The Effect of Brewing Time on the Antioxidant Activity of Tea Infusions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Material
2.2. Preparation of Tea Infusions
2.3. Reagents
2.4. Chemical Analyses
2.5. Antioxidant Content
2.6. Calculations and Statistical Analysis
3. Results
3.1. TPC
3.2. Flavonoids
3.3. Anthocyanins
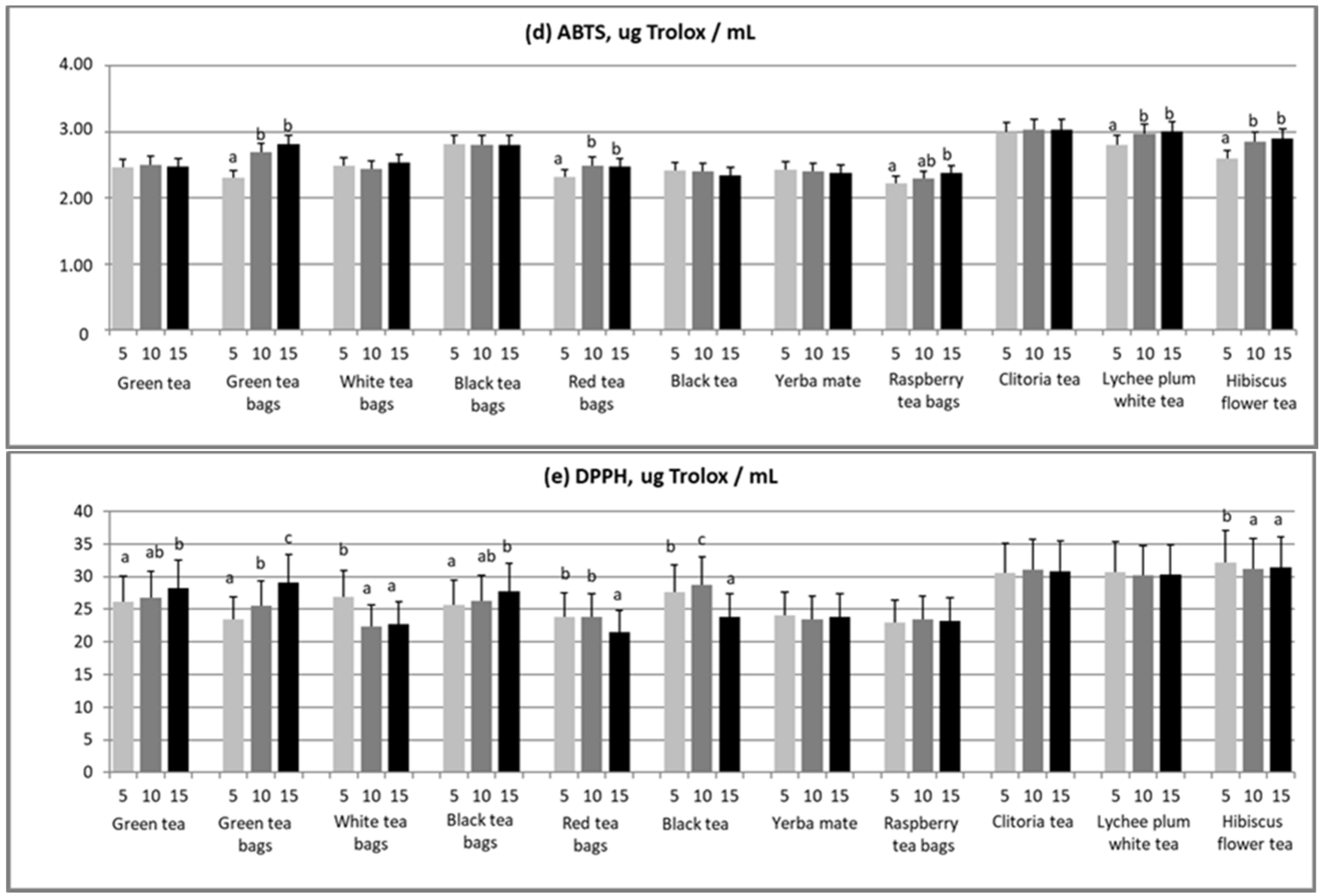
3.4. ABTS
3.5. DPPH
3.6. The Effect of Brewing Time of Particular Types of Tea on Changes in the Anti-oxidant Content of Tea Infusions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, W.; Xie, L.; Chen, Y.; Zhang, H. Purification, characterization and anticoagulant activity of the polysaccharides from green tea. Carbohydr. Polym. 2013, 2013, 1086–1090. [Google Scholar] [CrossRef]
- Sun, M.F.; Jiang, C.L.; Kong, Y.S.; Luo, J.L.; Yin, P.; Guo, G.Y. Recent Advances in Analytical Methods for Determination of Polyphenols in Tea: A Comprehensive Review. Foods 2022, 11, 1425. [Google Scholar] [CrossRef]
- Truong, V.L.; Jeong, W.S. Cellular Defensive Mechanisms of Tea Polyphenols: Structure-Activity Relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shokri, S.; Gao, B.; Xu, Z.; Li, B.; Zhu, T.; Wang, Y.; Zhu, J. Improving effects of three selected co-pigments on fermentation, color stability, and anthocyanins content of blueberry wine. LWT 2022, 156, 113070. [Google Scholar] [CrossRef]
- Rha, C.S.; Jeong, H.W.; Park, S.; Lee, S.; Jung, Y.S.; Kim, D.O. Antioxidative, Anti-Inflammatory, and Anticancer Effects of Purified Flavonol Glycosides and Aglycones in Green Tea. Antioxidants 2019, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Tomaszewska, E.; Jachimowicz, K. Antioxidant, anti-inflammatory, and immunomodulatory properties of tea—The positive impact of tea consumption on patients with autoimmune diabetes. Nutrients 2021, 13, 3972. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.Y.; Li, Q.S.; Lin, X.M.; Qiao, R.Y.; Yang, R.; Li, X.M.; Dong, Z.B.; Xiang, L.P.; Zheng, X.Q.; Lu, J.L.; et al. Antidiabetic Effects of Tea. Molecules 2017, 22, 849. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A. Protective effect of tea against lead and cadmium-induced oxidative stress—A review. Biometals 2018, 31, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Liu, C.; Dong, S.L.; Ou, C.S.; Lu, J.L.; Ye, J.H.; Liang, Y.R.; Zheng, X.Q. Anticarcinogenic potentials of tea catechins. Front. Nutr. 2022, 5, 1060783. [Google Scholar] [CrossRef] [PubMed]
