Abstract
The study aimed to evaluate the antioxidant potential and microbial composition of edible fermented vegetable roots available on the Polish market, including celeriac, carrot, beetroot, radish, and white radish. The microbiological determinations were conducted according to European Standards. The total phenol content (TPC) was measured by the Folin–Ciocalteu (FC) method, while the total antioxidant capacity (TAC) was analyzed by CUPRAC and DPPH assays. The mean recovery for FC, CUPRAC, and DPPH was in the range of 104–105%, 97–102%, and 96–108%, while the precision amounted to 2.3, 2.75, and 5.99, respectively. The average antioxidant potential among all fermented roots decreased in the following order: beetroot > celeriac > radish > carrot > white radish. In the case of microbiological analyses, no bacteria were found in pasteurized products. However, among unpasteurized vegetables, 7 out of 11 products met the FAO/WHO criteria for probiotic foods, as they contained a sufficient number of lactic acid bacteria and lacked Escherichia coli. None of the tested products were contaminated with Bacillus cereus, Salmonella spp., Enterococcus spp., or spore-forming anaerobic bacteria. Moreover, chemometric techniques such as the Kruskal–Wallis test and cluster analysis were used to differentiate samples in view of their antioxidant potential. These analyses demonstrated the similarity of vegetable samples from the Apiaceae and Brassicaceae families while highlighting differences in antioxidant potential compared to samples from the Amaranthaceae family.
1. Introduction
Root vegetables have a variety of characteristics and can be categorized according to their botanical origin, temperature hardiness, time, and cultivation methods [1]. They are characterized by several health-promoting effects, including antioxidant potential [2,3]. The process that is believed to make vegetables even healthier is fermentation [4]. Fermented vegetables, including kimchi, sauerkraut, or cucumbers as well as pickled edible roots, are becoming increasingly popular. The first three mentioned products are characterized by a high total antioxidant content (TAC) and total phenol content (TPC) [5,6,7]. There are studies that suggest that lactic acid bacilli (LAB) multiplying during fermentation leads to higher antioxidant potential [8]. Moreover, it was shown that fermented vegetables can be called probiotic foods according to the criteria set by the Food and Agriculture Organization and World Health Organization (FAO/WHO) [9,10,11]. The aforementioned organization established that the name probiotic is reserved only for products containing a minimum of 106 colony-forming units (CFU) per 1 mL [11].
Lactic acid bacilli fulfill an important part in food processing [12]. These microorganisms improve food safety and flavor profiles and may synthesize bioactive substances, like gamma-aminobutyric acid (GABA) [13]. Biological detoxification by LAB is believed to be a promising approach to reduce the risk related to the presence of chemical, microbiological, and environmental contaminants in food [14]. It has been demonstrated that lactic acid bacteria (LAB) have the ability to bind or degrade various pollutants, thereby reducing their intestinal absorption and facilitating the excretion of toxic substances. The effectiveness of contaminant binding by LAB is influenced by factors such as temperature, pH, the presence of bile salts, and the concentrations of both probiotics and toxic substances [13,14]. As a result, the incorporation of LAB cultures and the use of food fermentation represent a safe, environmentally friendly, and cost-effective approach to mitigating or eliminating hazardous substances. Moreover, the fermentation process enhances microbiological safety. This effect is attributed to the production of lactic acid, the primary metabolite of LAB, which lowers the pH of the environment. The reduced pH inhibits the proliferation of pathogenic microorganisms, thereby contributing to improved food safety [15]. Nonetheless, a study has demonstrated that microbiological pathogens, such as E. coli or Staphylococcus species, can persist in environments with a pH of less than 4 [16]. Moreover, microorganisms during fermentation produce alcohols, aldehydes, and organic acids, which affect the sensory quality of food [17,18].
The antioxidant potential of edible plants, including vegetables and root crops, is commonly evaluated using various analytical methodologies [3,19,20,21,22]. However, the comparability of results across different studies is often limited due to the diversity of methods employed [23]. Among the available techniques, the Folin–Ciocalteu (FC) assay is the most widely used for determining total phenolic content (TPC) [24,25,26]. This method is well-established and recognized as a reliable analytical approach. To assess total antioxidant capacity (TAC), the cupric ion reducing antioxidant capacity (CUPRAC) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays are frequently applied [25,27,28]. These two methods are complementary: the DPPH assay is primarily used to measure antioxidants with hydrophobic properties, while the CUPRAC assay can also detect compounds with hydrophilic structures [23,29]. Notably, the CUPRAC assay offers an advantage over the DPPH method due to its use of reagents with a pH closer to physiological conditions (pH 7) compared to the DPPH assay (pH 5). Consequently, the CUPRAC assay is considered to provide a more accurate representation of the behavior of antioxidants within the human body [23,30]. Furthermore, the DPPH assay has certain limitations, as the instability of the DPPH radical can lead to variability in methodologies, results, and their interpretation [31].
The primary objective of this study was to evaluate the potential of fermented root vegetables to contribute to a balanced diet and support human health. Specifically, the research focused on assessing the antioxidant potential and microbiological composition of commercially available fermented root vegetables on the Polish market, i.e., carrot (Daucus carota L. var. sativus [Hoffm.] Arcang.), radish (Raphanus sativus L. var. sativus), white radish (Raphanus sativus L. var. lingipinnatus Bailey), celery root (Apium graveolens L. var. rapaceum [Mill.] Gaud.), and beetroot (Beta vulgaris L.). The selection of products was determined by their availability on the Polish market and the limited range of products offered by domestic producers. These root vegetables represent some of the most commonly fermented plant materials in Poland, reflecting both traditional culinary practices and consumer preferences. Their selection also aligns with the current market trends, where these vegetables are readily accessible in fermented form, either pasteurized or unpasteurized. The antioxidant potential of the fermented vegetables was compared with values reported in the literature for their fresh counterparts. In addition, the microbiological quality of the fermented vegetables was examined, with particular emphasis on potential contamination. The hypothesis that pasteurized fermented vegetables have lower microbial counts and reduced antioxidant potential compared to non-pasteurized products was tested. Furthermore, the microbiological composition of the products was assessed against FAO/WHO criteria for probiotic food.
2. Materials and Methods
2.1. Samples Collection and Preparation
Five types of root vegetables were analyzed: carrot (Daucus carota L. var. sativus [Hoffm.] Arcang.), radish (Raphanus sativus L. var. sativus), white radish (Raphanus sativus L. var. lingipinnatus Bailey), celery root (Apium graveolens L. var. rapaceum [Mill.] Gaud.), and beetroot (Beta vulgaris L.), with three varieties from various sources included in the analysis (Table 1). The selection of products was dictated by their availability on the Polish market and the limited range offered by producers. Fermented root vegetables were collected from online (Poland) and stationary (Gdańsk, Poland) shops. All products were commercially available on the Polish market and were cultivated in a conventional way. Due to the significant time gap between the production of the products and their availability on the market in commercial form, raw materials used in the pickling process were not accessible for analysis.

Table 1.
Characteristics of the test material.
Microbiological examinations were conducted immediately after opening the jar. To determine antioxidant potential, products were drained from water and frozen at −20 °C. Then, the studied material was lyophilized in an Alpha 1–4D plus freeze dryer (Christ, Osterode am Harz, Germany). Finally, products were homogenized using an analytical mill (IKA®A11 basic, Warsaw, Poland).
Reagents and Standards
Reagents for the Folin–Ciocalteu assay were as follows: anhydrous sodium carbonate (purity > 99.5%, Chempur®, Piekary Śląskie, Poland), Folin–Ciocalteu reagent (analytical grade, Chempur®, Piekary Śląskie, Poland), and gallic acid (purity > 98%, Sigma-Aldrich®, North Brunswick, NJ, USA). Reagents for the DPPH assay were as follows: methanol and 2,2-diphenyl-1-picrylhydrazyl (Sigma-Aldrich®, St. Louis, MO, USA). Reagents for the CUPRAC assay were as follows: ethanol 96%, 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (purity > 97%, Sigma-Aldrich®, St. Louis, MO, USA), neocuproine (purity > 98%, Sigma-Aldrich®, St. Louis, MO, USA), copper(II) chloride (purity > 99%, Sigma-Aldrich®, St. Louis, MO, USA), and ammonium acetate (purity > 98%, Sigma-Aldrich®, St. Louis, MO, USA).
Ultrapure water (18.2 MΩ·cm, Millipore Simplicity System, Billerica, MA, USA) was used for all aqueous solutions.
