Abstract
Black spot disease, caused by Alternaria alternata, results in enormous losses in broccoli production. The current measures to prevent black spot disease mainly rely on seed disinfection and chemical control, but excellent disease-resistance resources are relatively scarce. In this study, we screened primers for black spot disease identification and conducted black spot disease resistance identification of 173 lines, including 70 hybrid lines and 103 inbred lines. Based on the phenotype, we have set five grades to present different symptoms of illness: high disease resistance, disease resistance, disease tolerance, susceptibility, and high susceptibility (the disease resistance gradually weakens). According to our phenotypic evaluations, 3, 55, 65, 45, and 5 lines were classified into high disease resistance, disease resistance, disease tolerance, susceptible, and high susceptibility, respectively. By comparing the proportion of resistant lines between hybrid and inbred lines, we noticed that the frequency of hybrid varieties with high disease resistance and disease resistance (28.57%) was lower than that in inbred lines (36.89%), indicating that the resistance resources have not yet been effectively utilized in hybrid broccoli breeding. Therefore, our results identified the resistance resources to black spot disease in broccoli, which lays the foundation for the exploration of disease resistance genes as well as the analysis of disease resistance mechanisms in the future.
1. Introduction
Broccoli (Brassica oleracea L. var. italica) is a nutritious vegetable that is packed full of vitamin C, vitamin E, and glucosinolates, and is well-received by consumers. Nowadays, broccoli is widely planted around the world. China and America occupy half of the broccoli planting area in the world. In China, the planting area of broccoli has rapidly increased to 76 khm2 in recent years [1]. Because the edible part of broccoli is its tender curds, which are delicate structures and vulnerable to mechanical damage, pathogen infection can destroy the appearance of broccoli as a commodity, leading to a lot of economic losses.
The most serious fungal disease in broccoli is black spot disease caused by A. alternata, which belongs to necrotrophic fungal pathogen. There are three species of A. alternaria fungi that infect cruciferous vegetables in the nature environment, Alternaria brassicae, Alternaria brassicicola, and Alternaria raphanin [2]. A. brassicicola mainly infects cabbage, while A. brassicae and A. raphanin cause black spot disease in Chinese cabbage and radish vegetables, respectively [3]. During the process of pathogen infection, the surface of leaves, stems, pods, and curds of broccoli appears with brown and black spots, and then quickly etiolates and rots, leading to accelerated aging. Humid environments are conducive to the growth and transmission of bacteria. Therefore, black spot disease generally occurs during rainy seasons, especially concentrating on plants in unfavorable environments such as poor ventilation and insufficient light [4,5,6]. Additionally, the fungus can infest host plant seeds and be disseminated as a seed-borne fungus to next-generation plants. If this disease cannot be effectively controlled in cultivation, it will result in a severe decrease in the quality and yield of broccoli.
The process of Alternaria genus invading the host can be divided into three stages: A. Alternaria spores are dispersed and arrive on the surface of the host, pathogens and hosts recognize each other to form adherent cells that penetrate the cell wall, and finally the pathogens reproduce additional infectious particles in the host [7,8,9,10]. During the process of pathogen infection, multiple transcription factors regulate the biosynthesis of enzymes to influence the pathogenicity of A. alternata. For example, AbVf19 influences the formation of cell wall degrading enzymes, and AbSte12 regulates the production of mature spores. When the mutational damage occurs, the mycelia will not penetrate the plant epidermis, and therefore cannot infect the plants [11,12]. The mutation of Acpg1 in A. citri also suspends the synthesis of cell wall degrading enzymes to reduce its pathogenicity [13]. AbrePsy1 encodes nonribosomal peptide synthetase; its expression level is related to the pathogenicity of A. brassicae [14]. Melanin is closely related to the fungus pathogenicity; it can regulate the number of mediastina, hyphal morphology, and the cell wall integrity of spores. Cmr1 is a key transcription factor in the synthesis pathway of melanin; it regulates the contents of melanin to influence the pathogenicity [15,16,17].
In order to resist pathogen infection, plants have established a set of autoimmune mechanisms during their evolution, including systemic acquired resistance (SAR) and induced systemic resistance (ISR) [18]. SAR is often induced by harmful factors, while ISR is induced by beneficial bacteria in soil [19,20]. When pathogens attack plants and cause trauma, the plants induce SAR to ensure plants develop broad-spectrum disease resistance. ISR also has broad-spectrum resistance, depending on the interaction between plants and pathogens. Additionally, some enzymes play crucial roles in the recognition and response to plant pathogens, such as peroxidases (PRXs), mitogen-activated protein kinase (MPK), and callose synthase. These enzymes take part in the regulation pathways of signal transduction, cell wall formation, synthesis of reactive oxygen species (ROS), and hormone response [21,22,23]. MPK4 participates in the signaling pathway of SA and JA, and conversely, in mpk4 mutants, the signal transduction of JA is turned off, causing severe black spot disease in Arabidopsis [23]. PMR4 encodes glucan-type callose synthase, responsible for producing callose to prevent the invasion of pathogenic bacteria [24]. PRX33 and PRX34 negatively regulate the resistance to A. brassicicola in Arabidopsis, and thus the level of ROS and the plaques have been found to be decreased in prx33 and prx34 Arabidopsis mutants [25].