- Simos, Y.V.; Verginadis, I.; Toliopoulos, I.K.; Velalopoulou, A.P.; Karagounis, I.V.; Karkabounas, S.C.; Evangelou, A.M. Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity, in vivo. Redox. Rep. 2012, 17, 181–186. [Google Scholar] [CrossRef]
- Shannon, E.; Jaiswal, A.K.; Abu-Ghannam, N. Polyphenolic content and antioxidant capacity of white, green, black, and herbal teas: A kinetic study. Food. Res. 2018, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A. The potential protective effect of green, black, red and white tea in fusions against adverse effect of cadmium and lead during chronic exposure—A rat model study. Regul. Toxicol. Pharm. 2015, 73, 521–529. [Google Scholar] [CrossRef]
- Hilal, Y.; Engelhardt, U. Characterisation of white tea—Comparison to green and black tea. J. Verbrauch Lebensm. 2007, 2, 414–421. [Google Scholar] [CrossRef]
- Wachira, F.N.; Areba, G.O.; Ngure, R.M.; Khalid, R.; Maloba, F.; Nyaga, N.; Moseti, K.O.; Ngotho, M.; Wanyoko, J.K.; Karori, S.M. Neuroprotective Effects of Tea Against Cadmium Toxicity. Bioact. Compd. Health Dis. 2019, 2, 230–246. [Google Scholar] [CrossRef]
- Khalaf, A.A.; Moselhy, W.A.; Abdel-Hamed, M.I. The protective effect of green tea extract on lead induced oxidative and DNA damage on rat brain. Neurotoxicology 2012, 33, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Mehana, E.; Meki, A.R.; Fazili, K.M. Ameliorated effects of green tea extract on lead induced liver toxicity in rats. Exp. Toxicol. Pathol. 2012, 64, 291–295. [Google Scholar] [CrossRef]
- Sivakumaran, K.; Amarakoon, S. Bioactivity of fruit teas and tisanes—A review. J. Pharmacogn. Phytochem. 2018, 7, 323–327. [Google Scholar]
- Pękal, A.; Dróżdż, P.; Biesaga, M.; Pyrzynska, K. Evaluation of the antioxidant properties of fruit and flavoured black teas. Eur. J. Nutr. 2011, 50, 681–688. [Google Scholar] [CrossRef]
- Pękal, A.; Dróżdż, P.; Biesaga, M.; Pyrzynska, K. Screening of the antioxidant properties and polyphenol composition of aromatised green tea infusions. J. Sci. Food. Agric. 2012, 92, 2244–2249. [Google Scholar] [CrossRef]
- Zujko, M.E.; Witkowska, A.; Mirończuk-Chodakowska, I. Antioxidant potential of fruit teas. Bromat. Chem. Toksykol. 2011, 3, 615–619. [Google Scholar]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Dmowski, P.; Śmiechowska, M.; Deja, B. Influence of the tea brewing conditions on the content of tannins and choosen parameters of colour. Sci. J. Gdyn. Marit. Univ. 2011, 68, 5–12. [Google Scholar]
