1. Introduction
Polyesters (PES) play an important role in the production of food contact materials (FCM), being among the most used type of polymers in the field. Different types of PES can be found in the market as base materials for several FCM applications, with polyethylene terephthalate (PET) as the most relevant. This polymer has been used for years due to its advantageous technological properties and characteristics over other polymers, such as possessing generally low levels of migration and being an efficient functional barrier against organic molecules [
1,
2,
3,
4]. These properties make PET one of the most inert food-contact polymers and the polymer of choice for applications such as water bottles [
5]. Another notable PES in use nowadays as FCM is the polybutylene terephthalate (PBT), in applications such as coffee capsules, kitchenware utensils, microwaveable dishware and beverage cups [
5].
PET and PBT have a huge recyclability potential, with the former being currently the most recycled food plastic in EU [
4]. There is a growing interest in the determination and quantification of potential contaminants that can originate due to or during recycling processes. In fact, for new recycling processes to be approved in EU, they must (1) be in accordance with Regulation (EU) 2022/2016 that replaced the Commission Regulation (EU) 282/2008 on recycled plastic materials intended to come into contact with foods [
6,
7], and (2) be submitted to the European Food Safety Authority (EFSA). This agency then carries out assessments of the risks originating from the migration of substances from a given recycled food contact plastic material into food and to deliver a scientific opinion on related recycling process. The aforementioned regulatory framework clearly highlights an absolute need not only for the identification of potential migrants (currently estimated in the several thousands), but even for a proper definition of all the categories to which those substances belong [
8].
Among the most studied plastic migrants are a group of substances known as oligomers. In 2016, Hoppe and colleagues hypothesized that polymeric oligomers (
oligo = a few and
mers = parts) could be defined as molecules consisting of a few of the monomer units used in the production of the plastic [
9]. In other fields an oligomer is usually considered to contain between two and forty repeating units [
10] (depending on the chemical composition of the building block), but it is not entirely clear (1) from how many repeating units a substance can be considered an oligomer and (2) up to what number.
Some oligomers can be used as additives in the production of plastic polymers, but the majority are considered as non-intentionally added substances (NIAS). They can be formed through the degradation of the polymers due to thermal effects during production or recycling, by degradation due to various thermo-mechanical processes and as byproducts of the polymeric synthesis [
1,
4,
9,
11,
12,
13,
14]. The use of irradiation energy during sterilization treatments of packed foods (gamma or beta radiation), as well as high pressure, UV-light or ozone, can also promote the formation of oligomers [
15]. Generally, species with a molecular weight below 1000 Da are considered as relevant potential migrants [
9]. The 1000 Da threshold has been set as a non-binding limit based on toxicological interest in their absorption in the gastrointestinal system. Nevertheless, the absorption can be related and correlated with several factors (i.e., molecular mass, polarity), though in general, the smaller the molecule the higher its diffusion rate [
3,
12]. Several proficiency tests have been organized by the Joint Research Centre of the European Commission (JRC-EC) in recent years on the migration of PET and PBT oligomers, which highlights the importance of this category of organic molecules [
16,
17,
18].
Molecular chain length odd-even effect is an important phenomenon in physical chemistry and materials science, closely related to surface structures and functions of these materials. It mostly describes an alteration of materials structures which reflect on physical and chemical properties depending on the odd or even number of structural units in a molecule [
19,
20]. This frequent but not so well-known effect may lead to unexpected changes in solubility (K) of substances and melting points (T
m), hindering their chemical analysis and proper quantification.
The objectives of this work are to address the lack of a clear definition for oligomers and investigate the behaviour and properties of PET cyclic oligomers, including the odd-even effect observed in their physical and chemical properties. The study also compares the similarities between PET and PBT oligomers, proposes a definition for oligomers in the synthetic polymeric field based on PET cyclic oligomers, and suggests an upper limit for oligomers before they start exhibiting properties similar to the polymer itself.
2. Materials and Methods
2.1. Chemicals
All the solvents used were supplied by Sigma Aldrich (Steinheim, Germany) and CHROMASOLV grade: acetone (Acet), acetonitrile (MeCN), dimethylformamide (DMF), ethanol (EtOH), methanol (MeOH), 2-propanol (IsOH), dimethylacetamide (DMA), dimethyl sulfoxide (DMSO), tetrahydrofuran (THF), dichloromethane (DCM), chloroform (CHCl3), 2,2,2-trifuoroethanol (TFE) and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP). Ultrapure water (18.2 MΩ) was used for all solutions. The formic acid used was LC-MS grade. All the PET oligomers used (cyclic dimer to heptamer) were acquired from Toronto Research Chemicals (TRC, Toronto, ON, Canada) with a purity of 97% or higher. Chloroform-d (99.8%) and trifluoroacetic acid (TFA) have also been supplied by Sigma Aldrich (Steinheim, Germany).