Germplasm resources contain rich genetic variations. It is effective to improve breeding abilities by using elite germplasm [26]. Many excellent alleles cloned from natural genetic populations have been widely used in modern breeding of rice, maize, and other major staple crops. The UPA2 allele in the wild ancestor of maize can reduce leaf angle, with previous reporting showed that modern hybrids (Nongda 108) with the wild UPA2 allele enhance high-density maize yields [27]. Waxy (Wx) gene encoding granule-bound starch synthase controls amylose synthesis in endosperm, and several Wx alleles have been utilized in modern rice quality improvement [28]. However, it is relatively weak in broccoli breeding programs. Black spot disease is a common disease during the growth period of broccoli, but current research on black spot disease is still limited. In order to explore excellent genetic materials for improving black spot resistance, it is also required to carry out phenotypic identification of vegetable germplasm resources. Additionally, black spot disease and black rot disease in broccoli curd are phenotypically indistinguishable in the field, because they both display black lesions. Therefore, it is necessary to develop sensitive molecular markers to identify A. alternata. To this end, we first developed a molecular marker for detecting A. alternata which infects broccoli. Then, we applied two techniques to identify the resistance to black spot disease in broccoli and screened out the optimal evaluation method. Additionally, we established the grade standards for the identification of black spot resistance in broccoli and evaluated the resistance to black spot disease in 173 broccoli germplasm resources. Furthermore, three high-resistance varieties and 55 resistance varieties were selected via phenotypic scoring, and these lines can be used to improve resistance to black spot disease in broccoli.
2. Materials and Methods
2.1. Pathogenic Strains and Cultivation Conditions
The pathogenic strain A. alternata used for inoculation isolated from broccoli was routinely grown in potato dextrose agar plates (Solarbio, P8931, Beijing, China) in a 22 °C constant-temperature incubator in the dark. Xanthomonas campestris pv. campestris, which causes black rot disease, was collected from the Institute of Plant Protection, Beijing Academy of Agriculture and Forestry Sciences. It was isolated from the leaves of broccoli and routinely grown in beef extract peptone medium in a 28 °C constant-temperature incubator in the dark.
2.2. Genomic DNA Extraction
The hypha of strains was sampled after growing on the culture medium for 10 days, and then the DNA was extracted using the CTAB method [29]. DNA concentration was checked using a NanoDrop 2000 (Thermo Scientific, Waltham, MA, USA), and DNA samples were dissolved in ddH2O and stored at −20 °C.
2.3. Primer Synthesis and PCR Detection
The primers were synthesized by Tianyi Huiyuan Biotech Company (Beijing, China), and Taq Master Mix (Vazyme, Nanjing, China) was used for PCR amplification reaction. Then, PCR amplification was conducted with denaturing at 95 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 15 s, annealing at 55 °C for 15 s, extension at 72 °C for 30 s, and final synthesis at 72 °C for 10 min. Then, the differences in PCR products were detected in 2% agarose gel.
2.4. Plant Materials and Growth Conditions
A panel of 173 broccoli accessions used in this study, collected from different regions around the world, including 73 hybrids and 103 inbred lines, was collected for identification (Supplementary Table S1). The material name was based on the field planting number, such as B1, B2, B6, etc. All accessions were planted in Sijiqing experimental farm of Beijing Vegetable Research Center in Beijing, China (39°9′ N, 116°3′ E), under normal cultivation conditions in 2023. At least five plants in the middle row were selected from each accession to test the resistance to black spot disease.
2.5. Methods for Inoculation of A. alternata in Broccoli
Alternaria sp. inoculation was performed as previously described [30]. The conidia of Alternaria sp. were dislodged into distilled water using pipette tips. The suspension was filtered by four layers of sterile gauze to remove sundries and residual culture medium. The concentration of spore suspension was adjusted to 5 × 105 spores per milliliter using a hemocytometer. To promote pathogen infection, 0.1% Tween 20 was added into the spore suspension. After filtration, the spore concentration was adjusted to 1 × 105 spores mL−1 for further use. The fresh leaves of plants were punched with four holes, and then, there were two inoculating methods used: Treat A, where 80 μL spore suspension was added to the wound of leaves and daubed evenly; Treat B, where the bacterial solution was sprayed on the leaves. Then, the leaves were placed on plastic film at the bottom of the box. To maintain sufficient humidity, all boxes containing leaf blades were supplemented with water under the plastic film. Additionally, the boxes were all enclosed with transparent polyethylene (PE) and stored indoors at 22 °C. At least five pathogen-challenged leaves from each variety were analyzed for the infection phenotype after 7-day treatments.
