Abstract
The study investigated the effect of Vinnex® (vinyl acetate polymer) and Joncryl® (epoxy-based copolymer) as compatibilizers on the mechanical properties of thermoplastic starch (TPS) and polybutylene adipate-co-terephthalate (PBAT) and polybutylene succinate-co-adipate (PBSA) films. Due to TPS’s hydrophilicity and brittleness, blending it with biodegradable polyesters like PBAT enhances its properties but may introduce compatibility challenges. This research evaluated three formulations (TPS/PBAT with Vinnex, TPS/PBAT with Joncryl, and TPS/PBAT with both additives) along with the inclusion of a polybutadiene succinate-co-adipate (PBSA) matrix to further improve performance. Mechanical testing (tensile strength, elongation at break, Young’s modulus) reveals that Vinnex and Joncryl enhance plasticization and polymer compatibility, positively impacting TPS/PBAT’s mechanical properties. The introduction of the PBSA matrix further improves tensile strength and elongation. Scanning electron microscopy (SEM) confirms better additive dispersion and interfacial adhesion within the blend. Complementary analysis includes melt flow index, melt density, DSC, and TGA, providing a comprehensive understanding of how these additives optimize TPS/PBAT compounds for sustainable applications. Mechanically, the compatibilized blends showed improved performance: Vinnex mainly enhanced stiffness, Joncryl primarily improved elongation, and a synergistic effect was observed with their combination.
1. Introduction
Plastic serves as an unavoidable and ubiquitous material in packaging. The packaging industry is responsible for 40% of the total plastic usage globally [1]. This extensive reliance on plastics has led to an unprecedented environmental crisis. Due to their resistance to natural degradation, plastics accumulate massively in oceans, rivers, and soils [2,3]. This accumulation severely impacts ecosystems and wildlife and poses potential risks to human health [4].
In the face of this problem, the search for sustainable alternatives to conventional plastics is imperative, balancing long-term properties with appropriate end-of-life management [5]. The development of biodegradable and compostable materials, as well as the implementation of circular economy strategies, represent promising approaches to mitigate the environmental impact of plastics [6,7,8]. These solutions not only have the potential to reduce waste accumulation but also to decrease reliance on non-renewable fossil resources and promote more responsible and sustainable practices in industry and consumption [9,10].
One of the most promising alternatives is thermoplastic starch (TPS). Derived from renewable resources such as corn, potatoes, and other starch-rich plants, TPS is an attractive candidate due to its low cost and abundant availability [11,12]. Thermoplastic starch can be processed using conventional plastic manufacturing techniques, facilitating its integration into existing production systems [13]. Additionally, TPS is biodegradable, reducing the environmental footprint associated with plastic waste [14,15]. Thermoplastic starch (TPS) is created by disrupting the granular structure of starch through the application of heat and mechanical shear in the presence of plasticizers such as water and glycerol. This process produces a material with properties akin to traditional thermoplastics but with the added advantages of being derived from renewable sources and fully biodegradable under suitable conditions [16,17]. These attributes make TPS a promising alternative for reducing the environmental impact of packaging materials [18].
However, TPS alone has certain limitations regarding its mechanical properties and sensitivity to moisture, which restrict its use in more demanding applications [19].
To overcome these limitations, it is common to blend thermoplastic starch with other biodegradable polymers, especially polyesters, which provide the necessary functionality. Biodegradable polyesters such as polylactic acid (PLA), polyhydroxyalkanoate (PHA), polybutylene succinate (PBS), and polyethylene adipate-co-terephthalate (PBAT) are some of the most common options for improving the properties of TPS [20,21].
Polybutylene adipate-co-terephthalate (PBAT) is an aliphatic–aromatic polyester that is highly flexible and resistant. Mixing PBAT with TPS results in a material that combines the flexibility and durability of PBAT with the biodegradability of TPS, making it an ideal choice for packaging films and other flexible products [22].
Polybutylene succinate (PBS) is another biodegradable polyester known for its excellent processability and heat resistance. Although the melting temperature of PBS is between 90 °C and 120 °C, it demonstrates stability under medium-temperature conditions due to its inherent molecular structure, which provides a balance between crystallinity and flexibility. This characteristic allows PBS to maintain its performance in applications where moderate thermal resistance is required [23]. The combination of PBS with TPS can produce materials with balanced mechanical properties and higher thermal stability, which is beneficial for applications requiring resistance to higher temperatures [24].
The blending of TPS with these polyesters is typically performed by extrusion and compounding, where starch and polymers are melted and mixed at high temperatures to form a homogeneous material. Plasticizers and other additives can be incorporated during this process to improve compatibility and the final properties of the composite material [24,25,26]. Among these additives, compatibilizers play a critical role in addressing the incompatibility challenges between TPS and biodegradable polyesters. For instance, reactive compatibilizers such as glycidyl methacrylate (GMA) and maleic anhydride (MA) have been shown to improve interfacial adhesion and phase dispersion, significantly enhancing mechanical and thermal properties [27,28].
In addition, although thermoplastic starch has great potential as a biodegradable material, its practical use is significantly enhanced when blended with biodegradable polyesters. These combinations not only improve the mechanical properties and stability of TPS but also allow its application in a wider range of products, contributing more effectively to the reduction of plastic pollution and the promotion of a more sustainable future [29]. For example, Vinnex, a maleic acid-based compatibilizer, improves compatibility by reacting with functional groups in both starch and polyesters, forming chemical bonds that promote interfacial adhesion [5]. Similarly, Joncryl, a reactive chain extender, reduces interfacial energy, enhancing dispersion and thermal stability [28,30].
Although TPS constitutes a smaller fraction of the blends (up to 25 wt%), its modification is essential to overcome its inherent limitations such as brittleness and water sensitivity. By tailoring TPS with compatibilizers, its inclusion modifies the properties of the matrix itself, such as its crystallinity, interfacial adhesion, and biodegradability. The compatibilizers used in this study, such as Joncryl and Vinnex, act not only to address TPS limitations but also to improve the homogeneity and performance of the PBAT or PBSA matrix. This interaction transforms the blend into a cohesive material where TPS, despite its lower percentage, acts as a structural modifier that significantly impacts the mechanical and thermal properties of the matrix. For instance, Joncryl acts as a chain extender, improving the dispersion of TPS in the polyester matrix and minimizing phase separation, as highlighted by Zhang et al. (2023) and Rasselet et al. (2019) [27,31].
