Radiomic-Based Biomarkers for the Evaluation of Prosthetic Heart Valve Infective Endocarditis in Non-Attenuation Correction [18F]FDG PET/CT Images
Abstract
1. Introduction
2. Materials and Methods
2.1. Image Database
2.2. Image Acquisition
2.3. Image Processing and Feature Extraction
2.4. Statistical Analysis
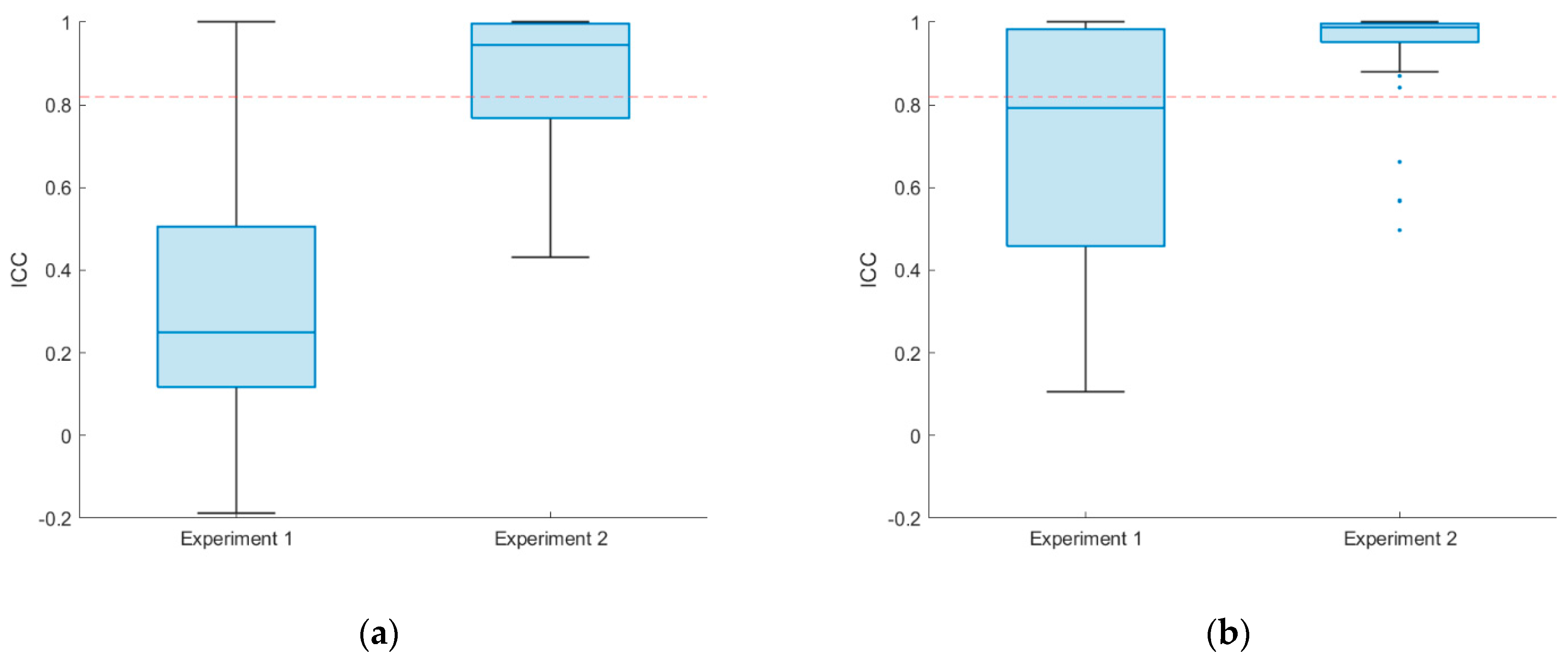
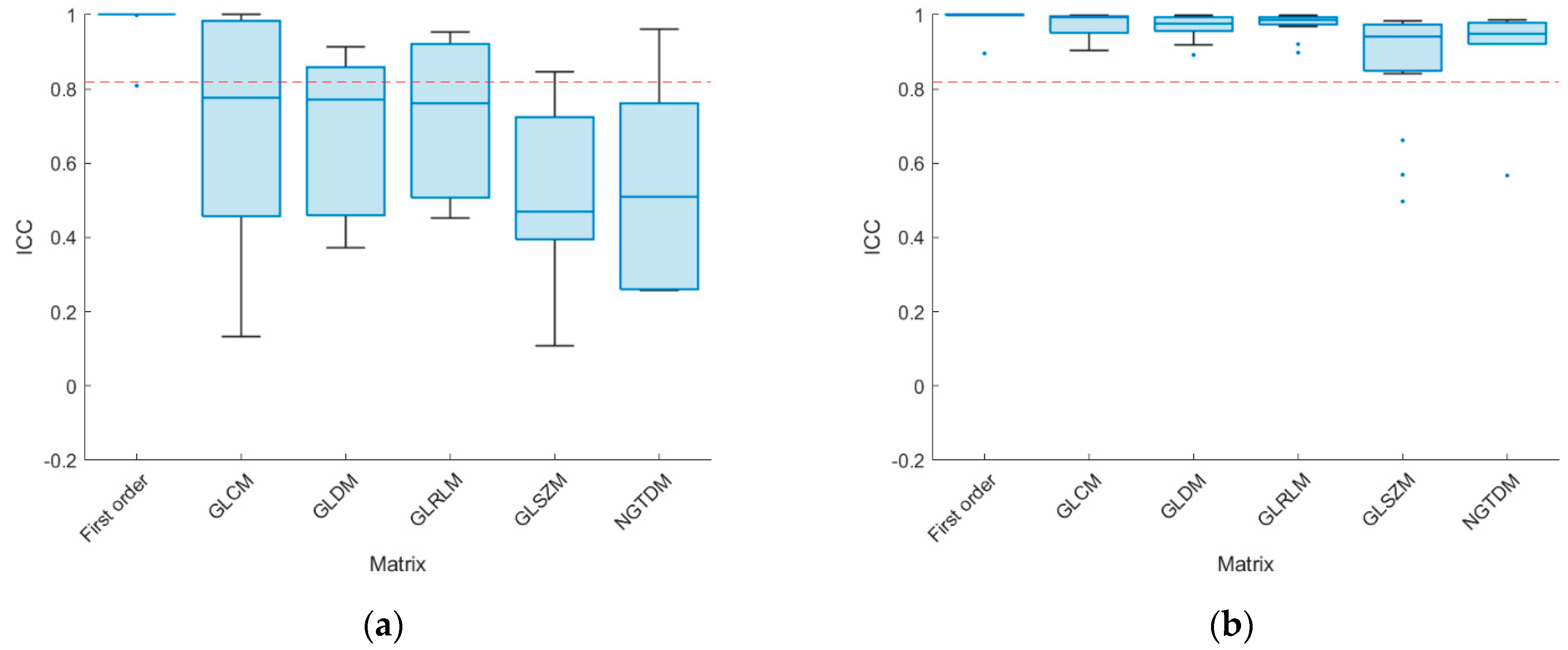
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holland, T.L.; Baddour, L.M.; Bayer, A.S.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr. Infective endocarditis. Nat. Rev. Dis. Primers 2016, 2, 16059. [Google Scholar] [CrossRef]
- Tanis, W.; Scholtens, A.; Habets, J.; Brink, R.B.v.D.; van Herwerden, L.A.; Chamuleau, S.A.; Budde, R.P. Positron Emission Tomography/Computed Tomography for Diagnosis of Prosthetic Valve Endocarditis. J. Am. Coll. Cardiol. 2014, 63, 186–187. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Habib, G.; Badano, L.; Tribouilloy, C.; Vilacosta, I.; Zamorano, J.L.; Galderisi, M.; Voigt, J.-U.; Sicari, R.; Cosyns, B.; Fox, K.; et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur. J. Echocardiogr. 2010, 11, 202–219. [Google Scholar] [CrossRef]
- Habib, G.; Derumeaux, G.; Avierinos, J.-F.; Casalta, J.-P.; Jamal, F.; Volot, F.; Garcia, M.; Lefevre, J.; Biou, F.; Maximovitch-Rodaminoff, A.; et al. Value and limitations of the duke criteria for the diagnosis of infective endocarditis. J. Am. Coll. Cardiol. 1999, 33, 2023–2029. [Google Scholar] [CrossRef]
- Raoult, D.; Casalta, J.P.; Richet, H.; Khan, M.; Bernit, E.; Rovery, C.; Branger, S.; Gouriet, F.; Imbert, G.; Bothello, E.; et al. Contribution of Systematic Serological Testing in Diagnosis of Infective Endocarditis. J. Clin. Microbiol. 2005, 43, 5238–5242. [Google Scholar] [CrossRef]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef]
- Ferro, P.; Boni, R.; Slart, R.H.; Erba, P.A. Imaging of Endocarditis and Cardiac Device-Related Infections: An Update. Semin. Nucl. Med. 2023, 53, 184–198. [Google Scholar] [CrossRef]