2.2. Solubility Tests
The solubility of all the studied cyclic oligomers was investigated. In order to assess the solubility of all oligomers, different organic solvents have been used so to obtain the maximum possible amount of substance in solution. The performed tests were carried out as previously reported for the PBT cyclic oligomers, from dimer up to pentamer [
11,
18].
2.3. DSC Analysis and Melting Points (Tm)
The DSC used was a TA Instruments Model Q100 (Newcastle, DE, USA), equipped with an auto-sampler. Every sample consisted of 3 to 5 mg, which were inserted in aluminum pans and sealed. After temperature equilibration, every sample was heated from −20 °C to 300 °C at 30 °C/min (1st cycle), followed by a cooling step back to −20 °C at 30 °C/min (2nd cycle), and then finally heated up to 300 °C at 30 °C/min (3rd cycle). Heating scans were carried under a constant gas flow of nitrogen at 50 mL/min. The melting points of the pure substances have been assessed using a Buchi melting point instrument (Buchi Labotechnik AG, Flawil, Switzerland). A program was set starting from a heating rate of 10 °C/min, up to temperature of 160 °C, followed by a rate of 1 °C/min.
2.4. NMR Analysis
All PET oligomers have been individually dissolved in a mixture of 600 μL of deuterated chloroform and TFA (8:1, v/v), which was then shaken in a vortex and transferred into 5 mm NMR tubes. The experiments were performed on a Bruker (Rheinstetten, Germany) Avance 600 (nominal frequency 600.13 MHz) equipped with a 5 mm cryo-probe. The spectra were recorded at 298 K using a 90° flip angle, with an acquisition time of 3.0 s (64k data points) and a total recycling time of 4.0 s. A spectral width of 20 ppm with 256 scans and no sample rotation in the DQD acquisition mode were applied. Prior to Fourier transformation a 0.5 Hz line-broadening factor was applied, and all spectra were phase- and baseline-corrected using Bruker Topspin 3.2 software. Chemical shifts (δ) for 1H and 13C NMR spectra are reported in parts per million (ppm) relative to the internal residual solvent signal of CDCl3-d: 7.2 ppm for 1H and 77 ppm for 13C APT.
2.5. Quadropole Time-of-Flight Mass Spectra
The UHPLC system (Agilent 1290) used was interfaced with a quadrupole Time-Of-Flight (TOF) MS detector (Agilent 6540 UHD Accurate-Mass, Agilent, Waldbronn, Germany) using an ESI. It operated in both positive and negative ionization modes. The source was set at 325 °C, with nitrogen being used as the drying gas at 2.8 bar. The nebulizing gas was also nitrogen, at a flow of 10 L/min. The used injection volumes were always 5 μL. The detector acquired MS data ranging from 100 to 1600 m/z, while the capillary voltage was set at 4 kV in positive and 3 kV in negative mode. Both single MS (Total Ion Chromatogram; TIC) and “all ion MS-MS” modes have been used, with collision energies ranging from 10 to 60 eV (optimization of these molecules in a previous work [
14]). The latter experiment was performed to assess and evaluate the fragmentation patterns of the studied molecules. An MS/MS fragmentation experiment was performed based on the precursor ion (m/z) of each one of the tested PET cyclic oligomers. The optimum collision energy (eV) for each of the target analytes was optimized, performing experiments ranging from 0 to 100 eV.
For the chromatographic part, a Waters analytical column BEH C18 100 × 2.1 mm, 1.7 μm particle size (Waters, Milford, MA, USA) has been used, with a flow rate set at 200 μL min−1. The chromatographic column was thermostabilized at 40 °C. The used mobile phase was a mixture of acidized water (0.1% formic acid, A) and acidized methanol (0.1% formic acid, B), and a gradient program was employed: 50% B changed linearly to 95% B when reaching 25 min, immediately followed by an isocratic elution during 4 min (until t = 29 min). An equilibration time of 1 min was used to reach initial mobile phase.
2.6. Chemoinformatic Processing
The ACD/Labs platform (ACD/Labs, Toronto, Canada) was employed in association with Agilent’s MassHunter (Agilent Technologies) for processing the chromatographic and HR-MS data and for the structural elucidation and identification of unknown compounds or potential degradation products. ACD/Labs allows both the confirmation and examination of the consistency of any suggested chemical structure, based on both NMR and/or HR-MS experimental data. It also allows assigning experimental spectra to chemical structures, hence facilitating the construction of central and fully searchable repository of acquired spectra.
3. Results and Discussion
3.1. Solubility Studies
The solubility of all the PET cyclic oligomers was investigated to identify behaviour and similarities, as well as to facilitate the selection of the most suitable solvent for further experiments. The used solubility study followed the one proposed by the Joint Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO) Expert Committee on Food Additives protocols [
21,
22]. The same document also provides different solubility classifications for each solubility range of amount of material per volume of solvent [
21,
22]. Obtained results are presented in
Table 1.
