Featured Application
2-Ethylhexyl nitrate (EHN) has demonstrated its effectiveness as a low-viscosity, high-cetane (LVHC) solvent for reducing the viscosity of straight vegetable oils (SVO) while improving their cetane number. This allows for better performance in diesel engines with comparable power output and stable emissions. Additionally, EHN could be produced in a renewable way using green hydrogen and biomass-derived components to enhance its environmental sustainability, making it an ideal candidate for biofuel blends in diesel engines, offering a partial replacement for fossil diesel.
Abstract
This study evaluates the performance of biofuels created from triple blends of fossil diesel, sunflower or castor oil (SVOs), and 2-Ethylhexyl Nitrate (EHN), a low-viscosity, high-cetane (LVHC) solvent. EHN reduces the viscosity of SVOs to enable their use in conventional diesel engines without compromising fuel properties. The results show that the power output from these blends is similar to or greater than that of fossil diesel, with comparable fuel consumption. Furthermore, the blends significantly reduce emissions of carbon monoxide (CO) and soot, though NOx emissions are slightly higher due to the nitrogen content in EHN. However, NOx levels remain within permissible limits. The substitution of fossil diesel could be further enhanced if EHN were produced using green hydrogen and lignocellulosic biomass, making it a renewable and sustainable biofuel component. These findings support the potential of EHN/SVO biofuel blends to replace a significant portion of fossil diesel in conventional diesel engines while maintaining performance and reducing harmful emissions, except for a slight increase in NOx.
1. Introduction
As a stronger action to reduce anthropogenic CO2 emissions, which are responsible for the rise in global temperatures linked to climate change, the systematic and sustained reduction of fossil fuel utilization in combustion engines is mandatory [1]. To achieve this, not only has been proposed the gradual replacement of combustion engines with electric motors but also the production of renewable fuels to replace fossil diesel in the global vehicle fleet, which currently exceeds one billion units [2].
Biomass is currently considered one of the safest sources for producing renewable fuels, commonly referred to as biofuels, which can be used in compression ignition (C.I.) engines. In this sense, it should be noted that, while for spark combustion engines ethanol is the most suitable biofuel, for C.I. engines, vegetable oils are the most appropriate raw materials to produce advanced or carbon-neutral fuels [3]. In the coming decades, as the transition from fossil fuels to renewable alternatives progresses, vegetable oils (VOs) will become the primary raw material for producing advanced biofuels for internal combustion engines. Triglycerides, which are abundant and low-cost, can be sourced in significant quantities from agriculture as well as from waste generated by the food and livestock industries and household food waste. Furthermore, the industrial production of these advanced biofuels does not depend on the development of new technologies, as current methods are already capable of addressing the major challenge of straight vegetable oils (SVOs), their high viscosity, which limits their direct use as a biofuel.
Currently, conventional biodiesel is practically the only biofuel being produced on an industrial scale. However, various technologies have been developed that also use vegetable oils as the primary raw material to produce biofuels to be used in C.I. engines. These new processes have emerged due to the economic limitations of conventional biodiesel production, the challenges associated with managing the excess of glycerol produced, and the difficulties in producing fatty acid methyl ester (FAME) blends that meet the European regulations standard EN 14,214 or the American ASTM D6751 standard [4]. The effective utilization of waste cooking oil and similar raw materials requires extensive pretreatment. This involves the removal of solid impurities, reduction of free fatty acid (FFA) content, and elimination of water to avoid complications during the transesterification process. Techniques like neutralization and acid esterification are essential for converting waste oils into viable biodiesel feedstocks, addressing both technical and sustainability challenges [5]. However, these methods are complex and expensive, which significantly limits the economic feasibility of biodiesel production through transesterification, particularly at an industrial scale. As a result, thermal conversion and/or hydrogenation of triglycerides from various sources are now considered the best alternative routes for obtaining renewable fuels with properties similar to those of fossil diesel. These biofuels, derived from triglycerides, are produced through catalytic processes such as cracking, pyrolysis, hydrodeoxygenation, or hydrotreatment. The resulting renewable fuels consist of hydrocarbon mixtures that are very similar to fossil fuels but with significant advantages, particularly in reducing greenhouse gas (GHG) emissions [6,7,8]. At present, biofuels derived from triglycerides share common characteristics, including the use of catalytic processes with relatively high energy costs and the loss of a portion of biofuel material due to the removal of oxygen molecules, such as H2O and CO2 [6,7,8]. However, these catalytic processes offer better management of gaseous wastes compared to the glycerol generated in transesterification, even though the mass loss of biofuel is similar. The current delay in replacing fossil fuels with biofuels in the diesel fleet is primarily due to the high economic costs of these processes. As a result, alternative methods that focus on reducing the viscosity of triglycerides to make them viable for use in existing C.I. engines are being explored for economic feasibility [9].
Various ongoing investigations aim to reduce the production costs of biodiesel using SVOs as a renewable fuel in current C.I. engines, which are traditionally operated with fossil diesel. One promising research direction involves reducing the viscosity of SVOs by blending them with low-viscosity organic solvents until the mixture falls within the range of 2.5 to 4.0 cSt, similar to that of fossil diesel. These low-viscosity solvents, ideally renewable, can be mixed in suitable proportions to achieve the desired viscosity, making them compatible with current C.I. engines. The organic solvents studied so far have a relatively low viscosity because they consist of short-oxygenated hydrocarbon chains. However, these short chains result in relatively low cetane numbers, leading to the classification of these solvents as LVLC (low viscosity and low cetane) solvents [10].
Over the past decade, numerous studies have explored the use of short-chain renewable alcohols as LVLC solvents for reducing the viscosity of various SVOs to levels acceptable for C.I. engines. These mixtures generally produce suitable biofuels with a relatively low cetane number due to the lower calorific value of these alcohols [11,12,13,14,15]. Additionally, other organic compounds with higher calorific values, more comparable to fossil diesel, have also been investigated as LVLC solvents. Their mixtures with SVOs exhibit better combustion characteristics and performance than short-chain alcohols, but they are typically more expensive. Oils such as Melaleuca cajuputi (MCO) [16], pine oil [17], eucalyptus oil [12,16], orange oil [12,18], and camphor oil [19] have all been evaluated as LVLC solvents. However, the high cost of these compounds means that short-chain alcohol remains the most viable option since they can be produced from a wide range of biomass transformation procedures, giving them the advantage of producing economical and renewable biofuels. Moreover, this method eliminates the need for chemical transformation to reduce the viscosity and allows the complete utilization of the oil. Consequently, this is the easiest and most economical method described to use vegetable oils as biofuels without generating any type of waste and reducing the cost to that of the SVO and the LVLC solvent used in the mixture.