The focus on improving TPS properties is driven by its critical role in providing biodegradability to the blends. PBAT and PBSA are already known for their flexibility and thermal stability, but without TPS, the blends would lack the required environmental benefits. By enhancing the properties of TPS through compatibilizers, the material becomes suitable for demanding applications, such as flexible packaging, without compromising valorisation routes [28,32].
While PBAT and PBSA provide the mechanical and thermal stability required for packaging applications, the incorporation of modified TPS enables a significant reduction in fossil-based content and improves the environmental profile of the blends. Compatibilizers ensure efficient integration, as reported by Ren et al., leading to enhanced phase dispersion and overall performance [28].
Despite the numerous advantages offered by biodegradable polyesters when combined with thermoplastic starch (TPS), it is important to note that compatibility between these materials can be challenging. The main reason for this possible incompatibility lies in the differences in chemical structure and physical properties of each component [33].
Starch is a natural polysaccharide composed mainly of glucose, which has an amorphous and semicrystalline structure. On the other hand, biodegradable polyesters such as polybutylene succinate-co-adipate (PBSA) and polybutylene adipate-co-terephthalate (PBAT) are synthetic polymers that have a more linear structure and are less polar than starch. This disparity in molecular structure can result in a lack of interaction between the components, making it difficult to form a homogeneous mixture and obtain improved properties in the composite material [34,35].
Additionally, the processability of these materials can also be affected by their possible incompatibility. During the extrusion and compounding process, it is crucial for the components to mix uniformly to ensure the homogeneity of the final material. However, if the polymers are not compatible with each other, phenomena such as phase separation or aggregate formation can occur, resulting in a non-uniform distribution of properties in the composite material [36].
Another factor to consider is the thermal stability of the mixture. Since starch is sensitive to heat and tends to degrade at high temperatures, compatibility with biodegradable polyesters can affect the thermal stability of the composite material. This may limit the processing conditions and the final applications of the material, especially if resistance to high temperatures is required [37].
In summary, the possible incompatibility between thermoplastic starch and biodegradable polyesters can pose significant challenges in the development of composite materials. However, overcoming these challenges is crucial to fully harness the advantages of each component and develop sustainable and functionally robust packaging solutions. In this study, this aspect will be addressed by optimizing processing conditions and selecting compatible additives to improve compatibility between the materials and obtain a composite material with improved properties and a wide range of potential applications [38,39].
These additives are specifically designed to enhance the interaction among the mixture components, promoting uniform dispersion of the polymers and facilitating the formation of a more effective interface between them. Among the most common compatibilizing additives are coupling agents and matrix compatibilizers. Coupling agents, such as silanes and isocyanates, function by chemically reacting with functional groups present in the polymers, promoting adhesion between them. On the other hand, matrix compatibilizers, such as block copolymers and graft copolymers, act by adsorbing onto the interface between the polymers, reducing interfacial energy, and improving compatibility between them [40,41].
The challenges arising from the incompatibility between thermoplastic starch (TPS) and biodegradable polyesters must be addressed to ensure homogeneous blending. To achieve this, compatibilizers like Vinnex® and Joncryl® can be used to improve the interaction between the components of the blend, enhancing the overall material properties.
Vinnex, a maleic acid-based additive, enhances compatibility by reacting with functional groups in both starch and polyesters, forming chemical bonds that promote interfacial adhesion. It also acts as a plasticizing agent, improving processability and enhancing the mechanical properties of the composite material [42,43,44,45]. On the other hand, Joncryl, a reactive chain extender, interacts onto the polymer interface, reducing interfacial energy and promoting uniform dispersion. It not only improves interfacial compatibility but also enhances the thermal stability and impact resistance of the material [31,44,45,46,47,48]. These improvements result in composite materials with a wider range of potential applications, particularly in the development of more sustainable packaging solutions [49,50].
In this study, Vinnex and Joncryl were selected as compatibilizing additives to improve the interaction between thermoplastic starch and biodegradable polyesters during the compounding process, and their effects on the properties of the composite material—such as interfacial compatibility, thermal stability, mechanical resistance, and biodegradability—were evaluated. The objective was to develop a polymer blend with enhanced properties that can contribute to the search for more sustainable solutions for packaging and other plastic products.
2. Materials and Methods
2.1. Materials
Native starch from potato was provided by Avebe S.L (Veendam, The Netherlands), and glycerol was purchased from Quimidroga (Valencia, Spain). Poly (butylene adipate-co-terephthalate) PBAT (Ecoflex F blend C1200) and Joncryl ADR 4468 (based on Glycidyl methacrylate and Methacrylic acid methyl ester) were purchased from BASF Chemical Company (Ludwigshafen am Rhein, Germany). Poly (butylene succinate-co-adipate) PBSA (polybutylene succinate—FD92PM/FD92PB) was provided by Mitsubishi (Washington, DC, USA). Talc (Mg3Si4O10(OH)2) was obtained from Imerys Performance Minerals EMEA (Paris, France), and Vinnex 2525 was provided by Wacker Chemie (Munich, Germany).
2.2. Film Preparation
A set of blends was prepared and labelled, according to the formulations detailed in Table 1, through a single-step extrusion process using a co-rotating twin-screw extruder (Leistritz ZSE 27 MAXX—60D, Nuremberg, Germany), with a temperature profile ranging from 80 °C to 150 °C and a residence time between 30 s and 3 min. The extruder consists of 14 different zones. In the first part, known as the feeding zone, the majority of the components are introduced, including starch along with glycerol, as well as Vinnex, and Joncryl. This initial zone contains shear elements designed to facilitate the destructurization of starch, enabling its plasticization, with a temperature progression from 80 °C to 120 °C. Following this, the subsequent zones of the extruder, with temperatures ranging from 130 °C to 150 °C, include side feeders through which the polymer matrices are introduced. These zones contain mixing elements that ensure adequate homogenization of the components. Particularly, the temperatures in the last zones (140–150 °C) optimize the reactivity of compatibilizers such as Joncryl by promoting chain extension and enhancing the dispersion of TPS in the polyester matrix. Finally, the material exits the extruder at the end, extruded in the form of a strand through the die, with the extruder operating at a production rate of 10 kg/h and a screw speed of 200 rpm, chopping pellets of 3–5 mm.

