Microbial Precipitation of Calcium Carbonate for Crack Healing and Stabilization of Sandy Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Cultivation
2.2. Preparation of Solutions for the MICP Experiment
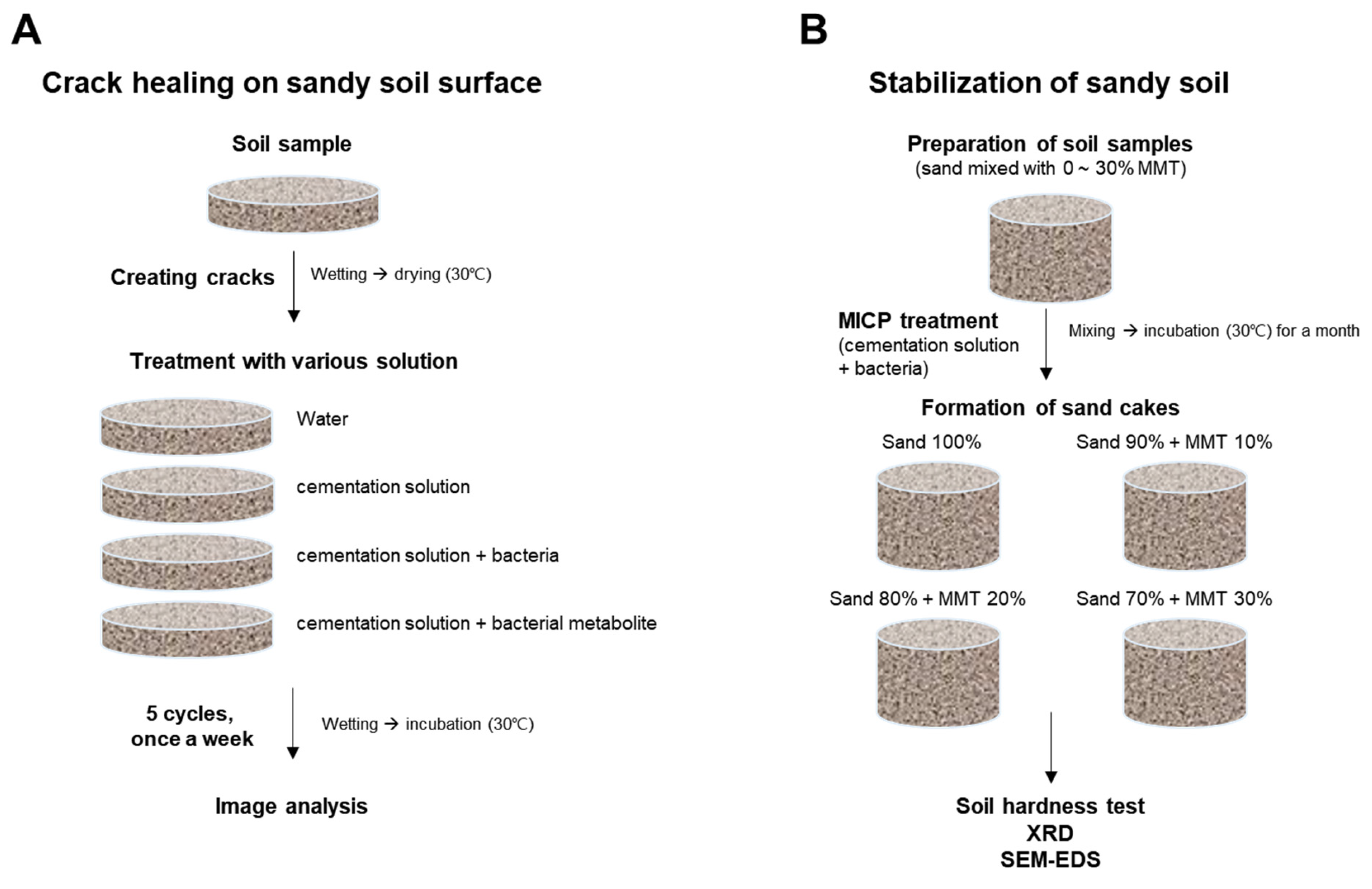
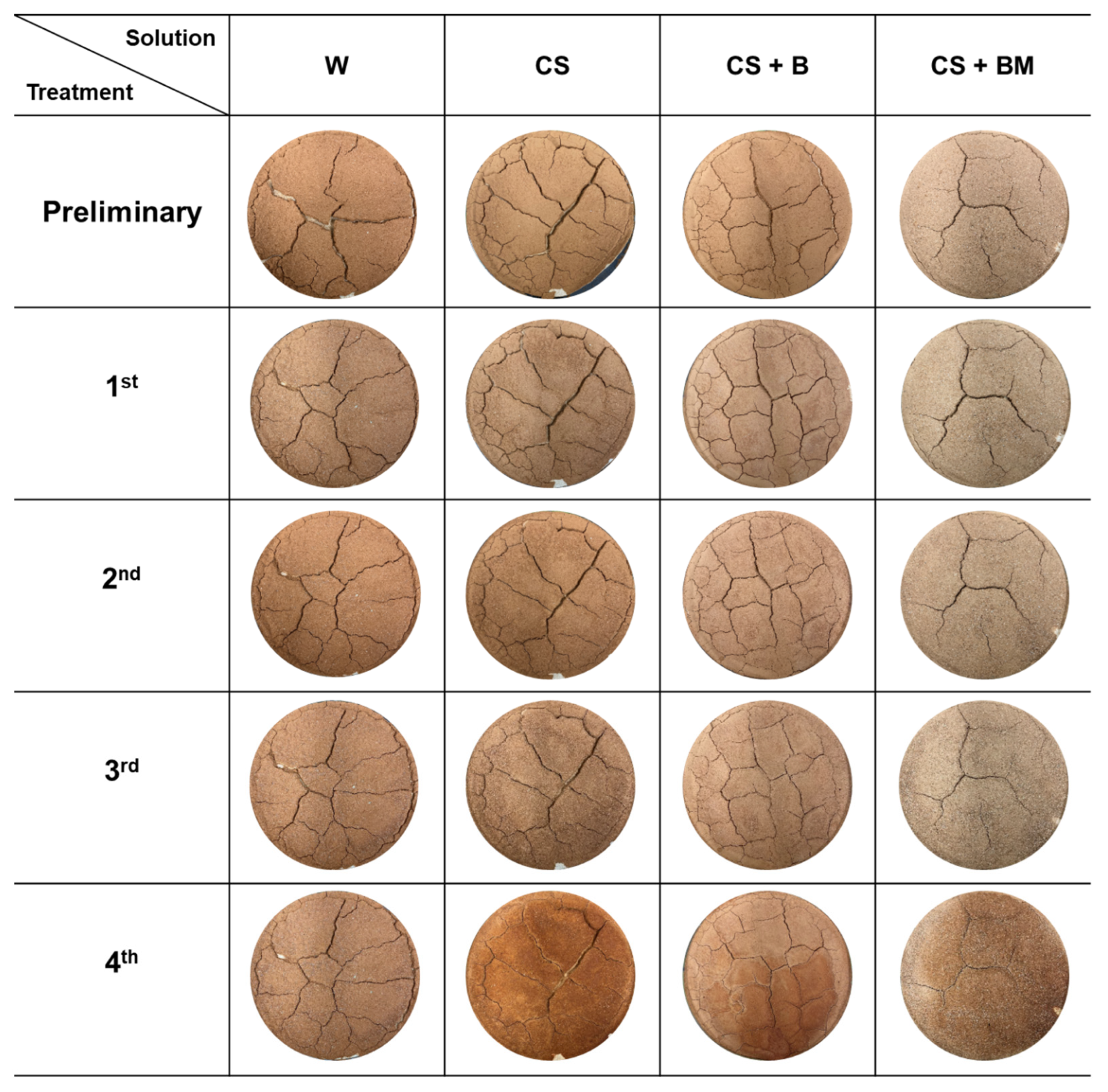
2.3. Healing of Desiccation Cracks on the Soil Surface Using S. pasteurii and Its Metabolites
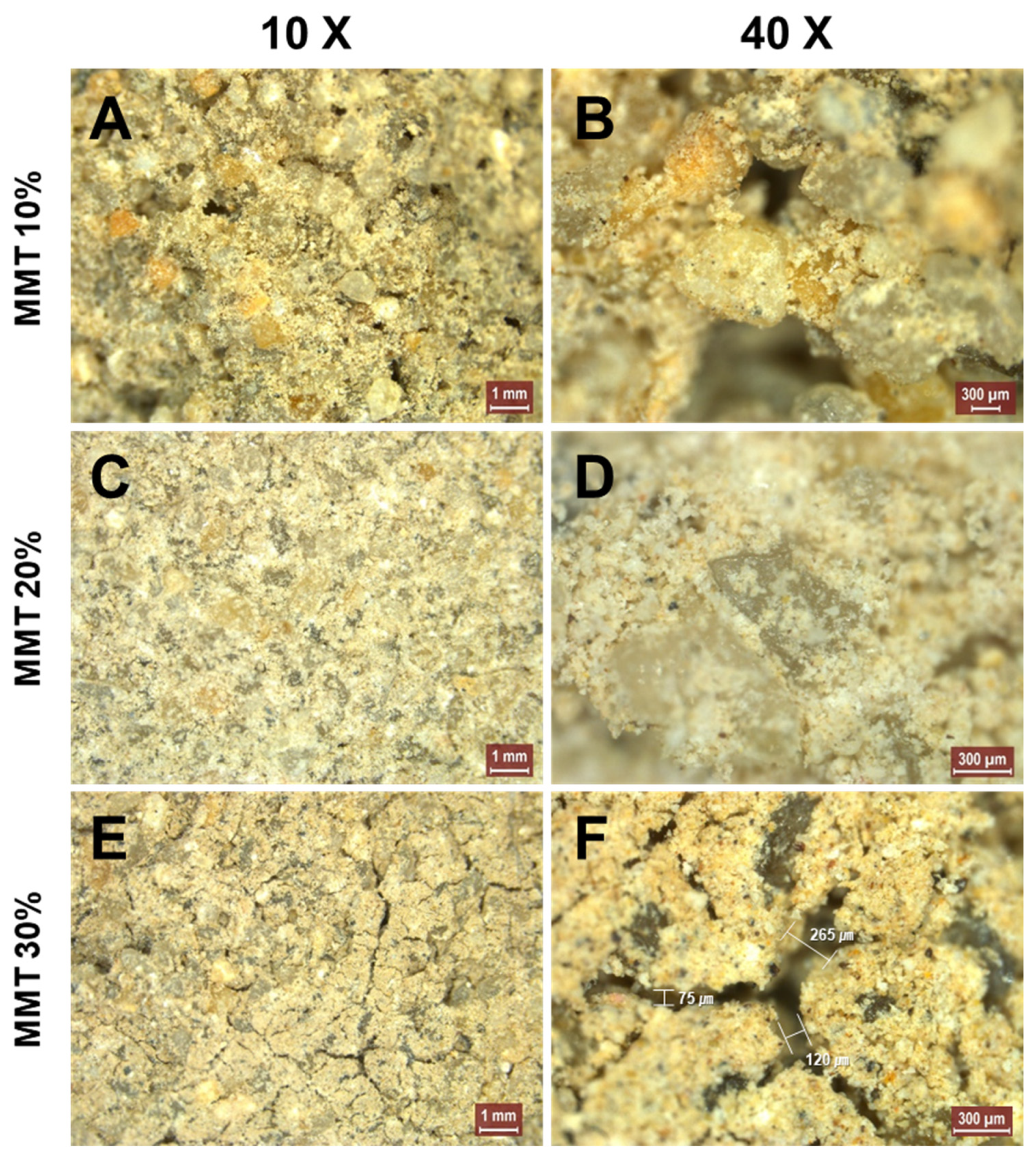
2.4. Effect of MMT on the Stabilization of Sandy Soil Using MICP
2.5. Analytical Methods
3. Results
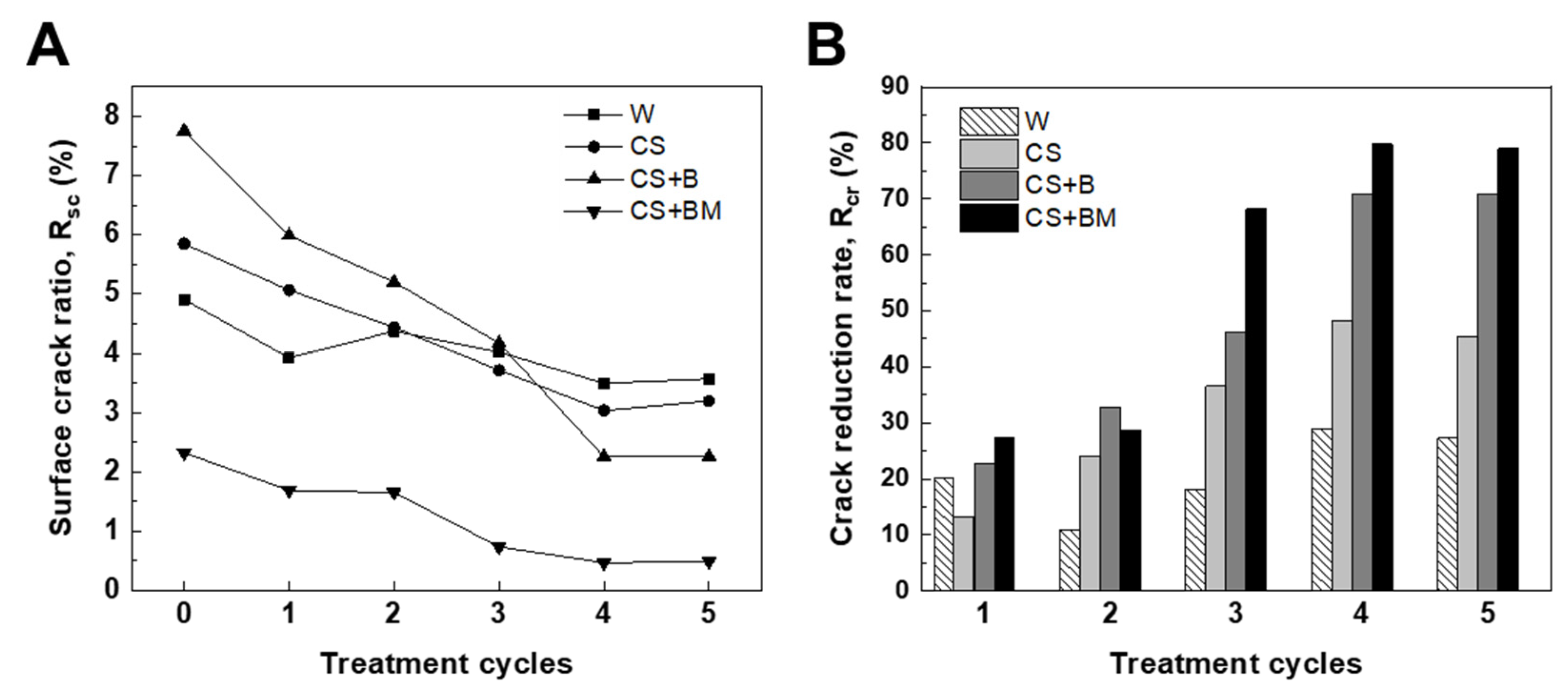
3.1. Healing of Desiccation Cracks on the Soil Surface via MICP
3.2. Stabilization of Sandy Soils Using MMT and MICP

4. Discussion
4.1. Healing of Desiccation Cracks on the Soil Surface Using MICP
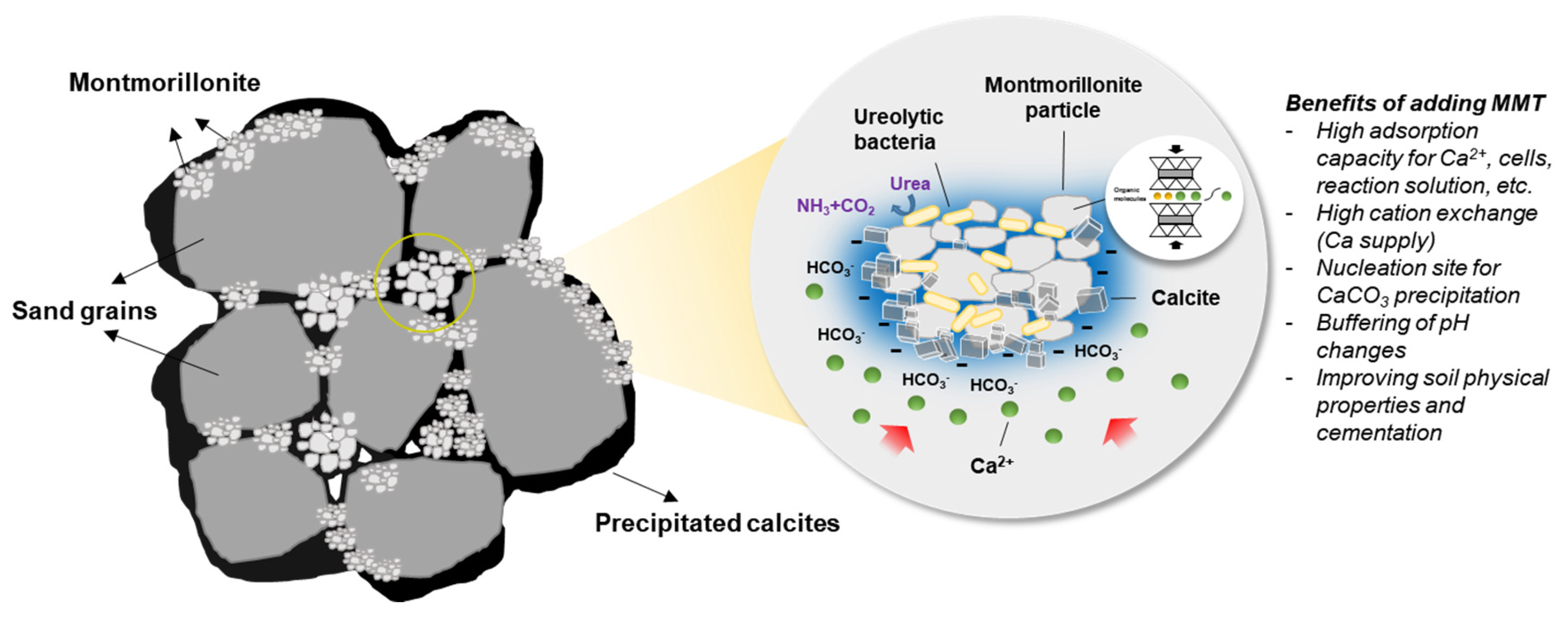
4.2. Combination of MMT and MICP for the Stabilization of Sandy Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, T.; Li, J.; Shi, P.; Li, Y.; Lei, J.; Zhou, J.; Hu, Z.; Duan, T.; Tang, Y.; Zhu, W. Effects of montmorillonite on the mineralization and cementing properties of microbiologically induced calcium carbonate. Adv. Mater. Sci. Eng. 2017, 2017, 7874251. [Google Scholar] [CrossRef]
- Brennan, S.T.; Lowenstein, T.K.; Horita, J. Seawater chemistry and the advent of biocalcification. Geology 2004, 32, 473–476. [Google Scholar] [CrossRef]
