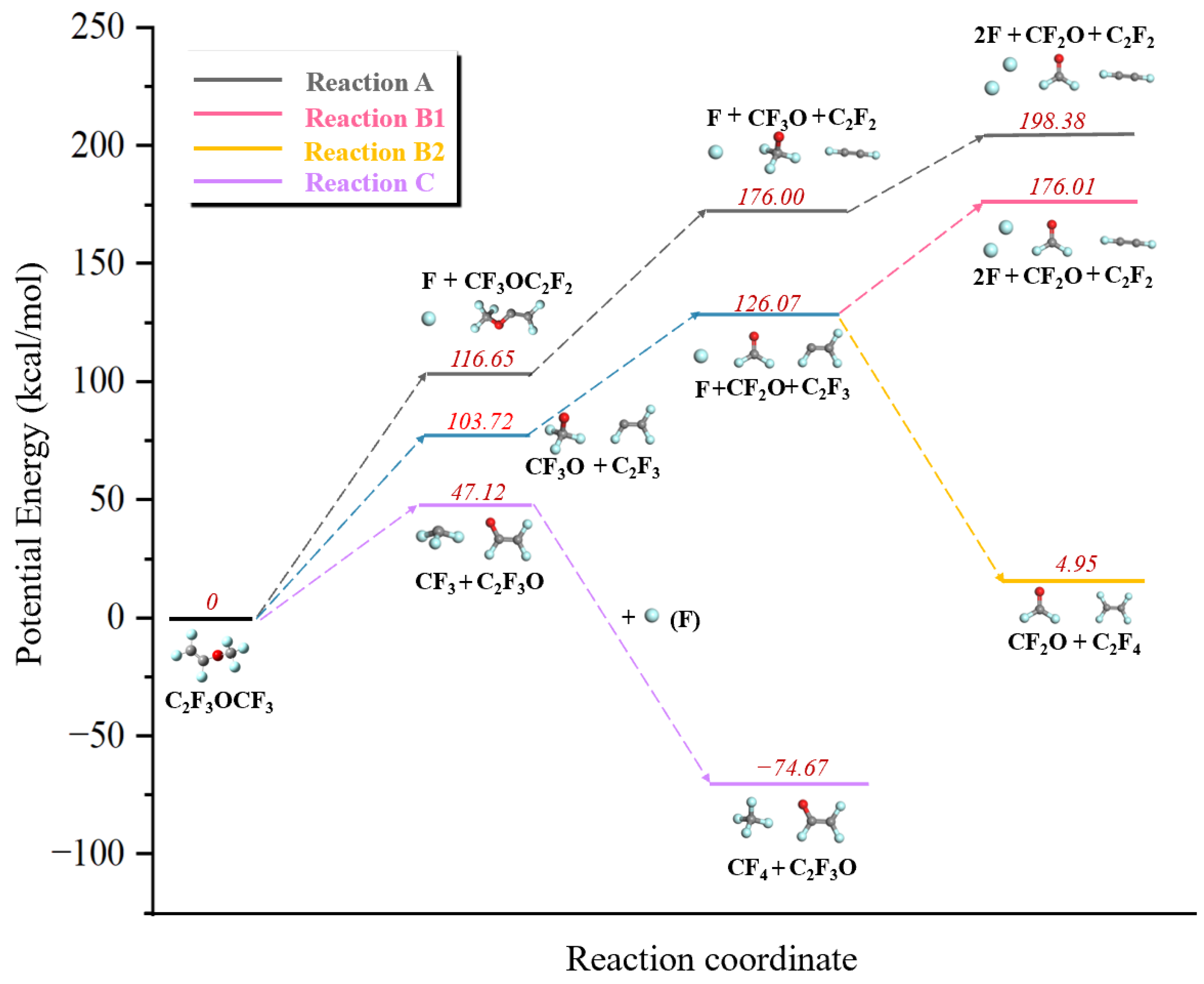
3.2. Decomposition Mechanism of PMVE/N2
3.3. Environmental Effects of PMVE/N2 Gas Mixture
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Zhang, X.; Tang, J.; Cui, Z.; Cui, H. Pristine and Cu decorated hexagonal InN monolayer, a promising candidate to detect and scavenge SF6 decompositions based on first-principle study. J. Hazard. Mater. 2019, 363, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Sulbaek Andersen, M.P.; Kyte, M.; Andersen, S.T.; Nielsen, C.J.; Nielsen, O.J. Atmospheric Chemistry of (CF3)2CF–C≡N: A Replacement Compound for the Most Potent Industrial Greenhouse Gas, SF6. Environ. Sci. Technol. 2017, 51, 1321–1329. [Google Scholar] [CrossRef]
- Fang, X.; Hu, X.; Janssens-Maenhout, G.; Wu, J.; Han, J.; Su, S.; Zhang, J.; Hu, J. Sulfur hexafluoride (SF6) emission estimates for China: An inventory for 1990–2010 and a projection to 2020. Environ. Sci. Technol. 2013, 47, 3848–3855. [Google Scholar] [CrossRef] [PubMed]
- Kieffel, Y.; Irwin, T.; Ponchon, P.; Owens, J. Green gas to replace SF6 in electrical grids. IEEE Power Energy Mag. 2016, 14, 32–39. [Google Scholar] [CrossRef]
- Obama, B. The irreversible momentum of clean energy. Science 2017, 355, 126–129. [Google Scholar] [CrossRef]
- Stoller, P.; Doiron, C.; Tehlar, D.; Simka, P.; Ranjan, N. Mixtures of CO2 and C5F10O perfluoroketone for high voltage applications. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2712–2721. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Tian, S.; Xiao, S.; Li, Y.; Chen, D. Insight into the decomposition mechanism of C6F12O-CO2 gas mixture. Chem. Eng. J. 2019, 360, 929–940. [Google Scholar] [CrossRef]
- Niemeyer, L. A systematic search for insulation gases and their environmental evaluation. In Gaseous Dielectrics VIII; Springer: Berlin/Heidelberg, Germany, 1998; pp. 459–464. [Google Scholar]