Microbiological media were as follows: buffered peptone water (Graso Biotech, Starogard Gdański, Poland), nutrient agar (Himedia, Mumbai, India), MRS agar (De Man–Rogosa–Sharpe agar; Biomaxima, Lublin, Poland), sabouraud agar with chloramphenicol and gentamicin (Graso Biotech, Starogard Gdański, Poland), iron sulphite agar (Graso Biotech, Starogard Gdański, Poland), MUG (4-methylumbelliferyl-β-D-glucuronide) Lauryl Sulpate Broth (Himedia, Mumbai, India), EC broth (Escherichia coli broth; Biomaxima, Lublin, Poland), Peptone water with tryptophan (Biomaxima, Lublin, Poland), Slanetz Barteley agar (Graso Biotech, Starogard Gdański, Poland), Muller–Kauffmann Tetrathionate-Novobiocin Broth (MKTTn), Rappaport–Vassiliadis soya (RVS) pepton broth, brilliant green agar (Biomaxima, Lublin, Poland), HiCrome Salmonella agar (Himedia, Mumbai, India), MYP agar (Mannitol egg Yolk Polymyxin agar; Biomaxima, Lublin, Poland), and HiCrom Bacillus agar (Himedia, Mumbai, India). Sucrose medium for Leuconostoc detection (meat extract 0.3%, peptone 1%, NaCl 0.5%, sucrose 10%, agar 2% were dissolved in 100 mL of distilled water, pH 7.0, sterile at 121 °C for 15 min) was also produced. Medium for detecting acetic fermentation bacteria (peptone 2%, yeast extract 1%, dissolved in 100 mL of distilled water, pH 6.8–7.0, sterile at 121 °C for 20 min, 0.8% ethyl alcohol) was added to the cooled medium.
2.2. The Total Antioxidant Capacity (TAC) and Total Phenolic Content (TPC)
2.2.1. Optimization of Extraction
The extraction procedure was optimized using the FC method. The separation of bioactive compounds from plant samples mainly depends on the nature of the solvent used [32]. In this study, methanol, ethanol, and acetone at different proportions with acidified water, 0.1% of formic acid (FA), were used. These are the most frequent solvents adopted for phenols’ determination in vegetables [28,33,34]. Optimization of extraction was performed using ten combinations of extractants in three variants of extraction (Figure 1). Each variant was examined in triplicate with a three-fold measurement. Three vegetables from different botanical families, i.e., carrot (C1), radish (R1), and beetroot (B1), were chosen for optimization to average the measurements. Based on the obtained results, one-stage ultrasound-assisted extraction with 75% methanol (MetOH) and 0.1% FA was found to be the most optimal extraction method. All extracts after preparation were kept at −20 °C until analysis.
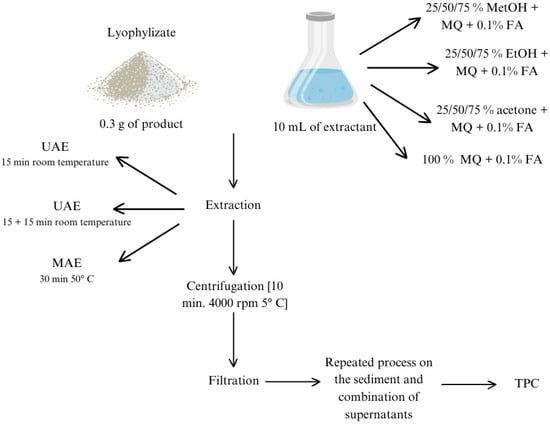
Figure 1.
Diagram of the performed extraction optimization procedure. MetOH—methanol, EtOH—ethanol, MQ—milli-Q water, FA—formic acid, TPC—total phenol content, UAE—ultrasound-assisted extraction, MAE—microwave-assisted extraction.
2.2.2. TPC Determination
The total phenolic content (TPC) in the extracted samples was determined using the optimized and validated FC method [28].
To begin, 200 µL of sample extract and 800 µL of Mili-Q water were combined in a test tube, and 5 mL of Folin–Ciocalteu reagent (FCR) was added. The sample was mixed in a laboratory vortex mixer (Lab Dancer, VWR®, Gdansk, Poland), and it was left for 3 min. Then, 10 mL of sodium carbonate solution (150 g/L) was added and re-mixed in a vortex. The absorbance was measured threefold at 760 nm (Genesys 10S, Thermo Fisher Scientific, Waltham, MA, USA) after incubation (30 min in darkness, room temperature). The results obtained in mg gallic acid equivalent (GAE)/g of dry weight (d.w.) were converted to mg GAE/100 g of fresh weight (f.w.) through the use of a water content percentage, specific for each vegetable (Table 1).
2.2.3. DPPH Assay
The protocol of the DPPH method was adapted from [35] with some modifications. The procedure was optimized in terms of concentration of the test DPPH solution, volumes of extract added, and wavelength. The variants tested were presented in Figure 2. According to Lambert–Beer’s law, a supersaturated solution may not give linear results [36]. The cut-off absorbance for saturated solutions should not exceed 0.8 ± 0.02 [31,35,37]. In our laboratory and under the prevailing environmental conditions, the concentration of the test DPPH solution was 73.7 mmol/L for absorbance 0.8 ± 0.02. The applied protocol is presented in Figure 3.
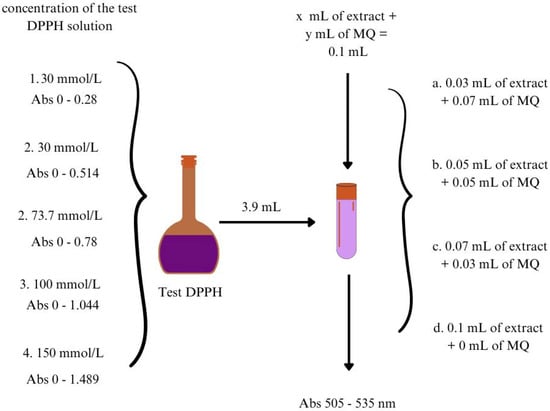
Figure 2.
Optimization of the DPPH assay procedure. MQ—milli—Q water.
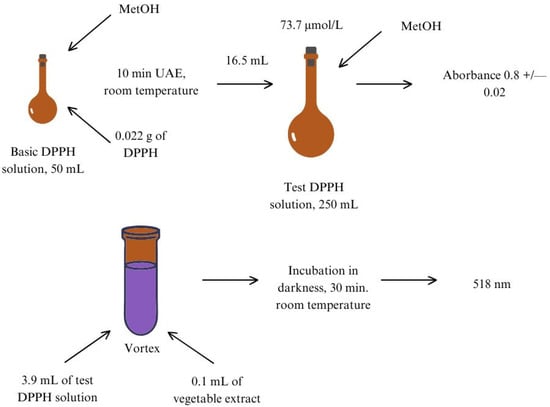
Figure 3.
The applied procedure of DPPH assay. UAE—ultrasonic-assisted extraction, MetOH—methanol.
2.2.4. CUPRAC Assay
The protocol of the CUPRAC assay was adapted from Apak et al. [38] with some modifications. The process was validated and optimized in terms of concentration and molar ratio of reagents, pH of ammonium buffer, time, and type of incubation (Figure 4). In the procedure, an aqueous solution of copper chloride (CuCl2), an ethanolic solution of neocuproine, and ammonium buffer were used in various proportions. While the molar ratio and volume of substrates should be invariable, incubation may be different and depend on the matrix of the analyzed material. Therefore, optimization of the molar ratio of substrates was conducted on Trolox’ solutions, and the type and time of incubation were verified for these solutions and for vegetables’ extracts.
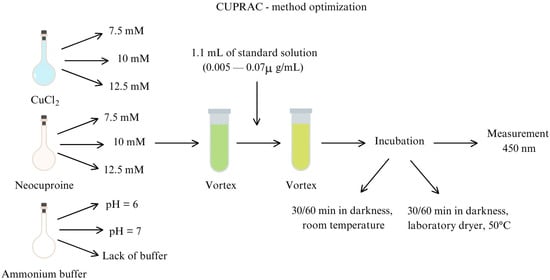
Figure 4.
Optimization of CUPRAC assay.
The optimal concentrations of reagents were as follows: 10 mM for CuCl2, 7.5 mM for neocuproine, and a buffer pH was 7. Incubation for 60 min in darkness in a laboratory dryer at 50 °C was chosen as the most favorable.
2.3. Determination of Microorganisms
Sample solution (100 mL) from ready-made fermented products was taken randomly, every time after mixing. Samples were collected aseptically. Then, a series of dilutions were prepared in geometric progression (from 10−1 to 10−6), according to the procedure described in PN-90 A-75052 [39].
