Comparison of Thermal and High-Pressure Pasteurization on Immunoglobulins, Lysozyme and Microbial Quality of Donkey Colostrum
Abstract
1. Introduction
2. Materials and Methods
2.1. Donkey Colostrum Collection and Processing
2.2. Microbial Load Analysis
2.3. Immunoglobulin Content and Lysozyme Activity Analyses
2.4. Kinetic Data Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Microbial Load Analysis
3.2. Immnunoglobulin Content and Lysozyme Activity in Raw Colostrum
3.3. Effect of Thermal and High-Pressure Pasteurization
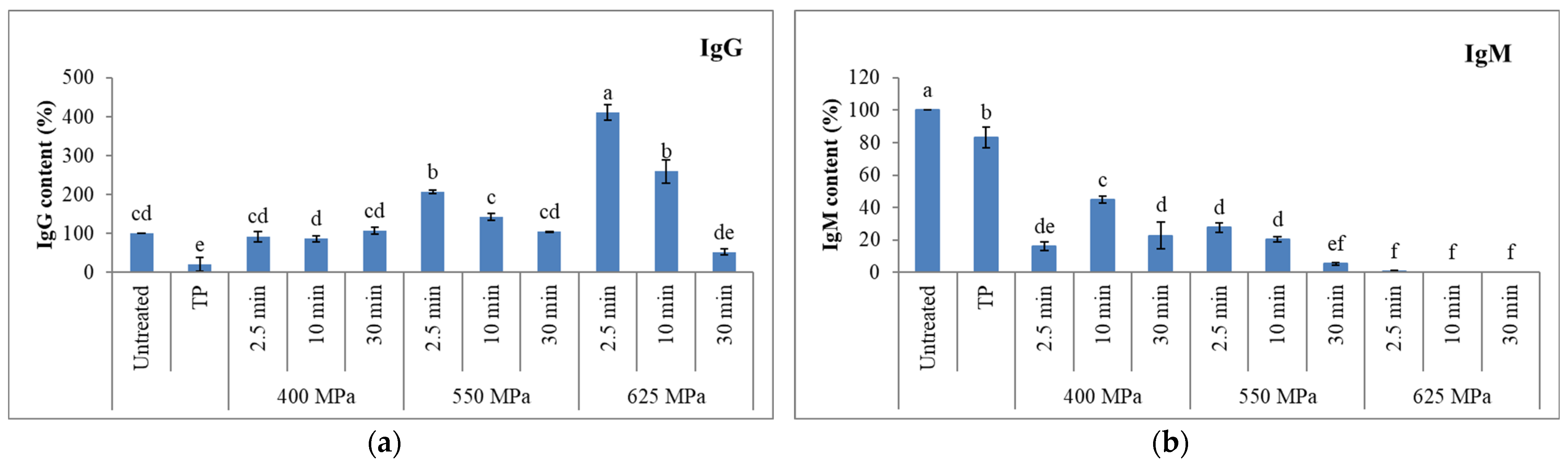
3.3.1. Immunoglobulins
- IgG
- IgM
- IgA
- Kinetics analysis for immunoglobulins
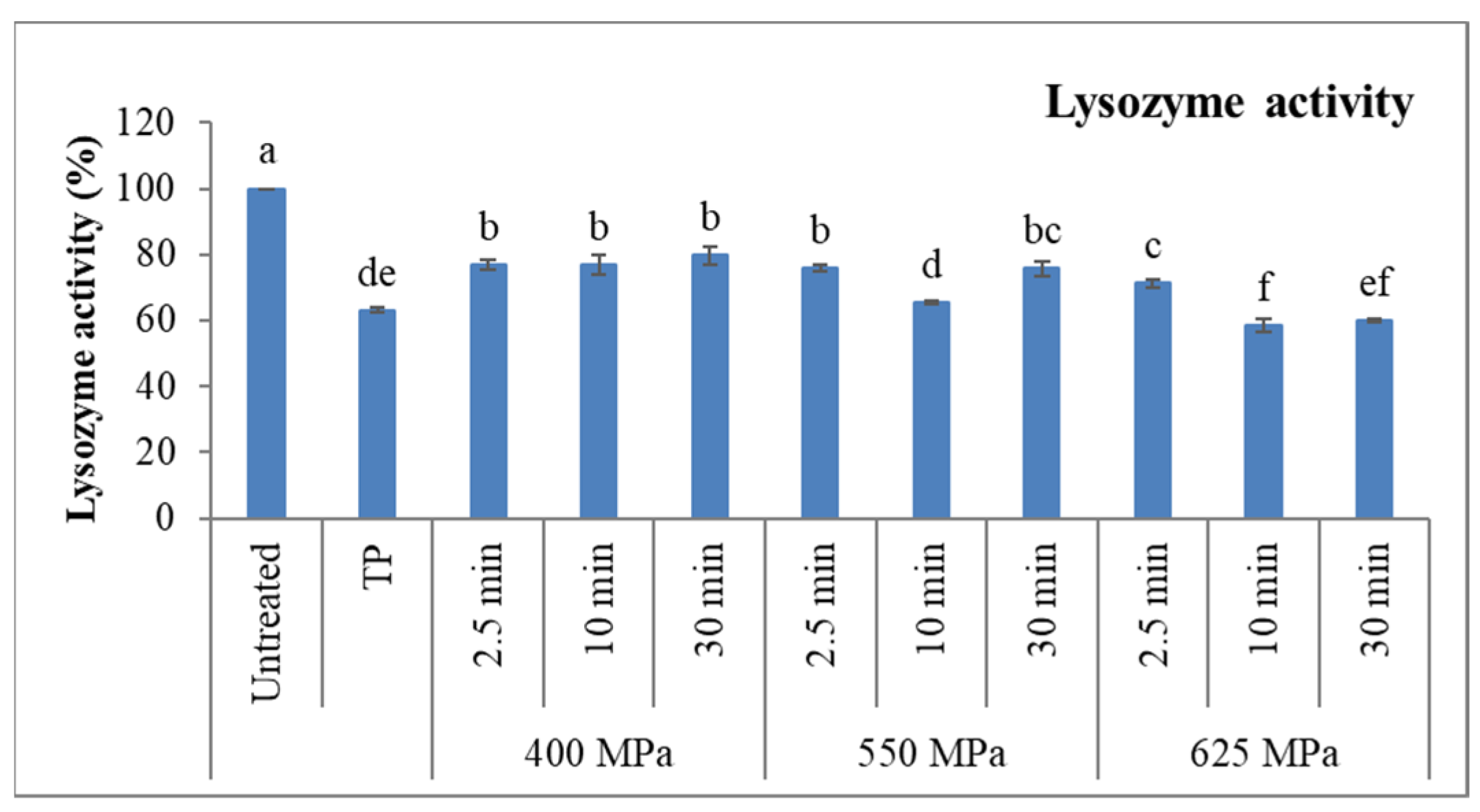
3.3.2. Lysozyme Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchis, Z.; Odagiu, A.; Coroian, A.; Oroian, I.; Mirza, M.; Burduhos, P. Analysis of Environmental Factors’ Impact on Donkeys’ Colostrum Quality. Sustainability 2018, 10, 2958. [Google Scholar] [CrossRef]
- Claeys, W.L.; Verraes, C.; Cardoen, S.; De Block, J.; Huyghebaert, A.; Raes, K.; Dewettinck, K.; Herman, L. Consumption of Raw or Heated Milk from Different Species: An Evaluation of the Nutritional and Potential Health Benefits. Food Control 2014, 42, 188–201. [Google Scholar] [CrossRef]
- Vincenzetti, S.; Pucciarelli, S.; Polzonetti, V.; Polidori, P. Role of Proteins and of Some Bioactive Peptides on the Nutritional Quality of Donkey Milk and Their Impact on Human Health. Beverages 2017, 3, 34. [Google Scholar] [CrossRef]
