Abstract
Breast cancer (BC) caused 685,000 deaths globally in 2020, earning the title of the most common type of tumor among females. With a multifactorial genesis, BC is influenced by several factors such as age, genetic and epigenetic predisposition, and an individual’s exposome, and its classification is based on morphological/histological, invasiveness, and molecular futures. Extracellular vesicles (EVs) are cell-derived lipid-bilayer-delimited nanoparticles, which are distinguishable by size, genesis, and the markers expressed in exosomes (40 to 150 nm), microvesicles (40 to 10,000 nm), and apoptotic bodies (100–5000 nm). Produced in physiological and pathological cellular contexts, EVs are shuttles of biological material and are implicated in cell-to-cell communications, thus attracting significant interest in diagnostic and drug delivery research. We report and discuss the latest evidence regarding the important role of EVs in BC, deepening their implication in tumorigenesis and metastatic mechanisms. On the other hand, the use of BC-derived EVs as prognostic biomarkers and therapeutic approaches is undergoing investigation. Hence, EVs have become new weapons in precision medicine; however, only with the support of advanced algorithms such as artificial intelligence (AI) can we develop a wide range of information. Looking ahead, it is possible to see the application of AI in the prognosis and diagnosis of different pathologies.
1. Introduction
1.1. Breast Cancer Epidemiology
The World Health Organization (WHO) reports noncommunicable diseases (NCDs), such as cardiovascular diseases and cancer, as leading causes of premature death (2019) [1]. In particular, breast cancer (BC) was reported to be one of the twenty global principal causes of death in a 2019 report [1,2]. BC is the most frequently diagnosed cancer worldwide, followed by lung cancer and colorectal cancer, with different percentages stratified by age group (40% in young people vs. 22% in older people) [2]. On 12 July 2023, the World Health Organization (WHO) asserted that by the end of 2020, breast cancer had been diagnosed in 7.8 million women over the past five years, causing 685,000 deaths globally in 2020 [3,4]. Male individuals can also have breast cancer, although with a very low incidence percentage (approximately 0.5–1% of breast cancers) [4]. Although global statistics on BC have not been published, different countries have reported their individual statistics. In Italy, the most recent report (2022) estimated 55,700 new diagnoses of BC in women, an incidence of deaths of 12,500 in 2021, and a net survival rate of 88% 5 years after diagnosis [5]. The American Cancer Society’s estimates for 2023 are that 297,790 new cases of invasive breast cancer will be diagnosed in women and 43,700 deaths will occur [6]; moreover, the COVID-19 pandemic might have caused a delay in cancer diagnosis and management [7,8]. BC occurs in women at any age after puberty and can occur in the absence of or in correspondence with different risk factors such as age, hormonal, reproductive, and metabolic factors, an unhealthy lifestyle, previous radiotherapy administration or breast dysplasia or neoplasm, and a family history of cancer, as it is heredity. In this context, it is important to remember that one of the Sustainable Development Goals (SDGs) of the 2023 Agenda is to reduce premature mortality from noncommunicable diseases (NCDs) by one-third with respect to the 2015 levels [9]. Therefore, reducing risk factors and improving early diagnosis and therapy strategies will be necessary to reach this goal. In this context, artificial intelligence (AI) could be a promising contributor [10].
1.2. Breast Cancer Classification
BC can be classified using morphological/histological features based on the cells giving rise to the tumor; lobule cells (lobular carcinoma) or milk ducts (ductal carcinoma) are the most frequent. Their ability to invade other tissues to metastasize classifies them as “non-invasive” or “invasive”. The in situ (noninvasive) forms of ductal and lobular carcinomas are abbreviated as DCIS (ductal carcinoma in situ) and LCIS (lobular carcinoma in situ), respectively. Ductal and lobular carcinomas can also reach the metastatic stage, such as tubular, papillary, mucinous, or cribriform carcinomas (less common forms). Using the TNM classification, the parameter “T” evaluates the size of the tumor on a scale of 1 to 4 (T1–T4), “N” refers to the involvement of lymph nodes adjacent to the tumor, and “M” indicates the presence of metastases. Moreover, the molecular characterization of invasive breast cancer involves the presence and dosage of specific hormonal cellular receptors (HRs), such as estrogen-positive (ER+), progesterone-positive (PR+), and human epidermal growth factor receptor2-positive (HER2+) receptors, and a triple-negative tumor is characterized by the absence of all the abovementioned receptors. HR overexpression induces a high replicative power in the tumor, with consequent aggressiveness, and is therefore useful for predicting its growth over time. Hormone-responsive tumors represent approximately 70%, HER2-positive tumors represent about 20%, and triple-negative breast cancer cases comprise 15% [11]. BC classifications and their relative prevalence percentages and invasiveness/colonization processes are reported in Figure 1.
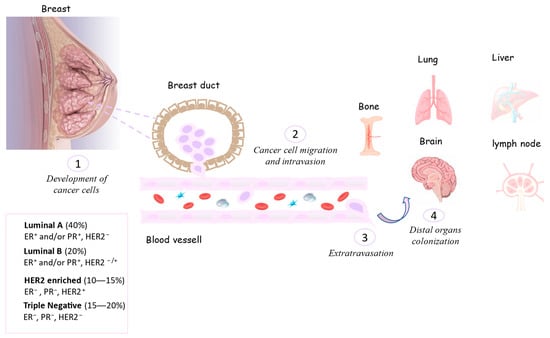
Figure 1.
Process of the invasiveness and colonization of breast tumor cells in other organs.
The evaluation of biological parameters, the cancer prognosis, the psycho-physical condition of the patient, and previous treatments received in a neoadjuvant/adjuvant context affect the choice of systemic treatment by a clinician. BC patients’ treatment is based on a surgical approach, preceded/followed by neoadjuvant/adjuvant treatment such as hormone therapy and targeted chemotherapy drugs, according to the type of tumor. Immunotherapies try to stimulate the lost immune system activity using vaccines, immune checkpoint inhibitors [11], and the modification of immune cells and microbiota, as occurs in endometrial diseases [12]. With awareness in the media of the topic and the regional distribution of screening campaigns as a preventive approach, accompanied by diagnostic–therapeutic implementation and the availability of new anticancer drugs, the overall survival of critical metastatic patients has significantly increased. There are many steps forward for the characterization and identification of BC and a therapeutic approach to its treatment, but there are still many points that need to be clarified. A classical approach investigated the alterations in canonical molecular pathways and the identification of new biomarkers such as miRNA involved in cancer progression, metastasis, and drug chemoresistance [13]. The identification of new pathways through the characterization of alternative forms of cell–cell communication that play a role in carcinogenesis and tumor aggressiveness is needed. In the context of microparticles, the involvement of extracellular vesicles (EVs) in many different types of tumors has already been investigated. The next section briefly introduces and describes EVs, the underlying reasons for their importance, and the success achieved in recent years.
1.3. Extracellular Vesicles (EVs)
EVs are very small lipid bilayer particles of a cellular origin, measuring in the order of nanometers to micrometers; they lack replicative power and express receptors that give them specificity and are produced by all three domains of Bacteria, Archaea, and Eukarya. Based on their size, the specific biomarkers expressed, and biogenesis mechanisms, they are divided into exosomes (40 to 150 nm), microvesicles (40 to 10,000 nm), and apoptotic bodies (100–5000 nm). According to the current classification, exosomes are subdivided into small and large (Exo-S, measuring 40–80 nm, and Exo-L, measuring 80–150 nm) exosomes, microvesicles are subdivided into ARMMs (arrestin domain-containing protein 1 (ARRDC1)-mediated microvesicles measuring 40–100 nm), which are distinguished from classical large microvesicles (~150–1000 nm) and large oncosomes (1–10 µm), and apoptotic EVs are subdivided into apoptotic bodies (1–5 µm) and apoptotic vesicles (~100–1000 nm) [14].
EVs’ cargo consists of several cell-derived components such as lipids, proteins, nuclei acids, and viral-derived particles (in case of infection), and their bilayer composition protects EVs’ cargo from aspecific cytotoxicity and consequent degradation. Figure 2 shows a typical exosome structure and main cargo component.
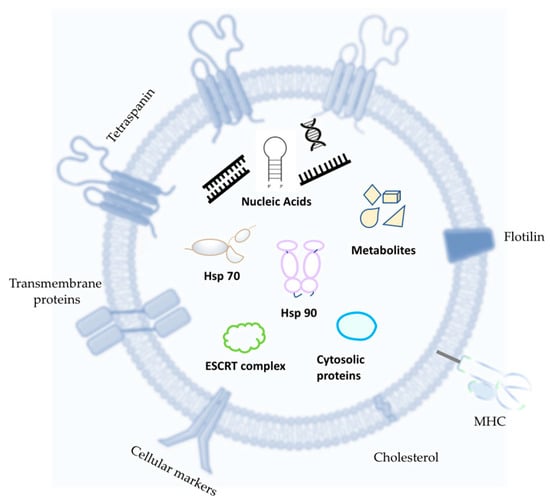
Figure 2.
Generic EV futures and cargo. The most common EV tetraspanins are Motility-Related Protein-1 (CD9), human leucocyte surface antigen (CD53), Cluster of Differentiation 36 (CD36), Cluster of Differentiation 81 (CD81), and Cluster of Differentiation 82 (CD82). Typical cytosolic proteins are tumor susceptibility gene 101 (TSG 101), ALG-2-interacting protein X (ALIX), annexins (ANXAS), C-X-C chemokine receptor type 4 (CXCR4), and epithelial cell adhesion molecule (EPCAM). Abbreviations: Major Histocompatibility Complex (MCH); heat shock protein 70 (Hsp70); heat shock protein 90 (Hsp90); Endosomal Sorting Complexes Required for Transport (ESCRT).
EVs are secreted by all cell types, both in physiological and pathological conditions, and are released by several biological fluids, such as blood, liquor, saliva, urine, milk, and others [15,16,17]. In the last decade, their characterization and functional studies have been implemented thanks to the use of highly technological and well-performing tools, reevaluating their importance. EVs have been identified as an alternative cell-to-cell communication strategy due to their cargo release to the receiving cell. Therefore, their contribution to homeostasis processes, tumoral context, and viral infections is well reported in the literature [18]. Thanks to their small size, ability to cross the blood–brain barrier, low toxicity, and nonimmunogenic phenotype, EVs can be engineered and directed to a specific target by the expression of particular surface structures (anchoring proteins and transmembrane), becoming valid candidates for drug delivery for precision medicine approaches.
1.3.1. EV Biogenesis
Exosomes are generated via the endo-lysosomal pathway by a process dependent on or independent from the endosomal sorting complex required for transport (ESCRT), through the intraluminal formation of multivesicular bodies and their fusion with the cell membrane, with consequent exocytosis, and they have a morphology typically defined as “cup-shaped” [19]. The chaperone Cdc37 assists protein kinase folding during exosome biogenesis and secretion, whereas autophagy-activating kinase ULK1, membrane-bound kinase SRC, and lipid kinase VPS34 intervene in exosome formation and release [20]. Microvesicles are generated by the extrusion of the cell membrane (budding/shedding), as well as apoptotic bodies when in an apoptotic cell. Apoptotic bodies can also derive from the endoplasmic reticulum, in which case they will not contain DNA. Although MVs originate as an extrusion of the plasma membrane, their arrangement in the bilayer of some lipids such as phosphatidylserine, aminophospholipids, and ethanolamine varies with respect to the structure of the mother membrane, resulting in their homogeneous (and not asymmetrical) distribution in the helix of the membrane [17].
