Characterization of Spent Grain from Irish Whiskey Distilleries for Biorefinery Feedstock Potential to Produce High-Value Chemicals and Biopolymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. DSG Proximate Composition Analysis
2.2.1. Determination of Moisture Content
2.2.2. Ash Content
2.2.3. Fat Content Determination
2.2.4. Protein Content Determination
2.2.5. Extraction and Determination of Total Phenolic Content
2.3. DSG Structural Composition Analysis
2.3.1. Quantification of Water and Ethanol Extractives in Biomass
2.3.2. Sample Preparation for Lignocellulosic Composition Analysis
2.3.3. Determination of Structural Carbohydrates and Lignin
2.4. Principal Component Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Composition Analysis
| Composition % | |||||||
|---|---|---|---|---|---|---|---|
| Protein | Cellulose | Hemicellulose | Lignin | Ash | Extractives | Reference | |
| DSG | 12.38–26.32 | 11.75–32.75 | 6.97–19.47 | 8.44–15.71 | 2.01–4.11 | 17.33–31.77 | This work |
| BSG | 15.25 | 16.8 | 28.4 | 27.8 | 4.6 | 5.82 | [54] |
| BSG | 20.3 | 11.1 | 22.7 | 13.8 | 4.7 | 35.5 | [43] |
| BSG | NR | 19.21 | 26.94 | 30.48 | NR | NR | [50] |
| BSG | 14.2 | 12 | 40 | 11.5 | NR | NR | [49] |
| BSG | 28 | 13.2 | 16.75 | NR | 4.5 | 34.8 | [43] |
| BSG | NR | 21.84 | 30.43 | 25.26 | 4.41 | NR | [51] |
| BSG | 22.54–30.24 | NR | NR | NR | 3.30–4.29 | NR | [26] |
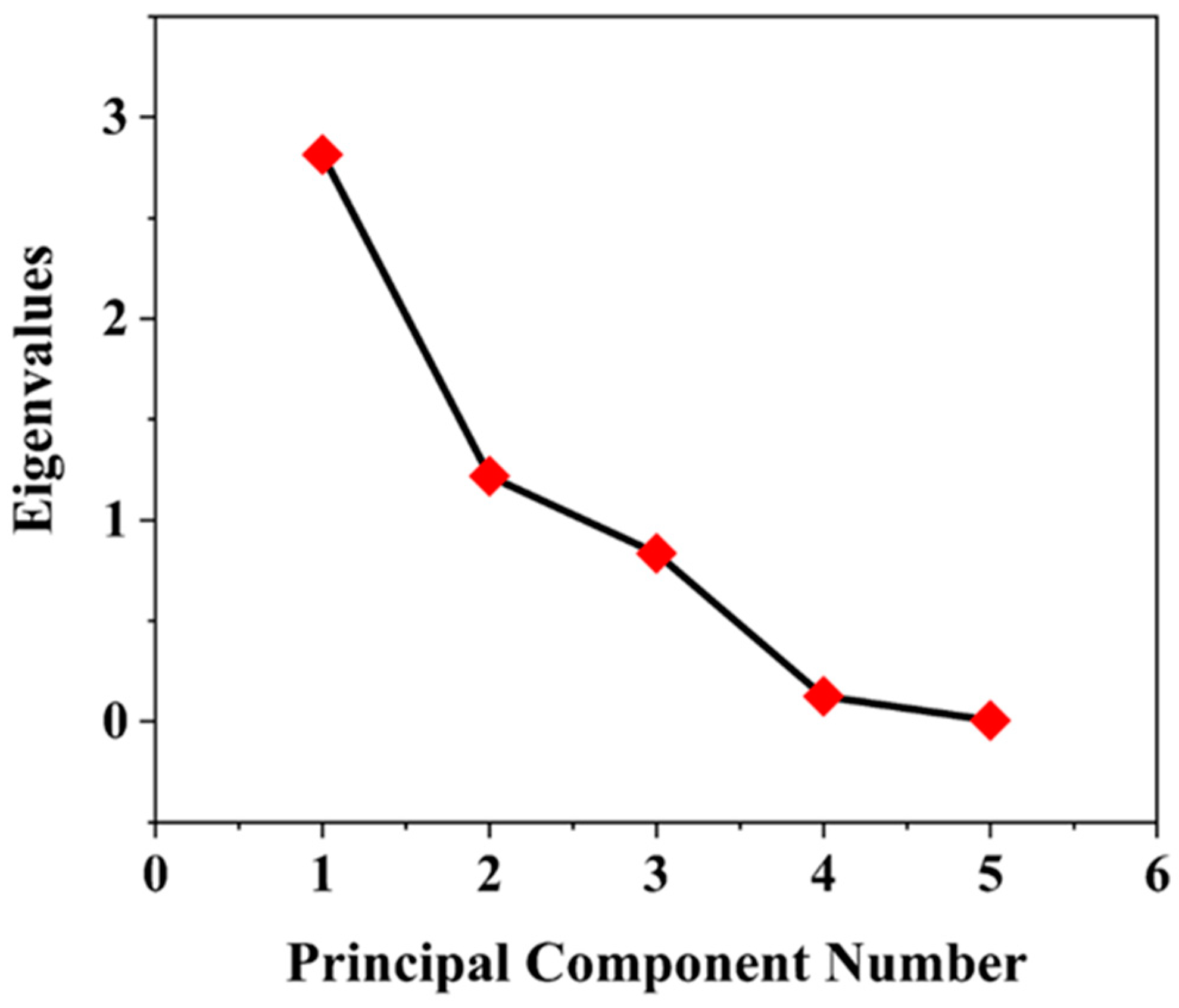
3.2. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Hames, B.R. Biomass Compositional Analysis for Energy Applications. Methods Mol. Biol. 2009, 581, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.U.; Qaisar, K.; Nawaz, A.; Akram, F.; Mukhtar, H.; Zohu, X.; Xu, Y.; Mumtaz, M.W.; Rashid, U.; Ghani, W.A.W.A.K.; et al. Advances in Valorization of Lignocellulosic Biomass towards Energy Generation. Catalysts 2021, 11, 309. [Google Scholar] [CrossRef]
- Department of Agriculture, Food and the Marine. Irish Whiskey Technical File. 2014. Available online: http://www.marketaccess.agriculture.gov.ie/media/marketaccess/content/Irish%20Whiskey%20Technical%20File.pdf (accessed on 29 November 2024).
- Price, R.; MacDonald, L.; Gillies, N.; Day, A.; Brightman, E.; Li, J. Utilisation and Valorisation of Distillery Whisky Waste Streams via Biomass Electrolysis: Electrosynthesis of Hydrogen. Faraday Discuss. 2023, 247, 268–288. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ Spent Grain: A Review with an Emphasis on Food and Health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Gunes, B.; Stokes, J.; Davis, P.; Connolly, C.; Lawler, J. Pre-Treatments to Enhance Biogas Yield and Quality from Anaerobic Digestion of Whiskey Distillery and Brewery Wastes: A Review. Renew. Sustain. Energy Rev. 2019, 113, 109281. [Google Scholar] [CrossRef]