- Mahmood, M.; Saleh, O.A. The Role of 18-F FDG PET/CT in Imaging of Endocarditis and Cardiac Device Infections. Semin. Nucl. Med. 2020, 50, 319–330. [Google Scholar] [CrossRef]
- Nuvoli, S.; Fiore, V.; Babudieri, S.; Galassi, S.; Bagella, P.; Solinas, P.; Spanu, A.; Madeddu, G. The additional role of 18F-FDG PET/CT in prosthetic valve endocarditis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1744–1751. [Google Scholar] [CrossRef]
- Roque, A.; Pizzi, M.N.; Fernández-Hidalgo, N.; Permanyer, E.; Cuellar-Calabria, H.; Romero-Farina, G.; Ríos, R.; Almirante, B.; Castell-Conesa, J.; Escobar, M.; et al. Morpho-metabolic post-surgical patterns of non-infected prosthetic heart valves by [18F]FDG PET/CTA: “normality” is a possible diagnosis. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 24–33. [Google Scholar] [CrossRef]
- Bartoletti, M.; Tumietto, F.; Fasulo, G.; Giannella, M.; Cristini, F.; Bonfiglioli, R.; Raumer, L.; Nanni, C.; Sanfilippo, S.; Di Eusanio, M.; et al. Combined computed tomography and fluorodeoxyglucose positron emission tomography in the diagnosis of prosthetic valve endocarditis: A case series. BMC Res. Notes 2014, 7, 32–36. [Google Scholar] [CrossRef]
- Spacek, M.; Belohlavek, O.; Votrubova, J.; Sebesta, P.; Stadler, P. Diagnostics of “non-acute” vascular prosthesis infection using 18F-FDG PET/CT: Our experience with 96 prostheses. Eur. J. Nucl. Med. 2009, 36, 850–858. [Google Scholar] [CrossRef]
- Kamani, C.H.; Allenbach, G.; Jreige, M.; Pavon, A.G.; Meyer, M.; Testart, N.; Firsova, M.; Vieira, V.F.; Boughdad, S.; Lalonde, M.N.; et al. Diagnostic Performance of 18F-FDG PET/CT in Native Valve Endocarditis: Systematic Review and Bivariate Meta-Analysis. Diagnostics 2020, 10, 754. [Google Scholar] [CrossRef]
- Lamas, C.C.; Fournier, P.-E.; Zappa, M.; Brandão, T.J.D.; Januário-Da-Silva, C.A.; Correia, M.G.; Barbosa, G.I.F.; Golebiovski, W.F.; Weksler, C.; Lepidi, H.; et al. Diagnosis of blood culture-negative endocarditis and clinical comparison between blood culture-negative and blood culture-positive cases. Infection 2016, 44, 459–466. [Google Scholar] [CrossRef]