EVs differ from each other in size, biogenesis process, density, and the presence/abundance of specific markers. However, their characterization becomes difficult due to the overlap of the size range of MVs and exosomes. The International Society for Extracellular Vesicles (ISEV) first published the Minimal Information for Studies of Extracellular Vesicles (MISEV) in 2014, updated in 2018, underlining the importance of defining the EV subcategories. Based on the latest evidence, the ISEV defines “extracellular vesicle (EV) as particles naturally released by cells, delimited by a lipid bilayer without the ability to replicate” [21].
1.3.2. EVs–Recipient Cell Interaction
The particular compositions of EV surface markers of different natures and structures (protein, lipid, etc.) are closely related to their original cell, and this makes EVs capable of specific interactions with recipient cells. In the presence of surface receptors and/or internalization, with the release of their cargo contents, EVs can influence the recipient cell’s physiological or pathological condition. Exosomes can interact with target cells through different mechanisms: interactions between proteins exposed by exosomes and the receptors of the target cell, activating them; modifications to exosomal membrane proteins whose fragments can act as ligands for cell surface receptors; and the fusion of exosomes with the target cell, resulting in the release of their contents.
Transmembrane proteins or adhesion molecules (CAM), integrins (ITG), tetraspanins (CD9, CD63, CD81) [22], growth factor receptors, heterotrimeric G proteins (GNA), and phosphatidyl-serine-binding MFGE8/lactadherin are able to mediate the interaction between the recipient cell and the extracellular vesicle, resulting in cargo release. Furthermore, cytosolic binding membrane receptor proteins, such as membrane-binding proteins (TSG101, annexins, Rabs), proteins responsible for signal transduction or scaffold proteins (syntenin), and extracellular proteins binding specifically or nonspecifically to membranes, such as fibronectin, acetylcholinesterase, collagen, and soluble secreted proteins (metalloproteinases, cytokines, growth factors), are implicated in EV uptake [23,24,25,26]. The mentioned EV uptake strategies are reported in Figure 3, such as EVs’ important role as diagnostic and prognostic biomarkers and their implication in therapeutic approaches and clinical applications (Figure 3).
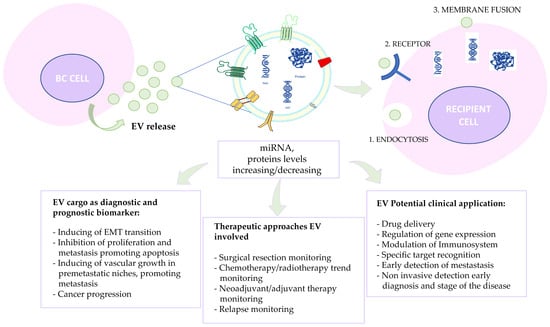
Figure 3.
Schematic representation of alternative exosome-mediated cell–cell communication via different modes: (1) endocytosis-mediated communication; (2) direct surface-bound receptor-mediated communication; and (3) exosome fusion with the recipient cell membrane. EVs feature as diagnostic and prognostic biomarkers, and their implications for therapeutic approaches and clinical applications are also reported.
1.4. EV Characterization
The most common EV isolation methods include ultracentrifugation, density gradient centrifugation, the size exclusion chromatography approach, ultrafiltration, and immunoprecipitation assays [27]. Once isolated, EV subpopulations are characterized using quantitative and qualitative criteria. EV morphology can be detected via transmission electron microscopy (TEM) and scanning electron microscope (SEM). To determine the vesicle size and concentration, single-particle interferometric reflectance (SP-IRIS) and nanoparticle tracking analyses (NTAs) are used, which are methods that are able to detect the particles’ intrinsic Brownian movement [28]. The number of particles can also be established via standard flow cytometry to evaluate large EVs and high-sensitivity flow cytometry (hsFCM) for small EVs [29], while resistive pulse sensing (RPS) is based on the pore size and adapted for a wide range of sizes [30]. Cryogenic electron microscopy (cryo-EM) and other technologies [31] are also used. EV characterization occurs via the identification of specific antigen–antibody recognition such as the use of an ELISA, Western blotting, specific antibodies for membrane proteins (tetraspanins) and cytosolic proteins (ALIX), peculiar markers of EV subgroups and the exclusion of other co-isolated materials, and the multiple-reaction-monitoring (MRM) approach [32]. The super-resolution microscopy approach, direct stochastic optical reconstruction microscopy (dSTORM) [33,34], is able to ascertain how and where EVs are clustered and localized; while with advanced fluorescence microscopy, it is possible to detect single EVs in real time, observe EVs during their interactions and uptake within living cells, and visualize their post-internalization movements in a time lapse. To better investigate this specific subject, the authors refer readers to the MISEV 2023 guidelines [35].
1.5. EVs in Cancer
As explained in the preceding section, EVs carry a significant amount of information and can also be used for drug delivery. These two hallmarks make them an important weapon against cancer. Their implications in cancer are very intricate and are still being characterized. Certainly, their role as cellular information mediators makes them capable of modifying both local and distant tumoral microenvironments [36]. Tumor-derived EVs (TEVs) carry cell-derived molecules, such as coding or noncoding RNAs and DNA fragments, contributing to tumor progression and malignancy [37]. So, in both a paracrine and systemic manner, TEVs promote cancer progression, transferring aggressive phenotype information and conferring drug resistance [37,38,39]. They also interfere with the antitumor immune response and mediate intercellular communication among stromal cells and bone marrow. On the other hand, TEVs are able to promote the formation of pre-metastatic niches to encourage the outgrowth of incoming cancer cells distant from the primary tumor site [37,40,41]. Microvesicles derived from tumoral endothelial miR-9 cells were reported to promote not only MV-induced endothelial cell migration but also tumor angiogenesis [42,43,44,45,46,47]. Some authors revealed that oral squamous cell carcinoma cell-derived extracellular vesicles (OSCC_EVs) were enriched in microRNA (miR)-21-5p and that EVs were associated with enhanced resistance to cisplatin, an increased metastatic phenotype, stemness, and a poor outcome in OSCC patients [43]. By conducting liquid biopsies in a minimally invasive or noninvasive way, it is easy to observe circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA), which are useful for monitoring patients and tumor evolution [45,46]. Similarly, EVs were found to be a source of prostate cancer biomarkers obtainable via liquid biopsies [47]. Another group correlated the pro-metastatic role of circulating EVs induced when undergoing neoadjuvant chemotherapy treatment with an enrichment in annexin A6 (ANXA6) in breast cancer patients [44].
2. EVs in Breast Cancer
An EV’s cargo is transferred from a parent cell to a recipient cell, so it is subsequently involved in tumor initiation and progression, metastasis formation via the dissemination of the oncogene in target cells, and remodeling the extracellular matrix [48,49,50,51,52]. The involvement of TEVs in BC has been reported in the literature for many years. In 2013, exosomes derived from the triple-negative breast cancer (TNBC) Hs578Ts(i)8 cell line were investigated, and their increased invasiveness in the recipient cells utilized was noted; for example, TNBC patients’ exosomes derived from sera were able to transfer phenotypic traits from the original cells to the receiving cells [53]. Certain authors have highlighted the role of exosomal miR-10b in tumoral invasiveness in the metastatic breast cancer MDA-MB-231 cell line [54]. Similar results were obtained for cancer-secreted miR-105, which regulated and induced cell migration via exosome-mediated transfer [55]. Tumor progression was investigated in response to hypoxic conditions; in particular, among the exosomal miRNAs, miR-16, let7a, miR-21, and miR-210 secreted by the MDA-MB-231 and T47D breast cancer cell lines, the latter was found to be significantly upregulated in hypoxic conditions and is thus a good target for a therapeutic approach [56]. Through the expression of peculiar exosomal integrins, exosomes can influence tumor cells’ tropism to enable metastases (integrins α6β4 and α6β1 are associated with pulmonary metastases, while αvβ5 is associated with liver metastases) [57]. Due to their own tendencies to be intrinsically biologically active and not only vectors of information, it has also been demonstrated that some precursor microRNAs (pre-miRNAs) present in BC-derived TEVs were processed into mature miRNAs within TEVs in a cell-independent manner. On the other hand, BC-EVs derived from the cells and sera of patients induced nontumorigenic epithelial cells to cause cancer in a Dicer-dependent manner, promoting tumorigenesis [57]. As shown in the current literature, the role of mesenchymal stem cells (MSCs) is gaining significant interest, and the results are often controversial, sometimes showing pro-tumor activity while demonstrating the opposite role in other cases [58]. In 2014, Ono et al. demonstrated that the coculture of a bone marrow metastatic human breast cancer cell line (BM2) with human donor-derived bone marrow mesenchymal stem cells (BM-MSCs) induced the acquisition of dormant phenotypes in the BM2 cells, causing unresponsiveness to traditional chemotherapy approaches. The same effect was achieved by culturing the cells with exosomes isolated from BM-MSC cultures. This evidence, together with the overexpression of miR-23b in BM-MSC-derived exosomes, suggests the role of exosomes in promoting BC cell dormancy in the metastatic niche [59]. Researchers demonstrated the anti-angiogenic effect of MSC-derived exosomes enriched in miR-16, which target vascular endothelial growth factor (VEGF) with a consequent inhibition of angiogenesis [60]. Some reported higher levels of circulating exosomal miR-373 in triple-negative carcinomas than in luminal carcinomas, such as in estrogen-negative and progesterone-negative tumors, suggesting a correlation of this exosomal mi-RNA with the aggressiveness of breast carcinomas [61]. Other authors demonstrated that BC-EVs could carry miR-182-5p, which is highly expressed in breast cancer tissues and cells, and were able to downregulate CKLF-like MARVEL transmembrane domain-containing 7 (CMTM7) expression and activate the EGFR/AKT signaling pathway, with a consequent aggravation of breast cancer and the promotion of breast cancer angiogenesis through EVs-miR-182-5p [62,63]. In 2022, Qi et al. promoted the involvement of the pluripotent factor Lin28B-high, which is particularly upregulated in triple-negative BC, and low-let-7s exosomes (sourced from Lin28B-induced breast cancer stem cells) to generate lung metastases in breast cancer patients via exosomes, supporting cancer progression [60]. Great progress has been made in recent years to characterize new pathways and identify new biomarkers in tumors using machine learning approaches. A very interesting example is a study using a machine learning approach that identified COL11A1 as a hub gene that is highly expressed in breast cancer samples and associated with a poor prognosis [64].
On the other hand, several studies reported EV proteomic signature profiling, which established a strong application of EV cargo in predicting clinical outcomes and monitoring disease progression and evolution. Using label-free quantitative phospho-proteomics, 144 phospho-proteins were identified in plasma EVs, the levels of which were found to be higher in BC-positive patients compared to healthy controls, showing the utility of EVs in cancer monitoring and screening [65]. Several proteins correlated with the tumor context were reported as EV cargo and found to be useful for tumor/health discrimination and tumor stage identification. For example, EGFR, epithelial cell adhesion molecule (EpCAM), and HER2 on circulating EVs were investigated as candidates to distinguish malignant BC patients from healthy controls; they were found to have sensitivities of 97.37% and 92.31% in detecting early stage I BC [66]. Moreover, a liquid biopsy was used to identify CD63/EpCAM/mucin 1-triple-positive circulating EVs in BC-positive patients, discriminating between patients and healthy samples with a sensitivity of 91% [67].