Table 1.
Compositions (%wt) of TPS/PBAT and TPS/PBAT-PBSA blends, modified with compatibilizers.
To prepare the films, the blended pellets were fed into an extruder (Collin single-spindle E25P, Maitenbeth, Germany), where the compound underwent fusion and homogenization, with a T profile between 150 and 160 °C and rotating speed of 33 rpm. Once the compound reached a uniform molten state, it was directed towards a blow head (width of 300–400 mm depending on material). In the blow head, the molten material was extruded as an inflated tube with air, which was subsequently flattened to form a thin film through cooling at room temperature for 120 min.
All samples were conditioned in a climatic chamber under controlled conditions at 23 °C and 50% relative humidity prior to testing. This conditioning process was consistently applied across all samples and experimental procedures to ensure uniformity and comparability of results. Sample preparation for subsequent DSC and TGA analyses involved grinding the material into a fine powder to ensure homogeneity.
2.3. Characterization
2.3.1. Thermogravimetric Analysis (TGA)
Thermogravimetric analysis (TGA Q5000 IR of TA Instrument, New Castle, DE, USA) was conducted from 35 to 900 °C, at a heating rate of 10 °C/min, with alumina pans, under N2 inert atmosphere. These measurements were repeated three times to ensure consistency and reliability. The reported values reflect the average of these replicates, with standard deviations indicating reproducibility.
2.3.2. Melt Density
The present study focused on conducting the melt density measurement of plastic materials according to the UNE-EN ISO 1183-1 standard [51]. The material was heated until it reached its molten state at 190 °C and was then transferred to a precise measuring cylinder.
2.3.3. Differential Scanning Calorimetry (DSC)
Differential scanning calorimetry (DSC Q-2000 of TA Instruments) was conducted from −50 to 160 °C at a heating/cooling/heating program with linear 10 °C/min ramps, with aluminium pans, and N2 inert atmosphere. Three replicates were considered in the analysis.
2.3.4. SEM
The microstructural analysis of the surface and cross-sections (fractured samples with liquid nitrogen) of the films was carried out using a scanning electron microscope (Philips XL 30 ESEM (Amsterdam, The Netherlands), EDAX PV 9760 (Mahwah, NJ, USA)). The film samples were placed on support stubs fixed with double-sided carbon tape and coated with gold/palladium. Observations were performed at 10.0 kV.
2.3.5. Mechanical Test
In accordance with the UNE-EN ISO 527 standard [52], the mechanical properties (Young’s modulus, yield stress, stress at break point, and elongation at break) of materials were measured through a tensile test using a Testometric M350-20CT (Rochdale, UK). This test involved gradually applying a uniaxial force until the sample fractured. The testing speed was set at 100 mm/min, adhering to the specific guidelines of the standard. The specimens used for testing were stamped and measured 2.5 × 10 cm and 120 ± 10 microns, were rectangular in shape, and, in accordance with the standard, a minimum of 10 repetitions were conducted for each sample to ensure consistency and comparability of the obtained results.
Elasto-plastic properties in the range from the yield strength until the tensile strength were analyzed by the Hollomon equation, expressed as σT = K⋅εTn. In this equation, σT denotes true stress, εT represents true strain, K is the strength coefficient, and n is the strain-hardening exponent. The Hollomon equation enables the understanding of how materials harden as they deform, providing essential insights for optimizing manufacturing processes and ensuring the structural integrity of materials.
3. Results and Discussion
3.1. Macroscopic Properties
Representative images of the obtained films are shown in Figure 1. Although all the films appear practically identical, which is relevant from the point of view of product commercialization, only one image has been included, as it is representative of all blends due to their similarity, as can be seen in Supplementary Materials Figure S1. The macroscopic appearance is quite homogeneous, with no visible heterogeneities. They have a good appearance, with no phase differentiation noticeable to the naked eye. They are practically opaque white, which must be due to the thickness (120 ± 10 microns, practically uniform). All of them have appropriate resistance.

Figure 1.
Representative image of a TPS-PBAT film. A specific picture for each composition can be found in Supplementary Materials Figure S1.
Table 2 shows the different melt densities of all formulations, revealing remarkably similar values among the various modifications. Regardless of the addition of Vinnex, Joncryl, or the blending with PBSA, the melt density remained consistently close to the baseline formulation. These consistent values suggested that the incorporation of compatibilizers effectively homogenizes the macromolecular distribution within the blends, reducing interfacial differences and promoting phase integration. This parameter is critical for extrusion and film-blowing processes, as uniform melt density minimizes defects such as uneven flow or phase separation during processing, ensuring consistent material properties in the final product. Although melt density values do not show significant differences between compatibilized and non-compatibilized blends, this parameter does not directly reflect the interfacial interactions promoted by compatibilizers. Instead, these interactions are better evidenced by the enhanced mechanical properties and phase integration observed in compatibilized formulations. This behavior is consistent with previous studies, such as those by Zhang et al. (2023) and Rasselet et al. (2019), which highlight that melt density is less sensitive to interfacial modifications while remaining critical for processing performance.

Table 2.
Melt density of the different formulations.
3.2. TGA (Thermogravimetric Analysis)
The thermogravimetric analysis (TGA) results for the different formulations are summarized in Table 3 and illustrated in Figure 2. The analysis was conducted to evaluate the thermal stability of TPS/PBAT and TPS/PBAT-PBSA bioplastic films, as well as the impact of the Vinnex and Joncryl additives in structural terms.