- Uruakpa, F.O.; Ismond, M.A.H.; Akobundu, E.N.T. Colostrum and Its Benefits: A Review. Nutr. Res. 2002, 22, 755–767. [Google Scholar] [CrossRef]
- Arslan, A.; Kaplan, M.; Duman, H.; Bayraktar, A.; Ertürk, M.; Henrick, B.M.; Frese, S.A.; Karav, S. Bovine Colostrum and Its Potential for Human Health and Nutrition. Front. Nutr. 2021, 8, 651721. [Google Scholar] [CrossRef]
- Escuder-Vieco, D.; Espinosa-Martos, I.; Rodríguez, J.M.; Fernández, L.; Pallás-Alonso, C.R. Effect of HTST and Holder Pasteurization on the Concentration of Immunoglobulins, Growth Factors, and Hormones in Donor Human Milk. Front. Immunol. 2018, 9, 2222. [Google Scholar] [CrossRef]
- Sousa, S.G.; Delgadillo, I.; Saraiva, J.A. Effect of Thermal Pasteurisation and High-Pressure Processing on Immunoglobulin Content and Lysozyme and Lactoperoxidase Activity in Human Colostrum. Food Chem. 2014, 151, 79–85. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. The Efficacy and Safety of High-pressure Processing of Food. EFSA J. 2022, 20, 1–195. [Google Scholar] [CrossRef]
- Dambrosio, A.; Capuozzo, F.; De Palo, P.; Mottola, A.; Storelli, M.M.; De Rosa, M.; Matrella, R.; Quaglia, N.C. Behaviour of Escherichia coli O157:H7 in Raw and Mild Pasteurised Donkey Milk Treated with High Pressure. Int. Dairy J. 2023, 136, 105486. [Google Scholar] [CrossRef]
- Giacometti, F.; Bardasi, L.; Merialdi, G.; Morbarigazzi, M.; Federici, S.; Piva, S.; Serraino, A. Shelf Life of Donkey Milk Subjected to Different Treatment and Storage Conditions. J. Dairy Sci. 2016, 99, 4291–4299. [Google Scholar] [CrossRef]