- Ranhotra, G.S.; Gelroth, J.A.; Torrence, F.A.; Bock, M.A.; Winterringer, G.L.; Bates, L.S. Nutritional Characteristics of Distiller’s Spent Grain. J. Food Sci. 1982, 47, 1184–1185. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and Sustainable Pretreatment Methods for Cellulose Extraction from Lignocellulosic Biomass and Its Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Mohana, S.; Acharya, B.K.; Madamwar, D. Distillery Spent Wash: Treatment Technologies and Potential Applications. J. Hazard. Mater. 2009, 163, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Akunna, J.C.; Walker, G.M. Co-Products from Malt Whisky Production and Their Utilisation; Abertay University: Dundee, UK, 2017; pp. 529–537. [Google Scholar]
- Wang, J.; Kong, B.; Feng, J.; Wang, H.; Zhang, R.; Cai, F.; Yu, Q.; Zhu, Z.; Cao, J.; Xu, J. A Novel Strategy for Comprehensive Utilization of Distillers’ Grain Waste towards Energy and Resource Recovery. Process Biochem. 2022, 113, 141–149. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Lucas, S.; García-Cubero, M.T.; Jiménez, J.J.; Coca, M. A Biorefinery Based on Brewer’s Spent Grains: Arabinoxylans Recovery by Microwave Assisted Pretreatment Integrated with Butanol Production. Ind. Crop. Prod. 2020, 158, 113044. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Z.; Li, X.; Lu, X.; Liu, G.; Qin, Y.; Zhao, J.; Qu, Y. Production of Single Cell Protein from Brewer’s Spent Grain through Enzymatic Saccharification and Fermentation Enhanced by Ammoniation Pretreatment. Bioresour. Technol. 2024, 394, 130242. [Google Scholar] [CrossRef] [PubMed]
- Gbenebor, O.P.; Olanrewaju, O.A.; Usman, M.A.; Adeosun, S.O. Lignin from Brewer’s Spent Grain: Structural and Thermal Evaluations. Polymers 2023, 15, 2346. [Google Scholar] [CrossRef]
- Tang, D.S.; Tian, Y.J.; He, Y.Z.; Li, L.; Hu, S.Q.; Li, B. Optimisation of Ultrasonic-Assisted Protein Extraction from Brewer’s Spent Grain. Czech J. Food Sci. 2010, 28, 9–17. [Google Scholar] [CrossRef]
- Rojas-Chamorro, J.A.; Cara, C.; Romero, I.; Ruiz, E.; Romero-García, J.M.; Mussatto, S.I.; Castro, E. Ethanol Production from Brewer’s Spent Grain Pretreated by Dilute Phosphoric Acid. Energy Fuels 2018, 32, 5226–5233. [Google Scholar] [CrossRef]