- Prendergast, B.D. Diagnostic criteria and problems in infective endocarditis. Heart 2004, 90, 611–613. [Google Scholar] [CrossRef]
- Mahmood, M.; Kendi, A.T.; Ajmal, S.; Farid, S.; O’horo, J.C.; Chareonthaitawee, P.; Baddour, L.M.; Sohail, M.R. Meta-analysis of 18F-FDG PET/CT in the diagnosis of infective endocarditis. J. Nucl. Cardiol. 2019, 26, 922–935. [Google Scholar] [CrossRef]
- Abikhzer, G.; Martineau, P.; Grégoire, J.; Finnerty, V.; Harel, F.; Pelletier-Galarneau, M. [18F]FDG-PET CT for the evaluation of native valve endocarditis. J. Nucl. Cardiol. 2020, 29, 158–165. [Google Scholar] [CrossRef]
- Granados, U.; Fuster, D.; Pericas, J.M.; Llopis, J.L.; Ninot, S.; Quintana, E.; Almela, M.; Paré, C.; Tolosana, J.M.; Falces, C.; et al. Diagnostic Accuracy of 18F-FDG PET/CT in Infective Endocarditis and Implantable Cardiac Electronic Device Infection: A Cross-Sectional Study. J. Nucl. Med. 2016, 57, 1726–1732. [Google Scholar] [CrossRef]
- Yeh, C.; Liou, J.; Chen, S.; Chen, Y. Infective endocarditis detected by 18F-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography in a patient with occult infection. Kaohsiung J. Med. Sci. 2011, 27, 528–531. [Google Scholar] [CrossRef]
- Aghayev, A. Utilization of FDG-PET/CT in the diagnosis of native valve endocarditis: There is a hope, but we need more data! J. Nucl. Cardiol. 2020, 29, 3455–3457. [Google Scholar] [CrossRef]
- Fukuchi, K.; Ishida, Y.; Higashi, M.; Tsunekawa, T.; Ogino, H.; Minatoya, K.; Kiso, K.; Naito, H. Detection of aortic graft infection by fluorodeoxyglucose positron emission tomography: Comparison with computed tomographic findings. J. Vasc. Surg. 2005, 42, 919–925. [Google Scholar] [CrossRef]
- Dilsizian, V.; Chandrashekhar, Y. Distinguishing Active Vasculitis from Sterile Inflammation and Graft Infection. JACC Cardiovasc. Imaging 2017, 10, 1085–1087. [Google Scholar] [CrossRef]