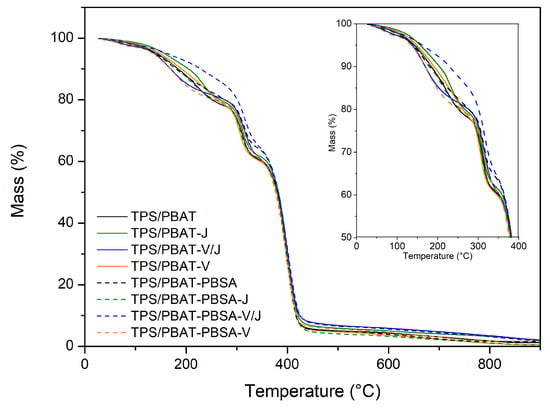
Figure 2.
Thermogravimetric curves of TPS/PBAT and TPS/PBAT-PBSA formulations. Insert zooms in the initial 50% mass-loss until 400 °C.
The TGA curves reveal that all samples exhibited a gradual weight loss as the temperature increased. The thermal decomposition process occurs in four main stages, characterized by distinct changes in the rate of weight loss:
- First stage (up to ~200 °C, T10%): This stage corresponds to the evaporation of residual moisture and the initial thermal degradation of glycerol, a plasticizer commonly used in TPS blends. This stage is critical for assessing the onset of thermal degradation, as it represents the temperature at which 10% of the sample’s initial mass is lost. The T10% values varied between 176 °C and 208.4 °C, indicating minor differences in the initial thermal stability among the formulations.
- Second stage (~200–300 °C, TmaxI): The primary decomposition of thermoplastic starch occurs here, including the breakdown of glycosidic linkages and depolymerization. The TmaxI values ranged from 207 °C to 225 °C, reflecting the impact of compatibilizers like Joncryl on delaying the degradation of starch by improving interfacial interactions and phase dispersion.
- Third stage (~300–400 °C, TmaxII): This stage is dominated by the degradation of PBAT and PBSA. The TmaxII values, ranging from 298 °C to 305 °C, showed relatively stable temperatures across all formulations, with PBSA contributing to slightly higher thermal resistance compared to TPS/PBAT blends.
- Fourth stage (~400 °C and above, TmaxIII): The final stage involves the degradation of carbonaceous residues, including any cross-linked or charred material. The TmaxIII values (389 °C to 400 °C) decreased slightly in formulations with Joncryl and Vinnex, suggesting minor effects on high-temperature stability.
The residue percentage after complete thermal decomposition ranged from 0.7% to 1.7%, indicating a minimal amount of remaining material. The final residue values primarily include contributions from partial decomposition of talc and carbonaceous residues [53,54].

Table 3.
Thermogravimetric results.
Table 3.
Thermogravimetric results.
| Reference | T10% (°C) | TmaxI (°C) | TmaxII (°C) | TmaxIII (°C) | Residue (%) |
|---|---|---|---|---|---|
| PBAT [55] | 310–340 | 400–420 | - | - | 0.5–1.0 |
| PBSA [56] | 290–320 | 370–390 | - | - | 0.5–2.0 |
| TPS/PBAT | 198.9 | 225 | 299 | 400 | 1.7 |
| TPS/PBAT-V | 180.0 | 207 | 302 | 397 | 0.7 |
| TPS/PBAT-J | 208.4 | 217 | 305 | 390 | 0.7 |
| TPS/PBAT-V/J | 187.8 | 222 | 303 | 389 | 1.2 |
| TPS/PBAT-PBSA | 196.0 | 213 | 298 | 396 | 1.6 |
| TPS/PBAT-PBSA-V | 176.0 | 222 | 299 | 390 | 0.8 |
| TPS/PBAT-PBSA-J | 196.0 | 212 | 298 | 392 | 1.2 |
| TPS/PBAT-PBSA-V/J | 201.6 | 217 | 298 | 398 | 1.3 |
3.3. DSC (Differential Scanning Calorimetry)
Thermal analysis is essential to understand the impact of formulations and processing on the polymer properties [54,55,56,57,58]. Table 4 and Figure 3 provide detailed information on the thermal transitions of TPS/PBAT and TPS/PBAT-PBSA films during the cooling and second heating cycles. The data include glass transition temperatures (Tg), gel temperature of starch (Tgel), melting points (Tm), and intrinsic melting enthalpies (Δhm) for each sample. Additionally, crystallization temperature (Tc) and intrinsic crystallization enthalpy (Δhc) during cooling are detailed. Tg was calculated at the midpoint of the transition while peak temperatures were considered for melting and crystallization events. The results indicated that the incorporation of additives such as Vinnex and Joncryl, as well as blending with PBSA, causes alterations in the thermal properties of the films.
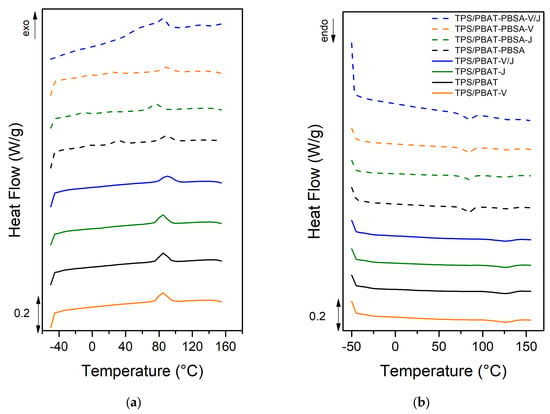
Figure 3.
DSC curves corresponding to (a) cooling cycle and (b) the second heating of the samples.
The analysis of fusion enthalpy reveals an increase, indicative of higher crystallinity, with the addition of additives and the inclusion of PBSA. This is consistent with findings from other studies where the incorporation of compatibilizing agents has been shown to enhance crystallinity in polymer blends due to improved molecular interactions and alignment during the crystallization process [53]. However, a decrease in melting enthalpy is observed when the additives are added simultaneously (Vinnex and Joncryl), suggesting an adverse effect on material crystallinity. Similar behavior has been reported in the literature, where the simultaneous addition of multiple additives can lead to competitive interactions that disrupt the crystallization process, resulting in reduced crystallinity [59]. It is also important to note that no significant changes are observed in the melting temperature, aligning with previous studies that show melting temperatures are often less sensitive to additive incorporation than other thermal properties like enthalpy and Tg [60].