- Matebie, B.Y.; Tizazu, B.Z.; Kadhem, A.A.; Venkatesa Prabhu, S. Synthesis of Cellulose Nanocrystals (CNCs) from Brewer’s Spent Grain Using Acid Hydrolysis: Characterization and Optimization. J. Nanomater. 2021, 1–10. [Google Scholar] [CrossRef]
- Cordis. Biological Upgrading of Brewer Spent Grain into High Added Value Products|BiOBreW|Project|Fact Sheet|HORIZON|CORDIS|European Commission. 2022. Available online: https://gotriple.eu/projects/horizon%3A101065428 (accessed on 29 November 2024).
- Ktenioudaki, A.; O’Shea, N.; Gallagher, E. Rheological Properties of Wheat Dough Supplemented with Functional By-Products of Food Processing: Brewer’s Spent Grain and Apple Pomace. J. Food Eng. 2013, 116, 318–324. [Google Scholar] [CrossRef]
- Birsan, R.I.; Wilde, P.; Waldron, K.W.; Rai, D.K. Recovery of Polyphenols from Brewer’s Spent Grains. Antioxidants 2019, 8, 380. [Google Scholar] [CrossRef]
- Chetrariu, A.; Dabija, A. Spent Grain: A Functional Ingredient for Food Applications. Foods 2023, 12, 1533. [Google Scholar] [CrossRef]
- Van Deventer, H.; Voogt, J.; Broeze, J.; Verkleij, T. New Application of Brewer’s Spent Grain for Food; Wageningen University & Research: Wageningen, The Netherlands, 2020. [Google Scholar]
- Qazanfarzadeh, Z.; Ganesan, A.R.; Mariniello, L.; Conterno, L.; Kumaravel, V. Valorization of Brewer’s Spent Grain for Sustainable Food Packaging. J. Clean. Prod. 2023, 385, 135726. [Google Scholar] [CrossRef]
- He, R.; Yang, Y.; Li, Y.; Yang, M.; Kong, L.; Yang, F. Recent Progress in Distiller’s Grains: Chemical Compositions and Biological Activities. Molecules 2023, 28, 7492. [Google Scholar] [CrossRef] [PubMed]
- Naibaho, J.; Korzeniowska, M. The Variability of Physico-Chemical Properties of Brewery Spent Grain from Eight Different Breweries. Heliyon 2021, 7, e06583. [Google Scholar] [CrossRef] [PubMed]
- Westreicher-Kristen, E.; Steingass, H.; Rodehutscord, M. Variations in Chemical Composition and In Vitro and In Situ Ruminal Degradation Characteristics of Dried Distillers’ Grains with Solubles from European Ethanol Plants. Arch. Anim. Nutr. 2012, 66, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Fox, G.P.; Bettenhausen, H.M. Variation in Quality of Grains Used in Malting and Brewing. Front. Plant Sci. 2023, 14, 1172028. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples. Laboratory Analytical Procedure (LAP). 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42621.pdf (accessed on 29 November 2024).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass. Laboratory Analytical Procedure (LAP). 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42622.pdf. (accessed on 29 November 2024).