- Trujillo, A.J.; Castro, N.; Quevedo, J.M.; Argüello, A.; Capote, J.; Guamis, B. Effect of Heat and High-Pressure Treatments on Microbiological Quality and Immunoglobulin G Stability of Caprine Colostrum. J. Dairy Sci. 2007, 90, 833–839. [Google Scholar] [CrossRef]
- Foster, D.M.; Poulsen, K.P.; Sylvester, H.J.; Jacob, M.E.; Casulli, K.E.; Farkas, B.E. Effect of High-Pressure Processing of Bovine Colostrum on Immunoglobulin G Concentration, Pathogens, Viscosity, and Transfer of Passive Immunity to Calves. J. Dairy Sci. 2016, 99, 8575–8588. [Google Scholar] [CrossRef]
- Masuda, T.; Rehinarudo, H.Y.; Suzuki, K.; Sakai, T.; Morichi, T. The Effect of High Hydrostatic Pressure Treatment on the Preservability and the Immunological Activity of Bovine Colostrum. Asian-Australas. J. Anim. Sci. 2000, 13, 1323–1328. [Google Scholar] [CrossRef]
- Wesolowska, A.; Sinkiewicz-Darol, E.; Barbarska, O.; Strom, K.; Rutkowska, M.; Karzel, K.; Rosiak, E.; Oledzka, G.; Orczyk-Pawiłowicz, M.; Rzoska, S.; et al. New Achievements in High-Pressure Processing to Preserve Human Milk Bioactivity. Front. Pediatr. 2018, 6, 323. [Google Scholar] [CrossRef] [PubMed]
- Koutchma, T. Advances in Ultraviolet Light Technology for Non-Thermal Processing of Liquid Foods. Food Bioprocess Technol. 2009, 2, 138–155. [Google Scholar] [CrossRef]
- Updegrove, K. Human Milk Banking in the United States. Newborn Infant Nurs. Rev. 2005, 5, 27–33. [Google Scholar] [CrossRef]
- NP 4405:2002; General Guidance for the Enumeration of Microorganisms. Colony Count Technique at 30 °C. Insituto Português da Qualidade: Lisboa, Portugal, 2002.