- Krausfeldt, L.E.; Farmer, A.T.; Castro Gonzalez, H.F.; Zepernick, B.N.; Campagna, S.R.; Wilhelm, S.W. Urea is both a carbon and nitrogen source for Microcystis aeruginosa: Tracking 13C incorporation at bloom pH conditions. Front. Microbiol. 2019, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.S.; Ferry, J.G. Prokaryotic carbonic anhydrases of Earth’s environment. Subcell. Biochem. 2014, 75, 77–87. [Google Scholar] [PubMed]
- Medina Ferrer, F.; Hobart, K.; Bailey, J.V. Field detection of urease and carbonic anhydrase activity using rapid and economical tests to assess microbially induced carbonate precipitation. Microb. Biotechnol. 2020, 13, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Decho, A.W. A laboratory investigation of cyanobacterial extracellular polymeric secretions (EPS) in influencing CaCO3 polymorphism. J. Cryst. Growth 2002, 240, 230–235. [Google Scholar] [CrossRef]
- Braissant, O.; Decho, A.W.; Dupraz, C.; Glunk, C.; Przekop, K.M.; Visscher, P.T. Exopolymeric substances of sulfate-reducing bacteria: Interactions with calcium at alkaline pH and implication for formation of carbonate minerals. Geobiology 2007, 5, 401–411. [Google Scholar] [CrossRef]
- Wei, S.; Cui, H.; Jiang, Z.; Liu, H.; He, H.; Fang, N. Biomineralization processes of calcite induced by bacteria isolated from marine sediments. Braz. J. Microbiol. 2015, 46, 455–464. [Google Scholar] [CrossRef]
- Mujah, D.; Shahin, M.A.; Cheng, L. State-of-the-art review of biocementation by microbially induced calcite precipitation (MICP) for soil stabilization. Geomicrobiol. J. 2016, 34, 524–537. [Google Scholar] [CrossRef]