- Pizzi, M.N.; Roque, A.; Fernández-Hidalgo, N.; Cuéllar-Calabria, H.; Ferreira-González, I.; Gonzàlez-Alujas, M.T.; Oristrell, G.; Gracia-Sánchez, L.; González, J.J.; Rodríguez-Palomares, J.; et al. Improving the Diagnosis of Infective Endocarditis in Prosthetic Valves and Intracardiac Devices with 18F-Fluordeoxyglucose Positron Emission Tomography/Computed Tomography Angiography. Circulation 2015, 132, 1113–1126. [Google Scholar] [CrossRef]
- Palomino-Fernández, D.; Gómez-Grande, A.; Fernández-Igarza, M.; Pilkington, P.; Seiffert, A.P.; Bueno, H.; Gómez, E.J.; Sánchez-González, P. CASSIA (cardiology software suite for image analysis): A potential new tool for the evaluation of [18F]FDG PET/CT in the setting of infective endocarditis. Int. J. Comput. Assist. Radiol. Surg. 2022, 18, 157–169. [Google Scholar] [CrossRef]
- Scholtens, A.M.; Swart, L.E.; Kolste, H.J.T.; Budde, R.P.J.; Lam, M.G.E.H.; Verberne, H.J. Standardized uptake values in FDG PET/CT for prosthetic heart valve endocarditis: A call for standardization. J. Nucl. Cardiol. 2018, 25, 2084–2091. [Google Scholar] [CrossRef]
- Roy, S.G.; Akhtar, T.; Bandyopadhyay, D.; Ghosh, R.K.; Hagau, R.; Ranjan, P.; Gerard, P.; Jain, D. The Emerging Role of FDG PET/CT in Diagnosing Endocarditis and Cardiac Device Infection. Curr. Probl. Cardiol. 2023, 48, 101510. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, C.; Niu, Y.; Yuan, R.; Zeng, X.; Ge, X.; Yang, Y.; Peng, X. The role of 18F-FDG PET/CT in infectious endocarditis: A systematic review and meta-analysis. Int. J. Clin. Pharmacol. Ther. 2016, 54, 337–342. [Google Scholar] [CrossRef]
- Hove, D.T.; Slart, R.; Sinha, B.; Glaudemans, A.; Budde, R. 18F-FDG PET/CT in Infective Endocarditis: Indications and Approaches for Standardization. Curr. Cardiol. Rep. 2021, 23, 130. [Google Scholar] [CrossRef]
- Guiot, J.; Vaidyanathan, A.; Deprez, L.; Zerka, F.; Danthine, D.; Frix, A.N.; Lambin, P.; Bottari, F.; Tsoutzidis, N.; Miraglio, B.; et al. A review in radiomics: Making personalized medicine a reality via routine imaging. Med. Res. Rev. 2022, 42, 426–440. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; De Jong, E.E.C.; Van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Rogers, W.; Seetha, S.T.; Refaee, T.A.G.; Lieverse, R.I.Y.; Granzier, R.W.Y.; Ibrahim, A.; Keek, S.A.; Sanduleanu, S.; Primakov, S.P.; Beuque, M.P.L.; et al. Radiomics: From qualitative to quantitative imaging. Br. J. Radiol. 2020, 93, 1108. [Google Scholar] [CrossRef]