- Park, Y.; Choi, Y.R.; Kim, D.-C.; Kim, Y.; Song, M.-Y.; Kim, Y.-W.; Cho, H.; Jang, H.-J.; Oh, Y.-H.; Song, K.-D. Total electron scattering cross section of C3F6O at the intermediate-energy region for developing an alternative insulation gas to SF6. Curr. Appl. Phys. 2022, 41, 111–115. [Google Scholar] [CrossRef]
- Sinha, N.; Song, M.-Y.; Chang, H.; Choi, H.; Jang, H.-J.; Oh, Y.-H.; Song, K.-D. Electron impact cross sections and transport studies of C3F6O. Appl. Sci. 2023, 13, 12612. [Google Scholar] [CrossRef]
- Xiao, S.; Chen, Y.; Tang, M.; Tian, S.; Xia, H.; Wang, Y.; Tang, J.; Li, Y.; Zhang, X. Characteristics of perfluoromethyl vinyl ether: A new eco-friendly alternative gas for SF6. High Volt. 2024, 9, 509–517. [Google Scholar] [CrossRef]
- Couperus, A. Annual Report on the Implementation of Council Regulation (EC) No 812/2004–2018; Centre for Fishery Research (CVO): Ijmuiden, The Netherlands, 2019. [Google Scholar]
- Luo, Z.; Wei, C.; Wang, T.; Su, B.; Cheng, F.; Liu, C.; Wang, Y. Effects of N2 and CO2 dilution on the explosion behavior of liquefied petroleum gas (LPG)-air mixtures. J. Hazard. Mater. 2021, 403, 123843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Li, Y.; Li, Y.; Chen, Q.; Zhang, G.; Xiao, S.; Tang, J. AC breakdown and decomposition characteristics of environmental friendly gas C5F10O/air and C5F10O/N2. IEEE Access 2019, 7, 73954–73960. [Google Scholar] [CrossRef]
- Raymond, W.J.K.; Illias, H.A.; Mokhlis, H. Partial discharge classifications: Review of recent progress. Measurement 2015, 68, 164–181. [Google Scholar] [CrossRef]
- Loeb, L.B. The mechanism of spark discharge in air at atmospheric pressure. Science 1929, 69, 509–512. [Google Scholar] [CrossRef]
- Sinha, N.; Choi, H.; Song, M.-Y.; Jang, H.-J.; Oh, Y.-H.; Song, K.-D. Perfluoro-methyl-vinyl-ether as SF6 alternative in insulation applications: A DFT study on the physiochemical properties and decomposition pathways. Comput. Theor. Chem. 2023, 1225, 114159. [Google Scholar] [CrossRef]
