Application of a Validated HPLC Method for the Determination of Resveratrol, Ferulic Acid, Quercetin, Retinol, and α-Tocopherol in a Cold Cream—Permeability Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Reagents, Solvents and Materials
2.3. Standard Solutions
2.4. Cream Preparation
2.5. In Vitro Permeability Studies—Franz Cells Apparatus
3. Results and Discussion
3.1. Investigation of the Chromatographic Method
3.2. Method Validation
3.2.1. System Suitability
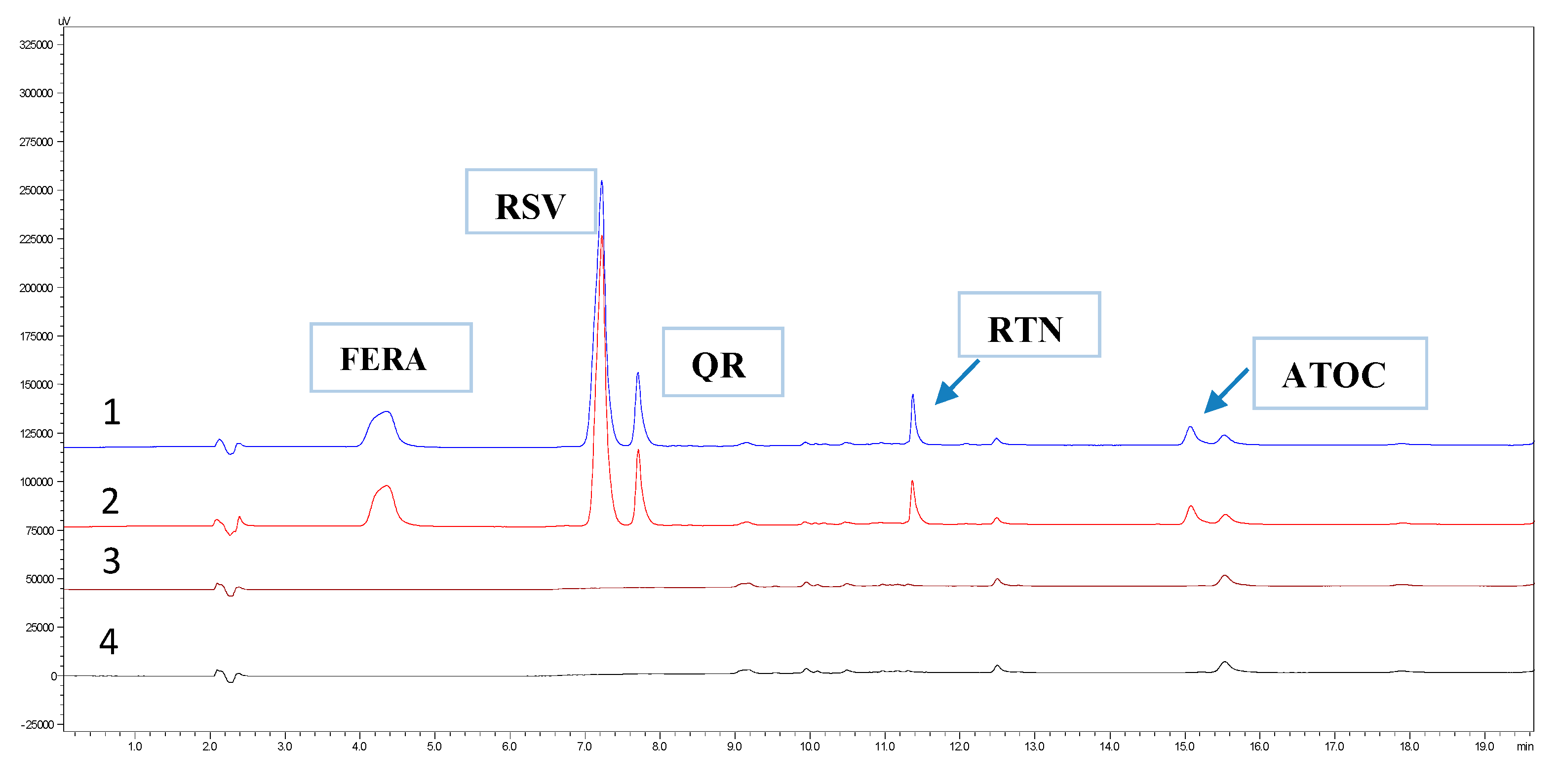
3.2.2. Specificity
3.2.3. Linearity
3.2.4. Precision
3.2.5. Accuracy
3.2.6. Limit of Detection (LOD) and Limit of Quantification (LOQ)
3.2.7. Robustness
3.3. Quantitative Determination of Resveratrol at the Plant Extract
3.4. Stability Study
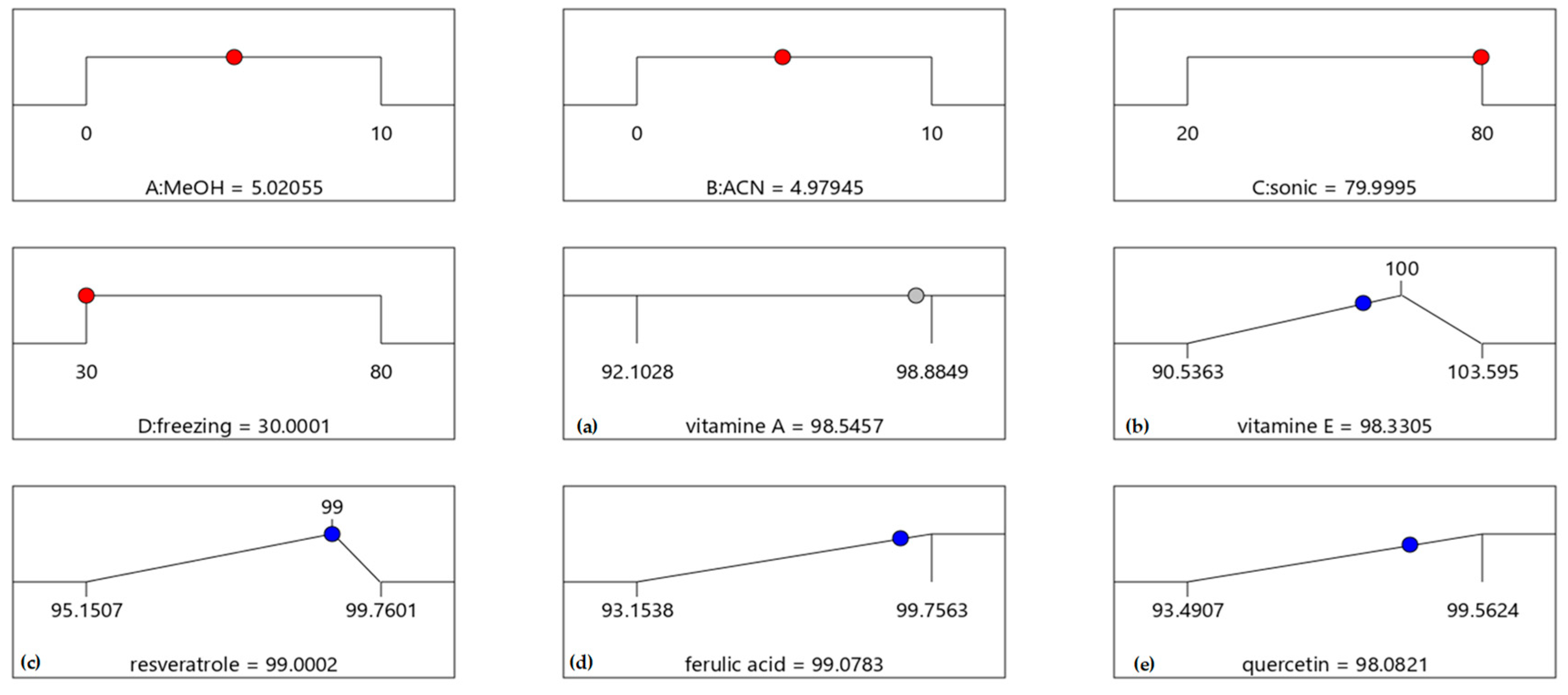
4. Extraction Procedure via Experimental Design
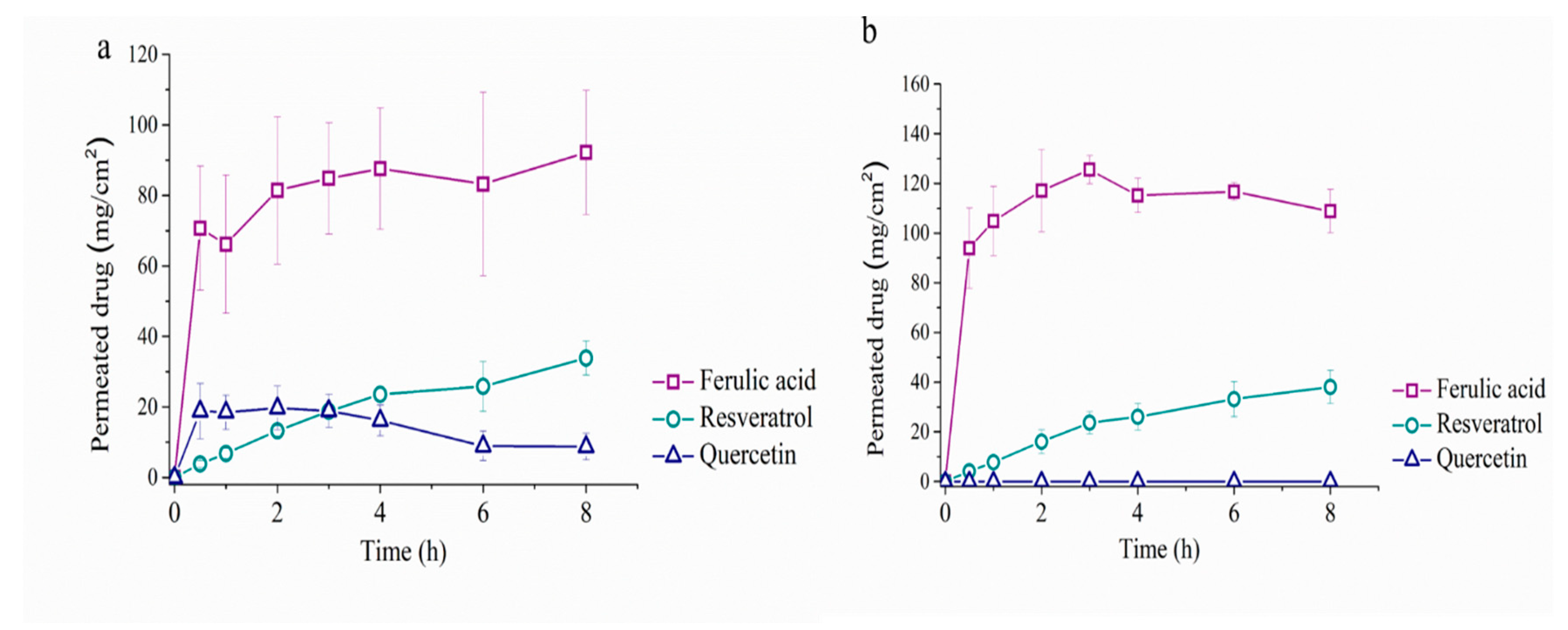
5. Permeability Study with Franz Cells
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallo, R.L. Human Skin Is the Largest Epithelial Surface for Interaction with Microbes. J. Investig. Dermatol. 2017, 137, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Bonté, F.; Girard, D.; Archambault, J.C.; Desmoulière, A. Skin Changes During Ageing. In Biochemistry and Cell Biology of Ageing: Part II Clinical Science; Springer: Singapore, 2019; pp. 249–280. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Lee, Y.I.; Almurayshid, A.; Jung, J.Y.; Lee, J.H. Effect of a topical antioxidant serum containing vitamin C, vitamin E, and ferulic acid after Q-switched 1064-nm Nd:YAG laser for treatment of environment-induced skin pigmentation. J. Cosmet. Dermatol. 2020, 19, 2576–2582. [Google Scholar] [CrossRef]