- Currie, G.; Hawk, K.E.; Rohren, E.; Vial, A.; Klein, R. Machine Learning and Deep Learning in Medical Imaging: Intelligent Imaging. J. Med. Imaging Radiat. Sci. 2019, 50, 477–487. [Google Scholar] [CrossRef]
- Erba, P.; Sollini, M.; Zanca, R.; Cavinato, L.; Ragni, A.; Hove, D.T.; Glaudemans, A.; Pizzi, M.; Roque, A.; Ieva, F.; et al. [18F]FDG-PET/CT radiomics in patients suspected of infective endocarditis. Eur. Heart J. Cardiovasc. Imaging 2022, 23, jeab289.443. [Google Scholar] [CrossRef]
- Godefroy, T.; Frécon, G.; Asquier-Khati, A.; Mateus, D.; Lecomte, R.; Rizkallah, M.; Piriou, N.; Jamet, B.; Le Tourneau, T.; Pallardy, A.; et al. 18F-FDG-Based Radiomics and Machine Learning. JACC Cardiovasc. Imaging 2023, 16, 951–961. [Google Scholar] [CrossRef]
- Williams, G.; Kolodny, G.M. Suppression of Myocardial 18F-FDG Uptake by Preparing Patients with a High-Fat, Low-Carbohydrate Diet. Am. J. Roentgenol. 2008, 190, W151–W156. [Google Scholar] [CrossRef]
- Scholtens, A.M.; Verberne, H.J. Attenuation correction and metal artifact reduction in FDG PET/CT for prosthetic heart valve and cardiac implantable device endocarditis. J. Nucl. Cardiol. 2018, 25, 2172–2173. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Palomino-Fernández, D.; Seiffert, A.P.; Gómez-Grande, A.; López-Guarch, C.J.; Moreno, G.; Bueno, H.; Gómez, E.J.; Sánchez-González, P. Robustness of [18F]FDG PET/CT radiomic analysis in the setting of drug-induced cardiotoxicity. Comput. Methods Programs Biomed. 2024, 244, 107981. [Google Scholar] [CrossRef]
- Jensen, L.J.; Kim, D.; Elgeti, T.; Steffen, I.G.; Hamm, B.; Nagel, S.N. Stability of Radiomic Features across Different Region of Interest Sizes—A CT and MR Phantom Study. Tomography 2021, 7, 238–252. [Google Scholar] [CrossRef]