These optimized SVO/LVLC blends can also be used in triple blends with conventional fossil diesel to improve the cetane number to the levels required for optimal diesel engine performance. While these triple blends do not completely eliminate anthropogenic CO2 emissions, they offer a significant reduction and represent a major step forward in the transition away from fossil fuels in the current diesel engine fleet. Ternary blends of SVO, diesel, and LVLC solvents have been shown to reduce smoke, CO, NOx, and unburned hydrocarbon emissions, particularly at high loads, highlighting their potential as a renewable energy source to partially or fully replace diesel fuel [20].
Consequently, considering the technical and economic feasibility of this approach, which enables the use of various vegetable oils and LVLC solvents as renewable fuels in current diesel engines, several studies have been conducted. Nevertheless, the selection of feedstocks for renewable fuel applications focuses on oils that do not compete with food supply and are feasible for large-scale industrial production. Castor oil, a non-edible vegetable oil, is particularly promising due to its established use in chemical industries and its global annual production of approximately 220,000 tons [21]. Its non-edible nature ensures no competition with the food chain, and its adaptability to grow in marginal lands further enhances its sustainability. Conversely, sunflower oil serves as a standard reference to study waste oils, primarily because it minimizes variability when compared to using waste oils of different origins. This makes it an excellent benchmark for research. Additionally, waste cooking oils, derived from over 190 million metric tons of globally generated waste vegetable oil annually [22], represent a highly sustainable and cost-effective option for biofuel production. As a result, exploring the potential of this abundant raw material as a biofuel is of great interest. The investigation of mixtures of these non-edible oils with various LVLC solvents presents a promising and cost-effective methodology for replacing fossil diesel, since based on raw material costs, castor oil ($1600/MT) and 2-EHN ($1700/MT), the costs are competitive with fossil diesel, making this an interesting approach to consider. The results so far indicate that a high percentage of fossil fuel can be substituted in diesel engines without sacrificing engine performance while achieving a significant reduction in pollutant emissions [23,24,25,26,27,28]. Some studies have explored blends with gasoline due to their lower viscosity [24] or with hydrocarbons derived from the pyrolysis of plastic waste [26,28]. Nevertheless, most research has focused on renewable LVLC solvents obtained from various alcohols [23,27,29]. However, to achieve a significant reduction in fossil diesel consumption using these mixtures, it is essential to identify a wide range of commercially available solvents. This is necessary to support the large-scale production of SVO/LVLC mixtures, given the vast quantity of fossil diesel currently in use. Moreover, identifying renewable, economically viable solvents with favorable cetane numbers is crucial. Ideally, solvents with low viscosity and high cetane numbers (LVHC) should be prioritized, as their use in blends with SVOs could further enhance results compared to those achieved with LVLC solvents investigated so far [30,31]. This strategy could lead to improved fuel properties, higher diesel substitution rates, and greater environmental benefits. The physicochemical properties of biodiesel play a crucial role in engine emissions, significantly influencing NOx, CO2, and CO outputs. Studies have identified oxygen content, aromatics, and lower heating value (LHV) as key predictors of NOx emissions. In contrast, viscosity is the most influential factor for CO emissions, while hydrogen content is critical for predicting CO2 emissions. Further analysis reveals notable correlations between viscosity, flash point, density, and hydrogen content with CO and CO2 emissions, although these properties have minimal impact on NOx emissions. Oxygen content demonstrates a strong negative correlation with NOx emissions, whereas properties such as boiling range, LHV, aromatics, and surface tension exhibit positive correlations [32]. These findings highlight the intricate relationship between fuel properties and emission behavior, underscoring the complexity of identifying optimal biodiesel blends to achieve enhanced performance with reduced pollutant emissions.
To address the limitations of LVLC solvents, particularly their potential to reduce the cetane number and negatively affect combustion performance, solvents with both low viscosity and high cetane numbers (LVHC) present a promising alternative. LVHC solvents can enhance the performance of blends with SVOs, improving combustion characteristics compared to LVLC solvents. Cetane improvers are widely used to enhance the cetane number of fuels, thereby reducing engine exhaust emissions and improving overall efficiency. For example, 2-ethylhexyl nitrate (EHN) is a well-established cetane improver capable of increasing the cetane number by 12–16 points while simultaneously reducing nitrogen oxides (NOx), carbon monoxide (CO), and unburned hydrocarbon emissions [33]. EHN has already been used as a cetane improver in biodiesel [34] and in blends with various organic compounds such as 2,5-dimethylfuran [35], dimethylcarbamate [33], and alcohols such as diesel-ethanol blends [36], n-pentanol-diesel blends [37], and even gasoline-ethanol mixtures for I.C. engines [38]. EHN has also been used to improve the cetane number in methanol-biodiesel blends [39], Karanja biodiesel [40], Yellow Oleander biodiesel [41], and SVO-based blends like hazelnut oil mixed with alcohol [42]. Globally, EHN is produced at an industrial scale, with an estimated annual production of approximately 100,000 tons [43].
The use of EHN as a cetane improver could be further used if we used EHN directly as a solvent. Leveraging EHN’s existing use as a cetane improver, this research explores its novel application as an LVHC solvent. Its potential benefits include enhanced fuel ignitability, reduced ignition time delay, lower combustion temperatures, and diminished combustion noise (cita 65). Furthermore, EHN could achieve renewable status if it is synthesized using green ammonia produced by sustainable processes such as water electrolysis powered by renewable energy sources. This would eliminate dependence on fossil fuel-derived ammonia, significantly reducing the environmental impact of EHN. As EHN is produced through the nitration of 2-ethylhexanol, the use of green ammonia would enable a fully renewable production pathway. This is not only in line with the principles of sustainable chemistry but also reinforces EHN’s potential as a green, high-performance fuel additive, paving the way for its integration into a circular bio-economy model [44].