- Sunder, S. Relevant Topical Skin Care Products for Prevention and Treatment of Aging Skin. Facial Plast. Surg. Clin. N. Am. 2019, 27, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Puizina-Ivic, N. Skin aging. Acta Dermatovenerol. Alp. Panon. Adriat. 2008, 17, 47–54. [Google Scholar]
- Sjerobabski-Masnec, I.; Situm, M. Skin aging. Acta Clin. Croat. 2010, 49, 515–518. [Google Scholar] [PubMed]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging—From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef]
- Prajakta, S.; Shahu, K. Formulation and Evaluation of Vanishing Herbal Cream of Crude Drugs. Asian J. Pharm. Res. Dev. 2020, 8, 66–69. [Google Scholar] [CrossRef]
- Niki, E.; Abe, K. Vitamin E: Structure, Properties and Functions. In Vitamin E: Chemistry and Nutritional Benefits; Food Chemistry, Function and Analysis Series; The Royal Society of Chemistry: London, UK, 2019; Chapter 1; pp. 1–11. [Google Scholar] [CrossRef]
- Janssens-Böcker, C.; Kerscher, M. Skin Anti-Aging Benefits of a 2% Resveratrol Emulsion. J. Cosmet. Dermatol. Sci. Appl. 2021, 11, 155–168. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Zasada, M.; Budzisz, E.; Kolodziejska, J.; Kalinowska-Lis, U. An evaluation of the physicochemical parameters and the content of the active ingredients in original formulas containing retinol. J. Cosmet. Dermatol. 2020, 19, 2374–2383. [Google Scholar] [CrossRef]
- Quan, T. Human Skin Aging and the Anti-Aging Properties of Retinol. Biomolecules 2023, 13, 1614. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, J.H. Protective effects of resveratrol on UVB-irradiated HaCaT cells through attenuation of the caspase pathway. Oncol. Rep. 2008, 19, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.G.; Byun, S. Quercetin Directly Targets JAK2 and PKCδ and Prevents UV-Induced Photoaging in Human Skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef]