- NP 4137:1991; Microbiologia Alimentar: Regras Gerais Para a Determinação de Enterobacteriaceae Sem Revitalização: Técnicas Do Número Mais Provável (NMP) e de Contagem de Colónias. Insituto Português da Qualidade: Lisboa, Portugal, 1991.
- Colavita, G.; Amadoro, C.; Salimei, E. Latte Di Asina: Aspetti Igienico-Sanitari e Normativi. Argom. SIVEMP 2011, 3, 61–70. [Google Scholar]
- Szteyn, J.; Wiszniewska, A.; Fus-szewczyk, M.M.; Cichosz, W.; Metais, V.Ū.R.R. Changes in Microbiological Quality of Raw Milk from the Region of Warmia and Mazury in 1998–2003. Vet. Zootech. 2005, 32, 2003–2006. [Google Scholar]
- Veronesi, M.C.; Dall’Ara, P.; Gloria, A.; Servida, F.; Sala, E.; Robbe, D. IgG, IgA, and Lysozyme in Martina Franca Donkey Jennies and Their Foals. Theriogenology 2014, 81, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Santana, C.; Pérez-Cano, F.J.; Audí, C.; Castell, M.; Moretones, M.G.; López-Sabater, M.C.; Castellote, C.; Franch, A. Effects of Cooling and Freezing Storage on the Stability of Bioactive Factors in Human Colostrum. J. Dairy Sci. 2012, 95, 2319–2325. [Google Scholar] [CrossRef]
- Yuen, J.W.M.; Loke, A.Y.; Gohel, M.-D.I. Nutritional and Immunological Characteristics of Fresh and Refrigerated Stored Human Milk in Hong Kong: A Pilot Study. Clin. Chim. Acta 2012, 413, 1549–1554. [Google Scholar] [CrossRef]
- Boudry, G.; Charton, E.; Le Huerou-Luron, I.; Ferret-Bernard, S.; Le Gall, S.; Even, S.; Blat, S. The Relationship between Breast Milk Components and the Infant Gut Microbiota. Front. Nutr. 2021, 8, 629740. [Google Scholar] [CrossRef]
- Carluccio, A.; De Amicis, I.; Panzani, S.; Tosi, U.; Faustini, M.; Veronesi, M. Electrolytes Changes in Mammary Secretions Before Foaling in Jennies. Reprod. Domest. Anim. 2008, 43, 162–165. [Google Scholar] [CrossRef]
- Živkov Baloš, M.; Ljubojević Pelić, D.; Jakšić, S.; Lazić, S. Donkey Milk: An Overview of Its Chemical Composition and Main Nutritional Properties or Human Health Benefit Properties. J. Equine Vet. Sci. 2023, 121, 104225. [Google Scholar] [CrossRef]
- Qureshi, A.S.; Enbergs, H. Studies on the Lysozyme Activity in the Milk of Female Donkeys (Equus asinus) in Relation to Reproductive Physiology. J. Anim. Plant Sci. 2012, 22, 70–74. [Google Scholar]
- Koenig, Á.; Diniz, E.M.D.A.; Barbosa, S.F.C.; Vaz, F.A.C. Immunologic Factors in Human Milk: The Effects of Gestational Age and Pasteurization. J. Hum. Lact. 2005, 21, 439–443. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chang, H.-M. Effect of Thermal Protectants on the Stability of Bovine Milk Immunoglobulin G. J. Agric. Food Chem. 1998, 46, 3570–3576. [Google Scholar] [CrossRef]
- Chen, C.-C.; Tu, Y.-Y.; Chang, H.-M. Thermal Stability of Bovine Milk Immunoglobulin G (IgG) and the Effect of Added Thermal Protectants on the Stability. J. Food Sci. 2000, 65, 188–193. [Google Scholar] [CrossRef]