Buffered peptone water (Graso Biotech, Starogard Gdański, Poland) was used as the diluent. The prepared dilutions were inoculated by the pour plate method using nutrient agar (Himedia, Mumbai, India) to determine the number of mesophilic bacteria (37 °C, 48 h, according to PN-EN 204 ISO4833:2004 [40]); the number of psychrophilic bacteria (4.5 °C, 5–7 days, according to PN-ISO 17410:2004 [41]); the number of thermophilic bacteria (55 °C, 48 h, according to PN-A-75052-06:1990 [42]); and the number of acidifying bacteria of the lactic type, according to PN-90 A-75052/07 208 [43], (37 °C, 72 h, elevated CO2 concentration; MRS agar–De Man–Rogosa–Sharpe agar; Biomaxima, Lublin Poland). The surface-spread method was used to determine the number of yeasts and molds, according to PN-90 A-75052/08; PN ISO 21527-1:2009; PN ISO 21527-2:2009 [44,45,46] (20–25 °C, 4–5 days; Sabouraud agar with chloramphenicol and gentamicin (Graso Biotech, Starogard Gdański, Poland); the presence of bacteria of the genus Enterococcus, according to PN-EN 15788:2009 217 [47] (37 °C, 48 h; Slanetz Barteley agar (Graso Biotech, Starogard Gdański, Poland)); and the number of Bacillus cereus bacteria, according to PN-EN ISO 7932:2005 [48] (30 °C, 48 h; MYP agar–Mannitol egg Yolk Polymyxin agar (Biomaxima, Lublin, Poland)). In order to determine the presence and number of bacteria of the genus Leuconostoc, inoculation was performed using the pour plate method with the sucrose medium (25 °C, for 5 days; home-made sucrose medium for Leuconostoc detection: meat extract 0.3%, peptone 1%, NaCl 0.5%, sucrose 10%, and agar 2% were dissolved in 100 mL of distilled water, pH 7.0, sterile at 121 °C for 15 min). In turn, the presence and number of mesophilic and thermophilic spore-forming anaerobic bacteria were detected according to PN-ISO 15213:2005 [49] (a 10 mL of initial sample was heated at 70 °C for 20 min). After cooling, the sample was diluted in a geometrical progression, and next, each dilution was inoculated in a 9:1 ratio with iron sulphite agar (Graso Biotech, Starogard Gdański, Poland). When the medium had set, approximately 1 mL of water agar was layered on the samples, and thus, prepared samples were incubated for 48 h at 37 °C (for mesophilic bacteria) and 55 °C (for thermophilic bacteria). The most probable number method was used to detect the presence and number of coliforms according to PN-EN ISO 4831:1998 [50], using MUG (4-methylumbelliferyl-β-D-glucuronide) Lauryl Sulpate Broth (Himedia, Mumbai, India). The initial sample and the next two tenfold dilutions were inoculated on the medium in a 9:1 ratio in triplicate and incubated at 37 °C for 48 h. Positive samples were checked for E. coli according to PN-EN ISO 7251:2005 [51], using EC broth (Escherichia coli broth; Biomaxima, Lublin, Poland) and peptone water with tryptophan (Biomaxima, Lublin, Poland). The samples mentioned above were identified biochemically with the API20E bioMérieux Polska Sp. z o.o. test. The presence of acetic fermentation bacteria was determined according to PN-90 A-75052/15 [52] on a home-made medium (peptone 2% and yeast extract 1% dissolved in 100 mL of distilled water, pH 6.8–7.0, sterilized at 121 °C for 20 min, and 0.8% ethyl alcohol was added to the cooled medium). Determination of the acetic fermentation bacteria was performed using the pour plate method, and the media were incubated at 30 °C for 5 days to determine the presence of Salmonella spp. (according to PN-EN ISO 6579:2003) [53]. The initial sample was mixed in a 1:10 ratio with buffered peptone water and then incubated at 37 °C for 24 h. Positive samples were inoculated on the following media: Muller–Kauffmann Tetrathionate–Novobiocin Broth (MKTTn) and Rappaport–Vassiliadis Soya (RVS) peptone broth (Biomaxima, Lublin Poland). Next, the above-mentioned samples were identified biochemically using the API20E test by bioMérieux Polska Sp. z o.o., Warsaw, Poland.
Moreover, the identification of isolated microorganisms was performed by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF).
2.4. Validation
Validation was carried out by the standard addition method using fermented carrot (sample C1). The following parameters were evaluated: limit of detection (LOD), limit of quantification (LOQ), linearity range, precision, and accuracy (Table 2). As a standard substance, gallic acid (GA) for the FC assay and Trolox for the DPPH and CUPRAC assays were used. Gallic acid was used in a concentration range of 0.1–10 μg/mL. Trolox was used in a concentration range of 5–70 µg/mL in the CUPRAC assay and 0.02–0.2 mg/mL in the DPPH assay. The LOD and LOQ were calculated from the formulas [54]:
LOD = (3.3 × BSD)/Amean
BSD—mean standard deviation of the y-intercept of three independent curves
Amean—mean of the slope of three independent curves
LOQ = 3 × LOD

Table 2.
The validation parameters of the applied methods.
Table 2.
The validation parameters of the applied methods.
| FC | CUPRAC | DPPH | |
|---|---|---|---|
| Standard substance | Gallic acid | Trolox | Trolox |
| Linearity range | 0.1–1 µg/mL | 5–70 µg/mL | 0.02–0.2 mg/mL |
| R2 | 0.9991 | 0.9990 | 0.9990 |
| LOD | 0.039 µg/mL | 0.043 µg/mL | 0.023 mg/mL |
| LOQ | 0.117 µg/mL | 0.129 µg/mL | 0.069 mg/mL |
| Precision | 2.3% | 2.75% | 5.99% |
| Accuracy | 104–105% | 97–102% | 96–108% |
2.5. Statistical Analysis
The data were reported as the mean ± SD of three independent samples. Statistical analyses were preceded by an analysis of the normality (the Shapiro–Wilk test) of the distribution of data. As the distribution was nonparametric, Spearman’s rank correlation analysis and ANOVA Kruskal–Willis tests were applied. To verify which of the analyzed groups are different from each other, a post-hoc Dunn’s test was used. Moreover, cluster analysis (CA) was applied. The data were processed using Statistica for Windows (version 13, Statsoft, Cracow, Poland).
3. Results and Discussion
All analyzed fermented root vegetables were characterized by a varied antioxidant potential, expressed as TPC and TAC. All the results are presented in Table 3.

Table 3.
The results from FC, CUPRAC, and DPPH assays, calculated for 100 g of f.w. and % of activity in the DPPH assay.
3.1. Total Phenols’ Content
The total phenolic content (TPC) was determined in all analyzed samples, ranging from 14.35 to 56.71 mg GAE/100 g fresh weight (f.w.) (Table 2). Among the fermented vegetables, beetroot exhibited the highest TPC values, while white radish had the lowest. The TPC values for the remaining pickled vegetables were similar, with average levels of 16.83 mg GAE/100 g f.w. for carrot, 19.45 mg GAE/100 g f.w. for radish, and 28.85 mg GAE/100 g f.w. for celeriac. Beetroot is widely regarded as a rich source of antioxidants, including phenolic compounds [55]. For fresh beetroot, TPC values reported in the literature range from 164 to 211 mg GAE/100 g f.w. [28,56]. In comparison, fermented beetroot exhibited significantly lower TPC values, ranging from 26.94 to 47.67 mg GAE/100 g f.w.
Similarly, fermented celeriac also showed lower TPC values compared to its fresh counterpart. The TPC of fermented celeriac ranged from 24.47 to 37.21 mg GAE/100 g f.w., whereas fresh celeriac was characterized by a TPC of 59.0 mg GAE/100 g f.w. [57]. For the other analyzed vegetables, TPC values were below 25 mg GAE/100 g f.w., which may be partially attributed to their high water content (Table 1). Additionally, radish, carrot, and white radish had statistically significant (p = 0.000) lower TPC values than beetroot. Celeriac, however, exhibited a higher TPC (p = 0.000) than radish. The TPC values for these three vegetables were similar, ranging from 18.69 to 19.84 mg GAE/100 g f.w. for radish, 12.33 to 26.61 mg GAE/100 g f.w. for carrot, and 10.89 to 10.16 mg GAE/100 g f.w. for white radish.
The order of TPC levels in the analyzed fermented vegetables was as follows: beetroot > celeriac > carrot > radish > white radish. By comparison, the reported TPC levels for their fresh counterparts follow a similar order: beetroot (164–211 mg GAE/100 g f.w.) [2,28,57], celeriac (59 mg GAE/100 g f.w.) [57], carrot (35.19 mg GAE/100 g f.w.) [58], and radish (29.45 mg GAE/100 g f.w.) [59]. The antioxidant potential and phenolic content of vegetables are strongly influenced by the raw material and cultivation conditions [60,61]. Data on root vegetables cultivated in Poland indicate relatively low antioxidant potential, with carrots containing 18.0 mg GAE/100 g fresh weight (f.w.) [62] and celeriac containing 32.2 mg of phenolic compounds per 100 g f.w. [63].