2.1. EVs’ Involvement in BC Drug Resistance
Therapeutic approaches in BC treatment are strongly related to the tumor classification, which provides information about the intrinsic future of drug resistance. The positive hormone receptors (HRs) in BC, such as the estrogen receptor (ER+) and the progesterone receptor (PR+), are generally sensitive to selective estrogen receptor modulators (SERMs), such as tamoxifen, and selective estrogen receptor downregulators (SERDs), such as fulvestrant [68]. Trastuzumab and lapatinib were found to be efficient in human epidermal growth factor receptor 2 (HER2)-positive BC treatment [69,70]. For both HR+ and HER2+ BC, chemotherapy and radiotherapy are suggested as neoadjuvant/adjuvant treatments to support a surgical approach. The cyclin-dependent kinase 4/6 inhibitor is now used in HR+/HER2-BC treatment and was recently proposed for TNBC treatment [71]. TNBCs cause low/absent expression levels of all HRs and present poor sensitivity to treatment, earning a reputation as a very aggressive and difficult-to-treat type of tumor. Regarding the intrinsic resistance phenomena, due to tumor heterogeneity, genetic and epigenetic mutations/futures, and the absence of expression of the drug target, acquired resistance is accompanied by a progressive loss of sensitivity to treatment over time, possibly due to alternative pathway expression or mutations that prevent drug efficiency. Alterations could occur in both cancer cells and/or at the expense of the tumor microenvironment (TME) [72]. The immunosuppressive microenvironment that can occur due to the presence of tumor-associated neutrophils (TANs), regulatory T cells (Tregs), and tumor-associated macrophages (TAMs), such as the secretion of immune suppression molecules, for instance, programmed cell death ligand 1 (PD-L1), leads to the acquisition of a treatment-resistant phenotype in BC. EVs are involved in therapy resistance; through their cargo, they influence the TME, although in a loop, TME cells communicate with BC cells via EVs. First, BC-derived EVs containing upregulated miR-146a are able to induce the transition of fibroblasts into cancer-associated fibroblasts (CAFs), a fundamental event in the formation of the TME [73]. CAFs promote more aggressive disease via several mechanisms, such as CAF-derived EVs enriched in miR-92 that induce increased PD-L1 expression in BC, inhibiting T cell performance with a consequent resistance to immunotherapies through immune suppression mechanisms [74].
MSCs are able to differentiate into adipocytes, chondrocytes, and osteoblasts, exhibiting plastic adherence, and they can be defined according to the presence/absence of the following biomarkers: CD73+, CD90+, CD105+ cells, CD14−, CD34−, CD45−, and human leukocyte antigen–DR isotype (HLA-DR) [75]. In physiological conditions, MSCs act as immunomodulators, but in BC-promoting tumor progression [76], EVs collected from MSCs promote increases in migration and proliferation in ER + BC via the wnt/β-catenin pathway, which is recognized to be implicated in BC drug resistance [77]. The controversial tumor-suppressor role of EV-derived MSCs in BC requires further research because they are reportedly able to suppress angiogenesis via the downregulation of VEGF, partially through miR-16 [60], and through the modulation of mTOR/HIF-1alpha/VEGF signaling suppression through miR-100 [78].
The involvement of RNA/proteins in EV cargo in the polarization of M2-type anti-inflammatory/immunosuppressive macrophages (alternatively activated) is well characterized and connected to cancer progression, lymph node metastasis, and responses to chemotherapy. An M2 immunosuppressive phenotype can be induced via an EV-mediated interplay between macrophages, cancer cells, and TME cells, which is associated with a poor prognostic outcome and lymph node metastasis [79,80,81,82,83,84]. Both M1-type pro-inflammatory (classically activated) and M2 macrophages were identified in the TME. In particular, the anticancer progression role of EVs was underlined in 2021 through the identification of miR-33-containing BC-derived EVs, which were able to induce M1 polarization in mouse macrophages [85], and the evidence that M1 macrophage-derived EVs cause BC sensitivity to chemotherapeutics [86]. Moreover, miRNA-205 is upregulated in tamoxifen-resistant MCF-7/TAMR-1 (M/T) cells and, also, in M/T cell-derived exosomes (M/T-Exo). Exosomal miRNA-205 could confer tamoxifen chemoresistance and tumorigenesis progress in recipient tumor cells by targeting E2F Transcription Factor 1 (E2F1) in human BC cells (BCCs) [87]. On the other hand, cancer stem cells (CSCs) are able to self-renew and differentiate into diverse cells, resulting in being resistant to chemotherapy; thus, they are a good therapeutic target. CD44+/CD24− surface markers were found to be minimum biomarkers for breast CSCs (BCSCs), and Her2- and CD44-positive EVs have been associated with tumor recurrence and metastasis [25].
EV-released biomolecules can change the metabolism/physiology of cells, conferring information capable of inducing a drug-resistant phenotype, such as the miR-181 family involved in drug resistance and metastasis progression in BC [88]. As shown in mouse models, CD63(+) cancer-associated fibroblasts (CAFs) confer tamoxifen resistance, and the sequestration of exosomal miR-22 induces overcoming tamoxifen resistance [89]. On the other hand, in an MCF7 xenograft mammary fat pad mouse model, Sansone et al. demonstrated the emergence from quiescence of metabolically dormant cells during hormonal treatment thanks to the transfer of exosome-derived mtDNA from CAFs to BC [90]. Previous evidence shows a controversial role for EVs, which, due to their content, are able to promote cancer progression and a drug resistance phenomenon or the inhibition of cancer progression [91,92,93,94,95,96,97,98,99,100]. Based on the evidence that EVs’ cargo is fundamental to cellular functions and also influences their responsiveness to drugs, the idea of engineering these nanovesicles into drug vehicles or molecules capable of directing cells towards a phenotype of sensitivity to the chosen treatment is currently a great challenge in precision medicine [101,102,103,104,105,106,107,108,109,110,111,112,113].
2.2. Exosomes as a Weapon in Precision Medicine
The field of EVs is highly speculative because the characterization of EVs’ contents, such as proteins and miRNAs, and their expression levels can be used as candidate markers for early detection, relapse, and metastasis prediction, such as susceptibility to therapeutic treatment in BC patients. Highly stratified by type and status, with a highly heterogeneous nature, BC is treated with typical approaches such as surgery and chemo/radio/hormone therapy but not personalized medicine [114,115,116,117,118,119,120,121,122,123,124,125,126]. The enrichment of protocols useful for obtaining and characterizing EVs through the arrival of cutting-edge technologies and the ease of isolating EVs from many biological matrices (blood, urine, milk, lymphatic exudate, etc.) in a noninvasive manner make them good candidates for biomarkers alone or in association with other emerging markers for different cancers [127,128,129,130,131,132,133,134,135]. The information obtained via the EVs’ fluid characterization can be useful and applied in BC diagnosis, monitoring after surgical resection, and during neoadjuvant/adjuvant therapy. The research led to the identification of several cancer-related markers (CD24, CD29, CD44, and CD146) and platelet markers (CD41b, CD42a, and CD62p) on EVs of potential interest as biomarkers; however, further characterizations are needed [66]. Many studies promote some markers EVs, in particular circulating EVs, as tools for several tumor diagnoses and prognostic biomarkers [120,121,122,123,124,125,126,127,128,129,130,131,132,133].
EVs’ low immune response and tolerability in the body, ease of internalization with the release of carried macromolecules, and ability to cross the blood–brain barrier due to their small size make them a good vehicle for drug delivery in precision medicine [136,137]. Although there are excellent prospects for the use of EVs, there still appear to be limitations, in part due to the half-life of the nanoparticles. In 2019, Saunderson et al. highlighted that the length of time for the clearance of exosomes from the blood of wild-type and CD169−/− mice was ~2 min, but a longer-lived reservoir of exosomes was present after 120 min in the spleen, although the majority of exosomes had been cleared from circulation [138]. The topical application of exosomes (buccal mucosa or ocular surface) shows a shorter exosome half-life caused by the rapid fluid turnover (saliva or tears) and the exposition of external elements. Due to the rapid clearance rate of circulating exogenous exosomes and consequently poor exosome enrichment in the target tissue, several studies in the literature promote strategies to increase the exosomes’ half-life in circulation. However, in vivo exosome half-life and clearance are closely associated with administrative methods, the availability of recipient cells for EV internalization, the size of the EVs, and the cell-type origin [139]. It is known that the exposition of CD47 proteins upon an exosome’s surface represents “don’t eat me” signaling, evading the monocytes’ phagocytosis with an increase in circulating exosomes [140]. The latest evidence suggests a hydrogel, an absorbable biological scaffold, as a vehicle able to achieve the stability and biological activity of exosomes [141]. In particular, hyaluronic-acid-based hydrogels have been used for a long time as an exosome delivery strategy in bone regeneration applications [142].
In particular, in Table 1, the main miRNAs and EV-derived biomarkers implicated in the diagnosis, prognosis, and drug sensitivity of BC and variations in their relative expression levels are shown.

Table 1.
miRNA and biomarkers derived from EVs implicated in BC diagnosis (a), prognosis (b), and chemoresistance (c).
Thus, EVs are identified as biomarkers, support cancer immunotherapies, and can act as a drug delivery mode for therapeutic agents. In fact, EVs can be genetically engineered to express specific monoclonal antibodies on their surfaces that are able to exert a host immune system response or the delivery of specific drugs for BC treatment (doxorubicin and reverse multidrug resistance) [143]. Some authors proposed HELA-Exos, a α-lactalbumin (α-LA)-engineered BC-derived exosome, to form an in situ dendritic cell (DC) vaccine to boost antitumor activity by promoting the activation of conventional DCs (cDC1s) in situ and tumor-reactive CD8+ T cell responses [144]. In Table 2, we report an actual clinical trial on BC exosomes, which was updated on 19 December 2023 [145].

Table 2.
Clinical trial: BC exosomes available at clinicaltrials.gov (last accessed on 19 December 2023).
3. An Artificial Intelligence Approach to Precision Medicine
Artificial intelligence (AI) is crucial in applying the precision medicine approach since it is able to elaborate on a significant amount of any kind of information. To better understand the reasons for the bond between AI and EVs and as a reason to keep investigating this kind of approach, we briefly report the definition of AI and its importance at present, focusing on health and breast cancer. AI, defined as a set of sciences, theories, and techniques, including mathematical logic, statistics, probabilities, computational neurobiology, and computer science, was created during the 1940s and experienced a new boom in 2010, mainly due to improvements in computing science and access to massive quantities of data [146]. AI tries to imitate human intelligence; therefore, it is necessary to develop a machine that has the basic abilities of vision, hearing, touch, induction, reasoning, knowledge acquisition, and the ability to think logically. A machine with these skills should be able to make optimal decisions to improve human life [147]. AI has a variety of applications in our daily lives, from smart watches that use AI to recognize motor activity to online shopping sites that suggest products based on previous purchasers’ records, but artificially intelligent systems have also made a great contribution to healthcare for patients’ diagnosis, prognosis, and care [148,149]. In particular, in recent decades, AI’s application for cancer diagnosis has increased quickly [150]. However, this kind of algorithm should be used carefully. In fact, AI helps in cancer detection, but only if it is combined with clinicians’ expertise [151]. In this sense, there seems to be a more interesting use of AI in the field, where the large number of parameters makes finding a correlation with cancer difficult. In this context, using living databases containing all kinds of patients’ data, from miRNAs to symptoms, extremely complex models can be customized for therapy selection, dose calculation, surveillance modality and scheduling, and so on [152].
