Synthesis and In Vitro Anticancer Activity of Pyrrolidone Derivatives Bearing 3,4,5-Trimethoxyphenyl Moiety as a Promising Anticancer Scaffold
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
- 5-Oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carboxylic acid (2)
- Methyl 5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carboxylate (3)
- 5-Oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (4)
- 5-Oxo-N′-(2-oxoindolin-3-ylidene)-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (5)
- N′-(7-bromo-2-oxoindolin-3-ylidene)-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (6)
- General procedure for the preparation of hydrazones 7, 8
- N′-((5-nitrofuran-2-yl)methylene)-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (7)
- N′-((5-nitrothiophen-2-yl)methylene)-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (8)
- General procedure for the preparation of hydrazones 9–18
- N′-benzylidene-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (9)
- N′-(4-chlorobenzylidene)-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (10)
- N′-(4-bromobenzylidene)-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (11)
- N′-(4-fluorobenzylidene)-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (12)
- N′-(4-methoxybenzylidene)-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (13)
- N′-(2,4-difluorobenzylidene)-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (14)
- 5-Oxo-N′-(3,4,5-trimethoxybenzylidene)-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (15)
- N′-(2-chlorobenzylidene)-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (16)
- N′-(3-chlorobenzylidene)-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (17)
- N′-(2-chloro-5-nitrobenzylidene)-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (18)
- General procedure for the synthesis of N-alkylated hydrazones 19–22
- N′-benzylidene-N-ethyl-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (19)
- N′-(4-chlorobenzylidene)-N-ethyl-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (20)
- N′-(4-bromobenzylidene)-N-ethyl-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (21)
- N-ethyl-N′-(4-fluorobenzylidene)-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carbohydrazide (22)
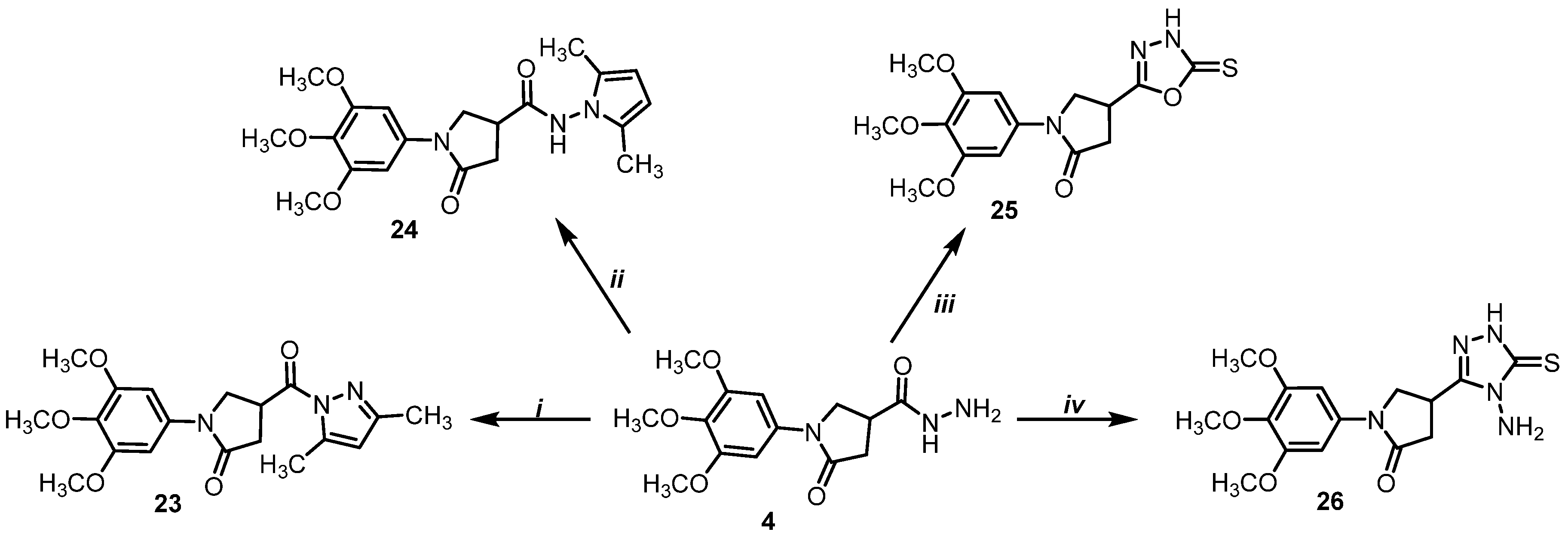
- 4-(3,5-Dimethyl-1H-pyrazole-1-carbonyl)-1-(3,4,5-trimethoxyphenyl)pyrrolidin-2-one (23)
- N-(2,5-dimethyl-1H-pyrrol-1-yl)-5-oxo-1-(3,4,5-trimethoxyphenyl)pyrrolidine-3-carboxamide (24)
- 4-(5-Thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)-1-(3,4,5-trimethoxyphenyl)pyrrolidin-2-one (25)
- 4-(4-Amino-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)-1-(3,4,5-trimethoxyphenyl)pyrrolidin-2-one (26)
2.2. Biology
2.2.1. Cell Lines and Culture Conditions