- Wang, L.; Weller, C.L.; Hwang, K.T. Extraction of Lipids from Grain Sorghum DDG. Trans. Am. Soc. Agric. Eng. 2005, 48, 1883–1888. [Google Scholar] [CrossRef][Green Version]
- Hach, C.C.; Bowden, B.K.; Kopelove, A.B.; Brayton, S.V. More Powerful Peroxide Kjeldahl Digestion Method. J. AOAC Int. 1987, 70, 783–787. [Google Scholar] [CrossRef]
- Chetrariu, A.; Dabija, A. Spent Grain from Malt Whisky: Assessment of the Phenolic Compounds. Molecules 2021, 26, 3236. [Google Scholar] [CrossRef] [PubMed]
- Matei, P.L.; Deleanu, I.; Brezoiu, A.M.; Chira, N.A.; Busuioc, C.; Isopencu, G.; Cîlțea-Udrescu, M.; Alexandrescu, E.; Stoica-Guzun, A. Ultrasound-Assisted Extraction of Blackberry Seed Oil: Optimization and Oil Characterization. Molecules 2023, 28, 2486. [Google Scholar] [CrossRef]
- Ayim, I.; Ma, H.; Alenyorege, E.A. Optimizing and Predicting Degree of Hydrolysis of Ultrasound-Assisted Sodium Hydroxide Extraction of Protein from Tea (Camellia sinensis L.) Residue Using Response Surface Methodology. J. Food Sci. Technol. 2018, 55, 5166–5174. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP) (Revised July 2011). 2008. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf#page=1.00&gsr=0 (accessed on 29 November 2024).
- Raman, J.K.; Gnansounou, E. LCA of Bioethanol and Furfural Production from Vetiver. Bioresour. Technol. 2015, 185, 202–210. [Google Scholar] [CrossRef]
- Thai, S.; Avena-Bustillos, R.J.; Alves, P.; Pan, J.; Osorio-Ruiz, A.; Miller, J.; Tam, C.; Rolston, M.R.; Teran-Cabanillas, E.; Yokoyama, W.H.; et al. Influence of Drying Methods on Health Indicators of Brewer’s Spent Grain for Potential Upcycling into Food Products. Appl. Food Res. 2022, 2, 100052. [Google Scholar] [CrossRef]
- Adu, O.B.; Ogundeko, T.O.; Ogunrinola, O.O.; Saibu, G.M.; Elemo, B.O. The Effect of Thermal Processing on Protein Quality and Free Amino Acid Profile of Terminalia Catappa (Indian Almond) Seed. J. Food Sci. Technol. 2015, 52, 4637–4641. [Google Scholar] [CrossRef]
- Terefe, G. Preservation Techniques and Their Effect on Nutritional Values and Microbial Population of Brewer’s Spent Grain: A Review. CABI Agric. Biosci. 2022, 3, 51. [Google Scholar] [CrossRef]