- Kim, Y.; Kwon, S.; Roh, Y. Effect of divalent cations (Cu, Zn, Pb, Cd, and Sr) on microbially induced calcium carbonate precipitation and mineralogical properties. Front. Microbiol. 2021, 12, 646748. [Google Scholar] [CrossRef]
- Li, M.; Cheng, X.; Guo, H. Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int. Biodeterior. Biodegrad. 2013, 76, 81–85. [Google Scholar] [CrossRef]
- Kang, C.H.; Han, S.H.; Shin, Y.; Oh, S.J.; So, J.S. Bioremediation of Cd by microbially induced calcite precipitation. Appl. Biochem. Biotechnol. 2014, 172, 2907–2915. [Google Scholar] [CrossRef]
- Song, C.; Wang, C.; Elsworth, D.; Zhi, S. Compressive strength of MICP-treated silica sand with different particle morphologies and gradings. Geomicrobiol. J. 2022, 39, 148–154. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Palombo, E.A.; Ong, D.E.L.; Nissom, P.M. Biocementation of sand by Sporosarcina pasteurii strain and technical-grade cementation reagents through surface percolation treatment method. Constr. Build. Mater. 2019, 228, 116828. [Google Scholar] [CrossRef]
- Xiao, J.Z.; Wei, Y.Q.; Cai, H.; Wang, Z.W.; Yang, T.; Wang, Q.H.; Wu, S.F. Microbial-induced carbonate precipitation for strengthening soft clay. Adv. Mater. Sci. Eng. 2020, 2020, 8140724. [Google Scholar] [CrossRef]