Therefore, the primary goal of this research is to evaluate the use of 2-ethylhexyl nitrate (EHN) as a low-viscosity, high-cetane (LVHC) solvent to enable castor oil and sunflower oil to be directly utilized as biofuels in diesel combustion engines. This study explores the potential of EHN in triple blends of Diesel/EHN/SVO, given its established role as a cetane enhancer in diesel fuels and its ability to reduce viscosity. Moreover, EHN could be classified as renewable if synthesized using green ammonia, as it is derived from the nitration of 2-ethylhexanol.
2. Materials and Methods
2.1. Preparation of Double Blends EHN/SVO and Triple Blends Diesel/EHN/SVO to Be Used as Advanced or Carbon-Neutral Fuels
2-Ethylhexyl Nitrate (purity ≥ 99.5%) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Castor oil was purchased from Panreac (Castellar Del Vallès, Spain), and sunflower oil (used as a reference for waste cooking oils) was sourced from a local market. Fossil diesel was acquired from a Repsol service station. For the preparation of the EHN/SVO double blends, different ratios of EHN were mixed with sunflower oil (SO) and castor oil (CO) in proportions of 20%, 40%, 60%, and 80% by volume. The double blends that exhibited viscosity values within the range specified by the European Regulation EN 590 and ISO-3104 standards for fossil diesel (2.0–4.5 cSt) were then used to prepare triple blends by mixing them with commercial fossil diesel (D) to form the D/EHN/SVO mixtures. These triple blends are called BioXXYY, where XX is the volume percentage of biofuel added to the fossil diesel, YY is the type of vegetable oil, SO for sunflower oil, and CO for castor oil. For example, Bio60SO corresponds to 60% sunflower oil biofuel and 40% of fossil diesel. All volumes were measured using a graduated cylinder at 25 °C to ensure precision. Blends were prepared by manual mixing with thorough agitation for 5 min using a vortex mixer to ensure homogeneity. Table 1 presents key physical and chemical properties of diesel, sunflower oil, castor oil, and 2-ethylhexyl nitrate relevant to their performance as fuels in modern diesel engines.

Table 1.
Physicochemical properties of the different compounds used as biofuels. Kinematic viscosity values were experimentally obtained in this research.
2.2. Characterization of the Rheological and Physicochemical Properties of the Biofuel Mixtures
The essential physicochemical properties of the biofuel blend to be utilized in diesel engines were identified and evaluated. These properties include kinematic viscosity, density, cloud point, and pour point, all of which were measured through experimental methods. Additionally, the calorific value and cetane index were determined using predictive models. Each data point represents the median of three independent measurements, with experimental errors calculated as standard deviations to ensure accuracy and reliability.
Determination of Calorific Value of Biofuel Samples
Theoretical calorific values (CV) were calculated using the Kay Mixing Rule, based on the volumetric concentration of each component in the blend, as shown in Equation (1):
where CVi represents the calorific value of each component in megajoules per liter (MJ/L), and Xi is the volumetric fraction of each component [45].
CV = Σi CViXi
2.3. Experimental Procedure for Testing the Mechanical and Environmental Performance of Biofuel Blends in a Diesel Engine–Electrogenerator Set
A C.I. engine (Model: AYERBE AY-4000 D, Vitoria, Spain), designed for electricity generation, was used to evaluate the energy performance and emissions of the blends, following a previously described methodology [23,26]. The main technical specifications of the engine are listed in Table S1 in Supplementary Materials. This engine operates with a 4-stroke, single-cylinder system, featuring a bore of 78 mm and a stroke of 67 mm. It utilizes a forced air-cooling system with a flywheel fan to maintain optimal operating temperatures.
The engine was operated at a constant crankshaft rotation speed, while the electrical energy demand was varied by connecting different numbers of 1000-watt heating plates. Since the engine maintained a fixed rotation speed and crankshaft torque, the variations in electrical power generated directly reflected the mechanical power produced from the combustion of the biofuels being tested. The electrical power generated was calculated by multiplying the voltage and current, as described in Equation (2). Both voltage and current were measured using a voltmeter-ammeter.
Electrical Power Generated (Watts) = voltage (Volts) × amperage (Amps)
Mechanical power was derived from electrical power measurements, and this methodology was applied to evaluate all double and triple blends with viscosity values suitable for use as biofuels. Fuel consumption was determined by measuring the volume consumed over time while operating the engine under different levels of electrical demand: low (1 kW), medium (3 kW), and high (5 kW). For each measurement, 600 mL of biofuel was used. This allowed us to calculate the fuel consumption under varying electrical loads, determining the engine consumption rate at each demand level. A key parameter for evaluating fuel efficiency is the brake-specific fuel consumption (BSFC), expressed in g/kW·h, which quantifies the amount of fuel consumed per hour per kW of power generated by the engine [46]. Accordingly, BSFC values were calculated based on the volume of fuel consumed while operating at low (1 kW), medium (3 kW), and high (5 kW) power demands. All measurements were performed in triplicate, with results presented as the average of three measurements.
The pollutant emissions of biofuels were assessed based on the opacity of the exhaust gases, as well as the concentrations of carbon monoxide (CO) and nitrogen oxides (NOx, including NO and NO2) emitted during combustion. Opacity measurements were performed using a TESTO 338 smoke densitometer according to the ASTM D-2156 standard method for measuring smoke density in gases from burning distillate fuels. Opacity is expressed using the Bosch number, which is calculated based on the soot level collected on filter paper. The scale ranges from 0 to 2.5, where 0 indicates complete clarity and 2.5 represents maximum soot blackening, equivalent to 100% blackening. Smoke emissions are reported as soot concentrations in mg/m3 [47]. The concentrations of CO and NOx were measured in parts per million (ppm) using a Testo 340 combustion analyzer. The emission sensor probe was placed directly in the exhaust gas outlet to ensure accurate detection. All measurements were performed as the average of three independent determinations, with an experimental error of less than 6%, as outlined in Table S2 in Supplementary Materials. These values were obtained following established methodologies [23,26].
3. Results
3.1. Physicochemical Properties of EHN/SVO Double Blends and D/EHN/SVO Triple Blends
Kinematic viscosity plays a fundamental role in combustion efficiency for fuels used in diesel engines. It affects key processes such as fuel atomization, the size and distribution of fuel droplets, and the uniformity of the air-fuel mixture within the combustion chamber. To ensure proper engine operation, the European standard EN 590 ISO 3104 specifies that the kinematic viscosity of diesel fuels should fall within the range of 2.0 to 4.5 cSt. Given its importance, viscosity was selected as a fundamental parameter for assessing the binary blends of EHN with sunflower oil and castor oil. Table 2 highlights the viscosity measurements for the EHN/SVO double blends, showing a notable decrease in viscosity with increasing EHN content. This reduction aligns the blends’ viscosity closer to that of fossil diesel, meeting the specifications outlined by the EN 590 ISO 3104 standard. This demonstrates the potential of EHN to enhance the suitability of straight vegetable oils (SVOs) for diesel engine applications.