2.6. Evaluation of Black Spot Disease Infection Levels
The phenotypic evaluation method refers to the rating system previously described [31], but with modifications. The leaves were evaluated according to the following rating system: 0—no lesions and no host reaction observed; 1—a lesion with a diameter of 0–0.2 cm appears at the inoculation hole; 3—a lesion with a diameter of 0.2–0.5 cm appears at the inoculation hole; 5—a lesion with a diameter of 0.5–1.0 cm appears at the inoculation hole, or a yellowing area less than 0.5 cm around the lesion; 7—a lesion with a diameter of 1.0–1.5 cm appears at the inoculation hole, or an existing yellowing area spread to 0.5–1.0 cm around the lesion; 9—a lesion with a diameter of 1.5–2.0 cm appears at the inoculation hole, or a yellowing area more than 1.0 cm around the lesion. The black spot susceptibility index (BSI) was calculated following the formula BSI (%) = (∑nX/9N) × 100, where X represents different disease severity level, n represents the leaves per level, and N represents the number of total leaves evaluated for each line. We further classified different plant resistance standards depending on the BSI. High disease resistance (BSI-1), 0–11.11%; disease resistance (BSI-2), 11.12–33.33%; disease tolerance (BSI-3), 33.34–55.55%; susceptible (BSI-4), 55.56–77.77%; high susceptibility (BSI-5), 77.78–100%. BSI-1 shows the strongest resistance to black spot disease, while BSI-5 represents the weakest resistance to black spot disease.
2.7. The Primers Used in This Study
The primers used in this study were the same as previous reports [32,33,34]. S1 and S2 are suitable for identifying the ITS sequence of A. alternata. S3, S4, and S5 are suitable for X. campestris identification.
S1-F: TCCGTAGGTGAACCTGCGG; S1-R: TCCTCCGCTTATTGATATGC;
S2-F: ATGCAGTTCACCACCATCGC; S2-R: ACGAGGGTGAYGTAGGCGTC;
S3-F: CCGTAGCACTTAGTGCAATG; S3-R: GCATTTCCATCGGTCACGATTG;
S4-F: AAATCAGGGGGATGCGGTGG; S4-R: TCCGGCCAGGGTCGATACAGTG;
S5-F: ATGGTTGTCGTCAGCTCGT; S5-R: TACGGCTACCTTGTTACGACTT.
3. Results
3.1. Identification of the Pathogen of Black Spot Disease and Black Rot Disease in Broccoli
Broccoli is a popular vegetable crop all over the world due to its rich nutrients. It is important to protect broccoli from pathogen infection. Black spot disease is one of the main diseases of broccoli that often occurs in cloudy and rainy environments. In order to obtain a healthy curd, the producer will spray bactericide to prevent disease occurrence in the field and the breeder will screen germplasm resources to develop disease resistant varieties. Black spot disease and black rot disease in broccoli curd are phenotypically indistinguishable in the field, because they both display black lesions. Therefore, it is necessary to develop sensitive molecular markers to identify A. alternata. In this study, we collected X. campestris, which causes similar pathogenic phenotypes in the curd to A. alternata, and we screened molecular markers that can easily distinguish between two pathogens. Firstly, we obtained two pairs of primers for the identification of A. alternata [32,33] and three pairs of primers for the identification of X. campestris [34]. We compared the differences between the black spot pathogen and the black rot pathogen using the primers mentioned above. The results showed significant differences between Alternaria sp. and Xanthomonas sp. when amplified with S1 and S2, but no difference could be detected when using S3, S4, and S5 (Figure 1A,B and Figure S1). S1 can produce a single band in A. alternata and X. campestris, but the fragment amplified in A. alternata was shorter than that in X. campestris. As to S2, only a single band can be detected in A. alternata, and the PCR products are nonspecific in X. campestris. The results indicated that the primers can clearly distinguish the difference between A. alternata and X. campestris. It can be used for field pathogen identification in the future.
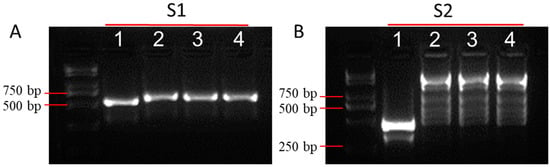
Figure 1.
PCR-analysis of A. alternata and X. campestris. (A) PCR-analysis of A. alternata and X. campestris using S1, (B) PCR-analysis of A. alternata and X. campestris using S2, where 1 represents A. alternata, and 2, 3, and 4 represent three kinds of X. campestris.
3.2. Evaluation of Different Inoculation Methods of A. alternata in Broccoli
There were two methods to evaluate the resistance of germplasm materials. Both created wounds on the leaves before infection, the difference between the two methods is the approach of applying the staining solution. Treat A is where 80 μL spore suspension was added to the wound of leaves and daubed evenly; Treat B is where the bacterial solution was sprayed on the leaves. Three varieties, B1, B2, and B51, with excellent resistance were used for testing. Based on their phenotypes after infections, we found that the leaves that were treated by Treat A showed homogeneous variations. However, the leaves treated by Treat B gave a differential performance (Figure 2). This result showed that Treat A is more suitable for disease resistance identification research.
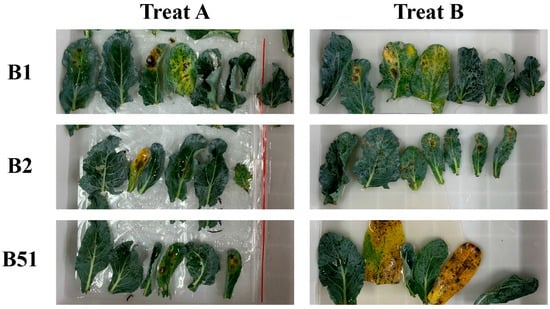
Figure 2.