- Rattanawiwatpong, P.; Wanitphakdeedecha, R.; Bumrungpert, A.; Maiprasert, M. Anti-aging and brightening effects of a topical treatment containing vitamin C, vitamin E, and raspberry leaf cell culture extract: A split-face, randomized controlled trial. J. Cosmet. Dermatol. 2020, 19, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Wolter, F.; Stein, J. Biological activities of resveratrol and its analogs. Drugs Future 2002, 27, 949–959. [Google Scholar] [CrossRef]
- Zinder, R.; Cooley, R.; Vlad, L.G.; Molnar, J.A. Vitamin A and Wound Healing. Nutr. Clin. Pract. 2019, 34, 839–849. [Google Scholar] [CrossRef]
- Lin, F.-H.; Lin, J.-Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic Acid Stabilizes a Solution of Vitamins C and E and Doubles its Photoprotection of Skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Hsieh, S.N.; Ekanayake-Mudiyanselage, S. Vitamin E: Critical review of its current use in cosmetic and clinical dermatology. Dermatol. Surg. 2005, 31, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, R.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; Vicentini, F.T.; Rafael, J.A.; Jabor, J.R.; Fonseca, M.J. Method validation and stability study of quercetin in topical emulsions. Química Nova 2009, 32, 1939–1942. [Google Scholar] [CrossRef]
- Fasolo, D.; Schwingel, L.; Holzschuh, M.; Bassani, V.; Teixeira, H. Validation of an isocratic LC method for determination of quercetin and methylquercetin in topical nanoemulsions. J. Pharm. Biomed. Anal. 2007, 44, 1174–1177. [Google Scholar] [CrossRef]
- Lai, J.F.; Franke, A.A. Analysis of circulating lipid-phase micronutrients in humans by HPLC: Review and overview of new developments. J. Chromatogr. B 2013, 931, 23–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gundersen, T.E.; Blomhoff, R. Qualitative and quantitative liquid chromatographic determination of natural retinoids in biological samples. J. Chromatogr. A 2001, 935, 13–43. [Google Scholar] [CrossRef] [PubMed]