- Kim, Y.; Hendrickson, R.; Mosier, N.S.; Ladisch, M.R.; Bals, B.; Balan, V.; Dale, B.E.; Dien, B.S.; Cotta, M.A. Effect of Compositional Variability of Distillers’ Grains on Cellulosic Ethanol Production. Bioresour. Technol. 2010, 101, 5385–5393. [Google Scholar] [CrossRef]
- Cervantes-Ramirez, J.G.; Vasquez-Lara, F.; Sanchez-Estrada, A.; Troncoso-Rojas, R.; Heredia-Olea, E.; Islas-Rubio, A.R. Arabinoxylans Release from Brewer’s Spent Grain Using Extrusion and Solid-State Fermentation with Fusarium Oxysporum and the Antioxidant Capacity of the Extracts. Foods 2022, 11, 1415. [Google Scholar] [CrossRef] [PubMed]
- de Crane Ravindran, S.; Bockstal, L.; Jacquet, N.; Schmetz, Q.; Richel, A. Potential for the Valorisation of Brewer’s Spent Grains: A Case Study for the Sequential Extraction of Saccharides and Lignin. Waste Manag. Res. 2022, 40, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Jiménez, J.J.; Bartolomé, B.; Gómez-Cordovés, C.; Del Nozal, M.J. Variability of Brewer’s Spent Grain within a Brewery. Food Chem. 2003, 80, 17–21. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Colpini, L.M.S. All-around Characterization of Brewer’s Spent Grain. Eur. Food Res. Technol. 2021, 247, 3013–3021. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s Spent Grain: A Valuable Feedstock for Industrial Applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, A.; Zannini, E.; Sahin, A.W.; Arendt, E.K. Barley Protein Properties, Extraction and Applications, with a Focus on Brewer’s Spent Grain Protein. Foods 2021, 10, 1389. [Google Scholar] [CrossRef]
- Waters, D.M.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Fibre, Protein and Mineral Fortification of Wheat Bread through Milled and Fermented Brewer’s Spent Grain Enrichment. Eur. Food Res. Technol. 2012, 235, 767–778. [Google Scholar] [CrossRef]
- Xiros, C.; Topakas, E.; Katapodis, P.; Christakopoulos, P. Evaluation of Fusarium Oxysporum as an Enzyme Factory for the Hydrolysis of Brewer’s Spent Grain with Improved Biodegradability for Ethanol Production. Ind. Crop. Prod. 2008, 28, 213–224. [Google Scholar] [CrossRef]