In summary, the material appears to become slightly more crystalline with the addition of additives. Regarding the glass transition temperature (Tg), an increase is observed in TPS/PBA-PBSA with the addition of additives, while for the TPS/PBAT blend, a slight decrease is observed. These findings are crucial for understanding how compatibilizers modify molecular mobility and phase interactions. Unique Tg values were observed in the non-compatibilized blends, suggesting some degree of miscibility between the phases. However, the presence of a single Tg does not guarantee complete homogeneity, especially in blends with significant differences in their chemical and physical properties. This behavior must be analyzed alongside SEM micrographs, which show evidence of partial immiscibility and phase separation. These observations support the hypothesis of partial miscibility driven by predominant interactions between PBAT and TPS or PBSA in the absence of compatibilizers. To further clarify this, additional analysis has been incorporated based on the values found in the literature of the Tg for individual components: approximately −30 °C for PBAT, 45–50 °C for PBSA, and 80–90 °C for TPS.

Table 4.
Differential scanning calorimetry (DSC) results for TPS/PBAT and TPS/PBAT-PBSA formulations (note that error intervals are not shown for the sake of clearness and lie in the 1–3% range.).
Table 4.
Differential scanning calorimetry (DSC) results for TPS/PBAT and TPS/PBAT-PBSA formulations (note that error intervals are not shown for the sake of clearness and lie in the 1–3% range.).
| Cooling | |||||
| Reference | Tc, PBSA (°C) | ∆hc, PBSA (J/kg) | Tc, PBAT (°C) | ∆hc, PBAT (J/kg) | |
| PBAT [61] | - | - | 88–91 | 35–50 | |
| PBSA [56] | 73–78 | 40–60 | - | - | |
| TPS/PBAT | - | - | 86 | 7.8 | |
| TPS/PBAT-V | - | - | 83 | 6.5 | |
| TPS/PBAT-J | - | - | 89 | 8.4 | |
| TPS/PBAT-V/J | - | - | 87 | 8.5 | |
| TPS/PBAT-PBSA | 32 | 3.9 | 78 | 8.2 | |
| TPS/PBAT-PBSA-V | 28 | 4.3 | 86 | 9.7 | |
| TPS/PBAT-PBSA-J | 33 | 4.2 | 91 | 9.6 | |
| TPS/PBAT-PBSA-V/J | 31 | 4.0 | 87 | 6.3 | |
| Second Heating | |||||
| Reference | Tg Polymer (°C) | Tm, PBSA (°C) | ∆hm, PBSA (J/kg) | Tm, PBAT (°C) | ∆hm, PBAT (J/kg) |
| PBAT [61] | −30 | - | - | 120–125 | 35–50 |
| PBSA [56] | −35 | 90–95 | 40–60 | - | - |
| TPS/PBAT | −31.0 | - | - | 124 | 3.7 |
| TPS/PBAT-V | −34.0 | - | - | 123 | 4.1 |
| TPS/PBAT-J | −32.6 | - | - | 124 | 3.8 |
| TPS/PBAT-V/J | −31.7 | - | - | 125 | 3.1 |
| TPS/PBAT-PBSA | −34.0 | 89 | 4.6 | 121 | 4.4 |
| TPS/PBAT-PBSA-V | −28.0 | 86 | 5.0 | 125 | 4.4 |
| TPS/PBAT-PBSA-J | −31.0 | 85 | 4.9 | 121 | 4.7 |
| TPS/PBAT-PBSA-V/J | −30.7 | 86 | 4.8 | 122 | 3.0 |
3.4. Microscopy (SEM)
Figure 4 shows 1000× transverse cross-sectional SEM images of the various TPS/PBAT and TPS/PBAT-PBSA composite formulations. In the micrographs corresponding to TPS/PBAT and TPS/PBAT-PBSA without additives, a relatively homogeneous structure with a smooth surface can be observed, although there are some starch granules that were not fully broken during the extrusion process.
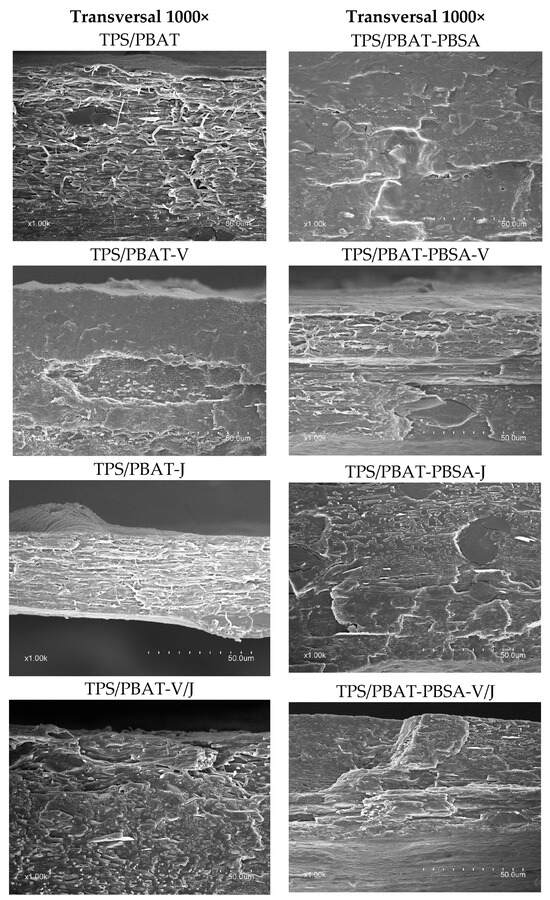
Figure 4.
Representative scanning electron microscopy (SEM) images of the surface of TPS/PBAT and TPS/PBAT-PBSA composites with and without the addition of Vinnex and Joncryl.
In contrast, the formulations containing Vinnex (TPS/PBAT-V and TPS/PBAT-PBSA-V) exhibit a rougher surface. This increased roughness could be attributed to the interaction between Vinnex and the polymer matrix, suggesting that compatibility between the components is not fully achieved. While Vinnex enhances interfacial adhesion and improves dispersion, the incomplete miscibility between the starch and polyester phases may lead to localized phase separation. This results in heterogeneities at the interface which manifest as increased surface roughness. Such behavior is common in partially compatibilized polymer blends, where molecular interactions are insufficient to achieve full homogeneity [62].