- Pungrasmi, W.; Intarasoontron, J.; Jongvivatsakui, P.; Likitlersuang, S. Evaluation of microencapsulation techniques for MICP bacterial spores applied in self-healing concrete. Sci. Rep. 2019, 9, 12484. [Google Scholar]
- Borrego-Sánchez, A.; Ignacio Sainz-Díaz, C. Chapter 17—Clay minerals as filters of drug compounds for green chemistry applications. In Green Chemistry and Computational Chemistry; Mammino, L., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 403–423. [Google Scholar]
- Tetsuka, H.; Katayama, I.; Sakuma, H.; Tamura, K. Effects of humidity and interlayer cations on the frictional strength of montmorillonite. Earth Planets Space 2018, 70, 56. [Google Scholar] [CrossRef]
- Tan, J.; Yi, H.; Zhang, Z.; Meng, D.; Li, Y.; Xia, L.; Song, S.; Wu, L.; Sáncheze, R.M.T.; Farías, M.E. Montmorillonite facilitated Pb(II) biomineralization by Chlorella sorokiniana FK in soil. J. Hazard. Mater. 2022, 423, 127007. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Chen, W.; Cai, P. Cd(II) Sorption on montmorillonite-humic acid-bacteria composites. Sci. Rep. 2016, 6, 19499. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhu, J.; Wang, H. Sorption of humic acid on Fe oxides, bacteria, and Fe oxide-bacteria composites. J. Soils Sediments 2014, 14, 1378–1384. [Google Scholar] [CrossRef]