The comparison of different identification methods for black spot disease in broccoli. Treat A: adding 80 μL bacterial solution to the wound and then wiping it evenly; Treat B: spraying bacterial solution on the leaves. B1, B2, and B51 were the three varieties used for testing.
3.3. Classification Criteria for Illness and Calculation of Illness Index
In order to screen excellent disease-resistant resources, at least five plants were selected from each variety to determine disease statistics. The new leaves growing from growth points were sampled and inoculated with pathogens. Based on the phenotype of the disease after infection, we determined the rating system by evaluating the spot area and leaf chlorosis area. There was a total of six levels to reflect the infection situation (see Methods). Level 0 indicates the best resistance, while level 9 indicates the weakest resistance (Figure 3). The black spot susceptibility index (BSI) for each line was calculated according to the formula previously reported [31]. In this study, we defined different resistance standards depending on the BSI (Table 1). Under pathogenic stress, varieties with different BSI values generally exhibited significantly different performances. The plants, whose BSI ranges from 0 to 11.11, have the highest disease resistance. As BSI values increase, the disease resistance of plants gradually weakens. Those susceptible plants often exhibit higher BSI values. This standard is crucial for identifying disease-resistant materials in germplasm resources.
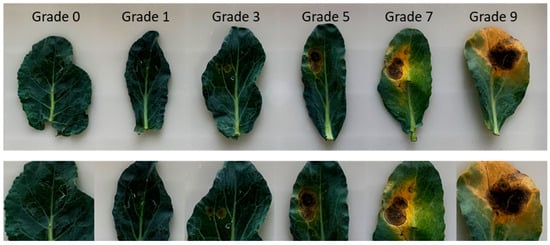
Figure 3.
The phenotypes of different disease levels. From grade 0 to grade 9, the disease susceptibility gradually increases.

Table 1.
Black spot disease grade.
3.4. Comparison of Phenotypes of Germplasm Materials before and after Inoculation
In this study, we conducted resistance identification for black spot disease and found plenty of excellent varieties, which show strong resistance to black spot disease in broccoli. Based on the standards of BSI calculation, 58 lines were resistant to black spot disease, accounting for 33.5% of total accessions evaluated. Additionally, 65 lines showed a certain level of tolerance to black spot disease, and 50 lines were found to be susceptible (Table 2; Supplementary Table S1). Meanwhile, we collected phenotype photos of all materials before and after infection and found there were significant phenotypic variations among differential resistance materials (Figure 4 and Figure 5). B230 and B238 both belong to highly resistant lines; there were no obvious changes on their leaves before and after infection. The leaves of B148 and B147 had slight spots after infection, indicating that these plants have resistance to pathogens. Compared to those varieties with disease resistance, B288 and B131 have an obvious susceptible phenotype, with several big circular spots distributed on the leaves. However, the lesions did not affect the morphology of the leaves, so we classified these lines into disease tolerance. In addition to the three categories mentioned above, there were two classes manifested as susceptible to A. alternata. The leaves of B253 and B181 had a noticeable change after pathogen treatment, with enlarged lesions and a yellow halo, reflecting weak disease resistance. Meanwhile, B346 and B314 had more severe infectious reactions and yellowing areas and the number of lesions had significantly increased. The results above showed significant differences among the five types of accessions and indicated the method is suitable for resistance identification.

Table 2.
Distribution of disease resistance types in 173 lines.
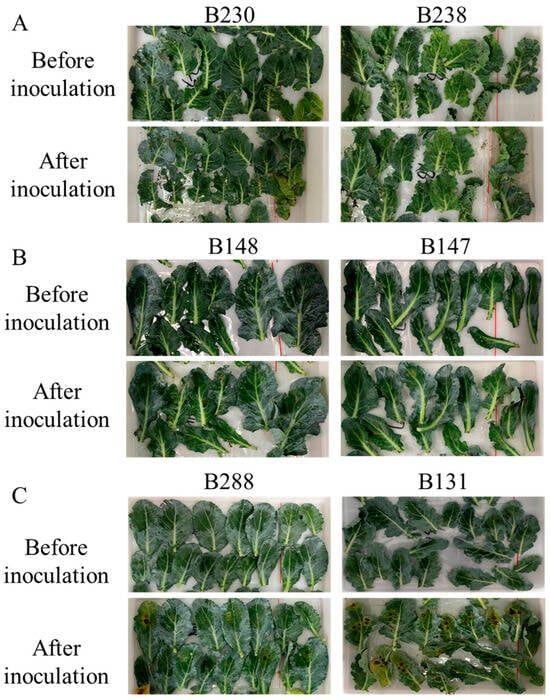
Figure 4.
Phenotypic comparison of resistant materials before and after inoculation. (A) Phenotypic changes in high disease resistance lines after infection, (B) Phenotypic changes in disease resistance lines after infection, (C) Phenotypic changes in disease tolerance lines after infection.

Figure 5.
