Featured Application
Encapsulation may be beneficial for citrus byproducts phenolics, according to low levels of bioactivity because of (i) unfavorable processing and storage conditions in food production and (ii) unfavorable conditions during transit through the gastrointestinal tract.
Abstract
This study evaluated Maltodextrin (MD), Gum Arabic (GA), and Carboxymethylcellulose (CMC) in different ratios as coating materials to encapsulate citrus pomace phenolic compounds. Citrus encapsulates were obtained by ultrasound-assisted extraction followed by the freeze-drying process and were characterized regarding the microencapsulation efficiency, physical, and chemical properties. Carrier material choice reflected a significant effect on encapsulation efficiency, phenolic compounds retention, and reconstitution properties of encapsulated extract. The encapsulation efficiency of prepared encapsulates ranked from 50.909% to 84.000%, and it was strongly dependent upon CMC addition. A wide range of reconstitution parameters (water absorption index-WAI and water solubility index-WSI) suggested possible release mechanism modifications. HPLC analysis revealed the presence of three main phenolic compounds, namely Hesperidin, Naringin, and Rutin. A wall material mixture of MD, GA, and CMC in the same proportions was optimal for freeze-drying. This combination resulted in encapsulates with a low moisture content (1.936 ± 0.012%) and a low water activity (0.110 ± 0.001), indicating prolonged stability. Based on the obtained results, freeze-drying as an encapsulation technique should be considered as a promising solution to recover compounds from industry byproducts and protect them from environmental and gastrointestinal circumstances.
1. Introduction
Polyphenols are a diverse group of naturally occurring compounds found in fruits, but in other parts of plants, they are widely known for their antioxidant, anti-inflammatory, antimicrobial, and other bioactivities. As bioactive compounds, polyphenols have gained significant attention due to their potential health benefits and protective effects against noncommunicable chronic diseases [1,2]. Citrus fruit is a rich source of polyphenols (phenolic acids, flavanones, flavanol, and flavones) [3]. Citrus fruit processing generates a significant number of byproducts, including peels, pulp, and rag, which are usually discarded. Despite the processing conditions, these byproducts are rich in polyphenols, which present an opportunity for their utilization. Transforming these byproducts into new products or materials both addresses environmental issues and produces new economic value [4,5]. Stability and bioavailability are recognized as one of the main challenges in utilizing polyphenols delivered from industry byproducts. Some phenolic compounds can easily degrade when exposed to elevated temperature, light, oxygen, and other non-desirable environmental conditions [6,7]. An increased number of authors recognized encapsulation as a possible solution to enhance the stability and preserve the bioactivity of polyphenol compounds [5,8,9,10]. Moreover, different combinations and modifications of encapsulating agents (coating) enable controlled, targeted, or postponed release of polyphenols from microcapsules or mask the unpleasant bitter and astringent taste [11,12,13]. On top of that, our previous results [12] showed that encapsulation using spray or freeze-drying can enhance the solubility and stability of phenolic compounds (hesperidin) and therefore broaden applications of phenolic compounds in food, food supplements, or pharmaceuticals. Many well-known techniques can serve as encapsulation techniques including spray drying, particle from gas-saturated solution processes, fluidized bed coating, coacervation, and freeze-drying [13,14,15]. Between various encapsulation techniques, freeze-drying has been recognized for effectively preserving heat-sensitive compounds [16]. Freeze-drying is an encapsulation technique based on the dehydration by sublimation of a liquid and semi-liquid mixture of core and carrier material. Carrier material (encapsulant) may be selected among various natural or synthetic materials, like Maltodextrins, dextrins, other polysaccharides, and gums. There are several requirements for compounds to be suitable for carrier material. Some of them are the ability to form films, desirable viscosity, safety, availability, low price, biocompatibility, and biodegradability [17,18]. The key feature of freeze-drying as an encapsulation technique is its performance at low temperatures where encapsulated compounds are not exposed to high temperatures, unlike spray-drying [19]. Despite the potential benefits, there is a lack of data on the encapsulation of polyphenols derived from mandarin juice byproducts using freeze-drying. Hu et al. [20] used a nanoencapsulation process to encapsulate mandarin peel polyphenols into whey protein coatings, while Mahdi et al. [21] used the same process for the encapsulation of mandarin essential oil. In an earlier study, Hu et al. [22] proved that during the encapsulation process, a synergistic effect between citrus peel extract and coating material (pectin) can occur and increase the bioaccessibility of phenolic compounds.