- Tee-ngam, P.; Nunant, N.; Rattanarat, P.; Siangproh, W.; Chailapakul, O. Simple and Rapid Determination of Ferulic Acid Levels in Food and Cosmetic Samples Using Paper-Based Platforms. Sensors 2013, 13, 13039–13053. [Google Scholar] [CrossRef] [PubMed]
- Barberousse, H.; Roiseux, O.; Robert, C.; Paquot, M.; Deroanne, C.; Blecker, C. Analytical methodologies for quantification of ferulic acid and its oligomers. J. Sci. Food Agric. 2008, 88, 1494–1511. [Google Scholar] [CrossRef]
- Marcato, D.C.; Spagnol, C.M.; Salgado, H.R.N.; Isaac, V.L.B.; Corrêa, M.A. New and potential properties, characteristics, and analytical methods of ferulic acid: A review. Braz. J. Pharm. Sci. 2022, 58, e18747. [Google Scholar] [CrossRef]
- Wang, J.; Shi, A.; Agyei, D.; Wang, Q. Formulation of water-in-oil-in-water (W/O/W) emulsions containing trans-resveratrol. RSC Adv. 2017, 7, 35917–35927. [Google Scholar] [CrossRef]
- Scalia, S.; Trotta, V.; Iannuccelli, V.; Bianchi, A. Bianchi Enhancement of in vivo human skin penetration of resveratrol by chitosan-coated lipid microparticles. Colloids Surf. B Biointerfaces 2015, 135, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.D.; Guerra, C.C.; Czermainski, A.B.C.; Ferrari, L.; Bergold, A.M. Validation of a chromatographic method to routine analysis of trans-resveratrol and quercetin in red wines. Pesqui. Agropecuária Bras. 2017, 52, 335–343. [Google Scholar] [CrossRef][Green Version]