- Zhao, D.; Yan, J.; He, R.; Geng, Y.; Liu, Z.; Wang, J. Decomposition mechanism of C4F7N/CO2 gas mixture based on molecular dynamics and effect of O2 content. J. Appl. Phys. 2024, 135, 024401. [Google Scholar] [CrossRef]
- Te Velde, G.t.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Van Duin, A.C.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Raymand, D.; Van Duin, A.C.; Baudin, M.; Hermansson, K. A reactive force field (ReaxFF) for zinc oxide. Surf. Sci. 2008, 602, 1020–1031. [Google Scholar] [CrossRef]
- Chenoweth, K.; Cheung, S.; Van Duin, A.C.; Goddard, W.A.; Kober, E.M. Simulations on the thermal decomposition of a poly (dimethylsiloxane) polymer using the ReaxFF reactive force field. J. Am. Chem. Soc. 2005, 127, 7192–7202. [Google Scholar] [CrossRef]
- Thielen, K.; Roth, P. N atom measurements in high-temperature N2 dissociation kinetics. AIAA J. 1986, 24, 1102–1105. [Google Scholar] [CrossRef]
- Paajanen, A.; Vaari, J. High-temperature decomposition of the cellulose molecule: A stochastic molecular dynamics study. Cellulose 2017, 24, 2713–2725. [Google Scholar] [CrossRef]
- Zheng, M.; Li, X.; Liu, J.; Wang, Z.; Gong, X.; Guo, L.; Song, W. Pyrolysis of Liulin coal simulated by GPU-based ReaxFF MD with cheminformatics analysis. Energy Fuels 2014, 28, 522–534. [Google Scholar] [CrossRef]
- Zheng, M.; Li, X.; Wang, M.; Guo, L. Dynamic profiles of tar products during Naomaohu coal pyrolysis revealed by large-scale reactive molecular dynamic simulation. Fuel 2019, 253, 910–920. [Google Scholar] [CrossRef]
- Berendsen, H.J.; Postma, J.v.; Van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Tian, S.; Xiao, S.; Chen, D.; Tang, J.; Zhuo, R. Decomposition mechanism of the C5-PFK/CO2 gas mixture as an alternative gas for SF6. Chem. Eng. J. 2018, 336, 38–46. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Xiao, S.; Tang, J.; Tian, S.; Deng, Z. Decomposition mechanism of C5F10O: An environmentally friendly insulation medium. Environ. Sci. Technol. 2017, 51, 10127–10136. [Google Scholar] [CrossRef]