- Vinci, G.; D’Ascenzo, F.; Maddaloni, L.; Prencipe, S.A.; Tiradritti, M. The Influence of Green and Black Tea Infusion Parameters on Total Polyphenol Content and ntioxidant Activity by ABTS and DPPH Assays. Beverages 2022, 8, 18. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wójcik, E.; Kukuła-Koch, W.; Kowalik, K.; Polak-Berecka, M.; Waśko, A. Evolution of the anticholinesterase, antioxidant, and anti-inflammatory activity of Epilobium angustifolium L. infusion during in vitro digestion. J. Funct. Foods 2021, 85, 104645. [Google Scholar] [CrossRef]
- Jośko, I.; Kusiak, M.; Różyło, K.; Baranowska-Wójcik, E.; Sierocka, M.; Sheteiwy, M.; Szwajgier, D.; Świeca, M. The life cycle study revealed distinct impact of foliar-applied nano-Cu on antioxidant traits of barley grain comparing with conventional agents. Food Res. Int. 2023, 164, 112303. [Google Scholar] [CrossRef]
- Teixeira, A.M.; Sousa, C.A. Review on the Biological Activity of Camellia Species. Molecules 2021, 26, 2178. [Google Scholar] [CrossRef]
- Kowalska, J.; Marzec, A.; Domian, E. Influence of Tea Brewing Parameters on the Antioxidant Potential of Infusions and Extracts Depending on the Degree of Processing of the Leaves of Camellia sinensis. Molecules 2021, 26, 4773. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Zargar, B.; Majeed, D.; Ganai, S.A.; Mir, S.A.; Dar, B.N. Effect of different processing parameters on antioxidant activity of tea. J. Food Meas. Charact. 2018, 12, 527–534. [Google Scholar] [CrossRef]
- Fernando, C.D.; Soysa, P. Extraction kinetics of phytochemicals and antioxidant activity during black tea (Camellia sinensis L.) brewing. Nutr. J. 2015, 14, 74–80. [Google Scholar] [CrossRef]
- McAlpine, M.D.; Ward, W.E. Influence of Steep Time on Polyphenol Content and Antioxidant Capacity of Black, Green, Rooibos, and Herbal Teas. Beverages 2016, 2, 17. [Google Scholar] [CrossRef]
- Pal, S.; Ghosh, D.; Dey, S.K.; Saha, C. Effect of infusion time and consecutive brewing on antioxidant status of black tea infusion. Int. J. Tea Sci. 2013, 9, 65–68. [Google Scholar]
- Alappat, B.; Alappat, J. Anthocyanin pigments: Beyond aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Aishah, B.; Nursabrina, M.; Noriham, A.; Norizzah, A.R.; Mohamad, S.H. Anthocyanins from Hibiscus sabdariffa, Melastoma malabathricum and Ipomoea batatas and its color properties. Int. Food Res. J. 2013, 20, 827–834. [Google Scholar]