These findings are different to other studies, which indicated that TPC increased in fermented vegetables compared to their fresh counterparts [64,65,66]. However, there was research that reported a decrease in phenols amount after fermentation. Moreover, the percentage decrease in TPC after the mentioned process was dependent on the specific bacteria type [66]. Nevertheless, the vegetables analyzed in our study were industrially fermented and purchased in this form. Therefore, there are no data about the TPC of fresh samples of analyzed vegetables. That is why we cannot unequivocally state what effect the fermentation process had on the antioxidant potential of the analyzed vegetables. Nevertheless, the analyses performed indicate that the pickled roots available on the Polish market are characterized by a decreased TPC compared to the fresh vegetables.
There are a notable lack of data on the TPC of fermented root vegetables in the scientific literature. Most available studies have focused on by-products of these pickles, such as fermented carrot juice (Salgam). For example, one study reported TPC values for Salgam ranging from 185.93 to 257.10 mg GAE/L [67], while another study indicated values between 627.0 and 748.5 mg GAE/L [68]. However, comparisons between these findings and the present study are challenging due to differences in sample matrices (juice versus whole vegetables).
Based on recommendations concerning consumption of phenols (500 mg GAE/day) [69], it was concluded that pickled roots constitute a moderate dietary source of the mentioned compounds. The consumption of 100 g of the analyzed fermented vegetables covered these requirements in a range of 2.8% for white radish, 3.36% for carrot, 3.89% for radish, 5.77% for celeriac, and 11.34% for beetroot.
3.2. Total Antioxidant Capacity
3.2.1. DPPH Assay Results
The results obtained by the DPPH assay were expressed as Trolox equivalent (TE) per 100 g of f.w. and % of activity. They were presented in Table 2. The highest value was 11.13 mmol TE/100 g f.w., which corresponded to 43.76% of activity, and was found for pickled beetroot. According to the literature, activity in scavenging the free radicals for fresh beetroot amounted to 63% [28]. Nevertheless, fermented beetroot was characterized by the greatest TAC of all the analyzed pickles. Radish was the root that was ranked second in terms of scavenging the free radicals (3.67–4.82 mmol TE/100 g f.w.). These results might be due to the high content of hydrophobic antioxidant compounds in radish, such as isothiocyanates [70]. However, apigenin from celeriac and carotenoids from carrots are hydrophobic antioxidants as well [71,72]. Despite their high amount in the mentioned vegetables, the results obtained in the DPPH assay for these roots were much lower. The lowest TAC was found for fermented carrot, i.e., 1.47–3.21 mmol of Trolox/100 g f.w., and celeriac—1.89–4.41 mmol TE/100 g f.w. Moreover, a statistically significant difference was observed between carrot and beetroot, as well as between white radish and beetroot (p = 0.000). No significant differences were found among the other root vegetables.
There is limited research on the antioxidant potential of fermented root vegetables. Furthermore, the DPPH assay is associated with considerable methodological discrepancies across studies [31]. Nevertheless, it has been demonstrated that the ability of fermented beetroot to scavenge free radicals increases during the first 7 days of fermentation, after which it begins to decline. Consequently, products stored for extended periods may exhibit lower total antioxidant capacity (TAC) compared to freshly fermented pickles [73]. Fresh counterparts of several roots cultivated in Poland were characterized by significantly lower levels of Trolox equivalents, as measured by the DPPH assay, i.e., 211 μmol TE/100 g f.w. in beetroot, 132 μmol TE/100 g f.w, in celeriac, and 42.5 μmol TE/100 g f.w. in carrot [74]. According to the cited study, the fermentation process may enhance the ability of processed products to scavenge free radicals.
3.2.2. CUPRAC Assay Results
Antioxidants capable of reducing Cu2+ are relevant to the CUPRAC assay, with phenols, tocopherols, and vitamin C being the primary compounds in vegetables [75]. However, the first two compounds mentioned are hydrophobic, while in CUPRAC, hydrophilic antioxidants are major reagents. Therefore, ascorbic acid may be an essential compound in this assay [76]. However, betalains present in beetroot are also hydrophilic compounds. Presumably, due to their high amount in the mentioned vegetable, the results obtained in the CUPRAC assay were the highest for beetroot (2.89–4.09 mmol TE/100 g f.w. In contrast, carrot exhibited the lowest TAC values (0.56–1.02 mmol TE/100 g f.w.), which corresponds with its low vitamin C content (5.9 mg/100 g f.w.) [72]. Celeriac and radish displayed intermediate TAC values in the CUPRAC assay, with results ranging from 0.94 to 2.14 mmol TE/100 g f.w. for celeriac and 1.06 to 1.21 mmol TE/100 g f.w. for radish. Vitamin C content in these vegetables was 8 mg/100 g f.w. in celeriac and 14.8 mg/100 g f.w. in radish [77], both of which are higher than the amount found in carrot. Celeriac, in particular, is rich in quercetin, which has a high molar absorptivity in the CUPRAC assay [78], potentially contributing to the higher average TAC observed for celeriac compared to radish.
The TAC values obtained for the fermented samples were lower than those observed for fresh roots. In this study, the TAC determined by the CUPRAC assay ranged from 1.89 to 4.09 mmol TE/100 g f.w., while literature values for fresh beetroot ranged from 1.52 to 6.87 mmol TE/100 g f.w. The lower results for fermented vegetables may be attributed to a reduction in vitamin C and betalain content during fermentation [4]. The CUPRAC assay results for beetroot cultivated in Poland showed a value of 2.74 mmol TE/100 g fresh weight (f.w.) [28]. Depending on the specific pickled product, this value may be either lower or higher. It is worth noting that the characteristics of the raw material are a critical determinant of antioxidant properties [61].
Statistically significant differences were observed between beetroot and carrot, as well as between white radish and beetroot, consistent with the results from the DPPH assay (p = 0.000).
3.3. Evaluation of Microorganisms’ Content
The composition of microorganisms in the analyzed products was varied (Table 4). However, no microorganisms were detected in pasteurized products. Therefore, pasteurized pickles cannot be recognized as probiotic foods. Unpasteurized products were characterized by the presence of LAB with no statistically significant differences. The total number of them was higher than 106 CFU/mL, except for one sample of pickled celeriac (CR2), where this value reached 6.2 × 105 CFU/mL. Moreover, microorganisms from the Lactobacillaceae family were identified. These included Levilactobacillus brevis in pickled celeriac (CR3) and radish (R2) samples, Lactobacillus plantarum in pickled radish (R1), carrot (C2), and white radish (W1) samples, and Lacticaseibacillus paracasei in fermented carrot (C2) and beetroot (B3). Both Levilactobacillus brevis and Lactobacillus plantarum are known to contribute to the safety and health-promoting properties of fermented foods through the production of gamma-aminobutyric acid (GABA) [79,80]. According to the literature, they were detected in fermented vegetables such as sauerkraut or cauliflower [79,80]. Lacticaseibacillus paracasei, a genus commonly associated with fermented dairy products [81,82], has also been found in pickled vegetables, including beetroot and fermented carrot juice [68,83,84]. This microorganism is of particular significance due to its potential to enhance the health properties of fermented products by positively influencing the gut microbiota and the gut-brain axis [82,85]. None of the tested products were microbiologically contaminated by Bacillus cereus, Salmonella, Enterococcus species, or spore-forming anaerobic bacteria.

Table 4.
Microbiological composition of fermented roots.
Unfortunately, some of the analyzed products may pose a potential health risk. In three out of the eleven unpasteurized samples, coliforms were detected, all of which were identified as Escherichia coli. The number of detected CFU/mL was greater than the maximal values established by the European Commission for Escherichia coli in fruits and vegetables, i.e., 102 [86]. Coliforms were detected in pickled radish (R1—1.1 × 105 CFU/mL), carrot (C1—2.4 × 102 CFU/mL), and beetroot (B3—1.1 × 105 CFU/mL).
The presence of coliforms in fermented products may be attributed to several factors, including the initial contact of the vegetables with soil, the presence of these bacteria in the irrigation system, the use of animal-based fertilizers, or the natural occurrence of the bacteria on fresh roots [87].
Due to the insufficient number of lactic acid bacteria (LAB) in sample CR2 and the detection of coliforms in three products, only seven out of the eleven unpasteurized pickled root samples can be classified as probiotic foods.
Comparing the microbiological properties of processed vegetables with their fresh counterparts is challenging when they originate from different crops. Nevertheless, fresh root vegetables are often characterized by greater microbiological diversity. This is largely due to the fermentation process and the resulting lower pH, which reduces the survival of many microorganisms [88,89]. For instance, while the total number of mesophilic bacteria was comparable in fresh celeriac, higher levels of psychrophilic bacteria, yeasts, molds, and coliforms were observed [63]. With regard to the microbiological safety of root vegetables in Poland, studies by Rutkowski et al. [90] have confirmed the absence of pathogenic bacteria such as Salmonella and Listeria monocytogenes in tested samples of Polish root vegetables. However, lactic acid bacilli are microorganisms which multiply during fermentation [4].