The images of the formulations with Joncryl (TPS/PBAT-J and TPS/PBAT-PBSA-J) also show an increase in roughness, although it is less pronounced compared to the Vinnex formulations. The surface texture appears more controlled, which could indicate better compatibility between Joncryl and the polymer matrix.
Finally, the formulations combining both additives (TPS/PBAT-V/J and TPS/PBAT-PBSA-V/J) exhibit the highest surface roughness, with a clearly more irregular structure. However, no pronounced interfaces between the phases are observed, suggesting that, despite the roughness, the additives are well integrated into the polymer matrix. This integration could enhance certain mechanical properties, as a rougher surface can improve adhesion between phases and potentially distribute stress more effectively, leading to better mechanical performance [63].
3.5. Mechanical Traction
As shown in Table 5 and Figure 5, significant differences are observed when comparing the mechanical properties of the TPS/PBAT and TPS/PBAT-PBSA formulations with the addition of Vinnex (V) and Joncryl (J). In the TPS/PBAT matrix, the addition of Vinnex increases the Young’s modulus from 73 MPa to 100 MPa, indicating greater material stiffness, along with an increase in yield stress from 3.3 MPa to 4.5 MPa. Joncryl, on the other hand, did not significantly affect the stiffness (the Young’s modulus remained at 74 MPa) but improved elongation at break, increasing it from 168% to 408%, and also raised the stress at break from 5.4 MPa to 11.2 MPa, indicating a greater ability of the material to deform before breaking. The combination of both additives (V/J) in TPS/PBAT resulted in a notable synergy, with an increase in elongation at break to 730% and stress at break to 16.9 MPa, highlighting a significant improvement in ductility and fracture resistance.

Table 5.
Mechanical properties of TPS/PBAT and TPS/PBAT-PBSA blends.
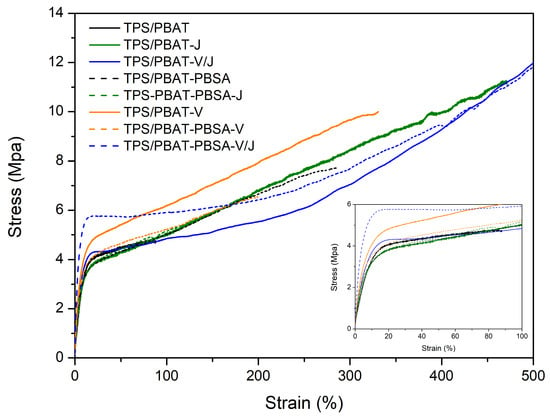
Figure 5.
Stress–strain mechanical curves of TPS/PBAT and TPS/PBAT-PBSA blends.
In the TPS/PBAT-PBSA matrix, Vinnex and Joncryl also enhanced mechanical properties, though less pronouncedly compared to the TPS/PBAT matrix (Figure 5, Table 5). Vinnex increased the Young’s modulus from 92 MPa to 98 MPa, while Joncryl slightly reduced it to 84 MPa, suggesting lower stiffness with Joncryl. However, elongation at break and stress at break were higher with Joncryl (500% and 9.1 MPa, respectively) compared to those with Vinnex. The combination of both additives (V/J) in TPS/PBAT-PBSA increased elongation at break to 610% and stress at break to 12 MPa, indicating improved material capacity to withstand larger deformations before fracturing. This is also corroborated by Hollomon’s coefficients, which show higher hardening in the elastic-to-plastic regime with the addition of the compatibilizers. In summary, while Vinnex primarily enhanced the stiffness and initial strength of the material, Joncryl significantly contributed to ductility and energy absorption capacity before breaking, with synergistic effects observed when both additives are combined, particularly in the TPS/PBAT formulation.
This synergistic behavior is consistent with previous studies that have shown how the incorporation of different additives and modifiers in polymer matrices can influence their mechanical and thermal properties [48].
4. Conclusions
The results presented in this study offer a comprehensive understanding of the effects of Vinnex (V) and Joncryl (J) on TPS/PBAT and TPS/PBAT-PBSA formulations. The films exhibited a macroscopically homogeneous appearance without visible phase separation, supported by uniform melt density values across formulations. However, SEM observations revealed a multiphasic morphology at the microscopic level, consistent with the immiscibility of the components. The compatibilizers improved interfacial interactions and phase dispersion, resulting in enhanced film performance. The incorporation of Vinnex and Joncryl caused minimal density changes, improved interfacial interactions, and phase dispersion, resulting in enhanced film performance. TGA results showed that the overall thermal stability remained comparable across formulations, with SEM observations of increased surface roughness also linked to crystallinity development, as described by DSC. Mechanically, the compatibilized blends showed improved performance as follows: (i) generally, roughness can help distribute stress; (ii) Vinnex mainly enhanced stiffness; (iii) Joncryl primarily improved elongation, and (iv) a synergistic effect was observed when both additives were combined, especially in the TPS/PBAT-V/J formulation, with higher hardening tendency during the elastic-to-plastic regime.
These findings suggest that strategically combining Vinnex and Joncryl is an effective approach for tailoring bioplastic film properties, achieving a balance of rigidity and flexibility suitable for specific applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15010456/s1, Figure S1: Representative images of (a) TPS/PBAT; (b) TPS/PBAT-V; (c) TPS/PBAT-J; (d) TPS/PBAT-V/J; (e) TPS/PBAT-PBSA; (f) TPS/PBAT-PBSA-V; (g) TPS/PBAT-PBSA-J and (h) TPS/PBAT-PBSA-V/J.