As explained in previous sections, prevention and screening are the first steps in reducing BC mortality. Currently, mammography is used as a method of early detection, and the introduction of mammography screening was proven to significantly reduce BC mortality [153], although it can lead to false negatives and false positives and thus unnecessary invasive exams [154]. Therefore, in recent years, liquid biopsy has become a good candidate to surpass this limitation. A liquid biopsy allows for noninvasive sample collection and enables an improvement in BC’s early diagnosis, screening, prognosis, relapse, disease monitoring, and response to therapy. Many components of tumor cells, such as circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), cell-free RNA, miRNA, tumor-educated platelets, and EVs, can be analyzed in a liquid biopsy [155,156]. In this scenario, miRNAs appear as liquid biopsy biomarkers for early and noninvasive BC detection and prognosis [157,158,159,160]. These biomarkers come from both free-circulating miRNAs or miRNAs within EVs [161,162,163]. Thanks to the implementation of high-throughput technologies, such as microarray and NGS, thousands of miRNAs in BC samples can be profiled. Therefore, discerning a causal correlation between miRNAs’ expression and a coincidental one is impossible for clinicians or researchers. Here, AI becomes the key to this limitation. Moreover, AI can integrate patients’ miRNA profiles with omics data to define a precise strategy for diagnosis and therapy [164,165]. As reported before, EVs’ miRNAs carry more personalized information with respect to the circulating miRNA. Here resides the indissoluble bond between AI and EVs. EVs’ importance was recognized only in recent decades; therefore, AI applications to EVs’ miRNA are still limited. However, it will have future applications. Therefore, we propose an overview of AI’s applications with regard to miRNAs in BC for diagnosis and therapy. Rehman et al., using ML algorithms integrated with feature-selection methods, demonstrated that with just three selected miRNAs, classifiers can still detect breast cancer in comparison to the use of many more miRNAs [166]. Other studies report interesting results from integrating the data of circulating miRNAs using multiple classification algorithms, an ensemble of decision trees, neural networks, and support vector machines [167,168]. In recent years, many models have been proposed to detect biomarkers in miRNAs, from a tree-based model to the most advanced algorithms such as deep learning (DL) or convolutional neural networks (CNNs), in different types of cancer, but their applications are still limited. On the other hand, machine learning algorithms are widely used in BC imaging diagnoses, for example, mammography [147,169,170,171]. AI can detect breast lumps (masses), mass segmentation, breast density, and the risk of breast cancer [172]. However, many scientists have identified challenging issues in these techniques [173]. miRNAs can also be useful for classifying different subtypes of BC; several studies in the literature have shown a particular pattern of expression of miRNAs for each subtype [174,175]. This is probably the most challenging field for clinicians and AI since miRNAs’ expression could also be influenced by a patient’s specific characteristics. Nowadays, few studies report the use of AI algorithms to detect miRNA as a subtype of biomarker [176]. Just as in BC early detection, the most common applications in BC subtypes’ detection are related to clinicopathologic or imaging parameters. Other authors implemented a predictive model based on an advanced ML algorithm using clinicopathological variables. Using a powerful ML algorithm and a support vector machine (SMV), which is ideal for categorization tasks, with the Firefly algorithm, they achieved excellent results compared to other algorithms. They were able to identify TNBC and nonTNBC with high accuracy [177]. Furthermore, a radiomics-based model proposed showed promising results in BC subtype detection, with an accuracy of 0.902 [178]. Nevertheless, this field of AI still has many limitations and necessitates further study. Few studies report the application of AI in prognosis using an miRNA profile [178]. Despite the challenges presented, the exploration of AI in the analysis of miRNA for cancer holds great promise as a research area. As AI technology continues to advance and larger miRNA expression datasets become available, it is poised to assume an increasingly significant role in the realms of cancer diagnosis, prognosis, and treatment. In the foreseeable future, improvements in AI algorithms and heightened collaboration among researchers, clinicians, and industry stakeholders are anticipated to further facilitate the seamless integration of AI into the routine management of BC. Finally, the amalgamation of miRNA analyses with other omics data has the potential to offer a more comprehensive understanding of BC biology, paving the way for the development of targeted therapeutic approaches in relation to other cancers.
4. Conclusions
Considered waste-disposal mechanisms in the past, nanometer-size lipid EVs derived from living or apoptotic cells provide essential biological functions in intercellular communication. Actually, EVs’ proteomic and miRNome profiles have become a popular way to detect and characterize BC, a promising approach for cancer detection and monitoring. Moreover, EV cargo analyses are useful to understand drug resistance mechanisms in cancers and to characterize a particular future, which is useful for an early diagnosis. A multiomics analysis of tumor-derived EVs, which collects information obtained from exosome cargo and uses a machine learning approach, should be attempted to develop an panel of tumor types (phenotypes). Subsequently, the next step should be optimizing the extraction and characterization of exosomes obtained from biological fluids directly at a patient’s bedside in order to rapidly obtain predictive factors about tumor type and/or sensitivity and drug therapy to better direct the clinical approach toward a personalized approach. To reach this gold standard, the integration of AI will be necessary. Improvements in AI algorithms and heightened collaboration among researchers, clinicians, and industry stakeholders will be fundamental for the routine management of BC. Moreover, EVs’ features, such as their small dimensions, nontoxicity, organotropism target, and low level of immunogenicity, position them as nanocarriers to drive drug delivery.
Author Contributions
Resources, writing—original draft, and writing—review and editing, E.S., A.S. (Annafrancesca Smimmo) and A.B.; investigation and supervision, D.P. and M.D.D.; investigation, resources, and writing—original draft, M.A. and P.B.; drawing of figures, tables, bibliographic research, A.B., A.S. (Annafrancesca Smimmo) and E.S.; investigation, data curation, and software, G.M. and A.S. (Antonella Sciarra); resources, formal analysis, and software, A.D.A. and P.D.M.; investigation and data curation, M.M.M.; critical revision of the manuscript for important intellectual content and final approval, M.D.D. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union—NextGenerationEU through the Italian Ministry of University and Research under PNRR—M4C2-I1.3 Project PE_00000019 “HEAL ITALIA HEALTH EXTENDED ALLIANCE FOR INNOVATIVE THERAPIES, ADVANCED LAB-RESEARCH, AND INTEGRATED APPROACHES OF PRECISION MEDICINE”.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 7 November 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Xu, Y.; Gong, M.; Wang, Y.; Yang, Y.; Liu, S.; Zeng, Q. Global trends and forecasts of breast cancer incidence and deaths. Sci. Data 2023, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 13 November 2023).
- Available online: https://www.aiom.it/wp-content/uploads/2022/12/2022_AIOM_NDC-web.pdf (accessed on 30 November 2023).
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Marty, S.; Lamé, G.; Guével, E.; Priou, S.; Chatellier, G.; Tournigand, C.; Kempf, E.; A CRAB* initiative. Impact of the Sars-Cov-2 outbreak on the initial clinical presentation of new solid cancer diagnoses: A systematic review and meta-analysis. BMC Cancer 2024, 24, 143. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Serretiello, E.; Troiano, G.; Smimmo, A.; Dioguardi, M.; Spirito, F.; Sasso, F.C.; De Vito, D.; Lo Muzio, L.; et al. Multiparametric correlation of laboratory biomarkers to multiorgan failure outcome in hospitalized COVID-19 patients: A retrospective observational study. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8962–8974. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/data/gho/data/themes/topics/sdg-target-3_4-noncommunicable-diseases-and-mental-health (accessed on 9 November 2023).
- Alimirzaie, S.; Bagherzadeh, M.; Akbari, M.R. Liquid biopsy in breast cancer: A comprehensive review. Clin. Genet. 2019, 95, 643–660. [Google Scholar] [CrossRef]
- Available online: https://www.salute.gov.it/portale/tumori/dettaglioContenutiTumori.jsp?lingua=italiano&id=5542&area=tumori&menu=screening (accessed on 27 November 2023).
- Cicinelli, E.; Ballini, A.; Marinaccio, M.; Poliseno, A.; Coscia, M.F.; Monno, R.; De Vito, D. Microbiological findings in endometrial specimen: Our experience. Arch. Gynecol. Obstet 2012, 285, 1325–1329. [Google Scholar] [CrossRef]
- Dioguardi, M.; Cantore, S.; Sovereto, D.; La Femina, L.; Caloro, G.A.; Spirito, F.; Scacco, S.; Di Cosola, M.; Lo Muzio, L.; Troiano, G.; et al. Potential Role of miR-196a and miR-196b as Prognostic Biomarkers of Survival in Head and Neck Squamous Cell Carcinoma: A Systematic Review, Meta-Analysis and Trial Sequential Analysis. Life 2022, 12, 1269. [Google Scholar] [CrossRef]
- Sheta, M.; Taha, E.A.; Lu, Y.; Eguchi, T. Extracellular Vesicles: New Classification and Tumor Immunosuppression. Biology 2023, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Serretiello, E.; Ballini, A.; Smimmo, A.; Acunzo, M.; Raimo, M.; Cantore, S.; Di Domenico, M. Extracellular Vesicles as a Translational Approach for the Treatment of COVID-19 Disease: An Updated Overview. Viruses 2023, 15, 1976. [Google Scholar] [CrossRef]
- Votteler, J.; Sundquist, W.I. Virus budding and the ESCRT pathway. Cell Host Microbe 2013, 14, 232–241. [Google Scholar] [CrossRef]
- Di Domenico, M.; Giordano, A. Signal transduction growth factors: The effective governance of transcription and cellular adhesion in cancer invasion. Oncotarget. 2017, 8, 36869–36884. [Google Scholar] [CrossRef][Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Belov, L.; Matic, K.J.; Hallal, S.; Best, O.G.; Mulligan, S.P.; Christopherson, R.I. Extensive surface protein profiles of extracellular vesicles from cancer cells may provide diagnostic signatures from blood samples. J. Extracell. Vesicles 2016, 5, 25355. [Google Scholar] [CrossRef] [PubMed]
- Ekström, K.; Crescitelli, R.; Pétursson, H.I.; Johansson, J.; Lässer, C.; Olofsson Bagge, R. Characterization of surface markers on extracellular vesicles isolated from lymphatic exudate from patients with breast cancer. BMC Cancer 2022, 22, 50. [Google Scholar] [CrossRef]
- Williams, C.; Palviainen, M.; Reichardt, N.C.; Siljander, P.R.; Falcón-Pérez, J.M. Metabolomics Applied to the Study of Extracellular Vesicles. Metabolites 2019, 9, 276. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Vestad, B.; Llorente, A.; Neurauter, A.; Phuyal, S.; Kierulf, B.; Kierulf, P.; Skotland, T.; Sandvig, K.; Haug, K.B.F.; Ovstebo, R. Size and concentration analyses of extracellular vesicles by nanoparticle tracking analysis: A variation study. J. Extracell. Vesicles 2017, 6, 1344087. [Google Scholar] [CrossRef]
- Cointe, S.; Judicone, C.; Robert, S.; Mooberry, M.J.; Poncelet, P.; Wauben, M.; Nieuwland, R.; Key, N.S.; Dignat-George, F.; Lacroix, R. Standardization of microparticle enumeration across different flow cytometry platforms: Results of a multicenter collaborative workshop. J. Thromb. Haemost. 2017, 15, 187–193. [Google Scholar] [CrossRef] [PubMed]
- De Vrij, J.; Maas, S.L.; van Nispen, M.; Sena-Esteves, M.; Limpens, R.W.; Koster, A.J.; Leenstra, S.; Lamfers, M.L.; Broekman, M.L. Quantification of nanosized extracellular membrane vesicles with scanning ion occlusion sensing. Nanomedicine 2013, 8, 1443–1458. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23384702 (accessed on 7 December 2023). [CrossRef]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.M.; Mornet, S.; Brisson, A.R. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Tan, S.S.; Yeo, R.W.Y.; Choo, A.B.; Reiner, A.T.; Su, Y.; Shen, Y.; Fu, Z.; Alexander, L.; Sze, S.K.; et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J. Extracell. Vesicles 2016, 5, 29828. [Google Scholar] [CrossRef] [PubMed]
- Endesfelder, U.; Heilemann, M. Direct stochastic optical reconstruction microscopy (dSTORM). Methods Mol. Biol. 2015, 1251, 263–276. [Google Scholar] [CrossRef]
- McNamara, R.P.; Zhou, Y.; Eason, A.B.; Landis, J.T.; Chambers, M.G.; Willcox, S.; Peterson, T.A.; Schouest, B.; Maness, N.J.; MacLean, A.G.; et al. Imaging of surface microdomains on individual extracellular vesicles in 3-D. J. Extracell. Vesicles 2022, 11, e12191. [Google Scholar] [CrossRef]
- Available online: https://www.isev.org/misev (accessed on 10 December 2023).