Phenotypic comparison of susceptible materials before and after inoculation. The upper part belongs to susceptible; the lower part belongs to high susceptibility.
4. Discussion
Black spot disease caused by A. alternata has devastating effects on broccoli cultivation. Black rot disease caused by X. campestris also results in a similar phenotype that is manifested by a black spot in broccoli leaves. In the fields, it is a big challenge to distinguish the two pathogens, which to some extent restricts the disease control. Therefore, it is necessary to develop a simple identification method. With the development of molecular detection technology, researchers can study the relationships among various fungal groups at the nucleic acid level, such as via an Internally Transcribed Spacer (ITS) located among 18S, 5.8S, and 28S rRNA, which have highly contained abundant genetic variations. Given that the sequence of ITS can truly reflects the differences in different strains intra and extra species, it has been widely used for pathogen identification. Previous reports showed that RAPD (Randomly Amplified Polymorphic DNA) technology contributed to distinguishing genera and intra-genus species of Alternaria [35,36]. Microsatellite primer pairs also have been used for pathogen identification, where five pairs of SSR markers can evaluate the genetic diversity of 64 isolates [37]. In this study, to compare the difference of A. Alternaria and X. campestris, we screened five pairs of fungal and bacterial identification primers and found that two pairs of primers can distinguish the differences through agarose electrophoresis (Figure 1). Compared to previous research, the method is convenient for broccoli disease identification, and it will help to detect disease types in the early stage. Then, the experts can provide control measures to prevent a large-scale occurrence of black spot disease.
Black spot disease can cause severe leaf yellowing and a decrease in yield, whether in crops or vegetables. Therefore, there are many researchers focused on the identification of resistance to black spot disease. The researchers identified black spot resistance in Chinese kale plants by spraying spore suspension (106 spores mL–1) on leaves. Then, the plants were grown under wet conditions in the greenhouse for 7 days. Compared to the control treatment, the plants with pathogenic induction showed serious diseases with large lesion areas [6]. Tao et al. performed black spot disease identification on two months old Chinese kale and added 100 μL spore suspension (5 × 105 spores mL–1) to the leaves to investigate the phenotype after 5 days [38]. In this study, we evaluated the black spot resistance of 173 broccoli accessions, but the identification method is different from that previously reported. We collected the leaves of mature plants and added 50 μL spore suspension (5 × 105 spores mL–1) to the artificial wounds of leaves. After cultivating the leaves in a wet environment at 22 °C for 5 days, we then evaluated the infection situation of each accession. Through these means, we not only accurately identified the disease resistance of mature plants but also saved a lot of manpower and material resources. It is suitable for large-scale germplasm disease resistance identification.
The current measures to prevent black spot disease in vegetables are seed disinfection treatment, biological control, and plant antibacterial active substances. The seeds of cauliflower treated with acetone and dichloromethane can completely inhibit the spore germination of A. brassicicola [39]. The mixture of iprodione and seeds also can effectively prevent black spot disease caused by A. alternata [40]. Although chemical reagents are effective in black spot disease control, they are harmful to humans and the environment and do not comply with green production standards. Additionally, several antagonists and plant antibacterial active substances have been reported that have disease resistance effects. Gliocladium roseum and Trichoderma harzianum, which antagonists separated from the seeds of cauliflower, can inhibit spore germination [41]. Indole alkaloids extracted from Alstonia venenata and emulsion extracted from Jatropha gossypiifolia both can repress the germination and growth of A. brassicicola [42,43]. However, the effect of biological control and active substances treatment is not better than fungicides in vegetable cultivation. In the present research, we conducted the identification of disease resistance to screen excellent germplasm materials. It is the most cost-effective method for disease prevention and control.
Based on the phenotypic survey after infection in the laboratory, the 173 lines were divided into five categories. There are two inbred lines and one hybrid line showing high disease resistance with no obvious changes at the wound sites (Figure 4A), indicating they are excellent disease-resistant donor materials. Five inbred lines have been identified as highly susceptible, but no highly susceptible hybrid was screened (Table 2). The results suggest that extreme susceptibility genes have been eliminated during the breeding process. Compared to the proportion of black spot disease-resistant lines in hybrid and inbred lines, the frequency of high disease resistance and disease resistance in hybrid lines is lower than that in inbred lines (Table 2), suggesting that the utilization rate of resistance genes is still insufficient. Therefore, the situation encourages us to screen candidate genes, develop molecular markers, and assist in black spot disease resistance breeding in broccoli. Nowadays, a lot of genes have been reported to participate in the regulation of black spot resistance in horticultural crops. 4-methoxy-indole-3-methylglucosinolate (4MI3G), a specific side chain modification product in indole glucosinolates, plays an important antibacterial role. WRKY33 directly regulates the expression level of MUB51 and CYP83B1 to influence indolic glucosinolate biosynthesis, further increasing the resistance to Alternaria brassicicola [38]. CmMLO17 is one of the Mildew Resistance Locus O genes; it is strongly induced to express in Chrysanthemum morifolium after A. alternata infection. Molecular and genetic experiments showed CmMLO17 can interact with CmKIC to accelerate the growth of A. alternata. Reducing the expression of CmMLO17 can effectively improve the resistance of black spot disease in Chrysanthemum morifolium [44]. Previous reports showed that CmNPR1, encoding a transcription factor, was expressed differently between Alternaria sp.-infected leaves and mock control leaves. Overexpression of CmNPR1 can enhance the resistance to Alternaria sp. infection in Chrysanthemum [45]. At present, our results enriched the resistance resources in broccoli, but there is still a long way to obtain resistance genes. Based on the results of our study, we need to further construct a genetic mapping population to research the natural variations in resistance genes.