Solubilities for PBT cyclic oligomers, from dimer up to pentamer, have been previously reported [
18]. Regarding PET cyclic oligomers, the current work’s results are in line with the aforementioned work for PBT oligomers: strong organic solvents, such as HFIP (considered as a strong Lewis acid) [
23], must be used in order to solubilize both oligomer types [
18].
Additionally, from
Table 1 it can be immediately seen that a particular trend for the solubility of the tested oligomers seems to exist. In fact, besides THF and DMF, that offered slight solubility for some of the oligomers, strong halogenated (fluorinated or chlorinated) solvents are required to achieve at least some dissolution degree for most of the PET cyclic oligomers, with the former presenting the best results. These observations seem to be in accordance with what has already been reported by Tsochatzis et al. for the solubility of the PBT cyclic oligomers [
18].
However, it can also be observed that both the PET cyclic tetramer and hexamer (even-numbered cyclic oligomers) presented lower solubilization rates than their odd-numbered counterparts, even when using halogenated solvents. A specific solubility behavior of the tetramer/hexamer seems to exist, in contrast with the trimer/pentamer/heptamer cyclic oligomers. In fact, both the tetramer and hexamer were practically insoluble, except when using HFIP and the mixture TFA/CHCl3 8:1 v/v, very different from what has been observed for the odd-numbered oligomers.
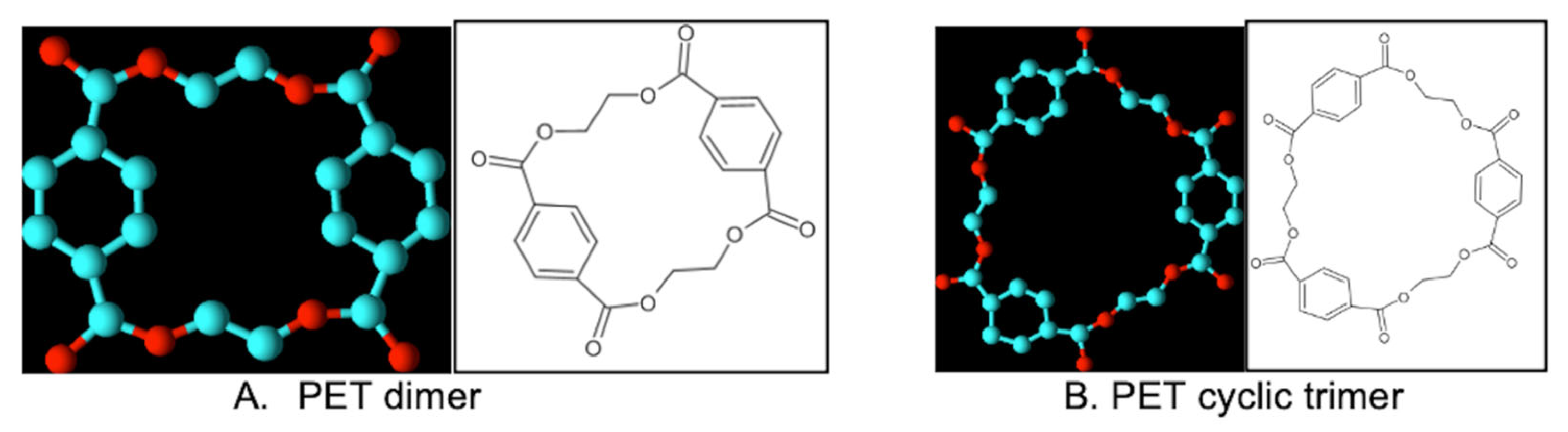
The exception seems to be the PET cyclic dimer, the smallest even-numbered cyclic oligomer: this molecule was soluble/freely soluble in almost all the tested solvents. It must be considered that the PET cyclic dimer is a relatively small molecule (M = 385 Da) and that the extent of the ring is considerably smaller than in case of tetramer (M = 769 Da) or hexamer (1153 Da). As expected, the size and the ring extension of the cyclic oligomers seems to affect their solubility. Both the tetramer and the hexamer are considered as large, almost macrocyclic molecules, when compared with typical small organic molecules. However, this may not be the only factor in place, as both the PET cyclic pentamer and heptamer, despite being even larger molecules/cycles, present slightly better solubility results than the tetramer/hexamer. The spatial configuration of the rings (symmetrical for the even-numbered oligomers, non-symmetrical for the odd-ones) may also play a role (see
Figure 1).