The aim of this study is to address this gap by conducting the encapsulation freeze-drying process to maximize polyphenol retention and evaluate the efficiency of encapsulation. For the first time, three encapsulating agents were compared and evaluated for valorization of underutilized byproducts. By exploring this approach, the study contributes to sustainable practices within the food industry, offering a novel method to enhance the value of mandarin juice byproducts.
2. Materials and Methods
2.1. Materials
Mandarin juice processing byproducts comprised of peel (flavedo and albedo), pulp (juice sac residue), and rag (membranes and cores) were obtained from Regius Company, Čapljina, Bosnia and Herzegovina immediately after processing (juice pressing). Samples were freeze-dried and stored in a dark place at 4 °C.
Carrier materials (Maltodextrin, DE:16-19; Gum Arabic, Carboxymethylcellulose) were obtained from Sigma-Aldrich, Saint Louis, MO, USA. Ethanol was obtained from JT Baker, Phillipsburg, NJ, USA. Standards used for HPLC analysis were obtained from Sigma-Aldrich and were of HPLC grade. All other chemicals were of analytical grade.
2.2. Citrus Pomace Extract: Obtaining and Characterization
Extraction of citrus polyphenols was performed using ultrasound-assisted extraction with optimal conditions defined by the study Montero-Calderon et al. [23] with a power of 400 W, temperature maximum of 40 °C using 50% aqueous ethanol solution (V/V) as a solvent, with 10 mL/g solvent solid ratio during 30 min of treatment. After the extraction procedure, the extract was filtered using vacuum filtration and concentrated using a rotary evaporator to remove ethanol. The concentration of the final extract was 74.72 mg/mL. The extract was characterized using UV-VIS spectrophotometer and HPLC.
2.3. Preparation for Encapsulation
Carrier materials were dissolved in the extract to achieve a concentration of 30% (w/V) according to the procedure described in Papoutsis et al. [24]. The prepared solution was homogenized for 60 min in a magnetic stirrer to achieve complete dissolution of carrier material. Three different carrier materials and a combination of them were used for the encapsulation of mandarin byproduct extract, as shown by the experimental plan in Table 1. Prepared mixtures were frozen in the laboratory freezer FORMA™ 88,000 Series (Thermo Scientific, Waltham, MA, USA) at a temperature of −80 °C for 24 h.

Table 1.
Experimental plan for encapsulating bioactive compounds from mandarin byproducts.
2.4. Encapsulation Using Freeze-Dryer
Following the preparation procedure, the feed was placed in a freeze-dryer (the Alpha LCS Plus system, Christ, Osterode am Harz, Germany) for 48 h. Vacuum during the main drying was set for 0.250 mbar. The maximum shelf temperature reached was 18 °C. Afterward, prepared encapsulates were collected and stored in a desiccator until further analysis.
2.5. Physical Properties of Prepared Encapsulates
Physical properties (color changes, water solubility index, water absorption index, moisture, and water activity) were determined by established procedures described previously [12].
The color measurements of the encapsulates were determined using a colorimeter (CS-10 portable 8 mm, Smart Color Solutions, Haryana, India). The equipment was previously calibrated using white and black calibration plates. The samples were placed in Petri dishes and measured in three parallels to obtain L* (lightness), a* (redness/greenness), b* (yellowness/blueness), C* (chroma), and hue angle (°h) parameters.