- Ravindran, R.; Sarangapani, C.; Jaiswal, S.; Lu, P.; Cullen, P.J.; Bourke, P.; Jaiswal, A.K. Improving Enzymatic Hydrolysis of Brewer Spent Grain with Nonthermal Plasma. Bioresour. Technol. 2019, 282, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Outeiriño, D.; Costa-Trigo, I.; Paz, A.; Deive, F.J.; Rodríguez, A.; Domínguez, J.M. Biorefining Brewery Spent Grain Polysaccharides through Biotuning of Ionic Liquids. Carbohydr. Polym. 2018, 201, 48–53. [Google Scholar] [CrossRef]
- Lara-Serrano, M.; Morales-delaRosa, S.; Campos-Martín, J.M.; Fierro, J.L.G. Fractionation of Lignocellulosic Biomass by Selective Precipitation from Ionic Liquid Dissolution. Appl. Sci. 2019, 9, 1862. [Google Scholar] [CrossRef]
- Mishra, P.K.; Gregor, T.; Wimmer, R. Utilising Brewer’s Spent Grain as a Source of Cellulose Nanofibres Following Separation of Protein-Based Biomass. Bioresources 2017, 12, 107–116. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Chemical Characterization and Liberation of Pentose Sugars from Brewer’s Spent Grain. J. Chem. Technol. Biotechnol. 2006, 81, 268–274. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Lynch, K.M.; Strain, C.R.; Johnson, C.; Patangia, D.; Stanton, C.; Koc, F.; Gil-Martinez, J.; O’Riordan, P.; Sahin, A.W.; Ross, R.P.; et al. Extraction and Characterisation of Arabinoxylan from Brewers Spent Grain and Investigation of Microbiome Modulation Potential. Eur. J. Nutr. 2021, 60, 4393. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and Chemical Modifications of Lignin: Towards Lignin-Based Nanomaterials for Biomedical Applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Cassoni, A.C.; Costa, P.; Mota, I.; Vasconcelos, M.W.; Pintado, M. Recovery of Lignins with Antioxidant Activity from Brewer’s Spent Grain and Olive Tree Pruning Using Deep Eutectic Solvents. Chem. Eng. Res. Des. 2023, 192, 34–43. [Google Scholar] [CrossRef]
- Verni, M.; Pontonio, E.; Krona, A.; Jacob, S.; Pinto, D.; Rizzello, C.G. Bioprocessing of Brewers’ Spent Grain Enhances Its Antioxidant Activity: Characterization of Phenolic Compounds and Bioactive Peptides. Front. Microbiol. 2020, 11, 1831. [Google Scholar] [CrossRef]