- Elfstrand, L.; Lindmark-Månsson, H.; Paulsson, M.; Nyberg, L.; Åkesson, B. Immunoglobulins, Growth Factors and Growth Hormone in Bovine Colostrum and the Effects of Processing. Int. Dairy J. 2002, 12, 879–887. [Google Scholar] [CrossRef]
- Watanabe, T.; Nagura, H.; Keiichi, W.; Brown, W.R. The Binding of Human Milk Lactoferrin to Immunoglobulin A. FEBS Lett. 1984, 168, 203–207. [Google Scholar] [CrossRef]
- Law, A.J.R.; Leaver, J.; Felipe, X.; Ferragut, V.; Pla, R.; Guamis, B. Comparison of the Effects of High Pressure and Thermal Treatments on the Casein Micelles in Goat’s Milk. J. Agric. Food Chem. 1998, 46, 2523–2530. [Google Scholar] [CrossRef]
| Treatment | Storage Days | Total Aerobic Mesophilic | Enterobacteriaceae | Total Coliforms | |
|---|---|---|---|---|---|
| Untreated | 0 | <1.0 | <1.0 | <1.0 | |
| 4 | 6.7 ± 0.1 | <1.0 | |||
| 7 | 5.1 ± 0.1 | 3.5 ± 0.1 | |||
| 11 | 5.3 ± 1.9 | 4.4 ± 0.1 | |||
| 18 | 8.0 ± 2.5 | 8.2 ± 0.1 | |||
| TP | 0 | <1.0 | <1.0 | <1.0 | |
| 4 | |||||
| 7 | |||||
| 11 | |||||
| 18 | |||||
| 21 | |||||
| 28 | |||||
| 40 | |||||
| HPP | 400 MPa | 0 | <1.0 | <1.0 | <1.0 |
| 4 | |||||
| 7 | |||||
| 11 | |||||
| 18 | |||||
| 21 | |||||
| 28 | |||||
| 40 | |||||
| 550 MPa | 0 | <1.0 | <1.0 | <1.0 | |
| 4 | |||||
| 7 | |||||
| 11 | |||||
| 18 | |||||
| 21 | |||||
| 28 | |||||
| 40 | |||||
| Protein | Pressure (MPa) | k (min−1) | R2 | D-Value (min) |
|---|---|---|---|---|
| IgG | 550 | 2.31 × 10−2 ± 7.93 × 10−3 | 0.910 | 99.68 ± 18.01 |
| 625 | 7.57 × 10−2 ± 3.83 × 10−3 | 0.997 | 30.42 ± 1.57 | |
| IgM | 550 | 6.05 × 10−2 ± 5.60 × 10−3 | 0.992 | 38.06 ± 3.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, M.S.; Fidalgo, L.G.; Sousa, S.G.; Queirós, R.P.; Castro, S.M.; Pinto, C.A.; Saraiva, J.A. Comparison of Thermal and High-Pressure Pasteurization on Immunoglobulins, Lysozyme and Microbial Quality of Donkey Colostrum. Appl. Sci. 2024, 14, 1592. https://doi.org/10.3390/app14041592
Gonçalves MS, Fidalgo LG, Sousa SG, Queirós RP, Castro SM, Pinto CA, Saraiva JA. Comparison of Thermal and High-Pressure Pasteurization on Immunoglobulins, Lysozyme and Microbial Quality of Donkey Colostrum. Applied Sciences. 2024; 14(4):1592. https://doi.org/10.3390/app14041592
Chicago/Turabian StyleGonçalves, Mafalda S., Liliana G. Fidalgo, Silvia G. Sousa, Rui P. Queirós, Sónia M. Castro, Carlos A. Pinto, and Jorge A. Saraiva. 2024. "Comparison of Thermal and High-Pressure Pasteurization on Immunoglobulins, Lysozyme and Microbial Quality of Donkey Colostrum" Applied Sciences 14, no. 4: 1592. https://doi.org/10.3390/app14041592
APA StyleGonçalves, M. S., Fidalgo, L. G., Sousa, S. G., Queirós, R. P., Castro, S. M., Pinto, C. A., & Saraiva, J. A. (2024). Comparison of Thermal and High-Pressure Pasteurization on Immunoglobulins, Lysozyme and Microbial Quality of Donkey Colostrum. Applied Sciences, 14(4), 1592. https://doi.org/10.3390/app14041592