All these observations show that the number of repeating units may play an important role when studying the solubility of cyclic oligomers that can be related to structural differences. Additionally, the solubility properties of the polymer itself (PET) are very similar with both the cyclic hexamer and heptamer. Only the unusual organic solvent HFIP, along with the mixture of TFA/CHCl3 8:1 v/v, seemed to be sufficiently efficient in solubilizing these molecules. The latter was finally selected to be used in the preparation of solutions for NMR and HR-MS analysis.
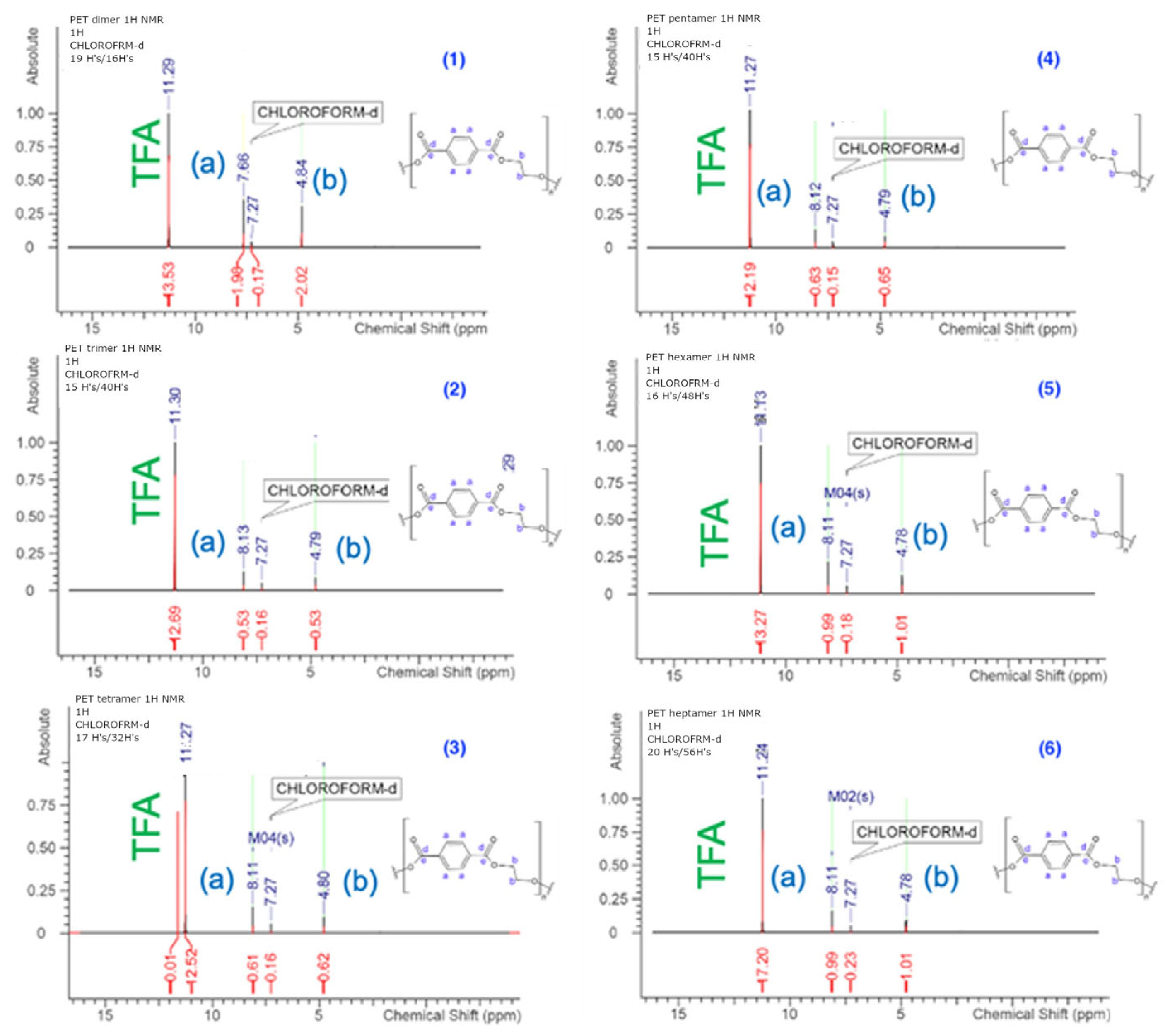
3.2. NMR Analysis
The chemical structures of the PET cyclic oligomers (dimer up to heptamer), the
1H NMR and
13C NMR spectra, as well as their respective signal assignments are presented in
Figure 1 and
Figure 2.