- Yildirim, R.M.; Ozulku, G.; Toker, O.S.; Baslar, M.; Durak, M.Z.; Sagdic, O. Modeling of bioactive compound content of different tea bags: Effect of steeping temperature and time. J. Food Process. Preserv. 2016, 41, e12773. [Google Scholar] [CrossRef]
- Nie, M.; Wang, L.; Lu, S.; Wang, Y.; Zheng, M.; Fang, Z. Protective effect of amino acids on the stability of bayberry anthocyanins and the interaction mechanism between l-methionine and cyanidin-3-O-glycoside. Food Chem. 2022, 396, 133689. [Google Scholar] [CrossRef]
- Sinela, A.; Rawat, N.; Mertz, C.; Achir, N.; Fulcrand, H.; Dornier, M. Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chem. 2017, 214, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Suzery, M.; Nudin, B.; Nurwahyu, B.D.; Cahyono, B. Effects of Temperature and Heating Time on Degradation and Antioxidant Activity of Anthocyanin from Roselle Petals (Hibiscus sabdariffa L.). Int. J. Sci. Technol. Manag. 2020, 1, 238–288. [Google Scholar] [CrossRef]
- Abdel-Aal, E.M.; Rabalski, I.; Mats, L.; Rai, I. Identification and Quantification of Anthocyanin and Catechin Compounds in Purple Tea Leaves and Flakes. Molecules 2022, 27, 6676. [Google Scholar] [CrossRef] [PubMed]
- Aboagye, G.; Tuah, B.; Bansah, E.; Tettey, C.; Hunkpe, G. Comparative evaluation of antioxidant properties of lemongrass and other tea brands. Sci. Afr. 2021, 11, e00718. [Google Scholar] [CrossRef]
- Braud, L.; Peyre, L.; de Sousa, G.; Armand, M.; Rahmani, R.; Maixent, J.M. Effect of Brewing Duration on the Antioxidant and Hepatoprotective Abilities of Tea Phenolic and Alkaloid Compounds in a t-BHP Oxidative Stress-Induced Rat Hepatocyte Model. Molecules 2015, 20, 14985–15002. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Manzocco, L.; Nunez, M.J.; Nicoli, M.C. Interaction among phenols in food fortification: Negative synergism on antioxidant capacity. J. Agric. Food Chem. 2004, 52, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Nikniaz, Z.; Mahdavi, R.; Ghaemmaghami, S.J.; Lotfi, Y.N.; Nikniaz, L. Effect of different brewing times on antioxidant activity and polyphenol content of loosely packed and bagged black teas (Camellia sinensis L.). Avicenna J. Phytomed 2016, 6, 313–321. [Google Scholar] [PubMed]

| Tea | Number of Teas | Characteristic | Serving Size per 200 mL of Water | Steeping Temperature |
|---|---|---|---|---|
| Green tea | n = 7 | Leafy | 2 g | 75 °C |