Author Contributions
Conceptualization, C.M.-P., J.D.B.-V. and J.P.C.-F.; methodology, J.D.B.-V. and J.P.C.-F.; validation, C.M.-P., J.D.B.-V. and J.P.C.-F.; formal analysis, C.M.-P.; investigation, C.M.-P.; visualization, C.M.-P.; resources, C.M.-P.; writing—original draft preparation, C.M.-P.; writing—review and editing, C.M.-P., J.D.B.-V. and J.P.C.-F.; supervision, J.D.B.-V. and J.P.C.-F.; project administration, C.M.-P.; funding acquisition, C.M.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by BZERO.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Technological Institute of Plastics (AIMPLAS) and Packaging, Transport & Logistics Research Institute (ITENE) are acknowledged for sample preparation. Central Research Services from Universitat de València (SCIE) are thanked for SEM analysis.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could appear to influence the work reported in this paper. However, Cristina Martín-Poyo is employee of BZERO, which provided funding and technical support for this work.
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; van der Zwet, J.; Damsteeg, J.W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef]
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and human health: A micro issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Badia, J.D.; Gil-Castell, O.; Ribes-Greus, A. Long-term properties and end-of-life of polymers from renewable resources. Polym. Degrad. Stab. 2017, 137, 35–57. [Google Scholar] [CrossRef]
- Rujnić-Sokele, M.; Pilipović, A. Challenges and opportunities of biodegradable plastics: A mini-review. Waste Manag. Res. 2017, 35, 132–140. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Ellen MacArthur Foundation. The New Plastics Economy: Rethinking the Future of Plastics & Catalysing Action; Ellen MacArthur Foundation: Cowes, UK, 2016. [Google Scholar]
- Avérous, L. Biodegradable multiphase systems based on plasticized starch: A review. J. Macromol. Sci. Part C Polym. Rev. 2004, 44, 231–274. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J. Starch-based materials in food packaging: Processing methods and applications. Crit. Rev. Food Sci. Nutr. 2008, 48, 601–608. [Google Scholar]
- Mali, S.; Grossmann, M.V.E.; Garcia, M.A.; Martino, M.N.; Zaritzky, N.E. Barrier, mechanical and optical properties of plasticized yam starch films. Carbohydr. Polym. 2004, 56, 129–135. [Google Scholar] [CrossRef]
- Chiellini, E.; Solaro, R. Biodegradable polymeric materials. Adv. Mater. 2003, 12, 1353–1363. [Google Scholar] [CrossRef]
- Chiellini, E.; Cinelli, P.; Chiellini, F.; Imam, S.H. Environmentally degradable bio-based polymeric blends and composites. Macromol. Biosci. 2004, 4, 218–231. [Google Scholar] [CrossRef]
- Shogren, R.L.; Lawton, J.W.; Doane, W.M.; Tiefenbacher, K.F. Starch-plastic materials—Preparation, physical properties, and biodegradability. J. Environ. Polym. Degrad. 1998, 6, 1–7. [Google Scholar]
- De Carvalho, A.J.F.; Zambon, M.D.; Curvelo, A.A.S.; Gandini, A. Thermoplastic starch modification during melt processing: Hydrolysis catalyzed by carboxylic acids. Carbohydr. Polym. 2003, 52, 283–287. [Google Scholar] [CrossRef]
- Rosa, D.S.; Guedes, C.G.F. Mechanical, thermal and morphological characterization of starch/ethylene vinyl alcohol copolymers blends. Carbohydr. Polym. 2003, 52, 215–220. [Google Scholar]
- Willett, J.L. Mechanical properties of starch-based plastics. Starch/Stärke 1994, 46, 293–298. [Google Scholar]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of bioplastic packaging materials: An overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef]
- Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Bioplastics with a green agenda. Curr. Opin. Microbiol. 2010, 13, 321–326. [Google Scholar] [CrossRef]
- Gross, R.A.; Kalra, B. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef]
- Li, F.; Zhang, J.; Sun, Z. Thermal degradation behavior of PBS: A review. Polym. Degrad. Stab. 2020, 182, 109379. [Google Scholar]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Soroudi, A.; Jakubowicz, I. Recycling of bioplastics, their blends and biocomposites: A review. Eur. Polym. J. 2013, 49, 2839–2858. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Morreale, M. Green composites: A brief review. Compos. Part A Appl. Sci. Manuf. 2011, 42, 579–584. [Google Scholar] [CrossRef]
- Zhang, J.F.; Sun, X. Mechanical properties of TPS/PBAT blends compatibilized by glycidyl methacrylate. Biomacromolecules 2023, 24, 455–467. [Google Scholar]
- Ren, J.; Xu, Q.; Wang, S. Compatibilizing effect of vinyl acetate on TPS-based blends. Carbohydr. Polym. 2022, 276, 118808. [Google Scholar]
- Averous, L.; Boquillon, N. Biocomposites based on plasticized starch: Thermal and mechanical behaviours. Carbohydr. Polym. 2004, 56, 111–122. [Google Scholar] [CrossRef]
- Weber, C.J.; Haugaard, V.; Festersen, R.; Bertelsen, G. Production and applications of biodegradable packaging materials from renewable resources. Trends Food Sci. Technol. 2006, 13, 106–117. [Google Scholar]
- Rasselet, D.; Caro-Bretelle, A.S.; Taguet, A.; Lopez-Cuesta, J.M. Reactive compatibilization of PLA/PA11 blends and their application in additive manufacturing. Materials 2019, 12, 485. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Silva, K.; Jordán-Silvestre, A.; Cháfer, A.; Muñoz-Espí, R.; Gil-Castell, O.; Badia, J.D. Ultrasonic chemo-thermal degradation of commercial poly(butylene adipate-co-terephthalate) (PBAT) and thermoplastic starch (TPS) blends. Polym. Degrad. Stab. 2024, 232, 111133. [Google Scholar] [CrossRef]