AACC Method 88–04 was used to determine the water absorption index (WAI) and water solubility index (WSI) with minimal modifications reported previously [12]. A total of 0.8 g of encapsulates were mixed with 10 mL of water, left standing for 30 min with periodic mixing and centrifuged for 15 min at 5000 rpm. Separated supernatant was dried at 105 °C until a constant mass was reached. WAI and WSI experiments were done in duplicates, calculated using Equations (1) and (2) expressed as % for WSI and grams of water per gram of encapsulates for WAI.
The dry matter of the encapsulates was determined using a halogen moisture analyzer (Mettler Toledo HR73, Columbus, OH, USA). A total of 0.13 g of encapsulates was weighed on an aluminum plate and dried at 105 °C with switch-off criteria 5 (mass loss less than 1 mg in 140 s interval).
Water activity (aw) was measured using a HygroPalm (Rotronic, Switzerland) at room temperature (22 °C). Approximately 10 g of the encapsulates was weighed into a special measuring cup and thermostated at room temperature (22 °C) under a cover for 10 min. Measurements were done in triplicate.
2.6. Encapsulation Efficiency Evaluation
Samples for calculating encapsulation efficiency were prepared following a previously established procedure by Tolun et al. [25]. Briefly, 15 mg of encapsulates was diluted with 3 mL of a 50:8:42, V/V/V ethanol/acetic acid solution, vortex mixed, and filtered through a 0.45 μm PTFE filter. Folin–Ciocalteu method was then applied to calculate the total phenolic content (TPC). 100 µL of Folin–Ciocalteu reagent, 40 µL of prepared solution, and 3160 µL of distilled water were combined. After 4 min of incubation, 300 µL of 20% (w/V) sodium carbonate was added and the mixture was incubated at 40 °C for 30 min. Gallic acid solutions (50–1000 mg/L) were used as a standard for establishing the calibration curve. The absorbance was measured at 765 nm in triplicates, and results are expressed as gallic acid equivalents. For the determination of surface phenol (SPC) content, 24 mg of encapsulates were washed for 5 min using 3 mL of ethanol/methanol (1:1, V/V) solution. The solution was filtered through a 0.45 μm PTFE filter and measured using the previously described Folin–Ciocalteu method.
Encapsulation efficiency (EE) was calculated using the following equation: Equation (3):
2.7. HPLC Analyses
The identification of individual phenolic compounds in encapsulates was performed by the high-performance liquid chromatography (HPLC) method with an injection volume of 20 μL. Samples were analyzed by HPLC (Agilent 1260 Infinity II (Agilent Technologies, Santa Clara, CA, USA), using a PLA detector (Maharashtra, India) (at 270 and 280 nm). Before analysis, encapsulates were extracted using a 50:8:42, V/V/V ethanol/acetic acid solution, vortex mixed, and filtered through a 0.45 μm PTFE filter. Separation was performed in an InfinityLab Inertsil ODS-3V (Noida, India) (250 × 4.6 mm inner diameter, 5 μm) column. The mobile phase was composed of solvent A (formic acid 0.1%) and solvent B (formic acid 0.1% in methanol). The elution was as follows: elution gradient started with 90% solvent A and 10% solvent B; solvent B is to reach 25% at 8 min, 45% at 25 min, and 80% at 45 min and then returned to the initial conditions by 10 min. The main phenolic compounds from encapsulates were identified by comparison against the retention times of the standards, their spectra, and the peak area of maximum absorption wavelength. OpenLab CDS software v. 2.5 (Agilent Technologies) was employed to process the chromatograms and spectra. Phenolic compounds were identified and quantified by measuring their absorption at characteristic wavelengths. All analyses were performed in triplicate.
2.8. Statistical Analysis
Data are presented as mean value ± SD of triplicate. Variances of mean values were statistically analyzed with a 95% confidence interval by one-way ANOVA followed by Tukey multiple comparisons HSD test using XLSTAT software 2014 1.04.