Table 2.
Kinematic viscosity (cSt) at 40 °C of EHN/SO and EHN/CO blends, with increasing EHN proportions. Values are averages of three measurements, with errors expressed as standard deviations of 75% for SO and 80% for CO.
To study the triple mixtures with fossil diesel, we selected the double blends with the highest SVO content that still comply with the viscosity values set by the European standard EN 590 ISO 3104. Specifically, we used the mixtures containing 65% of EHN solvent for sunflower oil and 75% for castor oil. The viscosity values of these triple blends, along with other key physicochemical properties such as density, cloud point, pour point, calorific value, and flash point, are presented in Table 3 and Table 4 for D/EHN/SO and D/EHN/CO, respectively.

Table 3.
Viscosity at 40 °C (cSt), cloud point, pour point, and calorific value of D/EHN/SO blends. Errors are calculated as the standard deviation from the average of three measurements.

Table 4.
Viscosity at 40 °C (cSt), cloud point, pour point, and calorific value of D/EHN/CO blends. Errors are calculated as the standard deviation from the average of three measurements.
As shown in Table 3 and Table 4, the addition of EHN/SVO biofuel mixtures to fossil diesel (ranging from B20 to B80) results in a progressive increase in the kinematic viscosity of the triple blends as the proportion of EHN/SVO increases. Both sunflower oil (SO) and castor oil (CO) mixtures exhibit higher viscosity with lower diesel content. Similarly, density values increase with the proportion of EHN/SVO increased in the blends, regardless of the SVO used.
Regarding cold flow properties, the blends showed notable improvements compared to pure fossil diesel, particularly in their cloud points, while maintaining similar pour point values. For instance, B20SO and B80CO exhibited cloud points of −12 °C, representing a significant enhancement of −6 °C relative to fossil diesel. This improvement suggests better usability in colder climates, as a lower cloud point reduces the risk of wax crystal formation at lower temperatures. However, pour point values remain largely unchanged, varying only within ±2 °C, indicating that while initial cold flow performance is improved, further enhancements in pour point may be necessary for optimal performance in extremely cold environments. Additionally, calorific values were similar for all the blends, with variations within ±1.5 MJ/kg, regardless of composition. This is important because calorific values indicate the energy content of a fuel, suggesting that all studied blends have comparable energy values to fossil diesel. Nevertheless, considering the physicochemical properties presented in Table 3 and Table 4, which include higher densities and relatively similar heat content values, it is essential to experimentally evaluate the engine performance using these different blends.
3.2. Performance of EHN/SVO Double Blends and D/EHN/SVO Triple Blends in a Diesel Engine Operating as an Electric Generator
The diesel engine performance using the triple blends was evaluated experimentally to determine the optimal ratios of EHN and straight vegetable oils (SVO) in these formulations, and it is shown in Table 3 and Table 4. Figure 1 provides a comparative analysis of the power output under varying engine loads for triple blends containing sunflower oil (Figure 1a) and castor oil (Figure 1b). Additionally, the performance of conventional fossil diesel and pure biofuels (Bio100SO and Bio100CO) is included for reference, offering a comprehensive overview of the blends’ effectiveness.
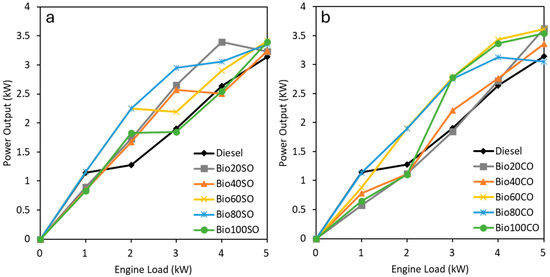
Figure 1.
Power generated (in watts) based on the power demanded (in kW) when operating with different triple blends: diesel/EHN/sunflower oil (a) and diesel/EHN/castor oil (b).
The results indicate that the type of SVO used in the triple blends has no significant effect on performance, as the power output increases nearly linearly with demand, from 1 kW to 5 kW. Furthermore, all the blends demonstrated higher power output compared to pure fossil diesel, suggesting that the addition of EHN and vegetable oils enhances the engine power generation capacity across various load levels.
It is noteworthy that the pure double blend Bio100CO outperforms all other blends, showing a 12.6% improvement in power output over fossil diesel. Similarly, the Bio100SO blend performs comparably to fossil diesel, achieving an 8% increase in power output. These results highlight the potential of both pure biofuels, Bio100SO and Bio100CO, as viable alternatives to fossil diesel, offering full replacement capabilities without sacrificing power generation. Additionally, all the triple blends also generate higher power output while enabling a significant reduction in fossil diesel utilization. Among these blends, Bio60SO and Bio60CO are the most efficient, delivering the highest power output across all the blends. This suggests that triple blends can offer an optimal balance between renewable content and engine performance. Despite the excellent results of the triple blends, both pure biofuels, Bio100SO and Bio100CO, remain viable for fully replacing fossil diesel without any reduction in power generation. The remarkable performance of Bio100CO also points to its potential for reduced fuel consumption, making it a particularly efficient and sustainable option.
3.3. Brake Specific Fuel Consumption (BSFC)
BSFC is a critical parameter for any biofuel aiming to replace fossil diesel in the current vehicle fleet, as lower BSFC values at a given power output indicate higher engine efficiency. Therefore, the efficiency of a fuel blend is largely dependent on its BSFC, making it a key factor in assessing the viability of biofuels as substitutes for conventional diesel. As shown in Figure 2, BSFC values are highest at lower engine loads (1 kW) and decrease as the load increases, which is due to the improved combustion efficiency at higher temperatures. Notably, the highest BSFC values are observed at 1 kW, where combustion is less efficient. The most significant increases in BSFC correspond to the Bio40SO and Bio100SO mixtures with sunflower oil and the Bio20CO and Bio100CO mixtures with castor oil.