4. Statistical Analysis Results
Statistical analyses were conducted to evaluate the relationship between the botanical families of the analyzed root vegetables and their antioxidant potential, as well as the assays employed. Additionally, the tests performed enabled the assessment of whether the pasteurization process influences the antioxidant potential of the pickled roots.
4.1. Correlation Analysis
The Spearman’s test revealed significant relationships (p < 0.001) among all analytical approaches used to determine the antioxidant potential. All correlations were positive and were characterized by a moderate strength (0.64–0.84) (Table 5). However, correlations between the number of LAB and the results from all antioxidant assays used were negative (Table 5). Moreover, botanical families were characterized by strong positive correlations in view of their antioxidant potential (Table 6).

Table 5.
The results of the Spearman’s rank correlation test between TAC and TPC results, and the LAB quantity obtained by different methods for root vegetables. Values that were statistically significant at p < 0.001 were marked in red, and those at p > 0.05 were marked in blue.

Table 6.
The results of the Spearman’s rank correlation test between TAC and TPC results obtained by all methods for analyzed botanical families. Values that were statistically significant at p < 0.001 were marked in red.
4.2. Kruskal–Wallis Test
It was found that botanical families were characterized by interdependencies of antioxidant potential, which were as follows: Apiaceae (H = 46.234; p = 0.000), Brassicaceae (H = 46.230; p = 0.000), and Amaranthaceae (H = 46.287; p = 0.000). Moreover, the Kruskal–Wallis test revealed relationships among the antioxidant methods used, i.e., FC (H = 25.677; p = 0.000), CUPRAC (H = 21.533; p = 0.000), DPPH (H = 21.842; p = 0.000). The pasteurization process appeared to be relevant to antioxidant potential. The Kruskal–Wallis test showed significant differences between pasteurized and unpasteurized pickled roots using the results from the FC assay (H = 9.967; p = 0.002) and the CUPRAC assay (H = 6.327; p = 0.012). Interestingly, pasteurized pickles and a lack of LAB were correlated with higher antioxidant potential. The strongest dependence was for FC and LAB (Figure 5). High temperatures induce a decrease in the content of some ingredients, e.g., vitamin C. Still, there are compounds that show greater antioxidant properties when subjected to processing, including short-term heating. At the same time, compounds such as phenols [91,92,93] and carotenoids [86] are chemically stable under temperatures ranging from 70 to 90 °C [85]. It was shown that the mentioned compounds are released into the analyzed extract in greater amounts at high temperatures than at low ones [94,95].
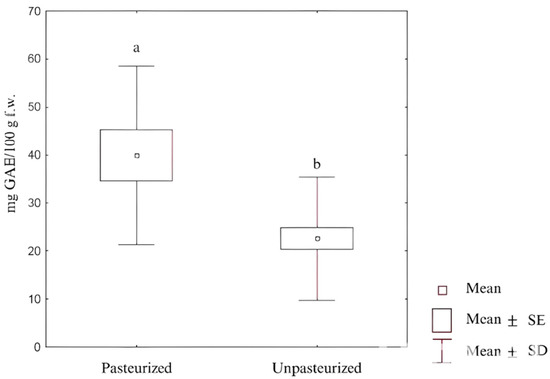
Figure 5.
Comparison of FC results for pasteurized and unpasteurized products. Various letters indicate statistically significant differences between groups (p = 0.0346). GAE—gallic acid equivalent, SE—standard error, SD—standard deviation.
There were no statistically significant differences between TPC and TAC in terms of the level of grinding. Unfortunately, the crop area was unknown. Therefore, it was not possible to determine any differences in the antioxidant potential of pickles depending on their cultivation characteristics.
The null hypothesis of the Kruskal–Wallis test was rejected, so a post-hoc Dunn’s test was applied, which allowed for differences’ identification. This test revealed statistically significant differences (p < 0.001) between the Amaranthaceae family (beetroot) and the other families, i.e., Apiaceae and Brassicaceae. There was no statistically significant difference between the Brassicaceae and Apiaceae families.
4.3. Cluster Analysis
Cluster analysis (CA) is an exploratory technique used to identify groups of objects within a dataset. It aims to reveal patterns or structures in the data without providing explanations for their occurrence. To assess the similarities between the samples, hierarchical clustering was performed using the Ward method and Euclidean distance as the distance measure. The final data matrix included the antioxidant potential of the analyzed root vegetables, measured by the Folin–Ciocalteu (FC), CUPRAC, and DPPH assays (Figure 6). Three main clusters can be distinguished. They enabled the botanical differentiation of samples. The first cluster was assigned to the CUPRAC assay and contained samples from the Amaranthaceae family, i.e., beetroot. Two remaining clusters were allocated to DPPH and FC methods and combined Apiaceae (celeriac and carrot) and Brassicaceae (radish and white radish) families.
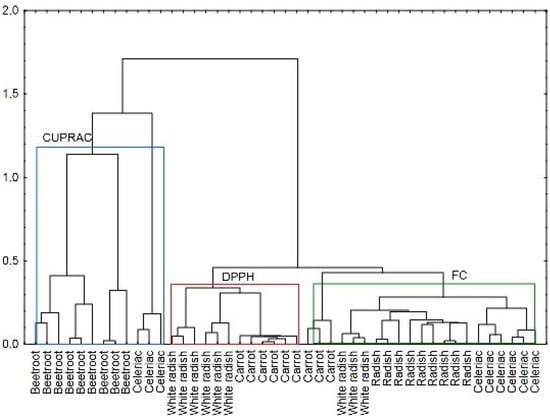
Figure 6.
Dendrogram of fermented roots in view of antioxidant assays used.
While the CA highlighted similarities between vegetables from the Apiaceae and Brassicaceae families, it also demonstrated significant differences between these families and the Amaranthaceae family. Interestingly, celeriac samples were assigned to two separate clusters. The product attributed to the first cluster was the sample CR3, which was characterized by the greatest values in DPPH, CUPRAC, and FC assays among all pickled celery roots. However, there could have been other similarities affecting the high antioxidant potential of sample CR3 (celeriac) and its connection with beetroot. These samples may share comparable mineral compositions, particularly elements known to influence antioxidant properties, such as zinc [96], copper [78], and iron [97]. CUPRAC is an assay in which the content of Cu may be relevant. Cluster analysis linked beetroot samples with the mentioned method, which might be in accordance with Cu content in the analyzed vegetables. The amount of Cu in fresh beetroot was 0.161 mg/100 g f.w. and 0.07 mg/100 g f.w. in celeriac, while in carrot it amounted to 0.061 mg/100 g f.w. and 0.05 mg/100 g in radish [77]. Moreover, higher content of compounds belonging to phenols relevant to the FC assay were identified in radish and carrot than in beetroot. Radish samples were characterized by a high content of flavonoids, mainly kaempferol. This substance is present in radish at a level of 0.63 mg/100 g f.w. [98]. Carrot is rich in hydroxycinnamic acids, mainly 5-caffeoylquinic acid (8.88 mg/100 g f.w.) [99]. Therefore, it can be concluded that CA allowed for the differentiation of samples based on their elemental composition and the bioactive compounds specific to each vegetable.
5. Conclusions
The present study demonstrates that fermented root vegetables, due to their antioxidant activity and microbiological composition, hold significant potential to enhance the human diet and contribute to maintaining optimal health. The analyzed pickles were characterized by various antioxidant potentials that were decreasing, as follows: beetroot > celeriac > radish > carrot > white radish. All spectrophotometric methods used in the study, i.e., FC, CUPRAC, and DPPH, were positively correlated. Pasteurized products were characterized by greater TPC than unpasteurized ones. Furthermore, there was a negative correlation between LAB and antioxidant potential. While the antioxidant potential of the pickled vegetables was lower than that of their fresh equivalents, all the analyzed products were found to be valuable sources of antioxidants.
The study also confirmed that pasteurization effectively eliminates microorganisms, thus precluding these products from being classified as probiotic foods. In contrast, unpasteurized products contained a diverse range of microorganisms, including LAB from the Lactobacillaceae family, specifically Lactobacillus plantarum, Levilactobacillus brevis, and Lacticaseibacillus paracasei. Moreover, seven out of the eleven unpasteurized products met the FAO/WHO criteria for probiotic food. However, microbiological contamination by coliforms was detected in three vegetable samples, with the number of Escherichia coli CFU/mL exceeding the European Commission’s established limit for fruits and vegetables.
Cluster analysis successfully differentiated vegetable samples based on their antioxidant potential, revealing similarities between the Apiaceae and Brassicaceae families, as well as distinct differences from the Amaranthaceae family. This classification correlates with the elemental composition and the bioactive substance content, particularly phenols, in the root vegetables. Vegetables with higher copper content were primarily associated with the CUPRAC method, while phenol-rich roots corresponded with the FC assay.