3. Results and Discussion
A total of 10 encapsulation experiments were carried out using three different coatings (MD, GA, and CMC) in varying proportions according to the experimental design described in Table 1. Visually, all obtained encapsulates were in the form of light yellowish-white free-flowing powder without agglomerate formation. Encapsulates quality is greatly affected by moisture content and water activity, significantly affecting shelf life. Water activity (aw) refers to the availability of free water in a food system, and it is responsible for biochemical reactions. In contrast, moisture content refers to the water composition of the food system. In the present study, the moisture content in the obtained encapsulates varied from 1.854 to 4.027%.
The highest moisture level was recorded for encapsulates encapsulated using pure CMC. Even the CMC is well-known for its hygroscopic properties [26]. A combination of CMC and GA gave very low moisture content (2.036 and 1.854%), while in combination with MD the moisture values were something higher (2.532 and 3.394%). Papoutsis et al. [24] reported moisture values between 1.150% and 2.105% for encapsulates delivered from lemon byproducts and encapsulated using Maltodextrin, soybean protein isolate, and ι-carrageenan. Water activity values varied between 0.110 and 0.240. According to aw values, all obtained encapsulates could be considered microbiologically and enzymatically stable since their water activity did not exceed 0.60 [27,28]. Functional rehydration properties of prepared encapsulated can be described by two properties, WAI and WSI, which are inversely proportional. In this study, both parameters were significantly affected by the addition of CMC to the carrier material, where the highest WAI values were obtained for pure CMC (11.316 g/g) and carriers with high levels of CMC (MD2CMC4 and GA2CMC4) (Table 2). An increase in WAI (g/g) led conversely to a decrease in WSI (%) (Table 2). Aside from CMC content, the decrease in WSI can be attributed to moisture content. However, in this case, moisture level was shown to be highly dependent on CMC content. Namely, CMC has a unique chemical structure where -CH2-COOH groups are attached to several hydroxyl groups of the cellulose units, which makes CMC soluble in water in contrast to insoluble cellulose [29]. High WSI values are especially desirable for application in the food and pharmaceutical industries, enabling even distribution and simple incorporation into the final product. On the opposite end, WAI values indicate immobilized water content and are more related to product microbial stability because with the increase in WAI values, the possibility of microbiological instability increases [30]. However, too high solubility can lead to undesirable fast release of core material. In general, formulations produced by freeze-drying have a more porous structure [31], which is the main reason for its high solubility properties. Nonetheless, solubility data presented in Table 2 indicate that with a careful selection of carriers, even encapsulates produced with freeze-drying can achieve a wide range of solubility, which could be suitable for controlled release. In our previous study [12], solubility of hesperidin encapsulates (pure or delivered from citrus peel) were between 88 and 99%. Lower-solubility parameters obtained in this study could be justified with CMC addition and higher WAI values. A similar trend was observed by Stabrauskiene et al. [19] for citrus paradisi extract encapsulated with different carriers with the addition of CMC.

Table 2.
The physical properties of prepared citrus byproduct encapsulates.
Color coordinates of encapsulates are shown in Table 3. The total color differences (ΔE) between the control (mixture of carrier material in 2:2:2 ratio) and encapsulates were greater than three units. Consequently, those changes are detectable by the human eye, which only detects color differences when ΔE is greater than 3 [32]. The sample MIX2:2:2 exhibited the highest lightness value (83.253), while MD4GA2 had the lowest L value (73.107), making it the darkest encapsulate. The significant differences identified by Tukey’s HSD test (Table 3) suggest that the carrier choice significantly impacts the lightness of the encapsulates. Obtained encapsulates were lighter than those obtained with spray drying and MD, GA, and sodium caseinate as carriers by Moawad et al. [33]. This could be justified by high temperatures applied during spray drying and possible non-enzymatic browning (caramelization of polysaccharide carriers), which was avoided by low temperatures during freeze-drying in the present study. The “a” parameter indicates the red/green value, with positive values towards red and negative towards green. Sample MD4GA2 had higher a and b values, which means that those encapsulated tend to be red and yellow, which could also indicate lower encapsulation efficiency where more of the core material remains non-encapsulated (on the surface of encapsulates).

Table 3.