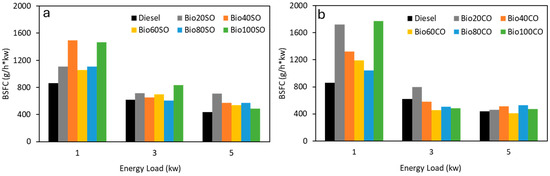
Figure 2.
Effect of (a) D/EHN/SO and (b) D/EHN/CO biofuels on BSFC (g/h·kW) at low, medium, and high engine loads (1, 3, 5 kW). Measurement errors are represented as standard deviations using error bars.
It is interesting to remark that at higher power outputs, the BSFC values for all the mixtures are quite similar, particularly for the castor oil blends, where the variation between mixtures is minimal. In contrast, the sunflower oil blends show more variability. The lower BSFC values observed with the castor oil blends, in comparison to those with sunflower oil, may be attributed to the slightly higher flash points, autoignition temperatures, and cetane numbers of castor oil. These properties are likely to extend the ignition delay, improving the premixed combustion phase. Furthermore, it is notable that at medium and high power levels (3 kW and 5 kW), there are no significant differences in BSFC compared to pure fossil diesel. This finding supports the potential for substituting fossil diesel with these biofuels in conventional engines without sacrificing efficiency or significantly increasing fuel consumption.
3.4. Exhaust Emissions from Diesel Engine
An extremely important characteristic of biofuels is their impact on emission levels, especially given the strict legal regulations in many countries for C.I. vehicle emissions. These regulations are periodically reviewed to meet quality standards, as the emissions not only contribute to climate change but also impact significantly public health.
3.4.1. Soot Emissions
Figure 3 illustrates the smoke opacity values versus engine load for the D/EHN/SO (Figure 3a) and D/EHN/CO (Figure 3b) triple blends. The results demonstrate that all biofuel blends significantly reduce smoke emissions compared to pure fossil diesel. The Bio100SO blend exhibited the lowest emissions, while among the triple blends with castor oil, Bio40CO, and Bio60CO showed the lowest smoke opacity levels, regardless of engine power demand. This reduction in emissions is strongly associated with the higher oxygen content in biofuel compounds compared to fossil diesel, which improves combustion efficiency and leads to a significant decrease in contaminant emissions. The additional oxygen in these biofuels promotes more complete combustion, thereby reducing the formation of pollutants and enhancing their potential as effective diesel substitutes.
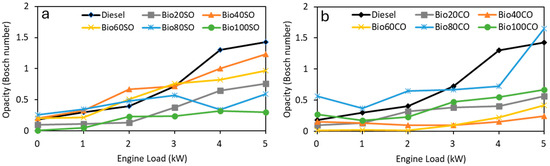
Figure 3.
Smoke opacity (Bosch number) produced at different engine power outputs (0 to 5 kW) for (a) D/EHN/SO and (b) D/EHN/CO triple blends.
3.4.2. Carbon Monoxide (CO) Emissions
The levels of carbon monoxide (CO) emissions produced by the different D/EHN/SVO mixtures are presented in Figure 4, which shows the detected CO concentrations in parts per million (ppm) at various engine power levels.
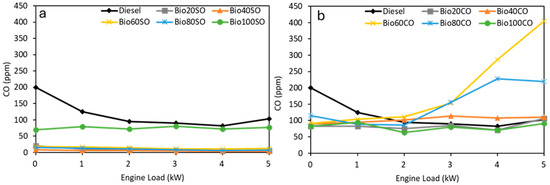
Figure 4.
Carbon monoxide (CO) emissions (ppm) generated at various engine load levels (0 to 5 kW) for (a) D/EHN/SO and (b) D/EHN/CO triple blends.
The CO emissions from the Diesel/EHN/SO blends show highly positive results, with all blends emitting lower CO levels than pure fossil diesel (Figure 4a). Most triple blends emit around 20 ppm of CO, except for Bio100SO, which emits about 100 ppm, regardless of engine power. However, even this value is still lower than that of fossil diesel. In the case of castor oil blends (Figure 4b), the Bio20CO, Bio40CO, and Bio100CO mixtures show CO emissions similar to fossil diesel, remaining fairly constant across different power levels. In contrast, the Bio60CO and Bio80CO blends show a marked increase in CO emissions, especially at higher power demands (4–5 kW). Importantly, all emissions are lower than the limits set by the European Union regulations (EU 2023/443), which cap CO emissions at 3000 ppm. The highest emission recorded in this study was only 405 ppm, far below the regulatory limit.
3.4.3. Nitrogen Oxides (NOx) Emissions
Figure 5 shows the NOx emissions (in ppm) recorded at different engine loads for the D/EHN/SO (Figure 5a) and D/EHN/CO (Figure 5b) triple blends. As expected, NOx emissions increase with higher power demands, which can be attributed to the rise in cylinder temperature and pressure, promoting NOx formation, as supported by previous studies [48]. Furthermore, NOx emissions increase as the biofuel concentration in the blend rises, with all biofuel blends producing higher NOx emissions than pure fossil diesel, regardless of the specific vegetable oil used.
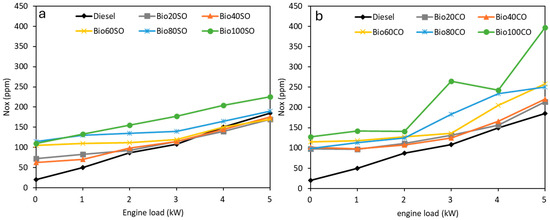
Figure 5.
Nitrogen oxides (NOx) emissions (ppm) generated at various engine load levels (0 to 5 kW) for (a) D/EHN/SO and (b) D/EHN/CO triple blends.
The observed increase in NOx emissions from the triple blends, compared to fossil diesel, is relatively modest. The highest NOx emissions were recorded for the B100SO and B100CO double blends, which are pure biodiesel blends without any fossil diesel content. Specifically, castor oil blends showed up to a 30% rise in NOx emissions, while sunflower oil blends exhibited values closer to those of fossil diesel. This trend aligns with previous findings for EHN-enhanced fuels, such as lemon peel oil, where improvements in other performance metrics were accompanied by elevated NOx levels [44]. The presence of nitrogen in 2-ethylhexyl nitrate (EHN) likely contributes to this increase, as it forms nitrogen oxides during combustion [44]. The nitrogen present in 2-ethylhexyl nitrate (EHN) likely contributes to this rise, as it forms nitrogen oxides during combustion [49].