- Tang, G.; Jia, C.; Wang, G.; Yu, P.; Zhang, H. Role of Na-montmorillonite on microbially induced calcium carbonate precipitation. Molecules 2021, 26, 6211. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Pang, A.P.; Luo, Y.; Lu, X.; Lin, F. Beneficial factors for biomineralization by ureolytic bacterium Sporosarcina pasteurii. Microb. Cell Factories 2020, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Jiang, J.; Sun, H.; Huang, Y.; Tao, F.; Lian, B. Carbonate mineral formation under the influence of limestone-colonizing Actinobacteria: Morphology and polymorphism. Front. Microbiol. 2016, 7, 366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, Y.; Roh, Y. Microbially induced carbonate precipitation using microorganisms enriched from calcareous materials in marine environments and their metabolites. Minerals 2019, 9, 722. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, C.; Tang, C.S.; Xie, Y.H.; Yin, L.Y.; Cheng, Q.; Shi, B. Bio-remediation of desiccation cracking in clayey soils through microbially induced calcite precipitation (MICP). Eng. Geol. 2020, 264, 105389. [Google Scholar] [CrossRef]
- Miller, C.J.; Rifai, S. Fiber reinforcement for waste containment soil liners. J. Environ. Eng. 2004, 130, 891–895. [Google Scholar] [CrossRef]
- Chae, S.H.; Chung, H.; Nam, K. Evaluation of microbially induced calcite precipitation (MICP) methods on different soil types for wind erosion control. Environ. Eng. Res. 2021, 26, 190507. [Google Scholar] [CrossRef]
- Cardoso, R.; Vieira, J.; Borges, I. Water retention curve of biocemented sands using MIP results. Appl. Sci. 2022, 12, 10447. [Google Scholar] [CrossRef]