5. Conclusions
In the present study, we have screened the primers that can be used to identify black spot disease and black rot disease in broccoli. Furthermore, we conducted in vitro black spot disease resistance identification on broccoli leaves using two methods and evaluated the stability of the infection. Treat A has been proven to be a more precise identification method for black spot disease in broccoli. Also, 173 varieties have been used to identify the resistance to black spot disease. Based on the infection phenotype of different varieties, we set a classification criterion for black spot disease in broccoli. The criterion provides a basis for identifying resistance to black spot disease. Additionally, we have screened 58 excellent resistant lines, which nearly have no impacts after infection. These lines can be used as donor parents to improve the disease resistance in future broccoli breeding. Our results lay the foundation for future research on broccoli black spot resistance and gene mining.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14072883/s1, Figure S1: PCR-analysis of A. alternata and X. campestris using S3, S4, and S5; Table S1: The black spot disease grade of 173 lines.
Author Contributions
Q.Z. and Y.D. designed the research and performed the experiments, F.B. reviewed the experimental plan and provided technical assistance. N.L. (Ning Li) and N.L. (Ning Liu) provided technical assistance for the identification of pathogenic bacteria, Q.Z., N.L. (Ning Liu), and Y.D. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Innovation and Development Program of Beijing Vegetable Research Center (KYCX202303), the Invitation Program of Foreign Experts of Beijing Vegetable Research Center (2023-06), the National Key Research and Development Program (2022YFF1003000) of China, the Technology Innovation Capacity Building Project of Beijing Academy of Agriculture and Forestry Sciences (KJCX20230813), the Youth Foundation of Beijing Academy of Agriculture and Forestry Sciences (QNJJ202432), and the Young Talent Supporting Program of Beijing Academy of Agriculture and Forestry Sciences (YCXTD00002-10).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Z.; Mei, Y.; Liu, Y.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H. The evolution of genetic diversity of broccoli cultivars in China since 1980. Sci. Hortic. 2019, 250, 69–80. [Google Scholar] [CrossRef]
- Jasalavich, C.A.; Morales, V.M.; Pelcher, L.E.; Seguin, S.G. Comparison of nuclear ribosomal DNA sequences from Alternaria species pathogenic to crucifers. Mycol. Res. 1995, 99, 604–614. [Google Scholar] [CrossRef]
- Huang, J.; Chung, W. Characteristics of cruciferous black spot pathogens, Alternaria brassicicola and A. brassicae. Plant Pathol. Bull. 1993, 2, 141–148. [Google Scholar]
- Lin, T.C.; Fan, M.C.; Wang, S.Y.; Huang, J.W. Identification of the Solanum nigrum extract component involved in controlling cabbage black leaf spot disease. J. Agric. Food Chem. 2011, 59, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, I.; Gilardi, G.; Ortu, G.; Gisi, U.; Gullino, M.L.; Garibaldi, A. Identification and characterization of Alternaria species causing leaf spot on cabbage, cauliflower, wild and cultivated rocket by using molecular and morphological features and mycotoxin production. Eur. J. Plant Pathol. 2017, 149, 401–413. [Google Scholar] [CrossRef]
- Nujthet, Y.; Jantasorn, A.; Dethoup, T. Biological efficacy of marine-derived Trichoderma in controlling chili anthracnose and black spot disease in Chinese kale. Eur. J. Plant Pathol. 2023, 166, 369–383. [Google Scholar] [CrossRef]
- Meng, S.; Torto-Alalibo, T.; Chibucos, M.C.; Tyler, B.M.; Dean, R.A. Common processes in pathogenesis by fungal and oomycete plant pathogens, described with Gene Ontology terms. BMC Microbiol. 2009, 9, S7. [Google Scholar] [CrossRef] [PubMed]
- Dhayanithy, G.; Subban, K.; Chelliah, J. Diversity and biological activities of endophytic fungi associated with Catharanthus roseus. BMC Microbiol. 2019, 19, 22. [Google Scholar] [CrossRef]
- Igbalajobi, O.; Gao, J.; Fischer, R. The HOG Pathway Plays Different Roles in Conidia and Hyphae During Virulence of Alternaria alternata. Mol. Plant-Microbe Interact. 2020, 33, 1405–1410. [Google Scholar] [CrossRef]