From the
1H NMR analysis results (
Table 2 and
Figure 3) we observed that the protons from the aliphatic chains and close to the carboxy groups presented a difference between the dimer and all the other structural cyclic oligomers (
Table 2). This observation can be attributed to the size and geometry of the dimer, which seem to differ from all the others, and this may be related to the increased solubility of this molecule in all organic solvents as already presented in
Table 1 and reported for other PBT cyclic oligomers [
18]. Moreover, of particular interest were the aromatic protons, as it could be observed that they have a different chemical shift (singlet) when compared with the other PET cyclic oligomers. The
1H NMR chemical shifts for PET oligomers are in accordance with the study by Tsochatzis et al. [
18], where a clear difference exists between the PBT cyclic dimer and all the other oligomers, suggesting a similar physicochemical behavior and a strong relation with the geometry of the molecule and its internal interactions.
Some differences were nevertheless observed on the chemical shifts, due to the different intermediate aliphatic chains connecting the terephthalic acid units [
18]. For
13C NMR (
Table 2,
Figure 3), we observed a clear difference between the cyclic PET dimer and all the other cyclic oligomers, where an increase of the chemical shift (δ) occurred for either aliphatic or aromatic carbons. In this case, the results for PET cyclic oligomers were in in accordance with previously reported work for PBT [
18].
3.3. Quadrupole Time-of-Flight Mass Spectra
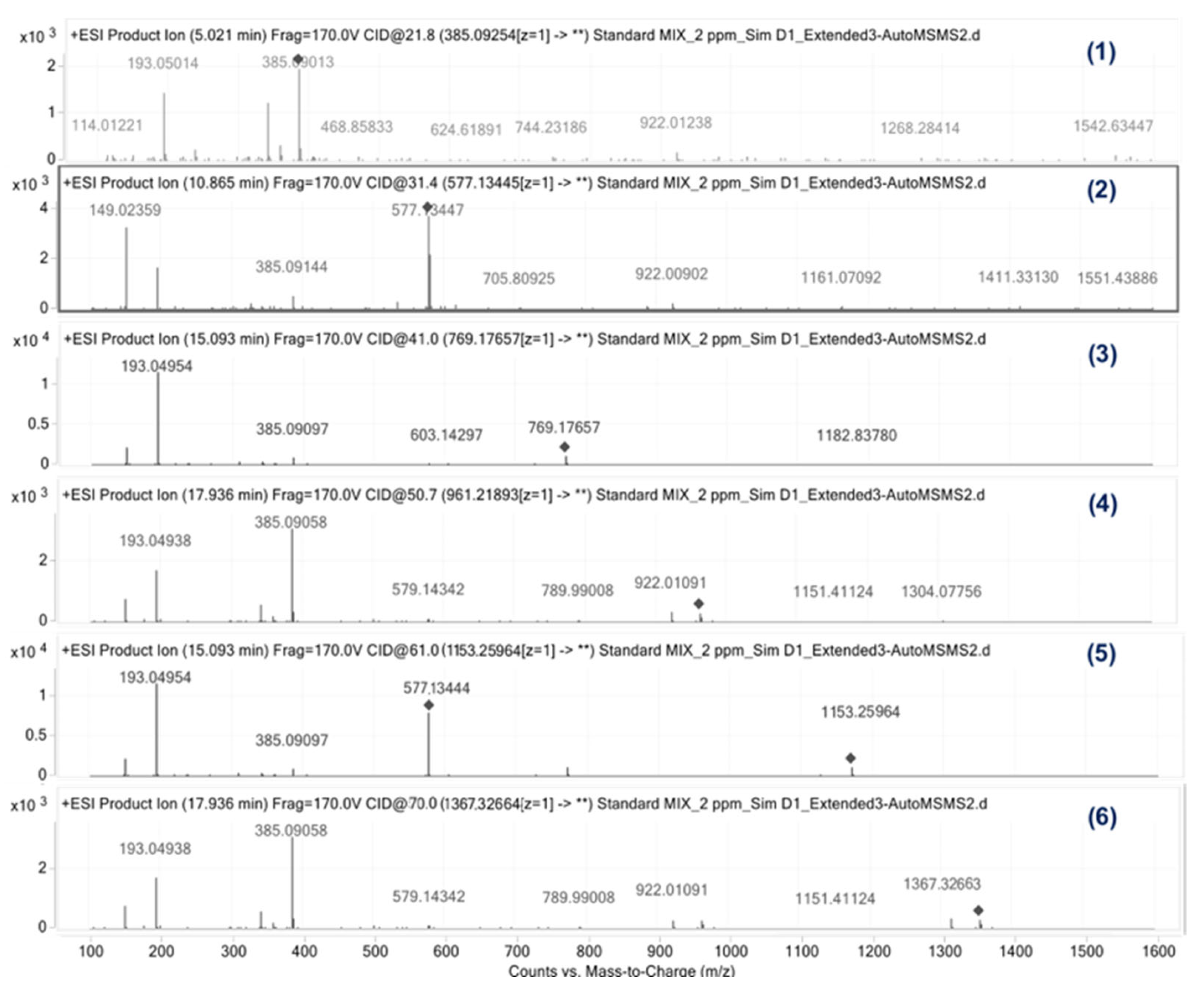
The obtained oligomer’s Total Ion Chromatogram (TIC) QTOF-MS spectra are depicted in
Figure 4, with additional information being provided in
Table 3.