- Martin, O.; Averous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Ren, J.; Fu, H.; Ren, T.; Yuan, W. Preparation, characterization, and properties of starch/PLA blend foams. Carbohydr. Polym. 2009, 77, 576–582. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J. Properties of biodegradable thermoplastic pea starch/carboxymethyl cellulose and pea starch/microcrystalline cellulose composites. Carbohydr. Polym. 2009, 76, 369–375. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Kennedy, J.F. Studies on the properties of natural fibers-reinforced thermoplastic starch composites. Carbohydr. Polym. 2005, 62, 19–24. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J. Properties of biodegradable pea starch/carboxymethyl cellulose composites modified by poly(ethylene glycol). Carbohydr. Polym. 2008, 72, 369–375. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, R.; Qian, Y.; Yin, J. Preparation and properties of thermoplastic starch/multi-walled carbon nanotubes composites. Carbohydr. Polym. 2012, 87, 3160–3166. [Google Scholar]
- De Carvalho, A.J.F.; Zambon, M.D.; Curvelo, A.A.S.; Gandini, A. Starch-based plastic materials. Compr. Rev. Food Sci. Food Saf. 2001, 20, 203–216. [Google Scholar]
- Zheng, Y.; Wang, S. Effects of compatibilizer and loading on the properties of starch/poly(lactic acid) composites. J. Appl. Polym. Sci. 1998, 67, 1323–1330. [Google Scholar]
- Ren, J.; Xu, Q.; Wang, S. Compatibilizing effect of maleic anhydride on the mechanical properties of starch/poly(lactic acid) composites. Carbohydr. Polym. 2012, 87, 3160–3166. [Google Scholar]
- Ke, T.; Sun, X.S. Starch, poly(lactic acid), and poly(vinyl alcohol) blends. J. Polym. Environ. 2000, 8, 19–27. [Google Scholar]
- Kagarise, C.; Xu, J.; Wang, Y.; Mahboob, M.; Koelling, K.W.; Bechtel, S.E. Rheological, morphological, and interfacial properties of compatibilized PLA/PBAT blends. Rheol. Acta 2010, 53, 501–517. [Google Scholar]
- Othman, N.; Mohamad, Z.; Khan, Z.I. Compatibility and miscibility of recycled polyethylene terephthalate/polyamide 11 blends with and without Joncryl® compatibilizer. Iran. Polym. J. 2023. [Google Scholar] [CrossRef]
- Singh, S.; Pal, K. Biodegradable polymers: Recent developments and new perspectives. Prog. Polym. Sci. 2011, 36, 1011–1047. [Google Scholar]
- Weber, M.; Haupert, F. Thermoplastic starch blends and their compatibilization with polyesters. Carbohydr. Polym. 2006, 66, 307–315. [Google Scholar]
- Gupta, A.P.; Sharma, V. Mechanical and thermal properties of polymer nanocomposites: A review. J. Appl. Polym. Sci. 2011, 120, 1145–1170. [Google Scholar]
- Corradini, E.; de Moura, M.R.; Mattoso, L.H.C. A preliminary study of the incorporation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2009, 4, 509–515. [Google Scholar] [CrossRef]
- Totaro, G.; Di Ludovico, S.; Cinelli, P. Thermoplastic starch blends: A study on processability and physical properties. J. Polym. Environ. 2019, 27, 1281–1290. [Google Scholar]
- ISO 1183-1:2019; Plastics—Methods for Determining the Density of Non-Cellular Plastics Part 1: Immersion Method, Liquid Pycnometer Method and Titration Method. 2019. Available online: https://www.iso.org/standard/74990.html (accessed on 21 December 2024).
- ISO 527-1:2019; Plastics—Determination of Tensile Properties. 2019. Available online: https://www.iso.org/standard/75824.html (accessed on 21 December 2024).
- Müller, C.M.O.; Laurindo, J.B.; Yamashita, F. Effect of nanoclay and plasticizer on the thermal properties of starch-based films. Carbohydr. Polym. 2011, 86, 300–306. [Google Scholar]
- Badia, J.D.; Vilaplana, F.; Karlsson, S.; Ribes-Greus, A. Thermal analysis as a quality tool for assessing the influence of thermo-mechanical degradation on recycled poly (ethylene terephthalate). Polym. Test. 2009, 28, 169–175. [Google Scholar] [CrossRef]
- Alves, T.S.; Pereira, A.C.; Costa, M.F.; Silva, R.B.; Souza, J.M.; Oliveira, L.G.; Santos, V.F.; Almeida, P.R.; Rodrigues, C.L.; Carvalho, J.P. Thermal and mechanical properties of PBAT-based composites reinforced with sugarcane bagasse fibers. J. Appl. Polym. Sci. 2012, 125, 141–149. [Google Scholar]
- Sivalingam, G.; Kumar, S.; Ramesh, K.; Rajan, M.; Varma, S.; Singh, A.; Prasad, D.; Sharma, P.; Gupta, R.; Nair, V. Study on thermal degradation and decomposition of biodegradable PBS and PBSA. J. Therm. Anal. Calorim. 2017, 127, 2145–2153. [Google Scholar]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. The role of crystalline, mobile amorphous and rigid amorphous fractions in the performance of recycled poly (ethylene terephthalate) (PET). Polym. Degrad. Stab. 2012, 97, 98–107. [Google Scholar] [CrossRef]
- Pascual-Jose, B.; Badia, J.D.; Múgica, A.; Addiego, F.; Müller, A.J. Analysis of plasticization and reprocessing effects on the segmental cooperativity of polylactide by dielectric thermal spectroscopy. Polymer 2020, 223, 123701. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, C.; Ma, L. Thermal stability and degradation kinetics of polylactic acid composites reinforced with inorganic nanoparticles. J. Therm. Anal. Calorim. 2018, 134, 179–187. [Google Scholar]
- Jiang, T.; Zhang, L. Thermal behavior and compatibility of poly(butylene succinate)/starch blends with and without compatibilizers. J. Appl. Polym. Sci. 2008, 108, 378–385. [Google Scholar]
- Jiang, L.; Zhang, J.; Wang, Y.; Chen, X.; Li, Q.; Zhao, H.; Liu, W.; Yang, Z.; Xu, M.; Huang, F. Preparation and characterization of poly(butylene adipate-co-terephthalate) (PBAT) nanocomposites with carbon nanotubes. Polym. Degrad. Stab. 2010, 95, 841–850. [Google Scholar]
- Liu, Q.; Liu, H.; Zhang, J. The influence of plasticizers on the thermal and mechanical properties of poly(lactic acid) blends. J. Appl. Polym. Sci. 2010, 116, 3142–3149. [Google Scholar]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizers on the thermal properties of biodegradable films from sugar palm (Arenga pinnata) starch. Polymers 2016, 8, 218. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).