However, sunflower oil blends demonstrated lower NOx emissions compared to castor oil blends. This difference could be attributed to the lower cetane number of sunflower oil, which delays combustion and reduces peak temperatures, thereby mitigating NOx formation. Although elevated NOx emissions are a known drawback of using EHN, this trade-off must be considered alongside the significant reductions in soot and CO emissions demonstrated by these blends. To address the challenges posed by increased NOx emissions, future research should focus on mitigation strategies. These may include the incorporation of NOx-reducing additives or the optimization of blend ratios, and exploring formulations that balance NOx emissions with reductions in CO and soot could provide a more comprehensive and sustainable solution.
4. Conclusions
2-ethylhexyl nitrate (EHN) has proven to be an effective solvent for reducing the viscosity of straight vegetable oils (SVOs), with blends of 65% EHN for sunflower oil and 75% for castor oil meeting viscosity requirements. Additionally, the combustion properties of these blends are similar to those of fossil diesel, positioning EHN as a promising candidate in a new category of low-viscosity, high-cetane (LVHC) solvents. Unlike traditional low-viscosity, low-cetane (LVLC) solvents, which tend to lower cetane numbers, EHN enhances both biofuel performance and diesel engine compatibility. A notable advantage of LVHC solvents is their higher calorific value, maintaining power output levels similar to fossil diesel. By increasing the cetane number, EHN also improves combustion efficiency, reducing ignition delay and ensuring stable emissions with only minor NOx increases.
Significantly, the pure Bio100CO blend achieved a notable 12.6% improvement in power output compared to fossil diesel, while Bio100SO showed an 8% increase, demonstrating both blends as viable substitutes for fossil diesel. Among the triple blends, Bio60SO and Bio60CO were the most efficient, achieving the highest power outputs and enabling substantial fossil diesel reduction. Specifically, Bio60SO also achieved significant reductions in soot and CO emissions by 30% and 80%, respectively, compared to fossil diesel, while maintaining comparable NOx emissions. These findings suggest that both pure biofuels and triple blends can serve as renewable and high-performance alternatives to fossil diesel. The Bio100CO blend, in particular, shows potential for reduced fuel consumption, underscoring EHN and other renewable nitrocompounds as promising LVHC solvents for sustainable diesel engine applications. To further advance the potential of LVHC solvents like EHN, future research could explore other renewable nitrocompounds and LVHC solvents to broaden the spectrum of biofuel compatibility. Additionally, long-term studies on engine performance and maintenance with these biofuel blends are essential to ensure durability and reliability, providing comprehensive insights into their practical applications in various engine types and conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app142411968/s1, Table S1: Technical description of the Diesel Engine Electric Generator Set: Table S2: Measurement accuracy of the different parameters.
Author Contributions
R.E.: Conceptualization, Supervision, Writing–review and editing, Investigation; F.J.L.-T.: Investigation, Writing–original draft, Writing—review and editing; V.M.: Investigation, Formal Analysis; A.A.R.: Formal Analysis; F.M.B.: Funding acquisition, Project administration, formal analysis; D.L.: Formal Analysis, Conceptualization, Supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are thankful to MCIN/AEI/10.13039/501100011033 (project PID2022/142275OB-I00), MCIN/AEI/10.13039/501100011033 and Unión Europea “NextGenerationEU/PRTR” (project TED2021/132224B-I00), and F.J.L.T. is thankful to the program FEDER-Andalucia 2021–2027, Junta de Andalucía (Project PP2F_L1_21).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are thankful for the technical assistance of the staff at the Central Service for Research Support (SCAI) and that provided by the Instituto Químico para la Energía y el Medioambiente (IQUEMA).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khalili, S.; Rantanen, E.; Bogdanov, D.; Breyer, C. Global Transportation Demand Development with Impacts on the Energy Demand and Greenhouse Gas Emissions in a Climate-Constrained World. Energies 2019, 12, 3870. [Google Scholar] [CrossRef]
- Senecal, P.K.; Leach, F. Diversity in Transportation: Why a Mix of Propulsion Technologies Is the Way Forward for the Future Fleet. Results Eng. 2019, 4, 100060. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; López-Tenllado, F.J.; Bautista, F.M.; Romero, A.A.; Luna, D. Internal Combustion Engines and Carbon-Neutral Fuels: A Perspective on Emission Neutrality in the European Union. Energies 2024, 17, 1172. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Bautista, F.M.; Luna, D.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A. Biodiesel at the Crossroads: A Critical Review. Catalysts 2019, 9, 1033. [Google Scholar] [CrossRef]
- Kosuru, S.M.Y.; Delhiwala, Y.; Koorla, P.B.; Mekala, M. A Review on the Biodiesel Production: Selection of Catalyst, Pre-Treatment, Post Treatment Methods. Green Technol. Sustain. 2024, 2, 100061. [Google Scholar] [CrossRef]