In conclusion, unpasteurized pickled root vegetables available on the Polish market can indeed be classified as probiotic foods. Nevertheless, there is still much unknown about fermented vegetables, especially roots. Further investigation is essential to comprehensively understand the effects of fermentation on the nutritional profile and health benefits of edible roots.
Author Contributions
Conceptualization, E.K. and M.G.; funding acquisition, E.K., M.G. and K.W.; methodology, E.K., M.G. and R.H.; validation, E.K.; formal analysis, E.K.; investigation, E.K., R.H. and K.T.; data curation, E.K. and R.H.; resources, M.G. and K.W.; project administration, E.K.; software, E.K.; writing—original draft preparation, E.K., K.K.-C. and J.O.; writing—review and editing, E.K., K.K.-C., J.O., K.T., R.H., K.W. and M.G.; visualization, E.K. and M.G.; supervision, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received financial support from Medical University of Gdańsk (Young Scientist Funding: 01-50022/0003381/01/643/504 and Statutory Research Funding 01-50023/0004927/01/504/504/0/2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Knez, E.; Kadac-Czapska, K.; Dmochowska-Ślęzak, K.; Grembecka, M. Root Vegetables—Composition, Health Effects, and Contaminants. Int. J. Environ. Res. Public Health 2022, 19, 15531. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Wilches-Pérez, D.; Hallmann, E.; Kazimierczak, R.; Rembiałkowska, E. Organic versus Conventional Beetroot. Bioactive Compounds and Antioxidant Properties. LWT 2019, 116, 108552. [Google Scholar] [CrossRef]
- Boadi, N.O.; Badu, M.; Kortei, N.K.; Saah, S.A.; Annor, B.; Mensah, M.B.; Okyere, H.; Fiebor, A. Nutritional Composition and Antioxidant Properties of Three Varieties of Carrot (Daucus carota). Sci. Afr. 2021, 12, 00801. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Effect of Fermentation on the Nutritional Quality of the Selected Vegetables and Legumes and Their Health Effects. Life 2023, 13, 655. [Google Scholar] [CrossRef]
- McMurtrie, E.K.; Johanningsmeier, S.D. Quality of Cucumbers Commercially Fermented in Calcium Chloride Brine without Sodium Salts. J. Food Qual. 2018, 2018, 8051435. [Google Scholar] [CrossRef]
- Peñas, E.; Pihlava, J.M.; Vidal-Valverde, C.; Frias, J. Influence of Fermentation Conditions of Brassica oleracea L. Var. Capitata on the Volatile Glucosinolate Hydrolysis Compounds of Sauerkrauts. LWT-Food Sci. Tech. 2012, 48, 16–23. [Google Scholar] [CrossRef]
- Jung, S.J.; Kim, M.J.; Chae, S.W. Quality and Functional Characteristics of Kimchi Made with Organically Cultivated Young Chinese Cabbage (Olgari-Baechu). J. Ethnic Foods 2016, 3, 150–158. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, N.K.; Paik, H.D. Health Benefits of Lactic Acid Bacteria Isolated from Kimchi, with Respect to Immunomodulatory Effects. Food Sci. Biotechnol. 2015, 24, 783–789. [Google Scholar] [CrossRef]
- Park, K.Y.; Jeong, J.K.; Lee, Y.E.; Daily, J.W. Health Benefits of Kimchi (Korean Fermented Vegetables) as a Probiotic Food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef]
- Tamang, J.P.; Lama, S. Probiotic Properties of Yeasts in Traditional Fermented Foods and Beverages. J. Appl. Microbiol. 2022, 132, 3533–3542. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on Fermented Foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hao, L.; Jin, Y.; Huang, J.; Zhou, R.; Wu, C. Functional Roles and Engineering Strategies to Improve the Industrial Functionalities of Lactic Acid Bacteria during Food Fermentation. Biotechnol. Adv. 2024, 74, 108397. [Google Scholar] [CrossRef] [PubMed]
- Jena, R.; Choudhury, P.K. Lactic Acid Bacteria in Fermented Dairy Foods: Gamma-Aminobutyric Acid (GABA) Production and Its Therapeutic Implications. Food Biosci. 2024, 62, 105276. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Mahmoud, A.R.; Abdelrahman, T.M.; Mustafa, M.A.; Atta, O.M.; Abdelmegiud, M.H.; Al-Asmari, F. Biodegradation of Chemical Contamination by Lactic Acid Bacteria: A Biological Tool for Food Safety. Food Chem. 2024, 460, 140732. [Google Scholar] [CrossRef]
- Schoustra, S.; van der Zon, C.; Groenenboom, A.; Moonga, H.B.; Shindano, J.; Smid, E.J.; Hazeleger, W. Microbiological Safety of Traditionally Processed Fermented Foods Based on Raw Milk, the Case of Mabisi from Zambia. LWT 2022, 171, 113997. [Google Scholar] [CrossRef]
- Byakika, S.; Mukisa, I.M.; Byaruhanga, Y.B.; Male, D.; Muyanja, C. Influence of Food Safety Knowledge, Attitudes and Practices of Processors on Microbiological Quality of Commercially Produced Traditional Fermented Cereal Beverages, a Case of Obushera in Kampala. Food Control 2019, 100, 212–219. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Xu, X.; Mu, X.; Fu, B.; Xu, J.; Ye, S.; Du, M. The Relationships between Flavor Substances and Microbial Communities during the Fermentation of Chinese Traditional Red Sour Soup. Food Biosci. 2024, 62, 105451. [Google Scholar] [CrossRef]
- Baralić, K.; Živančević, K.; Bozic, D.; Đukić-Ćosić, D. Probiotic Cultures as a Potential Protective Strategy against the Toxicity of Environmentally Relevant Chemicals: State-of-the-Art Knowledge. Food. Chem. Toxicol. 2023, 172, 113582. [Google Scholar] [CrossRef] [PubMed]
- Podsedek, A. Natural Antioxidants and Antioxidant Capacity of Brassica Vegetables: A Review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Cömert, E.D.; Gökmen, V. Evolution of Food Antioxidants as a Core Topic of Food Science for a Century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef]
- Ding, C.; Zhu, Y.; Huo, Z.; Yang, S.; Zhou, Y.; Yiming, A.; Chen, W.; Liu, S.; Qian, K.; Huang, L. Pt/NiFe-LDH Hybrids for Quantification and Qualification of Polyphenols. Mater. Today Bio. 2024, 26, 101047. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shu, W.; Zhao, L.; Wan, J. Advanced Mass Spectrometric and Spectroscopic Methods Coupled with Machine Learning for in Vitro Diagnosis. View 2023, 4, 20220038. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Lester, G.E.; Lewers, K.S.; Medina, M.B.; Saftner, R.A. Comparative Analysis of Strawberry Total Phenolics via Fast Blue BB vs. Folin–Ciocalteu: Assay Interference by Ascorbic Acid. J. Food Compos. Anal. 2012, 27, 102–107. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Güçlü, K.; Altun, M.; Özyürek, M.; Karademir, S.E.; Apak, R. Antioxidant Capacity of Fresh, Sun- and Sulphited-Dried Malatya Apricot (Prunus armeniaca) Assayed by CUPRAC, ABTS/TEAC and Folin Methods. Int. J. Food Sci. Technol. 2006, 41, 76–85. [Google Scholar] [CrossRef]
- Csicsor, A.; Tombácz, E.; Kulcsár, P. Antioxidant Potential of Humic Substances Measured by Folin-Ciocalteu, CUPRAC, QUENCHER-CUPRAC and ESR Methods. J. Mol. Liq. 2023, 391, 123294. [Google Scholar] [CrossRef]
- Brzezińska-Rojek, J.; Sagatovych, S.; Malinowska, P.; Gadaj, K.; Prokopowicz, M.; Grembecka, M. Antioxidant Capacity, Nitrite and Nitrate Content in Beetroot-Based Dietary Supplements. Foods 2023, 12, 1017. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Reprint of “Hurdles and Pitfalls in Measuring Antioxidant Efficacy: A Critical Evaluation of ABTS, DPPH, and ORAC Assays”. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- de Menezes, B.B.; Frescura, L.M.; Duarte, R.; Villetti, M.A.; da Rosa, M.B. A Critical Examination of the DPPH Method: Mistakes and Inconsistencies in Stoichiometry and IC50 Determination by UV–Vis Spectroscopy. Anal. Chim. Acta 2021, 1157, 338398. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H. Bin Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Mizgier, P.; Kucharska, A.Z.; Sokół-Łetowska, A.; Kolniak-Ostek, J.; Kidoń, M.; Fecka, I. Characterization of Phenolic Compounds and Antioxidant and Anti-Inflammatory Properties of Red Cabbage and Purple Carrot Extracts. J. Funct. Foods 2016, 21, 133–146. [Google Scholar] [CrossRef]
- Sharma, M.; Kaushik, P. Vegetable Phytochemicals: An Update on Extraction and Analysis Techniques. Biocatal. Agric. Biotechnol. 2021, 36, 102149. [Google Scholar] [CrossRef]
- Ravichandran, K.; Ahmed, A.R.; Knorr, D.; Smetanska, I. The Effect of Different Processing Methods on Phenolic Acid Content and Antioxidant Activity of Red Beet. Food Res. Int. 2012, 48, 16–20. [Google Scholar] [CrossRef]
- Li, L.; Zhao, H.; Ni, N.; Wang, Y.; Gao, J.; Gao, Q.; Zhang, Y.; Zhang, Y. Study on the Origin of Linear Deviation with the Beer-Lambert Law in Absorption Spectroscopy by Measuring Sulfur Dioxide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 275, 121192. [Google Scholar] [CrossRef]
- Casasanta, G.; Falcini, F.; Garra, R. Beer–Lambert Law in Photochemistry: A New Approach. J. Photochem. Photobiol. A Chem. 2022, 432, 114086. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- PN-A-75052-10:1990; Przetwory Owocowe, Warzywne i Warzywno-Mięsne—Metody Badań Mikrobiologicznych—Oznaczanie Obecności i Miana Bakterii Beztlenowych Przetrwalnikujących Mezofilnych i Termofilnych. Polski Komitet Normalizacyny: Warszawa, Poland, 1990.