- Mansour, F.R.; Abdallah, I.A.; Bedair, A.; Hamed, M. Analytical Methods for the Determination of Quercetin and Quercetin Glycosides in Pharmaceuticals and Biological Samples. Crit. Rev. Anal. Chem. 2023, 54, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Fiod Riccio, B.V.; Fonseca-Santos, B.; Colerato Ferrari, P.; Chorilli, M. Characteristics, Biological Properties and Analytical Methods of Trans-Resveratrol: A Review. Crit. Rev. Anal. Chem. 2020, 50, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Kamaris, G.; Dalavitsou, A.; Markopoulou, C.K. Development and Validation of an HPLC-DAD Method for the Determination of Seven Antioxidants in a Nano-Emulsion: Formulation and Stability Study. Separations 2024, 11, 43. [Google Scholar] [CrossRef]
- Thongchai, W.; Liawruangrath, B. Development of HPLC analysis for the determination of retinol and alpha tocopherol in corn oil nanoemulsion lotion. Int. Food Res. J. 2016, 23, 1367. [Google Scholar]
- Ahmed, O.A.; El-Bassossy, H.M.; El-Sayed, H.M.; El-Hay, S.S.A. Rp-HPLC Determination of Quercetin in a Novel D-α-Tocopherol Polyethylene Glycol 1000 Succinate Based SNEDDS Formulation: Pharmacokinetics in Rat Plasma. Molecules 2021, 26, 1435. [Google Scholar] [CrossRef]
- Benedetti, B.; Caponigro, V.; Ardini, F. Experimental Design Step by Step: A Practical Guide for Beginners. Crit. Rev. Anal. Chem. 2022, 52, 1015–1028. [Google Scholar] [CrossRef]
- Politis, S.N.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of experiments (DoE) in pharmaceutical development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef]
- Yadav, R.; Thakur, S.; Parihar, R.; Chauhan, U.; Chanana, A.; Chawra, H.S. Pharmaceutical Preparation and Evaluation of Cold Cream. Int. J. Innov. Sci. Res. Technol. 2023, 8, 1069–1075. [Google Scholar]
- ICH. ICH Q2(R2) Guideline on Validation of Analytical Procedures. 2023. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 5 November 2023).