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. Cell Physiol. 2020, 318, C29–C39. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.H.; Caires, H.R.; Abols, A.; Xavier, C.P.R.; Line, A. Extracellular vesicles as a novel source of biomarkers in liquid biopsies for monitoring cancer progression and drug resistance. Drug Resist. Updat. 2019, 47, 100647. [Google Scholar] [CrossRef]
- Arrigoni, R.; Ballini, A.; Santacroce, L.; Palese, L.L. The Dynamics of OXA-23 β-Lactamase from Acinetobacter baumannii. Int. J. Mol. Sci. 2023, 24, 17527. [Google Scholar] [CrossRef] [PubMed]
- Nardulli, P.; Ballini, A.; Zamparella, M.; De Vito, D. The Role of Stakeholders’ Understandings in Emerging Antimicrobial Resistance: A One Health Approach. Microorganisms 2023, 11, 2797. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, M.; Cantore, S.; Sovereto, D.; La Femina, L.; Spirito, F.; Caloro, G.A.; Caroprese, M.; Maci, M.; Scacco, S.; Lo Muzio, L.; et al. Does miR-197 Represent a Valid Prognostic Biomarker in Head and Neck Squamous Cell Carcinoma (HNSCC)? A Systematic Review and Trial Sequential Analysis. J. Pers. Med. 2022, 12, 1436. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Rani, S.; Corcoran, C.; Wallace, R.; Hughes, L.; Friel, A.M.; McDonnell, S.; Crown, J.; Chen, J.-H.; Wu, A.T.H.; et al. Ovatodiolide Suppresses Oral Cancer Malignancy by Down-Regulating Exosomal Mir-21/STAT3/β-Catenin Cargo and Preventing Oncogenic Transformation of Normal Gingival Fibroblasts. Cancers 2020, 12, 56. [Google Scholar] [CrossRef]
- Ricklefs, F.L.; Alayo, Q.; Krenzlin, H.; Mahmoud, A.B.; Speranza, M.C.; Nakashima, H.; Hayes, J.L.; Lee, K.; Balaj, L.; Passaro, C.; et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 2018, 4, eaar2766. [Google Scholar] [CrossRef] [PubMed]
- Keklikoglou, I.; Cianciaruso, C.; Güç, E.; Squadrito, M.L.; Spring, L.M.; Tazzyman, S.; Lambein, L.; Poissonnier, A.; Ferraro, G.B.; Baer, C.; et al. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat. Cell Biol. 2019, 21, 190–202. [Google Scholar] [CrossRef]
- Di Zazzo, E.; Feola, A.; Zuchegna, C.; Romano, A.; Donini, C.F.; Bartollino, S.; Costagliola, C.; Frunzio, R.; Laccetti, P.; Di Domenico, M.; et al. The p85 regulatory subunit of PI3K mediates cAMP-PKA and insulin biological effects on MCF-7 cell growth and motility. Sci. World J. 2014, 2014, 565839. [Google Scholar] [CrossRef]
- Amintas, S.; Bedel, A.; Moreau-Gaudry, F.; Boutin, J.; Buscail, L.; Merlio, J.P.; Vendrely, V.; Dabernat, S.; Buscail, E. Circulating tumor cell clusters: United we stand divided we fall. Int. J. Mol. Sci. 2020, 21, 2653. [Google Scholar] [CrossRef]
- Caponio, V.C.A.; Troiano, G.; Adipietro, I.; Zhurakivska, K.; Arena, C.; Mangieri, D.; Mascitti, M.; Cirillo, N.; Lo Muzio, L. Computational analysis of TP53 mutational landscape unveils key prognostic signatures and distinct pathobiological pathways in head and neck squamous cell cancer. Br. J. Cancer 2020, 123, 1302–1314. [Google Scholar] [CrossRef]
- Boccellino, M.; Ambrosio, P.; Ballini, A.; De Vito, D.; Scacco, S.; Cantore, S.; Feola, A.; Di Donato, M.; Quagliuolo, L.; Sciarra, A.; et al. The Role of Curcumin in Prostate Cancer Cells and Derived Spheroids. Cancers 2022, 14, 3348. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.M.; Nastri, B.M.; D’Agostino, M.; Risolo, R.; De Angelis, A.; Settembre, G.; Rienzo, M.; D’Esposito, V.; Abbondanza, C.; Formisano, P.; et al. Does Gut-breast Microbiota Axis Orchestrates Cancer Progression? Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Liu, Y.; Zhuang, X.; Zhang, S.; Michalek, S.; Taylor, D.D.; Grizzle, W.; Zhang, H.G. TLR2-mediated expansion of MDSCs is dependent on the source of tumor exosomes. Am. J. Pathol. 2010, 177, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- O’brien, K.; Rani, S.; Corcoran, C.; Wallace, R.; Hughes, L.; Friel, A.M.; McDonnell, S.; Crown, J.; Radomski, M.W.; O’driscoll, L. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Eur. J. Cancer 2013, 49, 1845–1859. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Pochampally, R.; Watabe, K.; Lu, Z.; Mo, Y.Y. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol. Cancer 2014, 13, 256. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, M.; Capasso, R.; Lombardi, A.; Di Domenico, M.; Fiorelli, A.; Feola, A.; Perna, A.F.; Santini, M.; Caraglia, M.; Ingrosso, D. Two Different Serum MiRNA Signatures Correlate with the Clinical Outcome and Histological Subtype in Pleural Malignant Mesothelioma Patients. PLoS ONE 2015, 10, e0135331. [Google Scholar] [CrossRef] [PubMed]
- King, H.W.; Michael, M.Z.; Gleadle, J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 2012, 12, 421. [Google Scholar] [CrossRef]
- Ayuko, H.; Bruno, C.S.; Tang-Long, S.; Goncalo, R.; Ayako, H.; Milica, T.M.; Henrik, M.; Shinji, K.; Angela, D.G.; Sophia, C. Tumour exosome integrins determine organo-tropic metastasis. Nature 2015, 527, 329–335. [Google Scholar]
- Melo, S.; Sugimoto, H.; O’Connell, J.T.; Kato, N.; Villanueva, A.; Vidal, A.; Qiu, L.; Vitkin, E.; Perelman, L.T.; Melo, C.A.; et al. Cancer exosomes perform cell-independent MicroRNA biogenesis and promote tumorigenesis. Cancer Cell 2014, 26, 707–721. [Google Scholar] [CrossRef]
- Ono, M.; Kosaka, N.; Tominaga, N.; Yoshioka, Y.; Takeshita, F.; Takahashi, R.U.; Yoshida, M.; Tsuda, H.; Tamura, K.; Ochiya, T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal. 2014, 7, ra63. [Google Scholar] [CrossRef]
- Lee, J.K.; Park, S.R.; Jung, B.K.; Jeon, Y.K.; Lee, Y.S.; Kim, M.K.; Kim, Y.G.; Jang, J.Y.; Kim, C.W. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE 2013, 8, e84256. [Google Scholar] [CrossRef] [PubMed]
- Polimeno, L.; Francavilla, A.; Piscitelli, D.; Fiore, M.G.; Polimeno, R.; Topi, S.; Haxhirexha, K.; Ballini, A.; Daniele, A.; Santacroce, L. The role of PIAS3, p-STAT3 and ALR in colorectal cancer: New translational molecular features for an old disease. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10496–10511. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhao, Y.; Wang, J.; Shi, W.; Dong, F.; Xin, Y.; Zhao, X.; Liu, C. Breast cancer cell-derived extracellular vesicles transfer miR-182-5p and promote breast carcinogenesis via the CMTM7/EGFR/AKT axis. Mol. Med. 2021, 27, 78. [Google Scholar] [CrossRef]
- Qi, M.; Xia, Y.; Wu, Y.; Zhang, Z.; Wang, X.; Lu, L.; Dai, C.; Song, Y.; Xu, K.; Ji, W.; et al. Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nat. Commun. 2022, 13, 897. [Google Scholar] [CrossRef]
- Shi, W.; Chen, Z.; Liu, H.; Miao, C.; Feng, R.; Wang, G.; Chen, G.; Chen, Z.; Fan, P.; Pang, W.; et al. COL11A1 as an novel biomarker for breast cancer with machine learning and immunohistochemistry validation. Front. Immunol. 2022, 13, 937125. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Annaratone, L.; Cascardi, E.; Vissio, E.; Sarotto, I.; Chmielik, E.; Sapino, A.; Berrino, E.; Marchio, C. The Multifaceted Nature of Tumor Microenvironment in Breast Carcinomas. Pathobiology 2020, 87, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, C.; Pan, W.; Shen, J.; Guo, J.; Luo, T.; Feng, J.; Situ, B.; An, T.; Zhang, Y.; et al. Facile fluorescent aptasensor using aggregation-induced emission luminogens for exosomal proteins profiling towards liquid biopsy. Biosens. Bioelectron. 2020, 168, 112520. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, J.; Zhang, H.; Zhu, Y.; Liu, W.; Zhang, K.; Zhang, Z. Localized fluorescent imaging of multiple proteins on individual extracellular vesicles using rolling circle amplification for cancer diagnosis. J. Extracell. Vesicles 2020, 10, e12025. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, J.; Wang, M.; Li, M. Potential Prospect of CDK4/6 Inhibitors in Triple-Negative Breast Cancer. Cancer Manag. Res. 2021, 13, 5223–5237. [Google Scholar] [CrossRef]
- Cosentino, G.; Plantamura, I.; Tagliabue, E.; Iorio, M.V.; Cataldo, A. Breast Cancer Drug Resistance: Overcoming the Challenge by Capitalizing on MicroRNA and Tumor Microenvironment Interplay. Cancers 2021, 13, 3691. [Google Scholar] [CrossRef]
- Yang, S.S.; Ma, S.; Dou, H.; Liu, F.; Zhang, S.Y.; Jiang, C.; Xiao, M.; Huang, Y.X. Breast cancer-derived exosomes regulate cell invasion and metastasis in breast cancer via miR-146a to activate cancer associated fibroblasts in tumor microenvironment. Exp. Cell Res. 2020, 391, 111983. [Google Scholar] [CrossRef]