- Burdalski, R.J.; Ribeiro, B.G.O.; Gomez, M.G.; Gorman-Lewis, D. Mineralogy, morphology, and reaction kinetics of ureolytic bio-cementation in the presence of seawater ions and varying soil materials. Sci. Rep. 2022, 12, 17100. [Google Scholar] [CrossRef]
- Alimova, A.; Katz, A.; Steiner, N.; Rudolph, E.; Wei, H.; Steiner, J.C.; Gottlieb, P. Bacteria-clay interaction: Structure changes in smectite induced during biofilm formation. Clays Clay Miner. 2009, 57, 205–212. [Google Scholar] [CrossRef]
- Zhu, W.; Mu, T.; Zhang, Y.; Duan, T.; Luo, X. Coating of microbially produced calcium carbonate onto stone materials. Sci. China Technol. Sci. 2014, 58, 266–272. [Google Scholar] [CrossRef]
- Tourney, J.; Ngwenya, B.T. Bacterial extracellular polymeric substances (EPS) mediate CaCO3 morphology and polymorphism. Chem. Geol. 2009, 262, 138–146. [Google Scholar] [CrossRef]
- Castro-Alonso, M.J.; Montañez-Hernandez, L.E.; Sanchez-Muñoz, M.A.; Macias Franco, M.R.; Narayanasamy, R.; Balagurusamy, N. Microbially Induced Calcium Carbonate Precipitation (MICP) and Its Potential in Bioconcrete: Microbiological and Molecular Concepts. Front. Mater. 2019, 6, 126. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Kröger, R. Biomineralization: Ion binding and nucleation. Nat. Mater. 2015, 14, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.A.; Ravi, K.; Mukherjee, A.; Sahoo, L.; Abiala, M.A.; Dhami, N.K. Biocementation mediated by native microbes from Brahmaputra riverbank for mitigation of soil erodibility. Sci. Rep. 2021, 11, 15250. [Google Scholar] [CrossRef] [PubMed]