- Tsanaktsidou, E.; Chatzitaki, A.T.; Chatzichristou, A.; Fatouros, D.G.; Markopoulou, C.K. A Comparative Study and Prediction of the Ex Vivo Permeation of Six Vaginally Administered Drugs across Five Artificial Membranes and Vaginal Tissue. Molecules 2024, 29, 2334. [Google Scholar] [CrossRef]
- Berben, P.; Bauer-Brandl, A.; Brandl, M.; Faller, B.; Flaten, G.E.; Jacobsen, A.C.; Brouwers, J.; Augustijns, P. Drug permeability profiling using cell-free permeation tools: Overview and applications. Eur. J. Pharm. Sci. 2018, 119, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Moghimipour, E.; Farsimadan, N.; Salimi, A. Ocular Delivery of Quercetin Using Microemulsion System: Design, Characterization, and Ex-vivo Transcorneal Permeation. Iran. J. Pharm.Res. IJPR 2022, 21, e127486. [Google Scholar] [CrossRef]
- Francioso, A.; Mastromarino, P.; Restignoli, R.; Boffi, A.; d’Erme, M.; Mosca, L. Improved stability of trans-resveratrol in aqueous solutions by carboxymethylated (1, 3/1, 6)-β-D-glucan. J. Agric. Food Chem. 2014, 62, 1520–1525. [Google Scholar] [CrossRef] [PubMed]


| Ingredients | Cream 1 | Cream 2 | Cream 3 | Cream 4 | Cream 5 |
|---|---|---|---|---|---|
| White wax | 2.0 g | 2.0 g | 2.0 g | 2.0 g | 2.0 g |
| Cetyl alcohol | 0.3 g | 0.3 g | 0.3 g | 0.3 g | 0.3 g |
| Coconut oil | 9.0 g | 3.0 g | 7.0 g | 4.5 g | 3.0 g |
| Almond oil | - | 3.0 g | - | - | - |
| Borax | 0.16 g | 0.16 g | 0.16 g | 0.16 g | 0.16 g |
| Deionized water | 5.16 g | 5.16 g | 5.16 g | 5.16 g | 5.16 g |
| Total | 16.62 g | 16.62 g | 14.62 g | 12.12 g | 10.62 g |
| Substances | Retention Time | Tailing Factor | Capacity Factor | Resolution | Theoretical Plates | HΕΤP mm × 10−3 |
|---|---|---|---|---|---|---|
| (min) | (Tf) | (k′) | (Rs) | (Ν) | ||
| FERA | 4.4 | 0.9 | 1.1 | 1109 | 225.4 | |
| RSV | 7.2 | 0.9 | 2.6 | 7.5 | 13,902 | 18.0 |
| QR | 7.2 | 1.6 | 2.9 | 2.5 | 43,334 | 5.8 |
| RTN | 11.4 | 1.8 | 4.7 | 25.0 | 99,790 | 2.5 |
| α-TOC | 15.0 | 1.7 | 6.5 | 20.0 | 71,317 | 3.5 |
| Analytes | Concentration Range (μg·mL−1) | Calibration Curves | %y-Intercept Values | Correlation Coefficient (r2) | LOD (μg·mL−1) | LOQ (μg·mL−1) |
|---|---|---|---|---|---|---|
| RSV | 0.4–3.2 | y = 186,124x − 1035.5 | 0.75 | 0.998 | 0.13 | 0.39 |
| FERA | 0.2–2.1 | y = 128,958x − 199.1 | 0.3 | 0.999 | 0.04 | 0.12 |
| QR | 1.2–9.4 | y = 98,150x − 1391.0 | 0.6 | 0.998 | 0.48 | 1.45 |
| RTN | 0.6–4.2 | y = 87,131x − 705.8 | 0.8 | 0.999 | 0.22 | 0.66 |
| ATOC | 6.4–27.0 | y = 4171.8x − 1260.8 | 2.0 | 0.998 | 2.28 | 6.91 |