- Kirschner, K.N.; Heiden, W.; Reith, D. Relative electronic and free energies of octane’s unique conformations. Mol. Phys. 2017, 115, 1155–1165. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Global Optimization by Basin-Hopping and the Lowest Energy Structures of Lennard-Jones Clusters Containing up to 110 Atoms. J. Phys. Chem. A 1997, 101, 5111–5116. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- McLean, A.; Chandler, G. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Q. Shermo: A general code for calculating molecular thermochemistry properties. Comput. Theor. Chem. 2021, 1200, 113249. [Google Scholar] [CrossRef]
- Slack, M.; Fishburne, E.; Johnson, A. Kinetics and Thermodynamics of the CN Molecule. II. The Dissociation of C2N2. J. Chem. Phys. 1971, 54, 1652–1658. [Google Scholar] [CrossRef]
- De Almeida, W.B.; Hinchliffe, A. An ab initio study of the C2N2 molecule: NCCN, CNNC and CNCN species. J. Mol. Struct. THEOCHEM 1990, 206, 77–87. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, L.; Yan, J.; Yang, A.; Han, G.; Han, G.; Wu, Y.; Rong, M. Investigation of dielectric properties of cold C3F8 mixtures and hot C3F8 gas as Substitutes for SF6. Eur. Phys. J. D 2015, 69, 240. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, X.; Li, X.; Yang, A.; Han, G.; Lu, Y.; Wu, Y.; Rong, M. Theoretical study of the decomposition pathways and products of C5-perfluorinated ketone (C5 PFK). Aip Adv. 2016, 6, 085305. [Google Scholar] [CrossRef]
- Hyrenbach, M.; Paul, T.A.; Owens, J. Environmental and safety aspects of AirPlus insulated GIS. Environ. Saf. Asp. Airplus Insul. GIS 2017, 2017, 132–135. [Google Scholar] [CrossRef][Green Version]



| Key Parameter | PMVE/N2 |
|---|---|
| N2 content (mix by mole ratio) | 40% |