- Schultze-Lam, S.; Fortin, D.; Davis, B.S.; Beveridge, T.J. Mineralization of bacterial surfaces. Chem. Geol. 1996, 132, 171–181. [Google Scholar] [CrossRef]
- Frankel, R.; Bazylinski, D. Biologically induced mineralization by bacteria. Rev. Mineral. Geochem. 2003, 54, 95–114. [Google Scholar] [CrossRef]
- Yi, J.H.; Kim, S.J. Comparison of properties of natural Ca-Montmorillonite and its Al-pillared montmorillonites. J. Miner. Soc. Korea 2002, 15, 273–282. [Google Scholar]
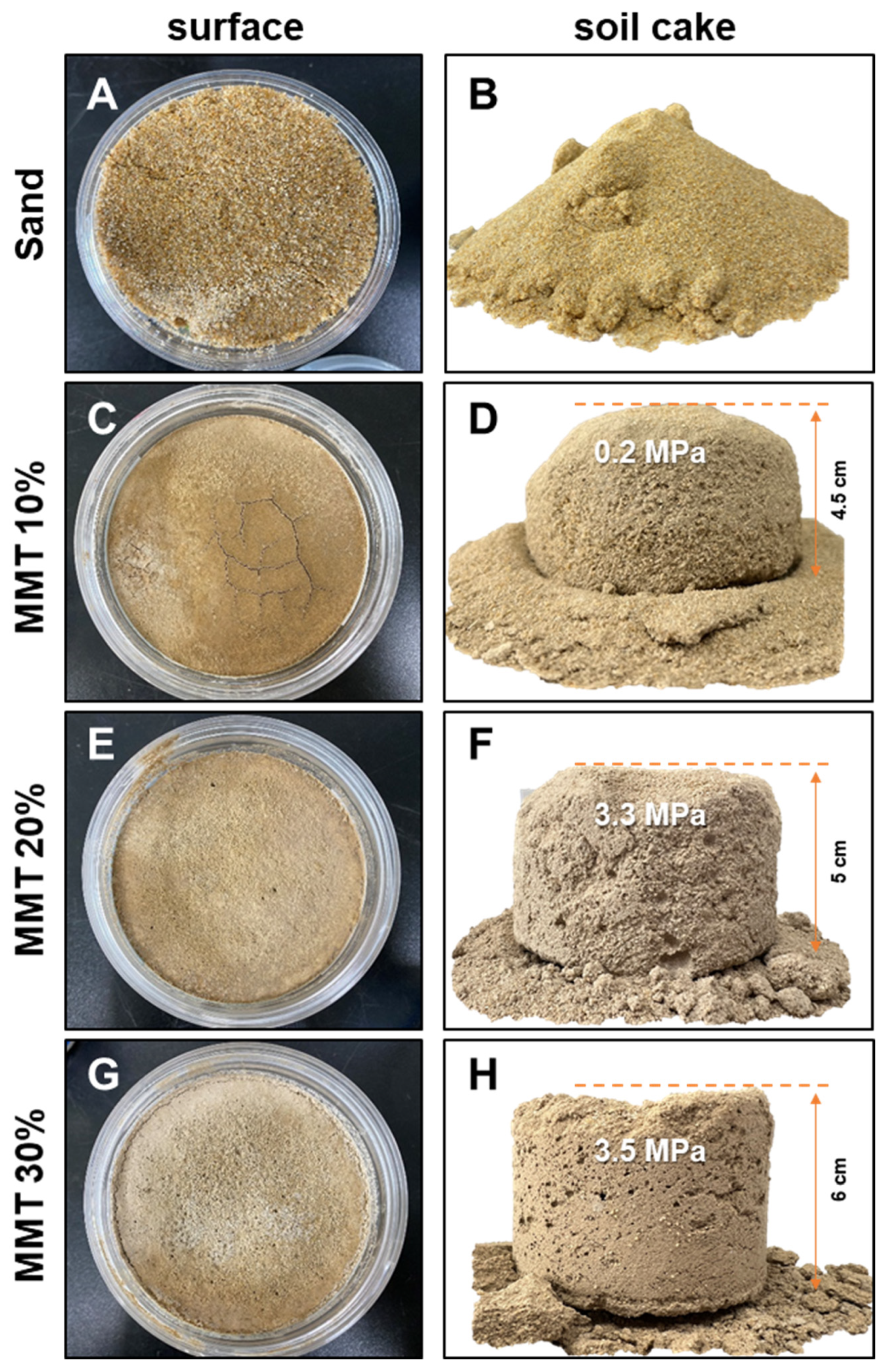

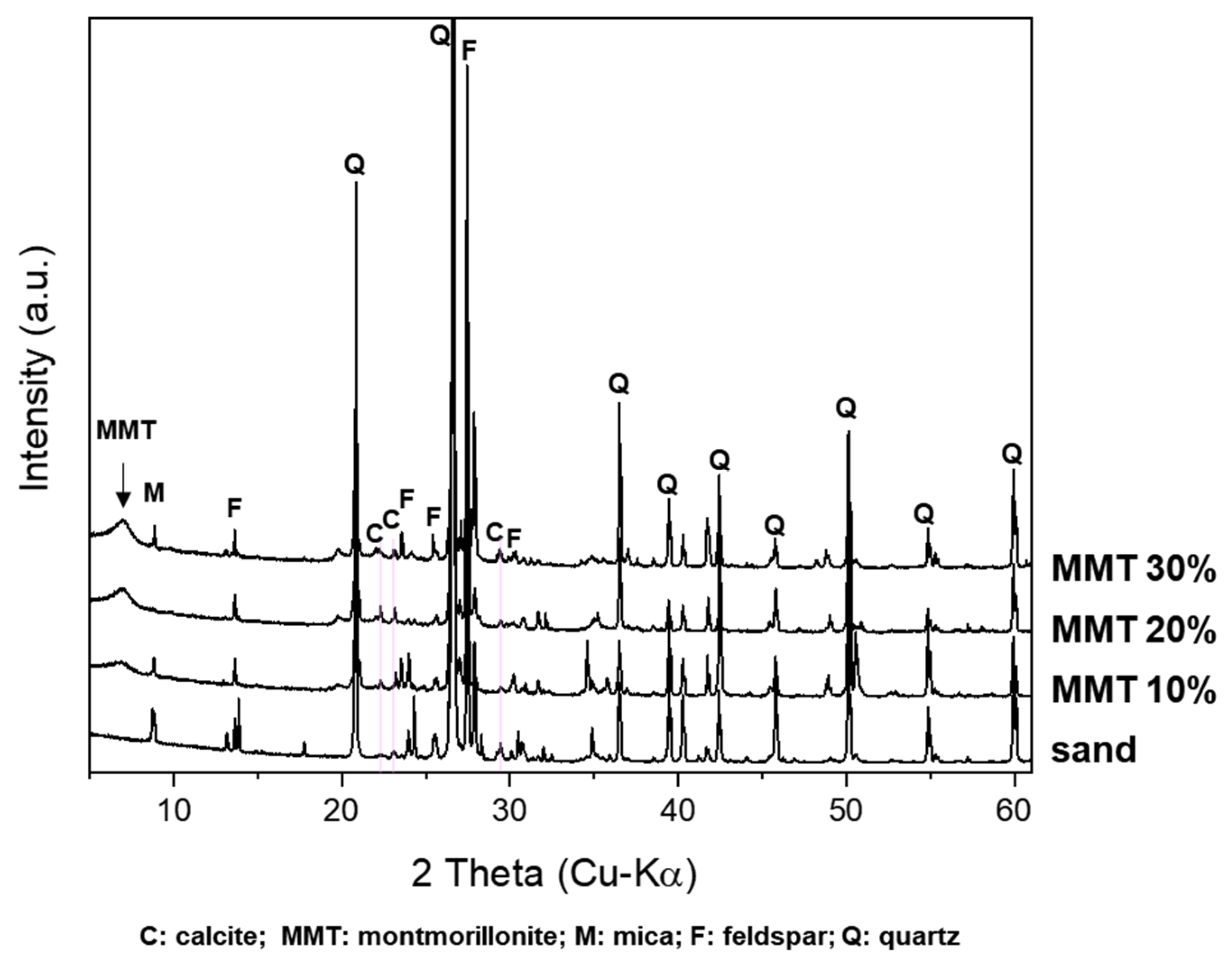
| No. | Samples | Calcium Carbonate in Soil (g) | MMT (001) | Compressive Strength | ||
|---|---|---|---|---|---|---|
| g | % | 2 Theta | d-Spacing (nm) | MPa | ||
| 1 | Sand | 0.014 | 1.428 | - | - | - |
| 2 | MMT 10% | 0.018 | 1.774 | 6.81 | 1.296 | 0.24 |
| 3 | MMT 20% | 0.022 | 2.232 | 6.90 | 1.278 | 3.33 (±0.4) |
| 4 | MMT 30% | 0.027 | 2.691 | 6.95 | 1.270 | 3.53 (±0.2) |
| This Study | Dubey et al. (2021) [37] | Tang et al. (2021) [22] | Chen et al. (2017) [1] | |
|---|---|---|---|---|
| Used microbes | Sporosarcina pasteurii (KCTC 3558) | Strains of Sporosarcina genera | Sporosarcina pasteurii (ATCC11859) | Bacillus pasteurii |
| Urea | 330 mM | 500 mM | 30.03 g | 0.05–0.15 mol/L (Ca2+/urea) |