CIELab colour parameters of the citrus byproduct encapsulates.
The encapsulation efficiency (calculated from total phenol content and surface phenols content) is shown in Figure 1. Encapsulation efficiency could be defined as the ability of the carrier material to encapsulate bioactive compounds. Also, it can be used as a determining factor for the integrity and porosity of produced encapsulates [34]. The encapsulation efficiency is affected by various parameters including carrier concentration, carrier solubility, as well as the rate of solvent evaporation during drying. The encapsulation efficiency of prepared encapsulates in this study ranged from 50.909% to 84.000%. Differences in encapsulation efficiency could be a result of polyphenol–carrier complex formation. According to Poomkokrak et al. [35] and Mazumder and Ranganathan [36], the formation of complex structures between phenolics and carriers (polysaccharides) depends on the molecular size and solubility of the carrier material. The highest encapsulation efficiency had an encapsulate prepared with all three carriers in the same proportions (MD2GA2CMC2), while the lowest had a sample prepared with pure MD.
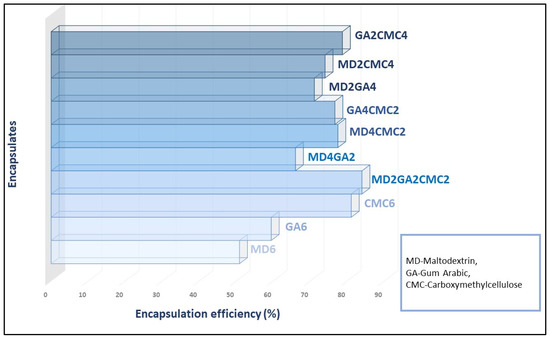
Figure 1.
Encapsulation efficiency of citrus byproduct encapsulates.
Additionally, a positive correlation between EE and WAI (r = 0.72, p = 0.01) and a negative between EE and WSI was observed, which could be explained by the CMC content in the carrier material. Namely, a slight positive correlation between CMC content and encapsulation efficiency was also observed (r = 0.69, p = 0.01). Surprisingly, there was no correlation between total color changes and encapsulation efficiency. However, upon comparing individual color properties a significant dependence between redness value (a) and encapsulation efficiency was observed. With an increase in the redness of the sample, a decrease in encapsulation efficiency was observed with a negative correlation of (r = −0.78, p = 0.01). This phenomenon is caused by lower encapsulation efficiency where more of the core material remains on the surface. It is evident that the increase of CMC in carrier material promotes encapsulation efficiency.
Retention of phenolic compounds was evaluated using spectrophotometric assay and HPLC analysis. Obtained encapsulates showed a high phenolic retention rate ranked from 5.641 to 12.821 mg/g. The highest retention rate had a sample encapsulated with a mixture of all three carriers in the same proportion (MD2GA2CMC2), while the lowest had a sample encapsulated with pure MD (MD6). The content of three major phenolic compounds (hesperidin, naringin, and rutin) was measured using HPLC and presented in Table 4. Hesperidin was ranked from 1.010 to 2.826 mg/g, naringin from 1.398 to 2.592 mg/g, and rutin from 0.156 to 1.590 mg/g. Hesperidin and naringin are phenolic compounds well-known for their hydrophobic and low-soluble properties. Those properties limit their bioavailability and application in the food and cosmetic industry [37]. However, encapsulation with hydrophilic carriers can enable better physical properties, as proven previously. Retention of hesperidin was dependent on carrier usage (particularly, CMC content), where an increase in CMC led to higher hesperidin retention. A similar trend for naringin content and CMC as a carrier can be observed [38,39]. It was previously shown that CMC can complex with phenolic compounds, enhancing physical properties and reducing interaction with proteins (reducing astringency). Hesperidin content also positively correlated with encapsulation efficiency (r = 0.86, p = 0.01), indicating that the main encapsulated phenolic compound is hesperidin while other phenolic compounds could be distributed on the surface of the encapsulates. Although an increase in carrier material can lead to the dilution of core material, surprisingly, other authors reported an increase in encapsulation efficiency probably due to an increase in the thickness of microparticle shell walls [40].