It can be seen that all but two oligomers present the [M + H]+ molecular ion, with the exceptions being the PET pentamer and heptamer. For these two the sodium adjunct is instead present, with the m/z 983.199 and 1367.282, respectively. In addition, using Electrospray Ionization mode in positive mode (ESI+), an on-source ionization of all the oligomers to their open linear dimer (m/z of 273.095) was observed. This identification was confirmed using the ACD/Labs platform, which resulted in a matching score of 89.3%.
The “all ion MS-MS” mass fragmentation patterns observed in the spectra of
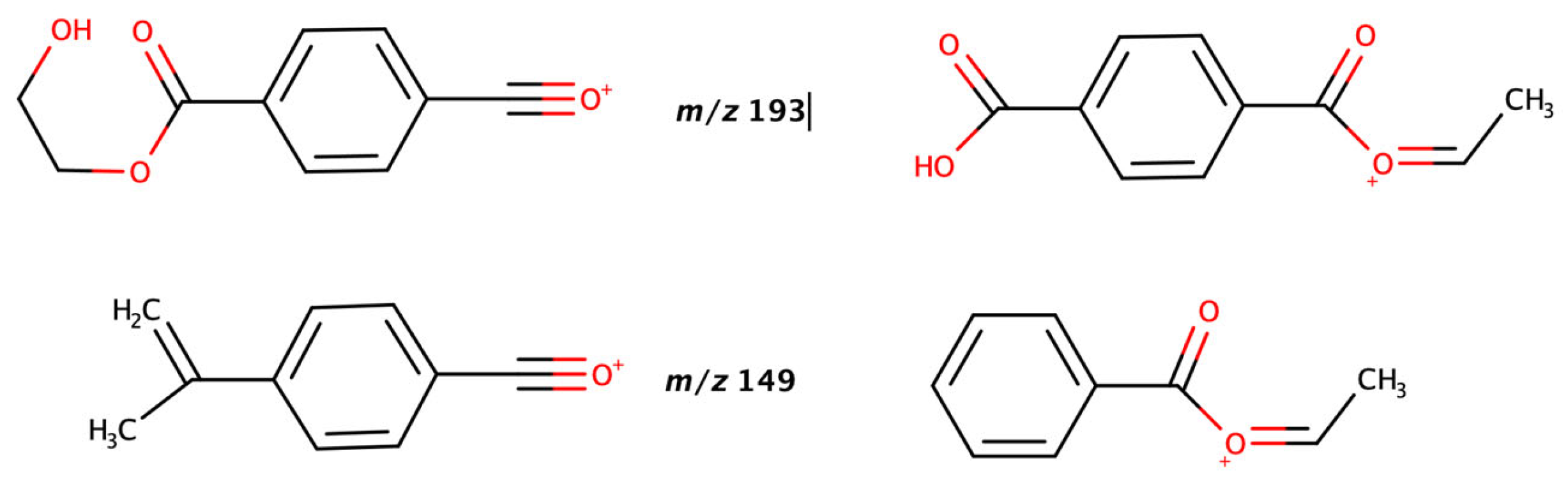
Figure 4 hint at a potential trend among the different PET cyclic oligomers. In fact, the even-numbered oligomers (dimer, tetramer and hexamer) seem to present specific patterns, fragmenting to the dimethyl terephthalate ion (the most abundant m/z 193.023,
Figure 5 and
Figure 6). The only difference is that, while the dimer and tetramer fragment directly to that ion, the hexamer instead is fragmented either directly or in two-steps via the trimer (m/z 577.134).
On the contrary, for all odd-numbered cyclic oligomers (trimer, pentamer and heptamer) it was observed that they are not fragmented directly to the dimer nor via the trimer. Instead, they are fragmented via the formation of the dimer’s ion (m/z 385.091), which is not present for the even numbered. This behavior clearly differentiates the higher number odd and even cyclic oligomers, but nothing can be concluded for the dimer and the cyclic trimer.
3.4. DSC Investigation
Many properties of dicarboxylic acids have been reported to be associated with their even-odd conformations [
20,
24,
25], such as solubility (K) and melting point (T
m). This one-to-one correspondence suggests that the same factors strained molecular conformations in odd acids and a balance between attractive dispersive forces and oxygen-oxygen repulsions governed T
m.