- PN-EN ISO 4833:2004; Mikrobiologia Żywności i Pasz—Horyzontalna Metoda Oznaczania Liczby Drobnoustrojów—Metoda Płytkowa w Temperaturze 30 Stopni C. Polski Komitet Normalizacyjny: Warszawa, Poland, 2004.
- PN-ISO 17410:2004; Mikrobiologia Żywności i Pasz—Horyzontalna Metoda Oznaczania Liczby Drobnoustrojów Psychrotrofowych. Polski Komitet Normalizacyjny: Warszawa, Poland, 2004.
- PN-A-75052-06:1990; Przetwory Owocowe, Warzywne i Warzywno-Mięsne—Metody Badań Mikrobiologicznych—Oznaczanie liczby Bakterii Tlenowych Termofilnych. Polski Komitet Normalizacyjny: Warszawa, Poland, 2004.
- PN-A-75052-04:1990; Przetwory Owocowe, Warzywne i Warzywno-Mięsne—Metody Badań Mikrobiologicznych—Sposób Pobierania i Przygotowanie Próbek do Badań Mikrobiologicznych. Polski Komitet Normalizacyjny: Warszawa, Poland, 1990.
- PN-ISO 21527-1:2009; Horyzontalna Metoda Wyznaczania Grzybów i Pleśni. Polski Komitet Normalizacyjny: Warszawa, Poland, 2009.
- PN-ISO 21527-2:2009; Horyzontalna Metoda Wyznaczania Grzybów i Pleśni. Polski Komitet Normalizacyjny: Warszawa, Poland, 2009.
- PN-A-75052-01:1990; Horyzontalna Metoda Wyznaczania Grzybów i Pleśni. Polski Komitet Normalizacyjny: Warszawa, Poland, 2009.
- PN-EN 15788:2009; Horyzontalna Metoda Wykrywania i Oznaczania Campylobacter spp. w Produktach Spożywczych. Polski Komitet Normalizacyjny: Warszawa, Poland, 2009.
- PN-EN ISO 7932:2005/A1:2020-09; Horyzontalna Metoda Oznaczania Liczby Przypuszczalnych Bacillus cereus—Metoda Liczenia Kolonii w Temperaturze 30 Stopni C. Polski Komitet Normalizacyjny: Warszawa, Poland, 2020.
- PN-ISO 15213:2005; Horyzontalna Metoda Wykrywania i Oznaczania Liczby Clostridium spp. Polski Komitet Normalizacyjny: Warszawa, Poland, 2005.
- PN-ISO 4831:1998; Horyzontalna Metoda Wykrywania i Oznaczania Liczby Bakterii z Grupy Coli. Polski Komitet Normalizacyjny: Warszawa, Poland, 1998.
- PN-ISO 7251:2006; Horyzontalna Metoda Wykrywania i Oznaczania Przypuszczalnych Bakterii Escheerichia coli w Produktach Spożywczych. Polski Komitet Normalizacyjny: Warszawa, Poland, 2006.
- PN-90 A-75052/15; Oznaczanie Obecności Bakterii Octowych w Produktach Spożywczych. Polski Komitet Normalizacyjny: Warszawa, Poland, 1990.
- PN-EN ISO 6579:2003; Horyzontalna Metoda Wykrywania, Oznaczania Liczby i Serotypowania Salmonella—Część 1: Wykrywanie Salmonella spp. Polski Komitet Normalizacyjny: Warszawa, Poland, 2003.
- Konieczka, P.; Namieśnik, J. Ocena i Kontrola Jakości Wyników Pomiarów Analitycznych, 1st ed.; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2017. [Google Scholar]
- Punia Bangar, S.; Sharma, N.; Sanwal, N.; Lorenzo, J.M.; Sahu, J.K. Bioactive Potential of Beetroot (Beta vulgaris). Food Res. Int. 2022, 158, 111556. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M.; Bacchiocca, M. Antioxidant Capacity of Vegetables, Spices and Dressings Relevant to Nutrition. Br. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, E.; Grȩda, A.; Adamus, W. Contents of Polyphenols in Fruit and Vegetables. Food Chem. 2006, 94, 135–142. [Google Scholar] [CrossRef]
- Chu, Y.F.; Sun, J.; Wu, X.; Liu, R.H. Antioxidant and Antiproliferative Activities of Common Vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.K.; Kim, D.O.; Smith, N.; Schroeder, D.; Han, J.T.; Chang, Y.L. Daily Consumption of Phenolics and Total Antioxidant Capacity from Fruit and Vegetables in the American Diet. J. Sci. Food Agric. 2005, 85, 1715–1724. [Google Scholar] [CrossRef]
- Sikora, M.; Hallmann, E.; Rembiałkowska, K. Zawartość Związków Bioaktywnych w Marchwi z Uprawy Ekologicznej i Konwencjonalnej w Kontekście Profilaktyki; Zakład Żywności Ekologiczne i Żywności Funkcjonalnej Towaroznawstwa Szkoła Główna Gospodarstwa Wiejskiego: Warszawa, Poland, 2011. [Google Scholar]
- Li, H.; Tsao, R.; Deng, Z. Factors Affecting the Antioxidant Potential and Health Benefits of Plant Foods. Can. J. Plant Sci. 2012, 92, 1101–1111. [Google Scholar] [CrossRef]
- Smoleń, S.; Sady, W. The Effect of Various Nitrogen Fertilization and Foliar Nutrition Regimes on the Concentrations of Sugars, Carotenoids and Phenolic Compounds in Carrot (Daucus carota L.). Sci. Hortic. 2009, 120, 315–324. [Google Scholar] [CrossRef]
- Radziejewska-Kubzdela, E.; Czapski, J.; Czaczyk, K.; Biegańska-Marecik, R. The Effect of Pre-Treatment and Modified Atmosphere Packaging on Contents of Phenolic Compounds and Sensory and Microbiological Quality of Shredded Celeriac. J. Sci. Food Agric. 2014, 94, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S. Valorization of Phenolic Compounds Delivery through Lactic Acid Fermentation of Fruit and Vegetable Juices: Innovations, Challenges, and Gaps. Adv. Phenol. Acids Drug Disc. 2024, 2024, 435–460. [Google Scholar] [CrossRef]
- Ye, J.H.; Huang, L.Y.; Terefe, N.S.; Augustin, M.A. Fermentation-Based Biotransformation of Glucosinolates, Phenolics and Sugars in Retorted Broccoli Puree by Lactic Acid Bacteria. Food Chem. 2019, 286, 616–623. [Google Scholar] [CrossRef]
- Liang, Z.; Huang, Y.; Zhang, P.; Fang, Z. Impact of Fermentation on the Structure and Antioxidant Activity of Selective Phenolic Compounds. Food Biosci. 2023, 56, 103147. [Google Scholar] [CrossRef]
- Tanguler, H.; Cankaya, A.; Agcam, E.; Uslu, H. Effect of Temperature and Production Method on Some Quality Parameters of Fermented Carrot Juice (Shalgam). Food Biosci. 2021, 41, 100973. [Google Scholar] [CrossRef]
- Agirman, B.; Settanni, L.; Erten, H. Effect of Different Mineral Salt Mixtures and Dough Extraction Procedure on the Physical, Chemical and Microbiological Composition of Şalgam: A Black Carrot Fermented Beverage. Food Chem. 2021, 344, 128618. [Google Scholar] [CrossRef]
- Williamson, G.; Holst, B. Dietary Reference Intake (DRI) Value for Dietary Polyphenols: Are We Heading in the Right Direction? Br. J. Nutr. 2008, 99, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Gamba, M.; Asllanaj, E.; Raguindin, P.F.; Glisic, M.; Franco, O.H.; Minder, B.; Bussler, W.; Metzger, B.; Kern, H.; Muka, T. Nutritional and Phytochemical Characterization of Radish (Raphanus sativus): A Systematic Review. Trends Food Sci. Technol. 2021, 113, 205–218. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J.; Lowe, G.M. Antioxidant and Prooxidant Properties of Carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef]
- Sawicki, T.; Wiczkowski, W. The Effects of Boiling and Fermentation on Betalain Profiles and Antioxidant Capacities of Red Beetroot Products. Food Chem. 2018, 259, 292–303. [Google Scholar] [CrossRef]
- Kosewski, G.; Górna, I.; Bolesławska, I.; Kowalówka, M.; Więckowska, B.; Główka, A.K.; Morawska, A.; Jakubowski, K.; Dobrzyńska, M.; Miszczuk, P.; et al. Comparison of Antioxidative Properties of Raw Vegetables and Thermally Processed Ones Using the Conventional and Sous-Vide Methods. Food Chem. 2018, 240, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Kondakçi, E.; Özyürek, M.; Güçlü, K.; Apak, R. Novel Pro-Oxidant Activity Assay for Polyphenols, Vitamins C and E Using a Modified CUPRAC Method. Talanta 2013, 115, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyûrek, M.; Gûçlû, K.; Bekdeşer, B.; Bener, M. The CUPRAC Methods of Antioxidant Measurement for Beverages. Process. Imp. Antioxidants Bev. 2014, 2014, 235–244. [Google Scholar] [CrossRef]
- FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 27 August 2023).