- Van Velden, F.H.P.; Kramer, G.M.; Frings, V.; Nissen, I.A.; Mulder, E.R.; de Langen, A.J.; Hoekstra, O.S.; Smit, E.F.; Boellaard, R. Repeatability of Radiomic Features in Non-Small-Cell Lung Cancer [18F]FDG-PET/CT Studies: Impact of Reconstruction and Delineation. Mol. Imaging Biol. 2016, 18, 788–795. [Google Scholar] [CrossRef]
- Sanchez, L.E.; Rundo, L.; Gill, A.B.; Hoare, M.; Serrao, E.M.; Sala, E. Robustness of radiomic features in CT images with different slice thickness, comparing liver tumour and muscle. Sci. Rep. 2021, 11, 8262. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J.W.L. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-sampling Technique. J. Artif. Intell. Res. 2002, 30, 321–357. [Google Scholar] [CrossRef]
- Huang, E.P.; O’connor, J.P.B.; McShane, L.M.; Giger, M.L.; Lambin, P.; Kinahan, P.E.; Siegel, E.L.; Shankar, L.K. Criteria for the translation of radiomics into clinically useful tests. Nat. Rev. Clin. Oncol. 2023, 20, 69–82. [Google Scholar] [CrossRef]
- Kocak, B.; Baessler, B.; Bakas, S.; Cuocolo, R.; Fedorov, A.; Maier-Hein, L.; Mercaldo, N.; Müller, H.; Orlhac, F.; dos Santos, D.P.; et al. CheckList for EvaluAtion of Radiomics research (CLEAR): A step-by-step reporting guideline for authors and reviewers endorsed by ESR and EuSoMII. Insights Imaging 2023, 14, 75. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Med. 2021, 31, 27–53. [Google Scholar] [CrossRef]
- Lin, W.-J.; Hsueh, H.-M.; Chen, J.J. Power and sample size estimation in microarray studies. BMC Bioinform. 2010, 11, 48. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
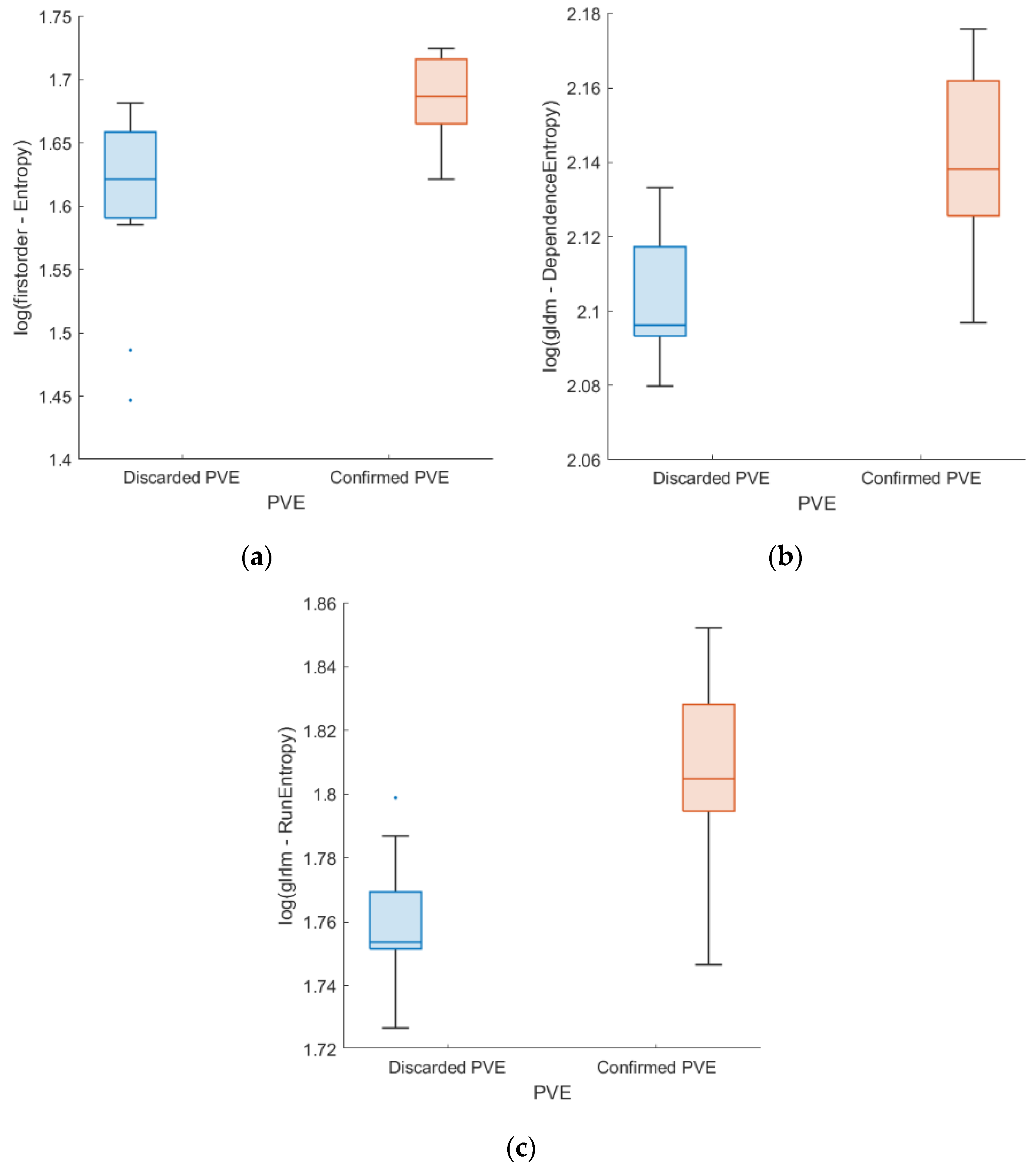
| Feature | Discarded PVE | Confirmed PVE | p-Value | ICC [95% CI] | |
|---|---|---|---|---|---|
| First order | Entropy | 5.00 ± 0.07 | 5.39 ± 0.03 | 0.003 * | 0.999 [0.999–1.000] |
| GLDM | DependenceEntropy | 8.18 ± 0.02 | 8.49 ± 0.02 | 0.003 * | 0.873 [0.767–0.942] |
| GLRLM | GrayLevelNonUniformity | 648.90 ± 0.29 | 452.78 ± 0.38 | 0.025 | 0.910 [0.835–0.959] |
| RunEntropy | 5.80 ± 0.02 | 6.09 ± 0.03 | 0.003 * | 0.892 [0.802–0.951] | |
| NGTDM | Coarseness | 0.0014 ± 0.001 | 0.002 ± 0.35 | 0.028 | 0.962 [0.930–0.983] |