| CaCl2 | 50 mM | 500 mM | 55.5 g | |
| Temperature | 30 °C | 32 ± 3 °C | 25 °C | 30 °C |
| Soil | sand | Sand, loamy sand | Sand | Uranium tailings |
| Additives | Montmorillonite | - | Na-montmorillonite | Montmorillonite |
| Metabolite | Bacterial suspension, soluble microbial products | - | Bacterial suspension | |
| Treatment method | Mixing | Spraying | - | Injection of mineralizing liquid |
| Treatment cycles | 1 | 1–3 | 1 | 1 |
| Minerals formed | Calcite | Calcite | Calcite | Calcite, vaterite |
| Soil compressive strength | 0.24–3.7 Mpa | 1.67–5.3 Mpa | - | 1.2–2.18 Mpa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Roh, Y. Microbial Precipitation of Calcium Carbonate for Crack Healing and Stabilization of Sandy Soils. Appl. Sci. 2024, 14, 1568. https://doi.org/10.3390/app14041568
Kim Y, Roh Y. Microbial Precipitation of Calcium Carbonate for Crack Healing and Stabilization of Sandy Soils. Applied Sciences. 2024; 14(4):1568. https://doi.org/10.3390/app14041568
Chicago/Turabian StyleKim, Yumi, and Yul Roh. 2024. "Microbial Precipitation of Calcium Carbonate for Crack Healing and Stabilization of Sandy Soils" Applied Sciences 14, no. 4: 1568. https://doi.org/10.3390/app14041568
APA StyleKim, Y., & Roh, Y. (2024). Microbial Precipitation of Calcium Carbonate for Crack Healing and Stabilization of Sandy Soils. Applied Sciences, 14(4), 1568. https://doi.org/10.3390/app14041568