- Horvat, D.; Šimić, G.; Drezner, G.; Lalić, A.; Tucak, M.; Zdunić, Z. Phenolic Acid Profiles and Antioxidant Activity of Major Cereal Crops. Antioxidants 2020, 9, 527. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Sologubik, C.A.; Fernández, M.B.; Manrique, G.D.; D’Alessandro, L.G. Extraction of Antioxidant Phenolic Compounds from Brewer’s Spent Grain: Optimization and Kinetics Modeling. Antioxidants 2018, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Arisekar, U.; Shalini, R.; Shakila, R.J.; Sundhar, S.; Afrin Banu, A.M.; Iburahim, S.A.; Umamaheshwari, T. Trace Metals in Commercial Seafood Products (Canned, Pickled and Smoked): Comparison, Exposure and Health Risk Assessment. Food Res. Int. 2024, 178, 113969. [Google Scholar] [CrossRef]
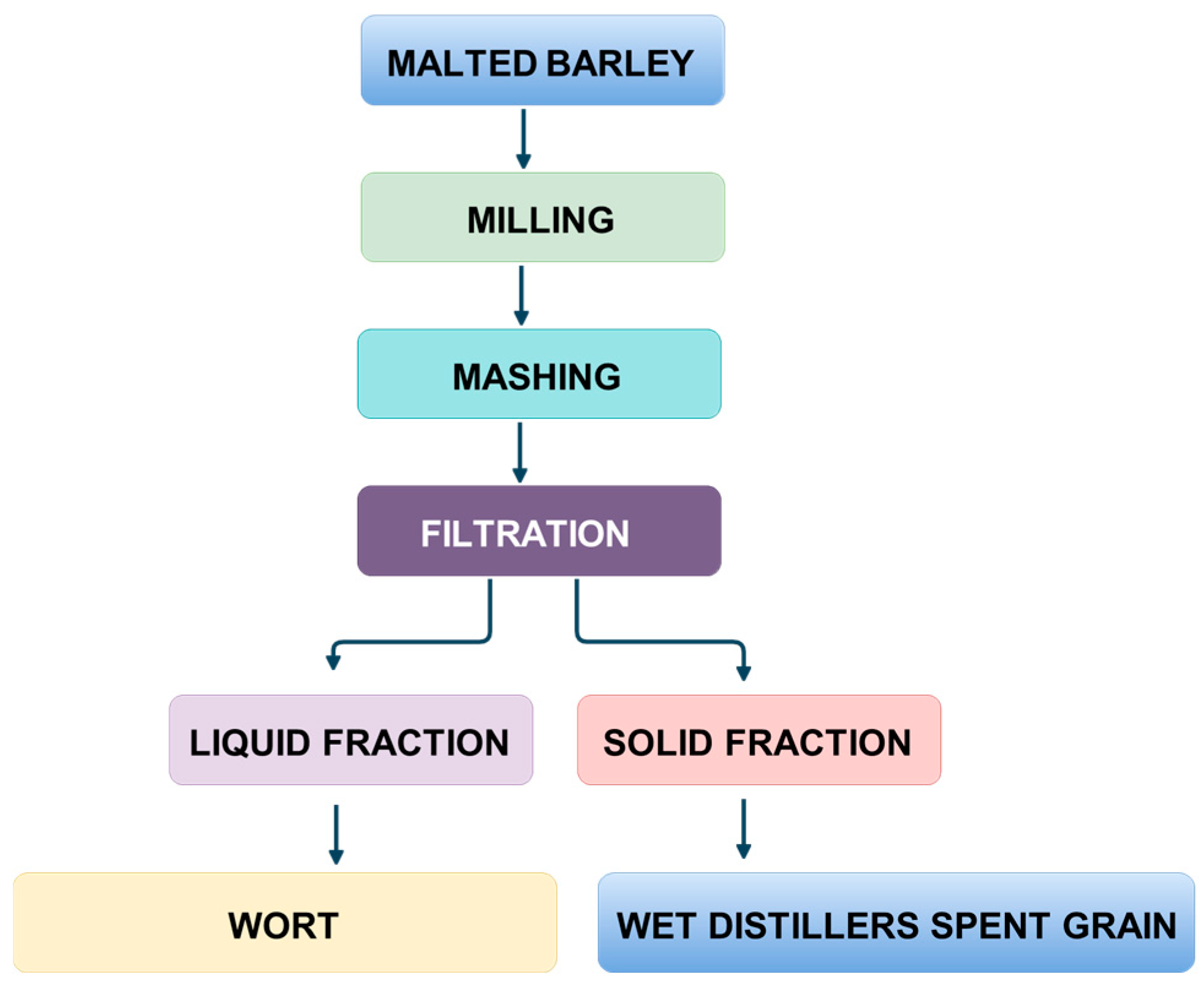

| Distillery Code | Sample Code | Malted Barley (%) | Unmalted Barley (%) | Oat (%) | Rye (%) | Whiskey Type |
|---|---|---|---|---|---|---|
| Distillery 1 | D1 | 100 | - | - | - | Malt Irish Whiskey |
| Distillery 2 | D2 | 60 | 35 | 5 | - | Single pot still whiskey |
| Distillery 3 | D3 | 60 | 40 | - | - | Single pot still whiskey |
| Distillery 4 | D4 | 40 | 60 | - | - | Single pot still whiskey |
| Distillery 5 | D5 | 100 | - | - | - | Malt Irish Whiskey |
| Distillery 6 | D6 | 60 | 40 | - | - | Single pot still whiskey |
| Distillery 7 | D7 | 100 | - | - | - | Malt Irish Whiskey |
| Distillery 8 | D8 | 100 | - | Malt Irish Whiskey | ||
| Distillery 9 | D9 | 40 | 55 | 3.25 | 1.75 | Single pot still whiskey |
| Sample Code | Moisture Content of Fresh DSG (%) | Fat (%) | Protein (%) | Ash (%) | Total Phenolics MgGAE/g DSG |
|---|---|---|---|---|---|
| D1 | 80.17 ± 1.57 a | 13.63 ± 0.47 b | 17.93 ± 1.06 c | 3.28 ± 0.10 a | 2.25 ± 0.17 ab |
| D2 | 75.54 ± 0.593 b | 13.54 ± 0.20 b | 18.28 ± 0.67 bc | 3.95 ± 0.32 a | 2.60 ± 0.05 ab |
| D3 | 75.02 ± 0.163 b | 10.98 ± 0.57 c | 24.73 ± 0.03 a | 3.53 ± 0.51 a | 2.61 ± 1.15 ab |
| D4 | 73.22 ± 0.192 b | 10.01 ± 0.02 cd | 18.46 ± 0.05 bc | 3.69 ± 0.06 a | 3.91 ± 0.01 a |
| D5 | 75.7 ± 0.367 b | 8.96 ± 0.023 d | 20.32 ± 0.65 b | 3.78 ± 0.13 a | 3.37 ± 0.46 a |
| D6 | 75.01 ± 0.085 b | 13.49 ± 0.29 b | 25.91 ± 0.01 a | 4.11 ± 0.06 a | 1.42 ± 0.15 b |
| D7 | 73.49 ± 0.645 b | 14.92 ± 0.11 a | 18.25 ± 0.21 bc | 3.64 ± 0.47 a | 3.74 ± 0.44 a |
| D8 | 74.71 ± 0.760 b | 13.01 ± 0.06 b | 12.38 ± 0.98 d | 2.01 ± 0.09 b | 2.27 ± 0.01 ab |