2.2.2. Cytotoxicity Assay
2.2.3. Statistical Analysis
3. Results and Discussion
3.1. Chemistry
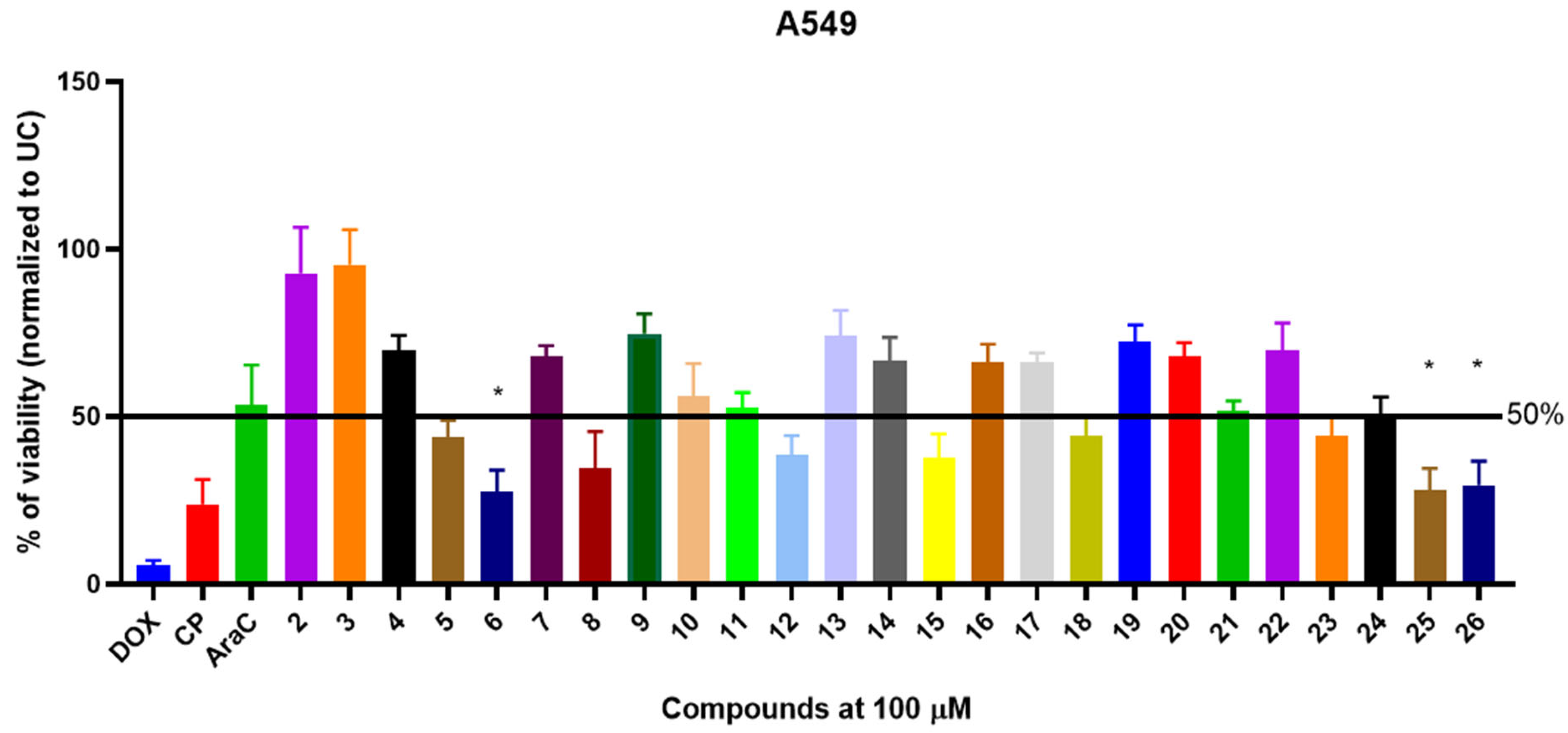
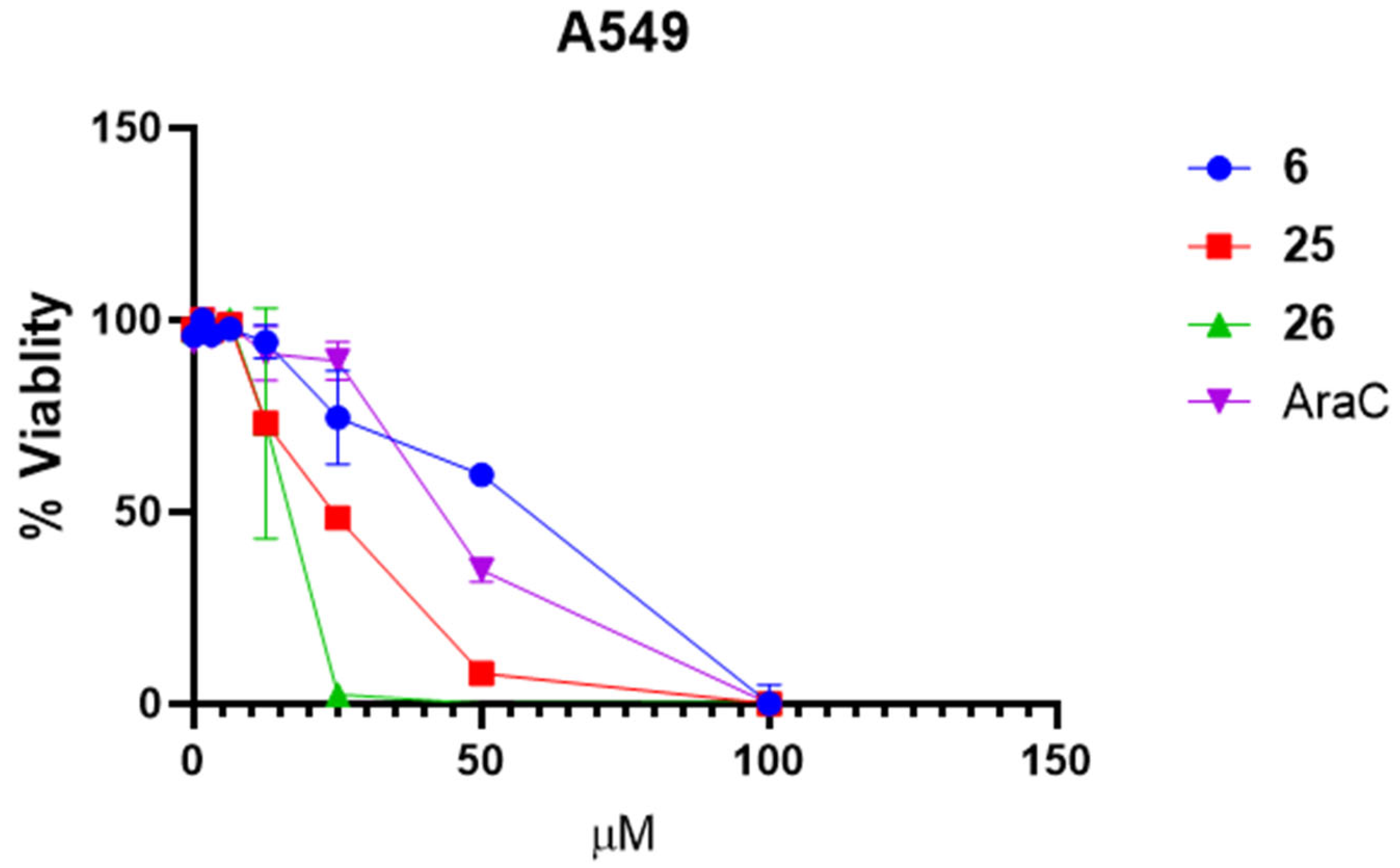
3.2. In Vitro Biological Activity Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamel, M.M.; Megally Abdo, N.Y. Synthesis of novel 1,2,4-triazoles, triazolothiadiazines and triazolothiadiazoles as potential anticancer agents. Eur. J. Med. Chem. 2014, 86, 75–80. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. (Eds.) Global Cancer Observatory: Cancer Today; Version 1.0; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 17 November 2024).
- World Health Organization (WHO). Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 17 November 2024).
- Ibrahim, H.S.; Albakri, M.E.; Mahmoud, W.R.; Allam, H.A.; Reda, A.M.; Abdel-Aziz, H.A. Synthesis and biological evaluation of some novel thiobenzimidazole derivatives as anti-renal cancer agents through inhibition of c-MET kinase. Bioorganic Chem. 2019, 85, 337–348. [Google Scholar] [CrossRef]
- Gravanis, I.; Vleminckx, C.; Jonsson, B.; Pignatti, F. The changing world of cancer drug development: The regulatory bodies’ perspective. Chin. Clin. Oncol. 2014, 3, 22–27. [Google Scholar]
- World Cancer Research Fund International. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data (accessed on 17 November 2024).