| Method | AUC | Accuracy | F1-Score | Precision | Recall | Specificity |
|---|---|---|---|---|---|---|
| Logistic Regression | 0.876 * | 0.864 * | 0.863 * | 0.867 * | 0.864 * | 0.864 * |
| Neural Network | 0.876 * | 0.818 * | 0.818 * | 0.818 * | 0.818 * | 0.818 * |
| SVM linear | 0.860 * | 0.818 * | 0.818 * | 0.818 * | 0.818 * | 0.818 * |
| Decision tree | 0.769 * | 0.682 | 0.681 | 0.683 * | 0.682 | 0.682 |
| Random Forest | 0.839 * | 0.818 * | 0.817 * | 0.829 * | 0.818 * | 0.818 * |
| Naive Bayes | 0.855 * | 0.773 * | 0.768 * | 0.795 * | 0.773 * | 0.773 * |
| Gradient Boosting | 0.851 * | 0.818 * | 0.818 * | 0.818 * | 0.818 * | 0.818 * |
| kNN | 0.835 * | 0.818 * | 0.818 * | 0.818 * | 0.818 * | 0.818 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomino-Fernández, D.; Gómez-Grande, A.; P. Seiffert, A.; Bueno, H.; J. Gómez, E.; Sánchez-González, P. Radiomic-Based Biomarkers for the Evaluation of Prosthetic Heart Valve Infective Endocarditis in Non-Attenuation Correction [18F]FDG PET/CT Images. Appl. Sci. 2024, 14, 2296. https://doi.org/10.3390/app14062296
Palomino-Fernández D, Gómez-Grande A, P. Seiffert A, Bueno H, J. Gómez E, Sánchez-González P. Radiomic-Based Biomarkers for the Evaluation of Prosthetic Heart Valve Infective Endocarditis in Non-Attenuation Correction [18F]FDG PET/CT Images. Applied Sciences. 2024; 14(6):2296. https://doi.org/10.3390/app14062296
Chicago/Turabian StylePalomino-Fernández, David, Adolfo Gómez-Grande, Alexander P. Seiffert, Héctor Bueno, Enrique J. Gómez, and Patricia Sánchez-González. 2024. "Radiomic-Based Biomarkers for the Evaluation of Prosthetic Heart Valve Infective Endocarditis in Non-Attenuation Correction [18F]FDG PET/CT Images" Applied Sciences 14, no. 6: 2296. https://doi.org/10.3390/app14062296
APA StylePalomino-Fernández, D., Gómez-Grande, A., P. Seiffert, A., Bueno, H., J. Gómez, E., & Sánchez-González, P. (2024). Radiomic-Based Biomarkers for the Evaluation of Prosthetic Heart Valve Infective Endocarditis in Non-Attenuation Correction [18F]FDG PET/CT Images. Applied Sciences, 14(6), 2296. https://doi.org/10.3390/app14062296