Additionally, a Tm is also related to intermolecular force in particular chemical structures. Hence, the stronger these forces are, the higher the Tm. It can also be an indicator of a stable thermodynamic state of a given system. Nevertheless, there is also the theory for substances of similar structure that, the greater the molecular mass, the higher the melting point. The observable melting and boiling points of different organic molecules provides an additional instance of the effects of noncovalent interactions. The main principle involved is simple and states that the stronger the noncovalent interactions between molecules, the more energy that is required (in the form of heat) to break them. Higher melting and boiling points imply stronger noncovalent intermolecular forces.
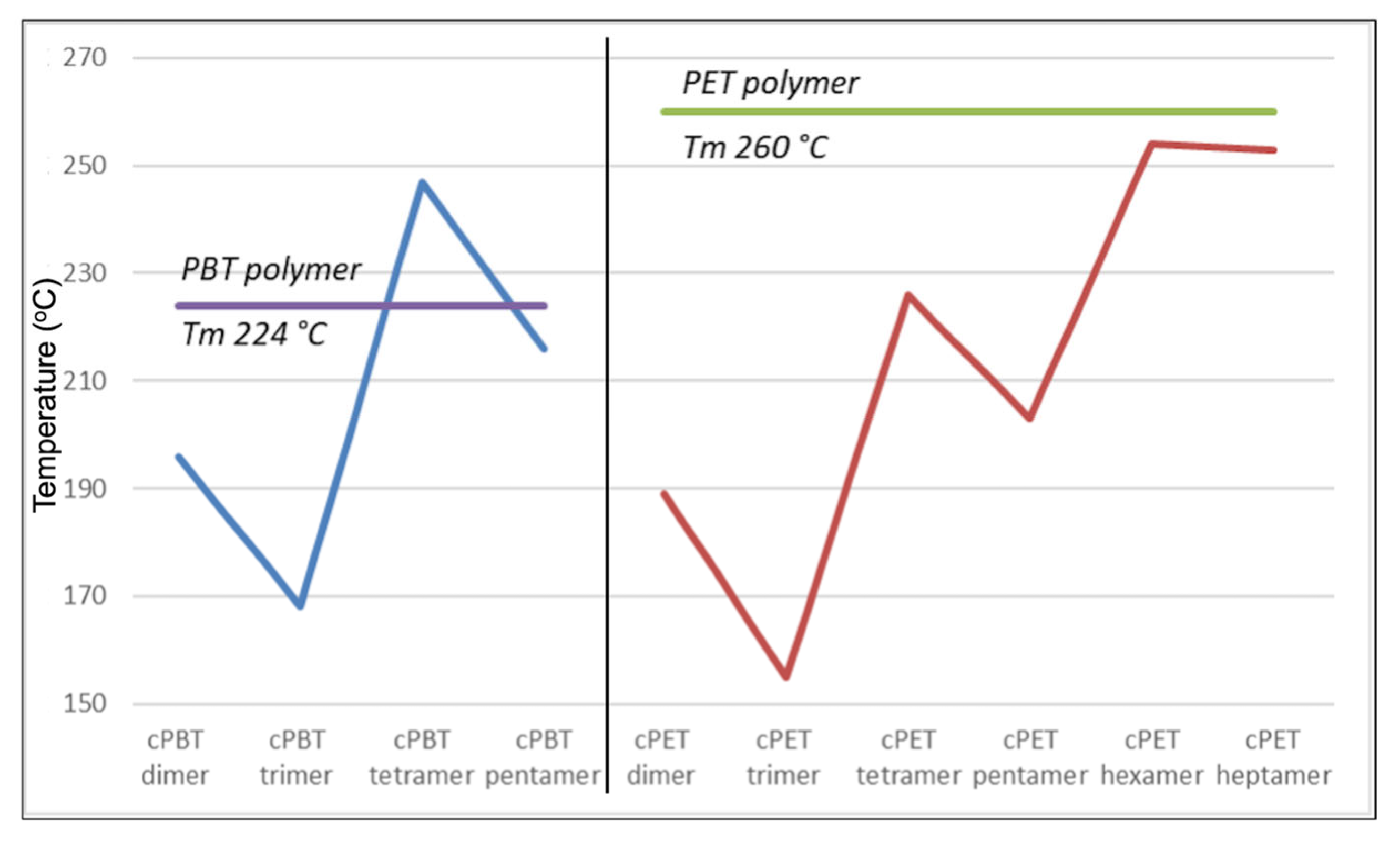
The results obtained for the PET cyclic oligomers which are multi-carboxylic cyclic acids) seem to be in line with the strained molecular conformation hypothesis for the odd-even T
m alternations proposed by Thalladi et al. [
26] and the observations presented in the previous section. In fact,
Figure 7 shows exactly that type of alternation: the PET cyclic dimer has a melting point of 189 °C that drops to 155 °C for the trimer. It then increases significantly to 226 °C for the tetramer and decreases again to 203 °C for the pentamer, before increasing to 253–254 °C for both the hexamer and the heptamer. The same alternation of T
m has also been observed for PBT cyclic oligomers [
11,
18] and are also depicted in
Figure 7 for comparison. It can also immediately be seen that, as expected due to an increase in molecular weight (MW), every PET cyclic oligomer has lower T
m’s than their PBT counterpart (dimers, trimers, etc.).