| APIs | Intraday Precision | Inter-Day Precision | |||||
|---|---|---|---|---|---|---|---|
| Concentrations | %RSD (n = 3) 1st Day | % Recovery | %RSD (n = 6) | %RSD (n = 3) | |||
| (μg·mL−1) | 2nd Day | 3rd Day | Total | ||||
| FERA | 0.1 | 2.0 | 102.0 | 2.0 | 1.2 | 2.0 | |
| 0.3 | 1.4 | 98.7 | 0.1 | 1.2 | 0.1 | 1.0 | |
| 2.1 | 0.1 | 99.6 | 0.3 | 1.5 | 0.2 | ||
| RSV | 0.2 | 0.1 | 101.0 | 0.8 | 1.4 | 2.0 | |
| 0.47 | 0.1 | 100.1 | 0.1 | 2.0 | 1.7 | 1.5 | |
| 3.1 | 1.6 | 100.5 | 0.3 | 0.4 | 1.0 | ||
| QR | 0.6 | 0.1 | 100.2 | 1.6 | 0.1 | 1.2 | |
| 1.4 | 1.3 | 97.1 | 0.1 | 1.4 | 1.6 | 2.0 | |
| 9.4 | 1.3 | 100.8 | 0.2 | 0.2 | 1.9 | ||
| RTN | 0.3 | 1.0 | 100.8 | 1.4 | 0.2 | 1.6 | |
| 0.6 | 0.5 | 100.7 | 0.2 | 0.3 | 0.5 | 1.2 | |
| 4.2 | 1.5 | 97.6 | 0.2 | 0.4 | 0.4 | ||
| α-TOC | 3.2 | 0.7 | 99.7 | 2.0 | 0.3 | 1.7 | |
| 8.1 | 1.6 | 100.0 | 0.6 | 0.0 | 0.4 | 1.3 | |
| 27 | 1.7 | 100.2 | 0.4 | 0.2 | 0.4 | ||
| % RSD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | AUC RSV | Tf RSV | AUC QR | Tf QR | Rs RSV-QR | AUC FERA | Tf FERA | AUC RTN | Tf RTN | AUC α-TOC | Tf α-TOC |
| According to USP | |||||||||||
| Flow rate mL/min | 5.7 | 4.9 | 5.9 | 2.9 | 5.5 | 3.7 | 5.0 | 5.9 | 1.0 | 4.7 | 6.5 |
| (1.4, 1.5, 1.6) | |||||||||||
| Column T (°C) | 1.0 | 4.5 | 0.8 | 5.7 | 3.5 | 0.8 | 4.9 | 5.1 | 2.6 | 3.8 | 4.5 |
| (39°,40°, 41°) | |||||||||||
| λmax | 0.4 | 1.1 | 0.1 | 1.2 | 2.5 | 0.5 | 1.2 | 2.3 | 4.9 | 2.0 | 1.4 |
| Mixture | Units | Limits | |
|---|---|---|---|
| A | MeOH | mL | 0–10 |
| B | ACN | mL | 0–10 |
| Process | |||
| C | sonic | min | 20–80 min |
| D | freezing | min | 30–80 min |
| Responses | |||
| R1 | RTN | % Recovery | |
| R2 | A-TOC | % Recovery | |
| R3 | RSV | % Recovery | |
| R4 | FERA | % Recovery | |
| R5 | QR | % Recovery | |
| ANOVA | RTN | RSV | FERA |
|---|---|---|---|
| R2 | 0.7931 | 0.8384 | 0.7279 |
| Adjusted R2 | 0.7488 | 0.8038 | 0.6916 |
| Predicted R2 | 0.5959 | 0.7367 | 0.6082 |
| Adeq. Precision | 11.36 | 9.81 | 10.03 |
| F | 17.89 | 24.21 | 20.06 |
| C.V. % | 1.03 | 0.63 | 1.63 |
| % Recovery | |||||
|---|---|---|---|---|---|
| Sample | RSV | FERA | QR | RTN | α-TOC |
| 1 | 98.9 | 99.9 | 98.6 | 100.9 | 99.3 |
| 2 | 99.4 | 99.4 | 99.5 | 102.9 | 100.8 |
| 3 | 99.3 | 100.0 | 98.9 | 102.5 | 100.0 |
| 4 | 99.1 | 99.7 | 98.5 | 101.3 | 99.7 |
| 5 | 98.5 | 99.1 | 97.7 | 101.1 | 99.0 |
| %RSD | 0.4 | 0.4 | 0.6 | 0.9 | 0.7 |
| Cell | J (μg/cm2/h) | Papp (h/cm2) × 10−3 | ||||
|---|---|---|---|---|---|---|