- Nikolopoulos, I.; Kordouli, E.; Mourgkogiannis, N.; Karapanagioti, H.K.; Lycourghiotis, A.; Kordulis, C. Valorization of Pyrolyzed Biomass Residues for the Transformation of Waste Cooking Oil into Green Diesel. Catalysts 2023, 13, 1004. [Google Scholar] [CrossRef]
- del Río, J.I.; Pérez, W.; Cardeño, F.; Marín, J.; Rios, L.A. Pre-Hydrogenation Stage as a Strategy to Improve the Continuous Production of a Diesel-like Biofuel from Palm Oil. Renew. Energy 2021, 168, 505–515. [Google Scholar] [CrossRef]
- d’Ambrosio, S.; Mancarella, A.; Manelli, A. Utilization of Hydrotreated Vegetable Oil (HVO) in a Euro 6 Dual-Loop EGR Diesel Engine: Behavior as a Drop-In Fuel and Potentialities along Calibration Parameter Sweeps. Energies 2022, 15, 7202. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; López-Tenllado, F.J.; Luna, C.; Calero, J.; Romero, A.A.; Bautista, F.M.; Luna, D. Biodiesel Is Dead: Long Life to Advanced Biofuels—A Comprehensive Critical Review. Energies 2022, 15, 3173. [Google Scholar] [CrossRef]
- Vallinayagam, R.; Vedharaj, S.; Yang, W.M.; Roberts, W.L.; Dibble, R.W. Feasibility of Using Less Viscous and Lower Cetane (LVLC) Fuels in a Diesel Engine: A Review. Renew. Sustain. Energy Rev. 2015, 51, 1166–1190. [Google Scholar] [CrossRef]
- Coughlin, B.; Hoxie, A. Combustion Characteristics of Ternary Fuel Blends: Pentanol, Butanol and Vegetable Oil. Fuel 2017, 196, 488–496. [Google Scholar] [CrossRef]
- Che Mat, S.; Idroas, M.Y.; Teoh, Y.H.; Hamid, M.F.; Sharudin, H.; Pahmi, M.A.A.H. Optimization of Ternary Blends among Refined Palm Oil-Hexanol-Melaleuca Cajuputi Oil and Engine Emissions Analysis of the Blends. Renew. Energy 2022, 196, 451–461. [Google Scholar] [CrossRef]
- Eiadtrong, S.; Maliwan, K.; Theppaya, T.; Kattiyawan, T.; Prateepchaikul, G.; Leevijit, T. An Investigation to Utilize Ternary Diesel-Palm Fatty Acid Distillate-10 wt% n-Butanol Blends as Simply Novel Diesel Substitutes. Fuel 2021, 289, 119965. [Google Scholar] [CrossRef]
- Atmanlı, A.; Yüksel, B.; İleri, E. Experimental Investigation of the Effect of Diesel–Cotton Oil–n-Butanol Ternary Blends on Phase Stability, Engine Performance and Exhaust Emission Parameters in a Diesel Engine. Fuel 2013, 109, 503–511. [Google Scholar] [CrossRef]
- Krishnamoorthy, V.; Dhanasekaran, R.; Rana, D.; Saravanan, S.; Rajesh Kumar, B. A Comparative Assessment of Ternary Blends of Three Bio-Alcohols with Waste Cooking Oil and Diesel for Optimum Emissions and Performance in a CI Engine Using Response Surface Methodology. Energy Convers. Manag. 2018, 156, 337–357. [Google Scholar] [CrossRef]
- Che Mat, S.; Idroas, M.Y.; Teoh, Y.H.; Hamid, M.F. Optimisation of Viscosity and Density of Refined Palm Oil-Melaleuca Cajuputi Oil Binary Blends Using Mixture Design Method. Renew. Energy 2019, 133, 393–400. [Google Scholar] [CrossRef]
- Prakash, T.; Geo, V.E.; Martin, L.J.; Nagalingam, B. Evaluation of Pine Oil Blending to Improve the Combustion of High Viscous (Castor Oil) Biofuel Compared to Castor Oil Biodiesel in a CI Engine. Heat Mass Transf. 2019, 55, 1491–1501. [Google Scholar] [CrossRef]
- Ashok, B.; Jeevanantham, A.K.; Vignesh, R.; Bhat Hire, K.R.; Prabhu, K.; Raaj Kumar, R.A.; Shivshankar, N.; Edwin Sudhagar, P. Calibration of Engine Parameters and Fuel Blend for Vibration and Noise Characteristics in CRDI Engine Fuelled with Low Viscous Biofuel. Fuel 2021, 288, 119659. [Google Scholar] [CrossRef]
- Gurusamy, M.; Ponnusamy, C. The Influence of Hydrogen Induction on The Characteristics of a CI Engine Fueled with Blend of Camphor Oil and Diesel with Diethyl Ether Additive. Int. J. Hydrogen Energy 2023, 48, 24054–24073. [Google Scholar] [CrossRef]
- Che Mat, S.; Idroas, M.Y.; Hamid, M.F.; Zainal, Z.A. Performance and Emissions of Straight Vegetable Oils and Its Blends as a Fuel in Diesel Engine: A Review. Renew. Sustain. Energy Rev. 2018, 82, 808–823. [Google Scholar] [CrossRef]
- Chidambaranathan, B.; Gopinath, S.; Aravindraj, R.; Devaraj, A.; Gokula Krishnan, S.; Jeevaananthan, J.K.S. The Production of Biodiesel from Castor Oil as a Potential Feedstock and Its Usage in Compression Ignition Engine: A Comprehensive Review. Mater. Today Proc. 2020, 33, 84–92. [Google Scholar] [CrossRef]
- Mannu, A.; Garroni, S.; Ibanez Porras, J.; Mele, A. Available Technologies and Materials for Waste Cooking Oil Recycling. Processes 2020, 8, 366. [Google Scholar] [CrossRef]
- Aguado-Deblas, L.; López-Tenllado, F.J.; Luna, D.; Bautista, F.M.; Romero, A.A.; Estevez, R. Advanced Biofuels from ABE (Acetone/Butanol/Ethanol) and Vegetable Oils (Castor or Sunflower Oil) for Using in Triple Blends with Diesel: Evaluation on a Diesel Engine. Materials 2022, 15, 6493. [Google Scholar] [CrossRef] [PubMed]
- Schubert, T. Production Routes of Advanced Renewable C1 to C4 Alcohols as Biofuel Components—A Review. Biofuels Bioprod. Biorefining 2020, 14, 845–878. [Google Scholar] [CrossRef]
- Karagöz, M. Investigation of Performance and Emission Characteristics of an CI Engine Fuelled with Diesel–Waste Tire Oil–Butanol Blends. Fuel 2020, 282, 118872. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; López-Tenllado, F.J.; Bautista, F.M.; Romero, A.A.; Luna, D. Study on the Performance and Emissions of Triple Blends of Diesel/Waste Plastic Oil/Vegetable Oil in a Diesel Engine: Advancing Eco-Friendly Solutions. Energies 2024, 17, 1322. [Google Scholar] [CrossRef]