Additionally, two significant facts can also be inferred from these results. Firstly, the different intermolecular forces in place, which are stronger in case of higher molecular weight molecules and longer ring lengths, generally lead to an increase of the T
m. But at the same time, those increases do not always lead to an increase of the T
m if we also face a change from an even-numbered chain into an odd one. That was observed in the T
m’s from the dimer to the trimer and from the tetramer to the pentamer, that decreased instead of increasing. Again, a similar effect had already been observed for the PBT cyclic oligomers [
11,
18].
Secondly, it can also be noticed that with the increase of the oligomer’s ring length it seems that there is a tendency to reach a Tm plateau very similar to the actual polymer Tm. This was observed for the hexamer and the heptamer, which Tm’s did not differ among them (253 °C and 254 °C, respectively) and are very close to the one of the PET (260 °C). These two oligomers were the only ones in which the odd-even effect on the Tm’s was not observed.
3.5. Odd-Even Effect and the Definition of Oligomers
The definition of oligomer is generically associated to a small (“oligo”) number of repeated organic molecules, and it is used in many fields. However, it is unclear from which number of repeated units and up to how many that nomenclature can be used. It also seems to be highly dependent on the field of application: proteins with thousands of Da can still be considered oligomers, while in the synthetic polymeric field, substances with similar MW would certainly be considered a polymer [
27,
28]. Additionally, associated to the increase of the number of repeated units, changes in the physical properties are also expected, which will also have implications in the analytical methods used [
29].
The results presented in this work for the PET cyclic oligomers showed that the progression of ring length from the smaller to the longer oligomers exhibited a behavior like what has been reported in the literature for other molecules, the so called odd-even effect. This affected not only their physical properties (solubility and T
m’s), chemical behavior and intermolecular forces, but could also be observed with two analytical techniques (HR-MS and NMR). Such as the case for other molecules, these clear differences may be related with the spatial molecular structure of the oligomers (
Figure 1). One theory states that the odd counterparts are more distorted from their equilibrium geometries, leading to a reduction of the O⋯O repulsions and consequent lowering of their melting point [
19,
20]. In fact, the even cyclic oligomers have an almost symmetric conformation, based also on the number of repeating units (e.g., di-, tri-, tetra-, etc.), which can justify the difficulty in solubilization (
Table 1), the higher observed T
m (
Figure 7) and the MS fragmentation patterns (
Figure 5). Especially, the different MS fragmentation for tetra- and hexa- (even number) which consists the formation of a new fragment (385.09058 Da) which presented at high intensity and it was confirmed to represent the dimer (even-numbered oligomer). It can be concluded that these even-numbered structures present a structural symmetry. The more disordered structures of an odd oligomer may facilitate its solubilization and a smaller need of energy (temperature) for its melting. This phenomenon also aligns with what has been observed for the PBT cyclic oligomers. However, in the latter case, only cyclic oligomers up to the pentamer were available. Previous works of the authors include additional information on solubility, chemical behavior, analytical data, and physical-chemical properties of PBT cyclic oligomers [
11,
17,
18].
Another interesting observation is related with the T
m’s of the longer PET cyclic oligomers, the hexamer and the heptamer. With MW higher than 1000 Da, these two oligomers not only had roughly the same T
m’s, but respective T
m’s were very close to the polymer itself, PET. Also, the solubility properties were very close, as can be seen in
Table 1. We can therefore hypothesize that the hexamer, for this type of oligomer, could be the upper limit in which an oligomer starts behaving and showing the physical properties of the polymer. Therefore, a potential definition for an oligomer in the synthetic polymeric field aligned with our observations could be “
oligomers are chemical substances that have a varying number of the same repeating unit up to a point in which their melting point reaches the melting point of the polymer itself”.
4. Conclusions
The definition of oligomer lacks a clear definition regarding the number of repeated units and the field of application. However, with an increase in the number of repeated units, there are changes in physical properties that will affect the analytical methods used. The PET cyclic oligomers showed an odd-even effect in physical properties, and this could be related to their spatial molecular structure. The hexamer could be the upper limit for oligomers to start showing the physical properties of this polymer. This would also align with the poor migration levels (low diffusion rates) observed for species with molecular weights above 1000 Da, as well as their absorption in the gastrointestinal system, also characteristic of polymers.
Hence, a proposed definition for oligomers in the polymeric field is suggested based on these observations. Additional studies are still needed, both including linear oligomers and other PES, such as PTT.