- Eseola, A.B.; Ryder, L.S.; Osés-Ruiz, M.; Findlay, K.; Yan, X.; Cruz-Mireles, N.; Molinari, C.; Garduño-Rosales, M.; Talbot, N.J. Investigating the cell and developmental biology of plant infection by the rice blast fungus Magnaporthe oryzae. Fungal Genet. Biol. 2021, 154, 103562. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, K.H.; La Rota, M.; Scott, D.; Santopietro, G.; Callihan, M.; Mitchell, T.K.; Lawrence, C.B. Identification of novel virulence factors associated with signal transduction pathways in Alternaria brassicicola. Mol. Microbiol. 2009, 72, 1316–1333. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Srivastava, A.; Ohm, R.A.; Lawrence, C.B.; Wang, K.H.; Grigoriev, I.V.; Marahatta, S.P. Transcription factor Amr1 induces melanin biosynthesis and suppresses virulence in Alternaria brassicicola. PLoS Pathog. 2012, 8, e1002974. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, A.; Akimitsu, K.; Yamamoto, M.; Yamamoto, H. Endopolygalacturonase Is Essential for Citrus Black Rot Caused by Alternaria citri but Not Brown Spot Caused by Alternaria alternata. Mol. Plant-Microbe Interact. 2001, 14, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Guillemette, T.; Sellam, A.; Simoneau, P. Analysis of a nonribosomal peptide synthetase gene from Alternaria brassicae and flanking genomic sequences. Curr. Genet. 2004, 45, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Yago, J.I.; Lin, C.H.; Chung, K.R. The SLT2 mitogen-activated protein kinase-mediated signalling pathway governs conidiation, morphogenesis, fungal virulence and production of toxin and melanin in the tangerine pathotype of Alternaria alternata. Mol. Plant Pathol. 2011, 12, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Kheder, A.A.; Akagi, Y.; Akamatsu, H.; Yanaga, K.; Maekawa, N.; Otani, H.; Tsuge, T.; Kodama, M. Functional analysis of the melanin biosynthesis genes ALM1 and BRM2-1 in the tomato pathotype of Alternaria alternata. J. Gen. Plant Pathol. 2011, 78, 30–38. [Google Scholar] [CrossRef]
- Kurahashi, A.; Shimoda, T.; Sato, M.; Fujimori, F.; Hirama, J.; Nishibori, K. A putative transcription factor Gf.BMR1 in Grifola frondosa, the homolog of BMR1 in Bipolaris oryzae, was strongly induced by near-ultraviolet light and blue light. Mycoscience 2015, 56, 177–182. [Google Scholar] [CrossRef][Green Version]
- Pieterse, C.M.; Van Loon, L.C. NPR1: The spider in the web of induced resistance signaling pathways. Curr. Opin. Plant Biol. 2004, 7, 456–464. [Google Scholar] [CrossRef]
- Park, D.S.; Sayler, R.J.; Hong, Y.G.; Nam, M.H.; Yang, Y.; Zeng, Y.; Dong, J.; Ji, Z.; Liang, Y.; Yang, C.; et al. A Method for Inoculation and Evaluation of Rice Sheath Blight Disease. Plant Dis. 2008, 92, 25–29. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, M.; Chhajed, S.; Yu, F.; Chen, S.; Zhang, Y.; Mou, Z. N-hydroxypipecolic acid triggers systemic acquired resistance through extracellular NAD (P). Nat. Commun. 2023, 14, 6848. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Chandrashekar, N.; Rawat, S.; Nayanakantha, N.M.C.; Mir, Z.A.; Manoharan, A.; Sultana, M.; Grover, A. Isolation and molecular characterization of pathogenesis related PR2 gene and its promoter from Brassica juncea. Biol. Plant. 2017, 61, 763–773. [Google Scholar] [CrossRef]
- Brodersen, P.; Petersen, M.; Bjørn Nielsen, H.; Zhu, S.; Newman, M.A.; Shokat, K.M.; Rietz, S.; Parker, J.; Mundy, J. Arabidopsis MAP kinase 4 regulates salicylic acid- and jasmonic acid/ethylene-dependent responses via EDS1 and PAD4. Plant J. 2006, 47, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Flors, V.; Ton, J.; Doorn, R.; Jakab, G.; García-Agustín, P.; Mauch-Mani, B. Interplay between JA, SA and ABA signalling during basal and induced resistance against Pseudomonas syringae and Alternaria brassicicola. Plant J. 2008, 54, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Kaman-Toth, E.; Danko, T.; Gullner, G.; Bozso, Z.; Palkovics, L.; Pogany, M. Contribution of cell wall peroxidase- and NADPH oxidase-derived reactive oxygen species to Alternaria brassicicola-induced oxidative burst in Arabidopsis. Mol. Plant Pathol. 2019, 20, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xie, J.; Zhu, X.; Ma, X.; Yang, T.; Khan, N.U.; Zhang, S.; Liu, M.; Li, L.; Liang, Y.; et al. Natural variation in Tiller Number 1 affects its interaction with TIF1 to regulate tillering in rice. Plant Biotechnol. J. 2023, 21, 1044–1057. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wang, C.; Xia, J.; Wu, L.; Xu, G.; Wu, W.; Li, D.; Qin, W.; Han, X.; Chen, Q.; et al. Teosinte ligule allele narrows plant architecture and enhances high-density maize yields. Science 2019, 365, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, Q.; Zhang, Q.; Chu, R.; Gu, Z.; Tan, H.; Zhao, D.; Fan, X.; Liu, Q. Creating novel Wx alleles with fine-tuned amylose levels and improved grain quality in rice by promoter editing using CRISPR/Cas9 system. Plant Biotechnol. J. 2020, 18, 2164–2166. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhao, Y.; Liu, Y.; Han, F.; Liu, W.; Li, Z. Genetic Diversity and DNA Fingerprinting in Broccoli Carrying Multiple Clubroot Resistance Genes Based on SSR Markers. Appl. Sci. 2022, 12, 4754. [Google Scholar] [CrossRef]