- Dou, D.; Ren, X.; Han, M.; Xu, X.; Ge, X.; Gu, Y.; Wang, X. Cancer-Associated Fibroblasts-Derived Exosomes Suppress Immune Cell Function in Breast Cancer via the miR-92/PD-L1 Pathway. Front. Immunol. 2020, 11, 2026. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Ahn, S.Y. The Role of MSCs in the Tumor Microenvironment and Tumor Progression. Anticancer Res. 2020, 40, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Wang, S.; Zhao, R.C. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol. Cell Biochem. 2013, 383, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; De Frenza, G.; Cantore, S.; Papa, F.; Grano, M.; Mastrangelo, F.; Tetè, S.; Grassi, F.R. In vitro stem cell cultures from human dental pulp and periodontal ligament: New prospects in dentistry. Int. J. Immunopathol. Pharmacol. 2007, 20, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; MatZin, A.A.; Ang, K.C.; Ch’ng, E.S. EvaluatingthePolarizationofTumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front. Oncol. 2019, 9, 1512. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Mandal, G.; RoyChowdhury, S.; Purohit, S.; Payne, K.K.; Anadon, C.; Gupta, A.; Swanson, P.; Yu, X.; Conejo-Garcia, J.R.; et al. Exosomes Produced by Mesenchymal Stem Cells Drive Differentiation of Myeloid Cells into Immunosuppressive M2-Polarized Macrophages in Breast Cancer. J. Immunol. 2019, 203, 3447–3460. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Song, X.; Li, Y.; Chen, B.; Zhao, W.; Wang, L.; Zhang, H.; Liu, Y.; Han, D.; Zhang, N.; et al. LncRNABCRT1promotes breast cancer progression by targeting miR-1303/PTBP3 axis. Mol. Cancer 2020, 19, 85. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, A.; Castoria, G.; de Falco, A.; Di Domenico, M.; Galdiero, M.; Nola, E.; Chambon, P.; Auricchio, F. In vitro phosphorylation and hormone binding activation of the synthetic wild type human estradiol receptor. J. Steroid. Biochem. Mol. Biol. 1991, 38, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.J.; Kim, H.S.; Hwang, E.H.; Woo, J.; Zhang, M.; Moon, W.K. Breast cancer cell-derived exosomes and macrophage polarization are associated with lymph node metastasis. Oncotarget 2018, 9, 7398–7410. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.X.; Wang, D.D.; Zhu, B.; Zhu, Y.Z.; Zheng, L.; Feng, Z.Q.; Qin, X.H. Exosomal miR-222 from adriamycin-resistant MCF-7 breast cancer cells promote macrophages M2 polarization via PTEN/Akt to induce tumor progression. Aging 2021, 13, 10415–10430. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Chaleshtori, M.; Bandehpour, M.; Heidari, N.; Mohammadi-Yeganeh, S.; MahmoudHashemi, S. Exosome-mediated miR-33 transfer induces M1 polarization in mouse macrophages and exerts antitumor effect in 4T1 breast cancer cell line. Int. Immunopharmacol. 2021, 90, 107198. [Google Scholar] [CrossRef]
- Walker, N.D.; Elias, M.; Guiro, K.; Bhatia, R.; Greco, S.J.; Bryan, M.; Gergues, M.; Sandiford, O.A.; Ponzio, N.M.; Leibovich, S.J.; et al. Exosomes from differentially activated macrophages influence dormancy or resurgence of breast cancer cells within bone marrow stroma. Cell Death Dis. 2019, 10, 59. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, L.J.; Zhang, X.Y. Exosomal miRNA-205 promotes breast cancer chemoresistance and tumorigenesis through E2F1. Aging 2021, 13, 18498–18514. [Google Scholar] [CrossRef]
- Wang, M.; Ji, S.; Shao, G.; Zhang, J.; Zhao, K.; Wang, Z.; Wu, A. Effect of exosome biomarkers for diagnosis and prognosis of breast cancer patients. Clin. Transl. Oncol. 2018, 20, 906–911. [Google Scholar] [CrossRef]
- Vinik, Y.; Ortega, F.G.; Mills, G.B.; Lu, Y.; Jurkowicz, M.; Halperin, S.; Aharoni, M.; Gutman, M.; Lev, S. Proteomic analysis of circulating extracellular vesicles identi es potential markers of breast cancer progression, recurrence, and response. Sci. Adv. 2020, 6, eaba5714. [Google Scholar] [CrossRef]
- Kibria, G.; Ramos, E.K.; Lee, K.E.; Bedoyan, S.; Huang, S.; Samaeekia, R.; Athman, J.J.; Harding, C.V.; Lötvall, J.; Harris, L.; et al. A rapid, automated surface protein pro ling of single circulating exosomes in human blood. Sci. Rep. 2016, 6, 36502. [Google Scholar] [CrossRef]
- Strotbek, M.; Schmid, S.; Sanchez-Gonzalez, I.; Boerries, M.; Busch, H.; Olayioye, M.A. miR-181 elevates Akt signaling by co-targeting PHLPP2 and INPP4B phosphatases in luminal breast cancer. Int. J. Cancer 2017, 140, 2310–2320. [Google Scholar] [CrossRef]
- Gao, Y.; Li, X.; Zeng, C.; Liu, C.; Hao, Q.; Li, W.; Zhang, K.; Zhang, W.; Wang, S.; Zhao, H.; et al. CD63+ Cancer-Associated Fibroblasts Confer Tamoxifen Resistance to Breast Cancer Cells through Exosomal miR-22. Adv. Sci. 2020, 7, 2002518. [Google Scholar] [CrossRef]
- Sansone, P.; Savini, C.; Kurelac, I.; Chang, Q.; Amato, L.B.; Strillacci, A.; Stepanova, A.; Iommarini, L.; Mastroleo, C.; Daly, L.; et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9066–E9075. [Google Scholar] [CrossRef]
- Lee, J.E.; Moon, P.G.; Cho, Y.E.; Kim, Y.B.; Kim, I.S.; Park, H.; Baek, M.C. Identifcation of EDIL3 on extracellular vesicles involved in breast cancer cell invasion. J. Proteomics 2016, 131, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Risha, Y.; Minic, Z.; Ghobadloo, S.M.; Berezovski, M.V. The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Sci. Rep. 2020, 10, 13572. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, J.; Tian, F.; Chang, J.; Zhang, W.; Sun, J. λ-DNA- and aptamer- mediated sorting and analysis of extracellular vesicles. J. Am. Chem. Soc. 2019, 141, 3817–3821. [Google Scholar] [CrossRef]
- Ciravolo, V.; Huber, V.; Ghedini, G.C.; Venturelli, E.; Bianchi, F.; Campiglio, M.; Morelli, D.; Villa, A.; Della Mina, P.; Menard, S.; et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J. Cell. Physiol. 2012, 227, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.H.; Xue, L.; Hsu, C.C.; Paez, J.S.; Pan, L.; Andaluz, H.; Wendt, M.K.; Iliuk, A.B.; Zhu, J.K.; Tao, W.A. Phosphopro-teins in extracellular vesicles as candidate markers for breast cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 3175–3180. [Google Scholar] [CrossRef]
- Chen, I.H.; Aguilar, H.A.; Paez Paez, J.S.; Wu, X.; Pan, L.; Wendt, M.K.; Iliuk, A.B.; Zhang, Y.; Tao, W.A. Analytical pipeline for discovery and veri cation of glycoproteins from plasma- derived extracellular vesicles as breast cancer biomarkers. Anal. Chem. 2018, 90, 6307–6313. [Google Scholar] [CrossRef]
- Ham, S.; Lima, L.G.; Chai, E.P.Z.; Muller, A.; Lobb, R.J.; Krumeich, S.; Wen, S.W.; Wiegmans, A.P.; Möller, A. Breast cancer-derived exosomes alter macrophage polarization via gp130/STAT3 signaling. Front. Immunol. 2018, 9, 871. [Google Scholar] [CrossRef]
- Menck, K.; Scharf, C.; Bleckmann, A.; Dyck, L.; Rost, U.; Wenzel, D.; Dhople, V.M.; Siam, L.; Pukrop, T.; Binder, C.; et al. Tumor- derived microvesicles mediate human breast cancer invasion through di erentially glycosylated EMMPRIN. J. Mol. Cell Biol. 2015, 7, 143–153. [Google Scholar] [CrossRef]
- Li, M.; Zou, X.; Xia, T.; Wang, T.; Liu, P.; Zhou, X.; Wang, S.; Zhu, W. A five-miRNA panel in plasma was identi ed for breast cancer diagnosis. Cancer Med. 2019, 8, 7006–7017. [Google Scholar] [CrossRef]
- Eichelser, C.; Stückrath, I.; Müller, V.; Milde-Langosch, K.; Wikman, H.; Pantel, K.; Schwarzenbach, H. Increased serum levels of circulating exosomal micro-RNA-373 in receptor-negative breast cancer patients. Oncotarget 2014, 5, 9650–9663. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, H.; Yang, F.; Qiu, R.; Zhao, X.; Gong, Z.; Yu, W.; Zhou, B.; Shen, B.; Zhu, W. miR-188-5p suppresses cellular proliferation and migration via IL6ST: A potential noninvasive diagnostic biomarker for breast cancer. J. Cell. Physiol. 2020, 235, 4890–4901. [Google Scholar] [CrossRef]
- Hannafon, B.N.; Trigoso, Y.D.; Calloway, C.L.; Zhao, Y.D.; Lum, D.H.; Welm, A.L.; Zhao, Z.J.; Blick, K.E.; Dooley, W.C.; Ding, W.Q. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016, 18, 90. [Google Scholar] [CrossRef]
- Qian, B.; Katsaros, D.; Lu, L.; Preti, M.; Durando, A.; Arisio, R.; Mu, L.; Yu, H. High miR-21 expression in breast cancer associated with poor disease-free survival in early stage disease and high TGF-beta1. Breast Cancer Res. Treat. 2009, 117, 131–140. [Google Scholar] [CrossRef]
- Shen, S.; Song, Y.; Zhao, B.; Xu, Y.; Ren, X.; Zhou, Y.; Sun, Q. Cancer-derived exosomal miR-7641 promotes breast cancer progression and metastasis. Cell Commun. Signal. 2021, 19, 20. [Google Scholar] [CrossRef]
- Kia, V.; Paryan, M.; Mortazavi, Y.; Biglari, A.; Mohammadi-Yeganeh, S. Evaluation of exosomal miR-9 and miR-155 targeting PTEN and DUSP14 in highly metastatic breast cancer and their effect on low metastatic cells. J. Cell Biochem. 2019, 120, 5666–5676. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.; Lima, N.D.S.; Sarian, L.O.; Matheu, A.; Ribeiro, M.L.; Derchain, S.F.M. Exosome-mediated breast cancer chemoresistance via miR-155 transfer. Sci. Rep. 2018, 8, 829. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer- secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Eichelser, C.; Flesch-Janys, D.; Chang-Claude, J.; Pantel, K.; Schwarzenbach, H. Deregulated serum concentrations of circulating cell-free microRNAs miR-17, miR-34a, miR-155, and miR-373 in human breast cancer development and progression. Clin. Chem. 2013, 59, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Iinuma, H.; Umemoto, Y.; Yanagisawa, T.; Matsumoto, A.; Jinno, H. Exosome-encapsulated microRNA-223-3p as a minimally invasive biomarker for the early detection of invasive breast cancer. Oncol. Lett. 2018, 15, 9584–9592. [Google Scholar] [CrossRef]