- Özyürek, M.; Bektaşoǧlu, B.; Güçlü, K.; Güngör, N.; Apak, R. Simultaneous Total Antioxidant Capacity Assay of Lipophilic and Hydrophilic Antioxidants in the Same Acetone–Water Solution Containing 2% Methyl-β-Cyclodextrin Using the Cupric Reducing Antioxidant Capacity (CUPRAC) Method. Anal. Chim. Acta 2008, 630, 28–39. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Liu, J.; Sui, Y.; Yu, W.; Kong, B.; Chen, Q. Improving the Bacterial Community, Flavor, and Safety Properties of Northeastern Sauerkraut by Inoculating Autochthonous Levilactobacillus Brevis. Food Chem. X 2024, 22, 101408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, D.; Zhang, C.; Niu, H.; Xin, X.; Chen, J.; Yi, H.; Liu, D. The Impact of Levilactobacillus Brevis YSJ3 and Lactiplantibacillus plantarum JLSC2-6 Co-Culture on Gamma-Aminobutyric Acid Yield, Volatile and Non-Volatile Metabolites, Antioxidant Activity, and Bacterial Community in Fermented Cauliflower Byproducts. Food Chem. 2024, 432, 137169. [Google Scholar] [CrossRef] [PubMed]
- Dehghani Champiri, I.; Bamzadeh, Z.; Rahimi, E.; Rouhi, L. Lacticaseibacillus Paracasei LB12, a Potential Probiotic Isolated from Traditional Iranian Fermented Milk (Doogh). Curr. Microbiol. 2023, 80, 333. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Dardis, C.; Garrote, G.L.; Abraham, A.G. Health-Promoting Properties of Lacticaseibacillus Paracasei: A Focus on Kefir Isolates and Exopolysaccharide-Producing Strains. Foods 2021, 10, 2239. [Google Scholar] [CrossRef]
- Duyar, S.M.; Sari, F.; Karaoglan, H.A. Production of Red Beetroot Juice by Different Methods: Kinetics of Microbial Growth, Sugar Consumption, and Acid Production. Heliyon 2024, 10, 30448. [Google Scholar] [CrossRef] [PubMed]
- Tanguler, H.; Erten, H. Occurrence and Growth of Lactic Acid Bacteria Species during the Fermentation of Shalgam (Salgam), a Traditional Turkish Fermented Beverage. LWT-Food Sci. Technol. 2012, 46, 36–41. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Zhang, M.; Ren, F.; Ren, Y.; Li, Y.; Liu, N.; Zhang, Y.; Zhang, Q.; Wang, R. Effects of Fermented Milk Containing Lacticaseibacillus Paracasei Strain Shirota on Constipation in Patients with Depression: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2238. [Google Scholar] [CrossRef]
- Commission Regulation No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. OJ L 283, 14/10/2006, p. 62–63 (EN). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02005R2073-20140601 (accessed on 10 April 2024).
- Diekman, C.M.; Cook, C.; Strawn, L.K.; Danyluk, M.D. Factors Associated with the Prevalence of Salmonella, Generic Escherichia Coli, and Coliforms in Florida’s Agricultural Soils. J. Food Prot. 2024, 87, 100265. [Google Scholar] [CrossRef]
- Oyedeji, A.B.; Green, E.; Jeff-Agboola, Y.A.; Olanbiwoninu, A.A.; Areo, E.; Martins, I.E.; El-Imam, A.M.A.; Adebo, O.A. Presence of Pathogenic Microorganisms in Fermented Foods. Indig. Fermented Foods Trop. 2023, 2023, 519–537. [Google Scholar] [CrossRef]
- Aljahani, A.H. Microbiological and Physicochemical Quality of Vegetable Pickles. J. Saudi Soc. Agric. Sci. 2020, 19, 415–421. [Google Scholar] [CrossRef]
- Rutkowski, P. Minimalnie Przetworzone Owoce i Warzywa; Instytut Ogrodnictwa: Skierniewice, Poland, 2020; ISBN 9788365903785. [Google Scholar]
- Pehlivan Karakas, F.; Sahin, G.; Ucar Turker, A.; Verma, S.K. Impacts of Heavy Metal, High Temperature, and UV Radiation Exposures on Bellis perennis L. (Common Daisy): Comparison of Phenolic Constituents and Antioxidant Potential (Enzymatic and Non-Enzymatic). S. Afr. J. Botany 2022, 147, 370–379. [Google Scholar] [CrossRef]
- Daliri, E.B.M.; Balnionytė, T.; Stankevičiūtė, J.; Lastauskienė, E.; Meskys, R.; Burokas, A. High Temperature Lacto-Fermentation Improves Antioxidant and Antidiabetic Potentials of Lithuanian Red Beetroot. LWT 2023, 185, 115122. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Gonzalez-Cavieres, L.; Trautmann-Saez, G.; Pavez-Guajardo, C.; Moreno, J. Unconventional Technologies as a Strategy to Improve the Phenolic and Antioxidant Potential of Zucchini Products Enriched with Blueberry Juice. Innov. Food Sci. Emerg. Tech. 2024, 95, 103738. [Google Scholar] [CrossRef]
- Achir, N.; Dhuique-Mayer, C.; Hadjal, T.; Madani, K.; Pain, J.P.; Dornier, M. Pasteurization of Citrus Juices with Ohmic Heating to Preserve the Carotenoid Profile. Innov. Food Sci. Emerg. Tech. 2016, 33, 397–404. [Google Scholar] [CrossRef]
- Bodoira, R.; Rossi, Y.; Montenegro, M.; Maestri, D.; Velez, A. Extraction of Antioxidant Polyphenolic Compounds from Peanut Skin Using Water-Ethanol at High Pressure and Temperature Conditions. J. Supercrit. Fluids 2017, 128, 57–65. [Google Scholar] [CrossRef]
- Mehdaoui, Y.; Yeddes, W.; Selmi, S.; Saidani-Tounsi, M.; Abdelly, C.; Ben Farhat, M. Variations of Nutritional and Antioxidant Contents of Lepidium sativum L. Sprouts as Affected by Zinc Biofortification. Sci. Hortic. 2024, 329, 112994. [Google Scholar] [CrossRef]
- Srivastava, U.; Saini, P.; Singh, A. Synergistic Enhancement of Iron, Folate, and Antioxidant Properties in Pearl Millet via RSM-Optimized Probiotic Fermentation with Lactiplantibacillus plantarum. Meas. Food 2024, 13, 100137. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Hollman, P.C.H.; Hertog, M.G.L.; Katan, M.B. Content of Potentially Anticarcinogenic Flavonoids of 28 Vegetables and 9 Fruits Commonly Consumed in The Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Alasalvar, C.; Grigor, J.M.; Zhang, D.; Quantick, P.C.; Shahidi, F. Comparison of Volatiles, Phenolics, Sugars, Antioxidant Vitamins, and Sensory Quality of Different Colored Carrot Varieties. J. Agric. Food Chem. 2001, 49, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).