Table 4.
Phenolic compound retention in citrus byproduct encapsulates.
4. Conclusions
Encapsulated polyphenols from citrus byproducts showed promising potential for use as a functional food due to their high polyphenol encapsulation efficiency, appealing color, and low water activity. The encapsulation efficiency of the prepared encapsulates was found to be strongly dependent on the addition of CMC and varied greatly between 50.909% and 84.000%. This observation suggests that CMC could potentially be used to modulate the controlled release of the encapsulated material. Further studies are required to gain a better insight into the structure of encapsulates and thermal properties. The focus of these studies should be on the stability of encapsulates in food products and simulated gastrointestinal conditions. Also, additional analyses (LC-MS) could be beneficial to confirm the presence of minor phenolic compounds, which could contribute to biochemical and physical properties of citrus byproduct encapsulates.
Author Contributions
Conceptualization, M.B. and S.J.; Methodology, K.A., J.I. and M.B.; Formal analysis, A.F., N.K., M.K., L.P. and M.B.; Investigation, K.A. and M.B.; Writing—original draft preparation, M.B.; Writing—review and editing, M.B., S.J., A.F. and A.I.; Visualization, M.B and K.A.; Funding acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Federal Ministry of Education and Science Bosnia and Herzegovina under the project, “Valorization of the by-product of citrus production and processing through the application of innovative extraction and encapsulation techniques (05-35-2120-2/23)”, and was supported by COST Action CA22134 “Sustainable Network for agrofood loss and waste prevention, management, quantification and valorisation (FoodWaStop)”, funded by the European Commission.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors would like to show our gratitude to Snježana Keleković for technical assistance during laboratory work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Vivarelli, S.; Costa, C.; Teodoro, M.; Giambò, F.; Tsatsakis, A.M.; Fenga, C. Polyphenols: A Route from Bioavailability to Bioactivity Addressing Potential Health Benefits to Tackle Human Chronic Diseases. Arch. Toxicol. 2023, 97, 3–38. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Marcillo-Parra, V.; Tupuna-Yerovi, D.S.; González, Z.; Ruales, J. Encapsulation of Bioactive Compounds from Fruit and Vegetable By-Products for Food Application: A Review. Trends Food Sci. Technol. 2021, 116, 11–23. [Google Scholar] [CrossRef]
- Caballero, S.; Li, Y.O.; McClements, D.J.; Davidov-Pardo, G. Encapsulation and Delivery of Bioactive Citrus Pomace Polyphenols: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8028–8044. [Google Scholar] [CrossRef]
- Teng, H.; Chen, L. Polyphenols and Bioavailability: An Update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Xiao, J. Available Technologies on Improving the Stability of Polyphenols in Food Processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Garavand, F.; Jalai-Jivan, M.; Assadpour, E.; Jafari, S.M. Encapsulation of Phenolic Compounds within Nano/Microemulsion Systems: A Review. Food Chem. 2021, 364, 130376. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A Review of Microencapsulation Methods for Food Antioxidants: Principles, Advantages, Drawbacks and Applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- Dadwal, M.; Gupta, M. Recent Developments in Citrus Bioflavonoid Encapsulation to Reinforce Controlled Antioxidant Delivery and Generate Therapeutic Uses: Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1187–1207. [Google Scholar] [CrossRef] [PubMed]