| FERA | RSV | QR | FERA | RSV | QR | |
| Cream | 2.344 ± 0.131 | 3.276 ± 0.674 | 1.890 ± 0.339 | 0.455 ± 0.026 | 0.323 ± 0.066 | 0.188 ± 0.034 |
| Solution | 3.303 ± 0.801 | 4.466 ± 0.566 | - | 0.578 ± 0.135 | 0.315 ± 0.065 | - |
| Amount of Drug in Suspension | Amount of Drug in Cream | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Reference Sample | Donor | Acceptor | Membrane | Reference Sample | Donor | Acceptor | Membrane | ||
| Loaded (μg) | Found (μg) | Loaded Sample (μg) | Final Found (μg) | Final Found (μg) | Loaded (μg) | Found (μg) | Loaded Sample (μg) | Final Found (μg) | Final Found (μg) | |
| RTN | 750.0 | 337.5 | 750.0 | - | 315.2 | 2040.0 | 2092.5 | 2040.0 | - | 1875.8 |
| α-TOC | 5000.0 | 1887.5 | 5000.0 | - | 1988.6 | 639.1 | 6590.0 | 639.1 | - | 5765.7 |
| RSV | 2600.0 | 545.0 | 2600.0 | 169.8 | 349.4 | 3235.3 | 3163.0 | 3235.3 | 155.5 | 2693.8 |
| FERA | 1300.0 | 565.0 | 1300.0 | 432.4 | 31.5 | 1641.6 | 1426.3 | 1641.6 | 397.7 | 726.2 |
| QR | 2500.0 | 2232.5 | 2500.0 | - | 2132.3 | 3417.0 | 3652.5 | 3417.0 | 22.7 | 2871.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karavalasi, A.; Almpani, S.; Tserkezou, P.; Chachlioutaki, K.; Kamaris, G.; Markopoulou, C.K. Application of a Validated HPLC Method for the Determination of Resveratrol, Ferulic Acid, Quercetin, Retinol, and α-Tocopherol in a Cold Cream—Permeability Study. Appl. Sci. 2024, 14, 11843. https://doi.org/10.3390/app142411843
Karavalasi A, Almpani S, Tserkezou P, Chachlioutaki K, Kamaris G, Markopoulou CK. Application of a Validated HPLC Method for the Determination of Resveratrol, Ferulic Acid, Quercetin, Retinol, and α-Tocopherol in a Cold Cream—Permeability Study. Applied Sciences. 2024; 14(24):11843. https://doi.org/10.3390/app142411843
Chicago/Turabian StyleKaravalasi, Athanasia, Sofia Almpani, Panagiota Tserkezou, Konstantina Chachlioutaki, Georgios Kamaris, and Catherine K. Markopoulou. 2024. "Application of a Validated HPLC Method for the Determination of Resveratrol, Ferulic Acid, Quercetin, Retinol, and α-Tocopherol in a Cold Cream—Permeability Study" Applied Sciences 14, no. 24: 11843. https://doi.org/10.3390/app142411843
APA StyleKaravalasi, A., Almpani, S., Tserkezou, P., Chachlioutaki, K., Kamaris, G., & Markopoulou, C. K. (2024). Application of a Validated HPLC Method for the Determination of Resveratrol, Ferulic Acid, Quercetin, Retinol, and α-Tocopherol in a Cold Cream—Permeability Study. Applied Sciences, 14(24), 11843. https://doi.org/10.3390/app142411843