| Green tea bags | n = 10 | Bags | 1 bag—2 g | 100 °C |
| White tea bags | n = 10 | Bags | 1 bag—2 g | 100 °C |
| Black tea bags | n = 11 | Bags | 1 bag—2 g | 100 °C |
| Red tea bags | n = 7 | Bags | 1 bag—2 g | 95 °C |
| Black tea | n = 10 | Leafy | 2 g | 95 °C |
| Yerba mate | n = 12 | Leafy | 2 g | 70 °C |
| Raspberry tea bags | n = 7 | Bags | 1 bag—2 g | 70 °C |
| Blue butterfly pea flowers (Clitoria) | n = 7 | Flowers | 2 g | 100 °C |
| White lichee plum | n = 7 | Leafy | 1 ball—5 g | 85 °C |
| Hibiscus | n = 10 | Flowers | 2 g | 100 °C |
| Tea | ΔTPC | ΔFlavonoids | ΔAnthocyanins | ΔABTS | ΔDPPH | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 min * | 15 min ** | 10 min * | 15 min ** | 10 min * | 15 min ** | 10 min * | 15 min ** | 10 min * | 15 min ** | |
| Green tea | ↑ 42 | ↑ 12 | ↓ 100 | ≈ | ↓ 13 | ↓ 113 | ≈ | ≈ | ≈ | ↑ 5 |
| Green tea bags | ↑ 35 | ↑ 37 | ↑ 400 | ↑ 20 | ↑ 23 | ↑ 21 | ↑ 15 | ≈ | ↑ 8 | ↑ 12 |
| White tea bags | ↑ 25 | ↑ 33 | ↑ 43 | ↑ 10 | ↑ 17 | ↑ 16 | ≈ | ≈ | ↓ 21 | ≈ |
| Black tea bags | ↑ 25 | ≈ | ↑ 20 | ≈ | ≈ | ↑ 15 | ≈ | ≈ | ≈ | ↑ 5 |
| Red tea bags | ↑ 37 | ≈ | ↑ 29 | ↑ 12 | ≈ | ≈ | ↑ 7 | ≈ | ≈ | ↓ 11 |
| Black tea | ↑ 46 | ≈ | ↑ 33 | ↑ 100 | ↑ 56 | ↑ 17 | ≈ | ≈ | ↑ 5 | ↓ 21 |
| Yerba mate | ↑ 92 | ↑ 49 | ≈ | ↑ 13 | ≈ | ≈ | ≈ | ≈ | ≈ | ≈ |
| Raspberry tea bags | ↑ 23 | ↑ 27 | ↑ 22 | ≈ | ≈ | ↑ 17 | ≈ | ↑ 4 | ≈ | ≈ |
| Blue butterfly pea flowers (Clitoria) | ≈ | ≈ | ↑ 82 | ≈ | ↑ 59 | ↑ 24 | ≈ | ≈ | ≈ | ≈ |
| White lichee plum | ↑ 12 | ↑ 24 | ≈ | ≈ | ↑ 91 | ↑ 44 | ↑ 5 | ≈ | ≈ | ≈ |
| Hibiscus | ↑ 5 | ≈ | ↓ 33 | ↓ 50 | ↓ 290 | ≈ | ↑ 9 | ≈ | ↓ 5 | ≈ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winiarska-Mieczan, A.; Baranowska-Wójcik, E. The Effect of Brewing Time on the Antioxidant Activity of Tea Infusions. Appl. Sci. 2024, 14, 2014. https://doi.org/10.3390/app14052014
Winiarska-Mieczan A, Baranowska-Wójcik E. The Effect of Brewing Time on the Antioxidant Activity of Tea Infusions. Applied Sciences. 2024; 14(5):2014. https://doi.org/10.3390/app14052014
Chicago/Turabian StyleWiniarska-Mieczan, Anna, and Ewa Baranowska-Wójcik. 2024. "The Effect of Brewing Time on the Antioxidant Activity of Tea Infusions" Applied Sciences 14, no. 5: 2014. https://doi.org/10.3390/app14052014
APA StyleWiniarska-Mieczan, A., & Baranowska-Wójcik, E. (2024). The Effect of Brewing Time on the Antioxidant Activity of Tea Infusions. Applied Sciences, 14(5), 2014. https://doi.org/10.3390/app14052014