- Banožić, M.; Krzywonos, M.; Aladić, K.; Pińkowska, H.; Mucha, I.; Złocińska, A.; Jokić, S. Physicochemical, Structural Characterization and Evaluation of Encapsulated Hesperidin from Natural Sources: Comparison of Two Encapsulation Techniques; Spray Drying and Freeze Drying. J. Drug Deliv. Sci. Technol. 2023, 90, 105098. [Google Scholar] [CrossRef]
- Banožić, M.; Vladić, J.; Banjari, I.; Velić, D.; Aladić, K.; Jokić, S. Spray Drying as a Method of Choice for Obtaining High-Quality Products from Food Wastes: A Review. Food Rev. Int. 2021, 39, 1953–1985. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Balanč, B.; Djordjević, V.; Bugarski, B. Encapsulation Techniques for Food Purposes. In Encapsulation in Food Processing and Fermentation; CRC Press: Boca Raton, FL, USA, 2022; pp. 37–80. [Google Scholar]
- Shaaban, H.A.; Farouk, A. Encapsulation of Essential Oils and Their Use in Food Applications; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and Delivery of Bioactive Compounds Using Spray and Freeze-Drying Techniques: A Review. Dry. Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Sosnowska, K.; Winnicka, K.; Morkuniene, R.; Bernatoniene, J. Freeze-Drying Technique for Microencapsulation of Elsholtzia Ciliata Ethanolic Extract Using Different Coating Materials. Molecules 2020, 25, 2237. [Google Scholar] [CrossRef]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of Grape (Vitis labrusca var. Bordo) Skin Phenolic Extract Using Gum Arabic, Polydextrose, and Partially Hydrolyzed Guar Gum as Encapsulating Agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Pudziuvelyte, L.; Bernatoniene, J. Optimizing Encapsulation: Comparative Analysis of Spray-Drying and Freeze-Drying for Sustainable Recovery of Bioactive Compounds from Citrus x paradisi L. Peels. Pharmaceutics 2024, 17, 596. [Google Scholar] [CrossRef]
- Hu, Y.; Kou, G.; Chen, Q.; Li, Y.; Zhou, Z. Protection and Delivery of Mandarin (Citrus reticulata Blanco) Peel Extracts by Encapsulation of Whey Protein Concentrate Nanoparticles. LWT 2019, 99, 24–33. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Mohammed, J.K.; Al-Ansi, W.; Ghaleb, A.D.S.; Al-Maqtari, Q.A.; Ma, M.; Ahmed, M.I.; Wang, H. Microencapsulation of Fingered Citron Extract with Gum Arabic, Modified Starch, Whey Protein, and Maltodextrin Using Spray Drying. Int. J. Biol. Macromol. 2019, 152, 1125–1134. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, W.; Ke, Z.; Li, Y.; Zhou, Z. In Vitro Release and Antioxidant Activity of Satsuma Mandarin (Citrus reticulata Blanco cv. unshiu) Peel Flavonoids Encapsulated by Pectin Nanoparticles. Int. J. Food Sci. Technol. 2017, 52, 2362–2373. [Google Scholar] [CrossRef]
- Montero-Calderon, A.; Cortes, C.; Zulueta, A. Green Solvents and Ultrasound-Assisted Extraction of Bioactive Orange (Citrus sinensis) Peel Compounds. Sci. Rep. 2019, 9, 16120. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Screening the Effect of Four Ultrasound-Assisted Extraction Parameters on Hesperidin and Phenolic Acid Content of Aqueous Citrus Pomace Extracts. Food Biosci. 2018, 21, 20–26. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of Grape Polyphenols Using Maltodextrin and Gum Arabic as Two Alternative Coating Materials: Development and Characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef]