- Dioguardi, M.; Spirito, F.; Sovereto, D.; La Femina, L.; Campobasso, A.; Cazzolla, A.P.; Di Cosola, M.; Zhurakivska, K.; Cantore, S.; Ballini, A.; et al. Biological Prognostic Value of miR-155 for Survival Outcome in Head and Neck Squamous Cell Carcinomas: Systematic Review, Meta-Analysis and Trial Sequential Analysis. Biology 2022, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.H.; Kim, J.Y.; Cho, E.Y.; Oh, J.M.; Lee, J.E.; Kim, S.W.; Nam, S.J.; Park, Y.H.; Ahn, J.S.; Im, Y.H. Elevated Level of Nerve Growth Factor (NGF) in Serum-Derived Exosomes Predicts Poor Survival in Patients with Breast Cancer Undergoing Neoadjuvant Chemotherapy. Cancers 2021, 13, 5260. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, W.; Bu, J.; Li, Y.; Li, R.; Nie, R.; Xiao, C.; Ma, K.; Huang, X.; Li, Y. Exosomal protein CD82 as a diagnostic biomarker for precision medicine for breast cancer. Mol. Carcinog. 2019, 58, 674–685. [Google Scholar] [CrossRef]
- Moon, P.G.; Lee, J.E.; Cho, Y.E.; Lee, S.J.; Jung, J.H.; Chae, Y.S.; Bae, H.I.; Kim, Y.B.; Kim, I.S.; Park, H.Y.; et al. Identifcation of Developmental Endothelial Locus-1 on Circulating Extracellular Vesicles as a Novel Biomarker for Early Breast Cancer Detection. Clin. Cancer Res. 2016, 22, 1757–1766. [Google Scholar] [CrossRef]
- Khan, S.; Bennit, H.F.; Turay, D.; Perez, M.; Mirshahidi, S.; Yuan, Y.; Wall, N.R. Early diagnostic value of survivin and its alternative splice variants in breast cancer. BMC Cancer 2014, 14, 176. [Google Scholar] [CrossRef]
- Ando, W.; Kikuchi, K.; Uematsu, T.; Yokomori, H.; Takaki, T.; Sogabe, M.; Kohgo, Y.; Otori, K.; Ishikawa, S.; Okazaki, I. Novel breast cancer screening: Combined expression of miR-21 and MMP-1 in urinary exosomes detects 95% of breast cancer without metastasis. Sci. Rep. 2019, 9, 13595. [Google Scholar] [CrossRef]
- Grassini, D.; Cascardi, E.; Sarotto, I.; Annaratone, L.; Sapino, A.; Berrino, E.; Marchiò, C. Unusual patterns of HER2 expression in breast cancer: Insights and perspectives. Pathobiology 2022, 89, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sueta, A.; Yamamoto, Y.; Tomiguchi, M.; Takeshita, T.; Yamamoto-Ibusuki, M.; Iwase, H. Di erential expression of exosomal miRNAs between breast cancer patients with and without recurrence. Oncotarget 2017, 8, 69934–69944. [Google Scholar] [CrossRef]
- Ni, Q.; Stevic, I.; Pan, C.; MuÃàller, V.; Oliveira-Ferrer, L.; Pantel, K.; Schwarzenbach, H. Different signatures of miR-16, miR-30b and miR-93 in exosomes from breast cancer and DCIS patients. Sci. Rep. 2018, 8, 12974. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Hu, J.; Yang, Y.; Hu, H.; Zhou, D.; Ma, M.; Xu, N. Plasma extracellular vesicle-packaged microRNAs as candidate diagnostic biomarkers for early-stage breast cancer. Mol. Med. Rep. 2019, 20, 3991–4002. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Hu, T.; Liu, J.; Su, J.; Sun, J.; Ming, Y.; Li, J.; Wu, N.; Chen, H.; Zhou, M. Genomic instability-derived plasma extracellular vesicle-microRNA signature as a minimally invasive predictor of risk and unfavorable prognosis in breast cancer. J. Nanobio-Technol. 2021, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Drucker, A.; Yoo, B.H.; Khan, I.A.; Choi, D.; Montermini, L.; Liu, X.; Jovanovic, S.; Younis, T.; Rosen, K.V. Trastuzumab-induced upregulation of a protein set in extracellular vesicles emitted by ErbB2-positive breast cancer cells correlates with their trastuzumab sensitivity. Breast Cancer Res. 2020, 22, 105. [Google Scholar] [CrossRef]
- Yang, S.J.; Wang, D.D.; Li, J.; Xu, H.Z.; Shen, H.Y.; Chen, X.; Zhou, S.Y.; Zhong, S.L.; Zhao, J.; Tang, J.H. Predictive role of GSTP1-containing exosomes in chemotherapy-resistant breast cancer. Gene 2017, 623, 5–14. [Google Scholar] [CrossRef]
- Ning, K.; Wang, T.; Sun, X.; Zhang, P.; Chen, Y.; Jin, J.; Hua, D. UCH-L1-containing exosomes mediate chemotherapeutic resistance transfer in breast cancer. J. Surg. Oncol. 2017, 115, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, Z.; Hua, D.; He, D.; Wang, L.; Zhang, P.; Wang, J.; Cai, Y.; Gao, C.; Zhang, X.; et al. Essential role for TrpC5-containing extracellular vesicles in breast cancer with chemo- therapeutic resistance. Proc. Natl. Acad. Sci. USA 2014, 111, 6389–6394. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, L.; Zhu, Y.; Chen, Z.; Qi, X.; Jin, L.; Jin, J.; Hua, D.; Ma, X. Breast cancer resistance protein (BCRP)-containing circulating microvesicles contribute to chemoresistance in breast cancer. Oncol. Lett. 2015, 10, 3742–3748. [Google Scholar] [CrossRef]
- Li, T.; Tao, Z.; Zhu, Y.; Liu, X.; Wang, L.; Du, Y.; Cao, J.; Wang, B.; Zhang, J.; Hu, X. Exosomal annexin A6 induces gemcitabine resistance by inhibiting ubiquitination and degradation of EGFR in triple-negative breast cancer. Cell Death Dis. 2021, 12, 684. [Google Scholar] [CrossRef]
- Kavanagh, E.L.; Halasz, M.; Dowling, P.; Withers, J.; Lindsay, S.; Higgins, M.J.; Irwin, J.A.; Rudd, P.M.; Saldova, R.; McCann, A. N-Linked glycosylation pro les of therapeutic induced senescent (TIS) triple negative breast cancer cells (TNBC) and their extracellular vesicle (EV) progeny. Mol. Omics. 2021, 17, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Boldrup, L.; Ardito, F.; Gu, X.; Lo Muzio, L.; Nylander, K. Circulating miRNAs from blood, plasma or serum as promising clinical biomarkers in oral squamous cell carcinoma: A systematic review of current findings. Oral. Oncol. 2016, 63, 30–37. [Google Scholar] [CrossRef]
- Lo Muzio, L.; Farina, A.; Rubini, C.; Pezzetti, F.; Stabellini, G.; Laino, G.; Santarelli, A.; Pannone, G.; Bufo, P.; de Lillo, A.; et al. Survivin as prognostic factor in squamous cell carcinoma of the oral cavity. Cancer Lett. 2005, 225, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Togni, L.; Caponio, V.C.A.; Zerman, N.; Troiano, G.; Zhurakivska, K.; Lo Muzio, L.; Balercia, A.; Mascitti, M.; Santarelli, A. The Emerging Impact of Tumor Budding in Oral Squamous Cell Carcinoma: Main Issues and Clinical Relevance of a New Prognostic Marker. Cancers 2022, 14, 3571. [Google Scholar] [CrossRef] [PubMed]
- Mascitti, M.; Togni, L.; Caponio, V.C.A.; Zhurakivska, K.; Bizzoca, M.E.; Contaldo, M.; Serpico, R.; Lo Muzio, L.; Santarelli, A. Lymphovascular invasion as a prognostic tool for oral squamous cell carcinoma: A comprehensive review. Int. J. Oral. Maxillofac. Surg. 2022, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.X.; Xu, L.Y.; Cheng, L.; Qian, Q.; He, X.; Peng, W.T.; Zhu, Y.L. Bioinformatics analysis of dysregulated microRNAs in exosomes from docetaxel- resistant and parental human breast cancer cells. Cancer Manag. Res. 2019, 11, 5425–5435. [Google Scholar] [CrossRef] [PubMed]
- Zhurakivska, K.; Troiano, G.; Caponio, V.C.A.; Dioguardi, M.; Arena, C.; Lo Muzio, L. The Effects of Adjuvant Fermented Wheat Germ Extract on Cancer Cell Lines: A Systematic Review. Nutrients 2018, 10, 1546. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.F.V.E.; Di Domenico, M.; Caponio, V.C.A.; Pérez-Sayáns, M.; Camolesi, G.C.V.; Rojo-Álvarez, L.I.; Ballini, A.; García-García, A.; Padín-Iruegas, M.E.; Suaréz-Peñaranda, J.M. Pyrosequencing Analysis of O-6-Methylguanine-DNA Methyltransferase Methylation at Different Cut-Offs of Positivity Associated with Treatment Response and Disease-Specific Survival in Isocitrate Dehydrogenase-Wildtype Grade 4 Glioblastoma. Int. J. Mol. Sci. 2024, 25, 612. [Google Scholar] [CrossRef]
- Almangush, A.; Alabi, R.O.; Troiano, G.; Coletta, R.D.; Salo, T.; Pirinen, M.; Mäkitie, A.A.; Leivo, I. Clinical significance of tumor-stroma ratio in head and neck cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 480. [Google Scholar] [CrossRef]
- Liu, J.; Ye, Z.; Xiang, M.; Chang, B.; Cui, J.; Ji, T.; Zhao, L.; Li, Q.; Deng, Y.; Xu, L.; et al. Functional extracellular vesicles engineered with lipid-grafted hyaluronic acid effectively reverse cancer drug resistance. Biomaterials 2019, 223, 119475. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Saunderson, S.C.; Dunn, A.C.; Crocker, P.R.; McLellan, A.D. CD169 mediates the capture of exosomes in spleen and lymph node. Blood 2014, 123, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mager, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Takahashi, Y.; Chang, H.Y.; Wu, Y.W.; Yamamoto, A.; Hibama, Y. Blood concentrations of small extracellular vesicles are determined by a balance between abundant secretion and rapid clearance. J. Extracell. Vesicles 2020, 9, 1696517. [Google Scholar] [CrossRef] [PubMed]
- Cantore, S.; Crincoli, V.; Boccaccio, A.; Uva, A.E.; Fiorentino, M.; Monno, G.; Bollero, P.; Derla, C.; Fabiano, F.; Ballini, A.; et al. Recent Advances in Endocrine, Metabolic and Immune Disorders: Mesenchymal Stem Cells (MSCs) and Engineered Scaffolds. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wang, J.; An, R. Hyaluronic acid-based hydrogels: As an exosome delivery system in bone regeneration. Front. Pharmacol. 2023, 14, 1131001. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.; Vasu, S.; Kumar, U.S.; Kumar, M. Surface-Engineered Extracellular Vesicles in Cancer Immunotherapy. Cancers 2023, 15, 2838. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Qi, P.; Hou, J.; Liu, W.; He, Y.; Gao, X.; Yuan, C.; et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol. Cancer 2022, 21, 45. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/search?cond=Breast%20Cancer&term=Exosome&viewType=Card&page=1&limit=25 (accessed on 20 November 2023).