- Özer, S. The Effect of Diesel Fuel-Tall Oil/Ethanol/Methanol/Isopropyl/n-Butanol/Fusel Oil Mixtures on Engine Performance and Exhaust Emissions. Fuel 2020, 281, 118671. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Veza, I.; Ampah, J.D.; Afrane, S.; Sarikoç, S.; Mujtaba, M.A.; Yahuza, I. Experimental Study on Emissions and Particulate Characteristics of Diesel Engine Fueled with Plastic Waste Oil, Acetone-Butanol-Ethanol and Diesel Blends. Process Saf. Environ. Prot. 2024, 191, 1419–1431. [Google Scholar] [CrossRef]
- Čedík, J.; Pexa, M.; Holúbek, M.; Aleš, Z.; Pražan, R.; Kuchar, P. Effect of Diesel Fuel-Coconut Oil-Butanol Blends on Operational Parameters of Diesel Engine. Energies 2020, 13, 3796. [Google Scholar] [CrossRef]
- Gurusamy, M.; Vijayaragavan, M.; Varuvel, E.G. Experimental Investigation of Features of CI Engine Fueled with Blends of Camphor Oil with Biomass Waste Simarouba Glauca Oil. Energy Sources Part A Recovery Util. Environ. Eff. 2024, 46, 3884–3901. [Google Scholar] [CrossRef]
- Sonthalia, A.; Varuvel, E.G.; Subramanian, T.; Kumar, N. NOx Emission Reduction in Low Viscous Low Cetane (LVLC) Fuel Using Additives in CI Engine: An Experimental Study. Clean. Technol. Environ. Policy 2024. [Google Scholar] [CrossRef]
- Kumar, M.; Karmakar, S.; Nimesh, V. Statistical Investigation of Combustion and Emission Characteristics of Biofuels According to Their Physical Properties: A Way to Explore Suitable Alternative Fuels. Fuel 2024, 358, 130242. [Google Scholar] [CrossRef]
- Qian, W.; Huang, H.; Pan, M.; Huang, R.; Tong, C.; Guo, X.; Yin, J. Effects of 2-Ethylhexyl Nitrate and Post-Injection Strategy on Combustion and Emission Characterizes in a Dimethyl Carbonate/Diesel Blending Engine. Fuel 2020, 263, 116687. [Google Scholar] [CrossRef]
- Simsek, S.; Uslu, S. Investigation of the Effects of Biodiesel/2-Ethylhexyl Nitrate (EHN) Fuel Blends on Diesel Engine Performance and Emissions by Response Surface Methodology (RSM). Fuel 2020, 275, 118005. [Google Scholar] [CrossRef]
- Zhang, Q.; Yao, M.; Luo, J.; Chen, H.; Zhang, X. Diesel Engine Combustion and Emissions of 2,5-Dimethylfuran-Diesel Blends with 2-Ethylhexyl Nitrate Addition. Fuel 2013, 111, 887–891. [Google Scholar] [CrossRef]
- Kuszewski, H. Effect of Adding 2-Ethylhexyl Nitrate Cetane Improver on the Autoignition Properties of Ethanol–Diesel Fuel Blend—Investigation at Various Ambient Gas Temperatures. Fuel 2018, 224, 57–67. [Google Scholar] [CrossRef]
- Pan, M.; Huang, R.; Liao, J.; Ouyang, T.; Zheng, Z.; Lv, D.; Huang, H. Effect of EGR Dilution on Combustion, Performance and Emission Characteristics of a Diesel Engine Fueled with n-Pentanol and 2-Ethylhexyl Nitrate Additive. Energy Convers. Manag. 2018, 176, 246–255. [Google Scholar] [CrossRef]
- Alemahdi, N.; Tuner, M. The Effect of 2-Ethyl-Hexyl Nitrate on HCCI Combustion Properties to Compensate Ethanol Addition to Gasoline. Fuel 2020, 270, 117569. [Google Scholar] [CrossRef]
- Li, R.; Wang, Z.; Ni, P.; Zhao, Y.; Li, M.; Li, L. Effects of Cetane Number Improvers on the Performance of Diesel Engine Fuelled with Methanol/Biodiesel Blend. Fuel 2014, 128, 180–187. [Google Scholar] [CrossRef]
- Chacko, N.; Johnson, C.; Varadarajan, P.; Sai Srinivas, S.; Jeyaseelan, T. A Comparative Evaluation of Cetane Enhancing Techniques for Improving the Smoke, NOx and BSFC Trade-off in an Automotive Diesel Engine. Fuel 2021, 289, 119918. [Google Scholar] [CrossRef]
- Sarmah, D.K.; Deka, D.C. Use of Yellow Oleander (Thevetia Peruviana) Seed Oil Biodiesel as Cetane and Lubricity Improver for Petrodiesel. Rasayan J. Chem. 2019, 12, 1547–1556. [Google Scholar] [CrossRef]
- Atmanli, A. Effects of a Cetane Improver on Fuel Properties and Engine Characteristics of a Diesel Engine Fueled with the Blends of Diesel, Hazelnut Oil and Higher Carbon Alcohol. Fuel 2016, 172, 209–217. [Google Scholar] [CrossRef]
- Insausti, M.; Fernández Band, B.S. Fast Determination of 2-Ethylhexyl Nitrate Diesel/Biodiesel Blends by Distillation Curves and Chemometrics. Energy Fuels 2016, 30, 5341–5345. [Google Scholar] [CrossRef]
- Ishaq, H.; Crawford, C. Review and Evaluation of Sustainable Ammonia Production, Storage and Utilization. Energy Convers. Manag. 2024, 300, 117869. [Google Scholar] [CrossRef]
- Razak, N.H.; Hashim, H.; Yunus, N.A.; Klemeš, J.J. Reducing Diesel Exhaust Emissions by Optimisation of Alcohol Oxygenates Blend with Diesel/Biodiesel. J. Clean. Prod. 2021, 316, 128090. [Google Scholar] [CrossRef]
- Mohite, S.; Maji, S. Biofuel Certification Performance: A Review & Analysis. Eur. J. Sustain. Dev. Res. 2020, 4, em0124. [Google Scholar]
- Sendzikiene, E.; Makareviciene, V.; Janulis, P. Influence of Composition of Fatty Acid Methyl Esters on Smoke Opacity and Amount of Polycyclic Aromatic Hydrocarbons in Engine Emissions. Pol. J. Environ. Stud. 2007, 16, 259–265. [Google Scholar]
- Çakmak, A.; Özcan, H. Analysis of Combustion and Emissions Characteristics of a DI Diesel Engine Fuelled with Diesel/Biodiesel/Glycerol Tert-Butyl Ethers Mixture by Altering Compression Ratio and Injection Timing. Fuel 2022, 315, 123200. [Google Scholar] [CrossRef]
- Vellaiyan, S.; Kandasamy, M.; Subbiah, A.; Devarajan, Y. Energy, Environmental and Economic Assessment of Waste-Derived Lemon Peel Oil Intermingled with High Intense Water and Cetane Improver. Sustain. Energy Technol. Assess. 2022, 53, 102659. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).