- Torres, M.D.; Moreira, R.; Chenlo, F.; Vázquez, M.J. Water Adsorption Isotherms of Carboxymethyl Cellulose, Guar, Locust Bean, Tragacanth and Xanthan Gums. Carbohydr. Polym. 2012, 89, 592–598. [Google Scholar] [CrossRef]
- Yamashita, C.; Chung, M.M.S.; dos Santos, C.; Mayer, C.R.M.; Moraes, I.C.F.; Branco, I. Microencapsulation of an Anthocyanin-Rich Blackberry (Rubus spp.) By-Product Extract by Freeze-Drying. LWT 2017, 84, 256–262. [Google Scholar] [CrossRef]
- Ramírez, M.J.; Giraldo, G.I.; Orrego, C.E. Modeling and Stability of Polyphenol in Spray-Dried and Freeze-Dried Fruit Encapsulates. Powder Technol. 2015, 277, 89–96. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Gholizadeh Vazvani, M.; Hassanisaadi, M.; Skorik, Y.A. Micro-/Nano-Carboxymethyl Cellulose as a Promising Biopolymer with Prospects in the Agriculture Sector: A Review. Polymers 2023, 15, 440. [Google Scholar] [CrossRef]
- Perković, G.; Martinović, J.; Šelo, G.; Bucić-Kojić, A.; Planinić, M.; Ambrus, R. Characterization of Grape Pomace Extract Microcapsules: The Influence of Carbohydrate Co-Coating on the Stabilization of Goat Whey Protein as a Primary Coating. Foods 2024, 13, 1346. [Google Scholar] [CrossRef]
- Da Silva Júnior, M.E.; Araújo, M.V.R.L.; Martins, A.C.S.; Dos Santos Lima, M.; Da Silva, F.L.H.; Converti, A.; Maciel, M.I.S. Microencapsulation by Spray-Drying and Freeze-Drying of Extract of Phenolic Compounds Obtained from Ciriguela Peel. Sci. Rep. 2023, 13, 15222. [Google Scholar] [CrossRef] [PubMed]
- Bodart, M.; De Peñaranda, R.; Deneyer, A.; Flamant, G. Photometry and Colorimetry Characterisation of Materials in Daylighting Evaluation Tools. Build. Environ. 2008, 43, 2046–2058. [Google Scholar] [CrossRef]
- Moawad, S.; El-Kalyoubi, M.; Khallaf, M.; Mohammed, D.M.; Mahmoud, K.F.; Farouk, A. Effect of Spray-Drying on the Physical, Sensory, and In-Vivo Parameters of Orange Peel Oil and Limonene. Egypt. J. Chem. 2022, 65, 353–368. [Google Scholar] [CrossRef]
- Amin, S.G.; Shah, D.A.; Dave, R.H. Formulation and Evaluation of Liposomes of Fenofibrate Prepared by Thin Film Hydration Technique. Int. J. Pharm. Sci. Res. 2018, 9, 3621–3637. [Google Scholar] [CrossRef]
- Poomkokrak, J.; Niamnuy, C.; Choicharoen, K.; Devahastin, S. Encapsulation of Soybean Extract Using Spray Drying. J. Food Sci. Agric. Technol. 2015, 1, 105–110. [Google Scholar]
- Mazumder, M.A.R.; Ranganathan, T.V. Encapsulation of Isoflavone with Milk, Maltodextrin and Gum Acacia Improves Its Stability. Curr. Res. Food Sci. 2019, 2, 77–83. [Google Scholar] [CrossRef]
- Chen, M.; Li, R.; Gao, Y.; Zheng, Y.; Liao, L.; Cao, Y.; Li, J.; Zhou, W. Encapsulation of Hydrophobic and Low-Soluble Polyphenols into Nanoliposomes by pH-Driven Method: Naringenin and Naringin as Model Compounds. Foods 2021, 10, 963. [Google Scholar] [CrossRef]
- Troszynska, A.; Narolewska, O.; Wolejszo, A.; Ostaszyk, A. Effect of Carboxymethyl Cellulose (CMC) on Perception of Astringency of Phenolic Compounds. Pol. J. Food Nutr. Sci. 2008, 58, 241. [Google Scholar]
- Ćorković, I.; Pichler, A.; Buljeta, I.; Šimunović, J.; Kopjar, M. Carboxymethylcellulose Hydrogels: Effect of Its Different Amount on Preservation of Tart Cherry Anthocyanins and Polyphenols. Curr. Plant Biol. 2021, 28, 100222. [Google Scholar] [CrossRef]
- Pai, D.A.; Vangala, V.R.; Ng, J.W.; Tan, R.B.H. Resistant Maltodextrin as a Shell Material for Encapsulation of Naringin: Production and Physicochemical Characterization. J. Food Eng. 2015, 161, 68–74. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).