- Available online: https://www.coe.int/en/web/artificial-intelligence/history-of-ai (accessed on 7 January 2024).
- Xu, Y.; Liu, X.; Cao, X.; Huang, C.; Liu, E.; Qian, S.; Liu, X.; Wu, Y.; Dong, F.; Qiu, C.-W.; et al. Artificial intelligence: A powerful paradigm for scientific research. Innov. 2021, 2, 100179. [Google Scholar] [CrossRef]
- Basu, K.; Sinha, R.; Ong, A.; Basu, T. Artificial Intelligence: How is It Changing Medical Sciences and Its Future? Indian J. Dermatol. 2020, 65, 365–370. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, H.; Wang, H. Machine Learning and AI in Cancer Prognosis, Prediction, and Treatment Selection: A Critical Approach. J. Multidiscip. Healthc. 2023, 16, 1779–1791. [Google Scholar] [CrossRef]
- Pantanowitz, L.; Quiroga-Garza, G.M.; Bien, L.; Heled, R.; Laifenfeld, D.; Linhart, C.; Sandbank, J.; Albrecht Shach, A.; Shalev, V.; Vecsler, M.; et al. An artificial intelligence algorithm for prostate cancer diagnosis in whole slide images of core needle biopsies: A blinded clinical validation and deployment study. Lancet Digit. Health 2020, 2, e407–e416. [Google Scholar] [CrossRef]
- Dembrower, K.; Crippa, A.; Colón, E.; Eklund, M.; Strand, F.; ScreenTrustCAD Trial Consortium. Artificial intelligence for breast cancer detection in screening mammography in Sweden: A prospective, population-based, paired-reader, non-inferiority study. Lancet Digit. Health 2023, 5, e703–e711, Erratum in Lancet Digit. Health 2023, 5, e646. [Google Scholar] [CrossRef]
- Sebastian, A.M.; Peter, D. Artificial Intelligence in Cancer Research: Trends, Challenges and Future Directions. Life 2022, 12, 1991. [Google Scholar] [CrossRef]
- Tabar, L.; Yen, M.F.; Vitak, B.; Chen, H.H.; Smith, R.A.; Duffy, S.W. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet 2003, 361, 1405–1410. [Google Scholar] [CrossRef]
- Majid, A.S.; de Paredes, E.S.; Doherty, R.D.; Sharma, N.R.; Salvador, X. Missed Breast Carcinoma: Pitfalls and Pearls. RadioGraphics 2003, 23, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.K.Y.; Tan, P.H. Liquid Biopsy in Breast Cancer: A Focused Review. Arch. Pathol. Lab. Med. 2021, 145, 678–686. [Google Scholar] [CrossRef] [PubMed]
- D’Oronzo, S.; Lovero, D.; Palmirotta, R.; Stucci, L.S.; Tucci, M.; Felici, C.; Cascardi, E.; Giardina, C.; Cafforio, P.; Silvestris, F. Dissection of major cancer gene variants in subsets of circulating tumor cells in advanced breast cancer. Sci. Rep. 2019, 9, 17276. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, Z.; Talkhabi, M.; Taleahmad, S. Identification of potential microRNA diagnostic panels and uncovering regulatory mechanisms in breast cancer pathogenesis. Sci. Rep. 2022, 12, 20135. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Kim, Y.S.; Kang, K.N.; Kim, K.H.; Park, Y.J.; Kim, C.W. Multiple microRNAs as biomarkers for early breast cancer diagnosis. Mol. Clin. Oncol. 2021, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, T.; Wadhwa, R.; Gupta, R.; Paudel, K.R.; Collet, T.; Chellappan, D.K.; Gupta, G.; Perumalsamy, H.; Mehta, M.; Satija, S.; et al. MicroRNAs as Biomarker for Breast Cancer. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1597–1610. [Google Scholar] [CrossRef]
- Słyk-Gulewska, P.; Kondracka, A.; Kwaśniewska, A. MicroRNA as a new bioactive component in breast milk. Non-Coding RNA Res. 2023, 8, 520–526. [Google Scholar] [CrossRef]
- Kim, M.W.; Park, S.; Lee, H.; Gwak, H.; Hyun, K.A.; Kim, J.Y.; Jung, H.I.; Kim, S.I. Multi-miRNA panel of tumor-derived extracellular vesicles as promising diagnostic biomarkers of early-stage breast cancer. Cancer Sci. 2021, 112, 5078–5087. [Google Scholar] [CrossRef]
- Borsos, B.N.; Páhi, Z.G.; Ujfaludi, Z.; Sükösd, F.; Nikolényi, A.; Bankó, S.; Pankotai-Bodó, G.; Oláh-Németh, O.; Pankotai, T. BC-miR: Monitoring Breast Cancer-Related miRNA Profile in Blood Sera-A Prosperous Approach for Tumor Detection. Cells 2022, 11, 2721. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Deng, P.; Li, Z.; Xu, Y.; Li, H.; Su, W.; Qin, J. Advances of Exosomal miRNAs in Breast Cancer Progression and Diagnosis. Diagnostics 2021, 11, 2151. [Google Scholar] [CrossRef]
- Fawaz, A.; Ferraresi, A.; Isidoro, C. Systems Biology in Cancer Diagnosis Integrating Omics Technologies and Artificial Intelligence to Support Physician Decision Making. J. Pers. Med. 2023, 13, 1590. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Zuo, F.; Shi, H.; Jing, J. Artificial intelligence-based multi-omics analysis fuels cancer precision medicine. Semin. Cancer Biol. 2023, 88, 187–200. [Google Scholar] [CrossRef]
- Rehman, O.; Zhuang, H.; Muhamed Ali, A.; Ibrahim, A.; Li, Z. Validation of miRNAs as Breast Cancer Biomarkers with a Machine Learning Approach. Cancers 2019, 11, 431. [Google Scholar] [CrossRef]
- Floares, A.G.; Zety, A.V.; Floares, C.; Calin, G.; Carvalho, E.K.; Radi, K. i-Biomarker CaDx: A circulating miRNA-based multi-cancer detection tool with explainable AI for breast cancer. JCO Glob. Oncol. 2023, 9, 5. [Google Scholar] [CrossRef]
- Muthamilselvan, S.; Ramasami Sundhar Baabu, P.; Palaniappan, A. Microfluidics for Profiling miRNA Biomarker Panels in AI-Assisted Cancer Diagnosis and Prognosis. Technol. Cancer Res. Treat. 2023, 22, 15330338231185284. [Google Scholar] [CrossRef]
- Sun, P.; Fan, S.; Li, S.; Zhao, Y.; Lu, C.; Wong, K.C.; Li, X. Automated exploitation of deep learning for cancer patient stratification across multiple types. Bioinformatics 2023, 39, btad654. [Google Scholar] [CrossRef]
- Parvathavarthini, S.; Karthikeyani Visalakshi, N.; Shanthi, S. Breast Cancer Detection using Crow Search Optimization based Intuitionistic Fuzzy Clustering with Neighborhood Attraction. Asian Pac. J. Cancer Prev. 2019, 20, 157–165. [Google Scholar] [CrossRef]
- Kühl, J.; Elhakim, M.T.; Stougaard, S.W.; Rasmussen, B.S.B.; Nielsen, M.; Gerke, O.; Larsen, L.B.; Graumann, O. Population-wide evaluation of artificial intelligence and radiologist assessment of screening mammograms. Eur. Radiol. 2023. [Google Scholar] [CrossRef]
- Khalid, A.; Mehmood, A.; Alabrah, A.; Alkhamees, B.F.; Amin, F.; AlSalman, H.; Choi, G.S. Breast Cancer Detection and Prevention Using Machine Learning. Diagnostics 2023, 13, 3113. [Google Scholar] [CrossRef]
- Lee, J.; Kang, B.J.; Kim, S.H.; Park, G.E. Evaluation of Computer-Aided Detection (CAD) in Screening Automated Breast Ultrasound Based on Characteristics of CAD Marks and False-Positive Marks. Diagnostics 2022, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Blenkiron, C.; Goldstein, L.D.; Thorne, N.P.; Spiteri, I.; Chin, S.F.; Dunning, M.J.; Barbosa-Morais, N.L.; Teschendorff, A.E.; Green, A.R.; Ellis, I.O.; et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007, 8, R214. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, Y.; Wang, R.; Zhou, W.; Zhao, Z.; Liang, H.; Qi, L.; Zhao, W.; Wang, H.; Wang, C.; et al. Identification of differentially expressed miRNAs in individual breast cancer patient and application in personalized medicine. Oncogenesis 2016, 5, e194. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Dovrolis, N.; Zografos, E.; Theodoropoulos, C.; Zografos, G.C.; Michalopoulos, N.V.; Gazouli, M. Circulating miRNA Expression Profiling in Breast Cancer Molecular Subtypes: Applying Machine Learning Analysis in Bioinformatics. Cancer Diagn. Progn. 2022, 2, 739–749. [Google Scholar] [CrossRef]
- Sarkar, S.; Mali, K. Firefly-SVM predictive model for breast cancer subgroup classification with clinicopathological parameters. Digit. Health 2023, 9, 20552076231207203. [Google Scholar] [CrossRef]
- Cho, S.; Joo, B.; Park, M.; Ahn, S.J.; Suh, S.H.; Park, Y.W.; Ahn, S.S.; Lee, S.K. A Radiomics-Based Model for Potentially More Accurate Identification of Subtypes of Breast Cancer Brain Metastases. Yonsei Med. J. 2023, 64, 573–580. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).