- Thomma, B.P.; Eggermont, K.; Penninckx, I.A.; Mauch-Mani, B.; Vogelsang, R.; Cammue, B.P.; Broekaert, W.F. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 1999, 95, 15107–15111. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, F.; Yu, R.; Zou, Y.; Qi, J.; Zhao, X.; Yu, Y.; Zhang, D.; Li, L. Genetic mapping and localization of a major QTL for seedling resistance to downy mildew in Chinese cabbage (Brassica rapa ssp. pekinensis). Mol. Breed. 2009, 23, 573–590. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Hong, S.G.; Cramer, R.A.; Lawrence, C.B.; Pryor, B.M. Alt a 1 allergen homologs from Alternaria and related taxa: Analysis of phylogenetic content and secondary structure. Fungal Genet. Biol. 2005, 42, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Burlakoti, R.R.; Chen, J.R.; Hsu, C.F.; Burlakoti, P.; Kenyon, L. Molecular characterization, comparison of screening methods, and evaluation of cross-pathogenicity of black rot (Xanthomonas campestris pv. campestris) strains from cabbage, choy sum, leafy mustard and pakchoi from Taiwan. Plant Pathol. 2018, 67, 1589–1600. [Google Scholar]
- Sharma, T.R.; Tewari, T.R. RAPD analysis of three Alternaria species pathogenic to crucifers. Mycol. Res. 1998, 102, 807–814. [Google Scholar] [CrossRef]
- Cooke, D.E.L.; Forster, J.W.; Jenkins, P.D.; Jones, D.G.; Lewis, D.M. Analysis of intraspecific and interspecific variation in the genus Alternaria by the use of RAPD-PCR. Ann. Appl. Biol. 2008, 132, 197–209. [Google Scholar] [CrossRef]
- Tran-Dinh, N.A.I.; Hocking, A. Isolation and characterization of polymorphic microsatellite markers for Alternaria alternata. Mol. Ecol. Notes 2006, 6, 405–407. [Google Scholar] [CrossRef]
- Tao, H.; Miao, H.; Chen, L.; Wang, M.; Xia, C.; Zeng, W.; Sun, B.; Zhang, F.; Zhang, S.; Li, C.; et al. WRKY33-mediated indolic glucosinolate metabolic pathway confers resistance against Alternaria brassicicola in Arabidopsis and Brassica crops. J. Integr. Plant Biol. 2022, 64, 1007–1019. [Google Scholar] [CrossRef]
- Otani, H.; Kohnobe, A.; Kodama, M.; Kohmoto, K. Production of a host-specific toxin by germinating spores of Alternaria brassicicola. Physiol. Mol. Plant Pathol. 1998, 52, 285–295. [Google Scholar] [CrossRef]
- Oka, K.; Akamatsu, H.; Kodama, M.; Nakajima, H.; Kawada, T.; Otani, H. Host-specific AB-toxin production by germinating spores of Alternaria brassicicola is induced by a gost-derived oligosaccharide. Physiol. Mol. Plant Pathol. 2005, 66, 12–19. [Google Scholar] [CrossRef]
- Pochon, S.; Simoneau, P.; Pigne, S.; Balidas, S.; Bataille-Simoneau, N.; Campion, C.; Jaspard, E.; Calmes, B.; Hamon, B.; Berruyer, R.; et al. Dehydrin-like proteins in the necrotrophic fungus Alternaria brassicicola have a role in plant pathogenesis and stress response. PLoS ONE 2013, 8, e75143. [Google Scholar] [CrossRef]
- Singh, U.P.; Sarma, B.K.; Mishra, P.K.; Ray, A.B. Antifungal activity of venenatine. an indole alkaloid isolated from Alstonia venenata. Folia Microbiol. 2000, 45, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Soledade, M.; Pedras, C.; Smith, K.C. Sinalexin, a phytoalexin from white mustard elicited by destruxin B and Alternaria brassicae. Phytochemistry 1997, 46, 833–837. [Google Scholar] [CrossRef]
- Xin, J.; Liu, Y.; Li, H.; Chen, S.; Jiang, J.; Song, A.; Fang, W.; Chen, F. CmMLO17 and its partner CmKIC potentially support Alternaria alternata growth in Chrysanthemum morifolium. Hortic. Res. 2021, 8, 101. [Google Scholar] [CrossRef]
- Zhao, X.; Song, L.; Jiang, L.; Zhu, Y.; Gao, Q.; Wang, D.; Xie, J.; Lv, M.; Liu, P.; Li, M. The integration of transcriptomic and transgenic analyses reveals the involvement of the SA response pathway in the defense of chrysanthemum against the necrotrophic fungus Alternaria sp. Hortic. Res. 2020, 7, 80. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).