| Number of PMVE | 100 |
| Number of N2 | 400 |
| Density (g/cm3) | 0.00227 |
| Box length (Å) | 273.0 |
| No | Reaction | Reaction Enthalpy (kcal/mol), T = 298.15 K |
|---|---|---|
| A1 | C3F6O → CF3OC2F2 + F | 116.67 |
| A2 | CF3OC2F2 → CF3O + C2F2 | 59.34 |
| A3 | CF3O → CF2O + F | 22.37 |
| B1 | C3F6O → C2F3 + CF3O | 103.70 |
| B2 | C2F3 → C2F2 + F | 72.30 |
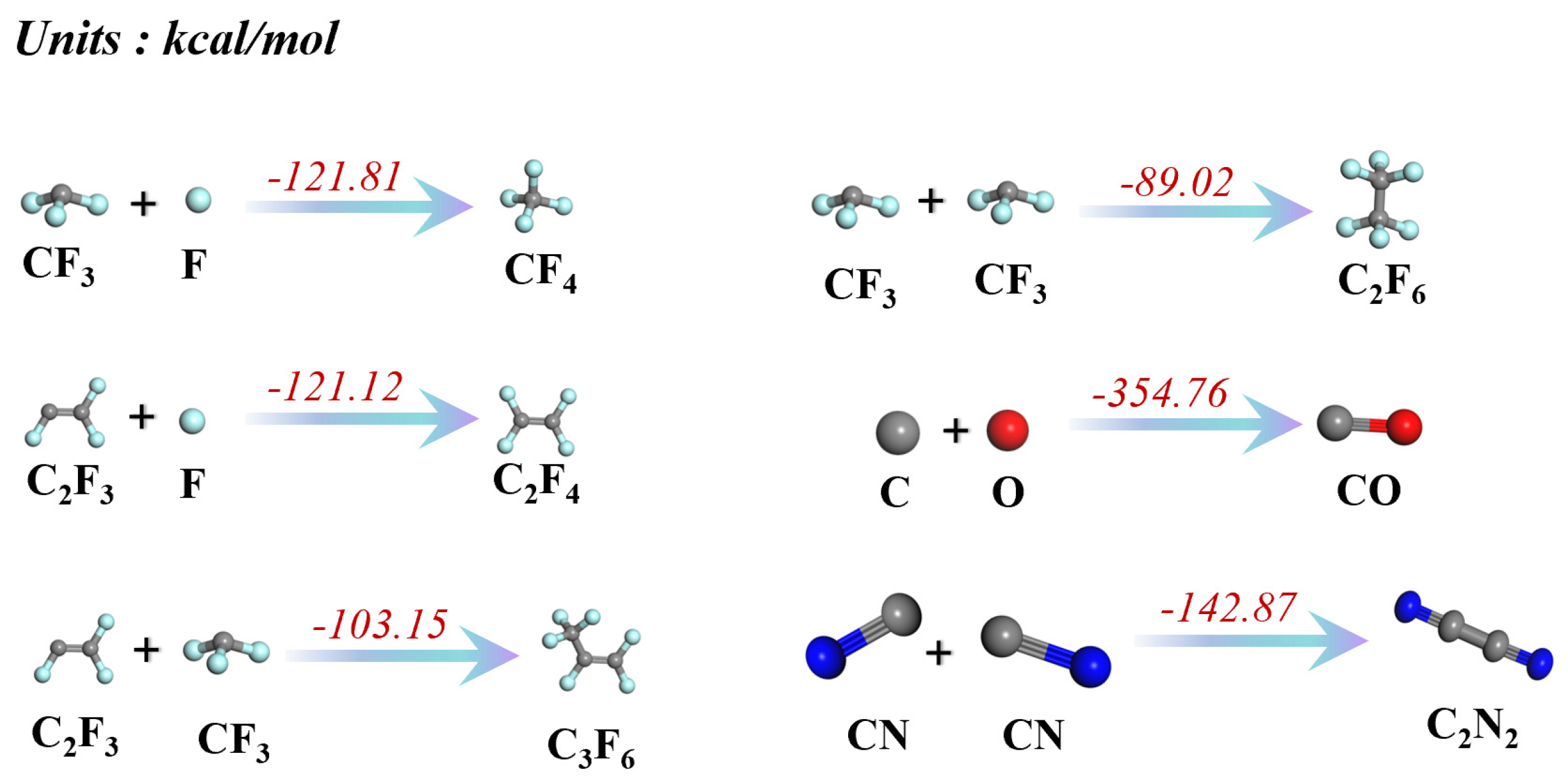
| B3 | C2F3 + F → C2F4 | −121.12 |
| C1 | C3F6O → C2F3O + CF3 | 47.12 |
| C2 | CF3 + F → CF4 | −121.81 |
| D | C2F3 + CF3 → C3F6 | −103.15 |
| E | 2CF3 → C2F6 | −89.02 |
| F | C + O → CO | −354.76 |
| G | 2CN → C2N2 | −142.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Chu, H.; Liu, Y.; Liu, S.; Ye, W.; Yan, J. Insights into the Pyrolysis Properties of Environmentally Friendly PMVE/N2 Gas Mixtures: A Collaborative Analysis Based on Density Functional Theory and Reaction Kinetics. Appl. Sci. 2025, 15, 5272. https://doi.org/10.3390/app15105272
Dong H, Chu H, Liu Y, Liu S, Ye W, Yan J. Insights into the Pyrolysis Properties of Environmentally Friendly PMVE/N2 Gas Mixtures: A Collaborative Analysis Based on Density Functional Theory and Reaction Kinetics. Applied Sciences. 2025; 15(10):5272. https://doi.org/10.3390/app15105272
Chicago/Turabian StyleDong, Haibo, Haonan Chu, Yunhao Liu, Shicheng Liu, Wenyu Ye, and Jiaming Yan. 2025. "Insights into the Pyrolysis Properties of Environmentally Friendly PMVE/N2 Gas Mixtures: A Collaborative Analysis Based on Density Functional Theory and Reaction Kinetics" Applied Sciences 15, no. 10: 5272. https://doi.org/10.3390/app15105272
APA StyleDong, H., Chu, H., Liu, Y., Liu, S., Ye, W., & Yan, J. (2025). Insights into the Pyrolysis Properties of Environmentally Friendly PMVE/N2 Gas Mixtures: A Collaborative Analysis Based on Density Functional Theory and Reaction Kinetics. Applied Sciences, 15(10), 5272. https://doi.org/10.3390/app15105272