| D9 | 74.95 ± 0.487 b | 10.17 ± 0.45 cd | 26.32 ± 0.16 a | 3.21 ± 0.11 a | 3.29 ± 0.13 a |
| Distillery | Protein | Water/Ethanol Extractives | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|---|---|
| D1 | 17.93 ± 1.06 c | 24.77 ± 0.61 bc | 20.2 ± 0.20 cd | 17.07 ± 3.03 ab | 14.04 ± 0.15 a |
| D2 | 18.28 ± 0.67 bc | 31.14 ± 2.98 a | 16.2 ± 1.93 e | 16.07 ± 2.79 abc | 12.89 ± 0.71 abc |
| D3 | 24.73 ± 0.03 a | 17.33 ± 0.41 d | 20.6 ± 0.12 c | 19.47 ± 0.49 a | 15.71 ± 0.65 a |
| D4 | 18.46 ± 0.05 bc | 30.28 ± 0.13 a | 16.5 ± 0.09 de | 13.27 ± 0.01 bc | 10.44 ± 0.27 bcd |
| D5 | 20.32 ± 0.65 b | 24.74 ± 0.52 bc | 22.6 ± 2.05 bc | 12.31 ± 0.71 bcd | 9.76 ± 1.39 cd |
| D6 | 25.91 ± 0 a | 30.63 ± 1.54 a | 11.8 ± 0.39 f | 11.07 ± 0.41 cd | 9.95 ± 1.01 cd |
| D7 | 18.25 ± 0.21 bc | 29.16 ± 1.46 ab | 19.2 ± 0.31 cde | 15.41 ± 0.48 abc | 13.45 ± 1.42 ab |
| D8 | 12.38 ± 0.98 d | 31.77 ± 0.27 a | 32.7 ± 0.49 a | 6.97 ± 0.15 d | 8.44 ± 0.51 d |
| D9 | 26.32 ± 0.16 a | 20.32 ± 0.96 cd | 25.48 ± 0.80 b | 12.18 ± 0.27 bcd | 13.99 ± 0.91 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abolore, R.S.; Pradhan, D.; Jaiswal, S.; Jaiswal, A.K. Characterization of Spent Grain from Irish Whiskey Distilleries for Biorefinery Feedstock Potential to Produce High-Value Chemicals and Biopolymers. Appl. Sci. 2024, 14, 11577. https://doi.org/10.3390/app142411577
Abolore RS, Pradhan D, Jaiswal S, Jaiswal AK. Characterization of Spent Grain from Irish Whiskey Distilleries for Biorefinery Feedstock Potential to Produce High-Value Chemicals and Biopolymers. Applied Sciences. 2024; 14(24):11577. https://doi.org/10.3390/app142411577
Chicago/Turabian StyleAbolore, Rasaq S., Dileswar Pradhan, Swarna Jaiswal, and Amit K. Jaiswal. 2024. "Characterization of Spent Grain from Irish Whiskey Distilleries for Biorefinery Feedstock Potential to Produce High-Value Chemicals and Biopolymers" Applied Sciences 14, no. 24: 11577. https://doi.org/10.3390/app142411577
APA StyleAbolore, R. S., Pradhan, D., Jaiswal, S., & Jaiswal, A. K. (2024). Characterization of Spent Grain from Irish Whiskey Distilleries for Biorefinery Feedstock Potential to Produce High-Value Chemicals and Biopolymers. Applied Sciences, 14(24), 11577. https://doi.org/10.3390/app142411577