- 9. Cancer Research UK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer (accessed on 17 November 2024).
- Avendano, C.; Menendez, J.C. Medicinal Chemistry of Anticancer Drugs, 1st ed.; Elsevier B.V.: Oxford, UK, 2008. [Google Scholar]
- Dudhe, R.; Sharma, P.K.; Verma, P.; Chaudhary, A. Pyrimidine as anticancer agent: A review. J. Adv. Sci. Res. 2011, 2, 10–17. [Google Scholar]
- Szafraniec, S.I.; Stachnik, K.J.; Skierski, J.S. New nucleoside analogs in the treatment of hematological disorders. Acta Pol. Pharm. 2004, 61, 223–232. [Google Scholar]
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. [Google Scholar] [CrossRef]
- Sangwan, K.; Sharma, V.; Goyal, P.K. Pharmacological Profile of Novel Anti-cancer Drugs Approved by USFDA in 2022: A Review. Curr. Mol. Med. 2024, 24, 734–750. [Google Scholar] [CrossRef]
- Sochacka-Ćwikła, A.; Mączyński, M.; Regiec, A. FDA-Approved Small Molecule Compounds as Drugs for Solid Cancers from Early 2011 to the End of 2021. Molecules 2022, 27, 2259. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Zhang, S.; Xu, Z.; Lv, Z.S.; Liu, M.L.; Feng, L.S. 4-Quinolone hybrids and their antibacterial activities. Eur. J. Med. Chem. 2017, 141, 335–345. [Google Scholar] [CrossRef]
- Zhang, G.F.; Zhang, S.; Pan, B.F.; Liu, M.L.; Feng, L.S. 4-Quinolone derivatives and their activities against Gram positive pathogens. Eur. J. Med. Chem. 2018, 143, 710–723. [Google Scholar] [CrossRef]
- Negi, A.S.; Gautam, Y.; Alam, S.; Chanda, D.; Luqman, S.; Sarkar, J.; Khan, F.; Konwar, R. Natural antitubulin agents: Importance of 3,4,5-trimethoxyphenyl fragment. Bioorganic Med. Chem. 2015, 23, 373–389. [Google Scholar] [CrossRef]
- Shawky, A.M.; Ibrahim, N.A.; Abdalla, A.N.; Abourehab, M.A.S.; Gouda, A.M. Novel pyrrolizines bearing 3,4,5-trimethoxyphenyl moiety: Design, synthesis, molecular docking, and biological evaluation as potential multi-target cytotoxic agents. J. Enzyme Inhib. Med. Chem. 2021, 36, 1312–1332. [Google Scholar] [CrossRef]
- Kovar, S.E.; Fourman, C.; Kinstedt, C.; Williams, B.; Morris, C.; Cho, K.-J.; Ketcha, D.M. Chalcones bearing a 3,4,5-trimethoxyphenyl motif are capable of selectively inhibiting oncogenic K-Ras signaling. Bioorganic Med. Chem. Lett. 2020, 30, 127–144. [Google Scholar] [CrossRef]
- Abdelall, E.K.A.; Lamie, P.F.; Aboelnaga, L.S.; Hassan, R.M. Trimethoxyphenyl containing compounds: Synthesis, biological evaluation, nitric oxide release, modeling, histochemical and histopathological studies. Bioorganic Chem. 2022, 124, 105806. [Google Scholar] [CrossRef]
- Romagnoli, R.; Baraldi, P.G.; Salvador, M.K.; Camacho, M.E.; Preti, D.; Tabrizi, M.A.; Bassetto, M.; Brancale, A.; Hamel, E.; Bortolozzi, R.; et al. Synthesis and biological evaluation of 2-substituted-4-(3,4,5-trimethoxyphenyl)-5-aryl thiazoles as anticancer agents. Bioorganic Med. Chem. 2012, 20, 7083–7094. [Google Scholar] [CrossRef]
- Raimondi, M.V.; Randazzo, O.; La Franca, M.; Barone, G.; Vignoni, E.; Rossi, D.; Collina, S. DHFR Inhibitors: Reading the Past for Discovering Novel Anticancer Agents. Molecules 2019, 24, 1140. [Google Scholar] [CrossRef]
- Pinza, M.; Farina, C.; Cerri, A.; Pfeiffer, U.; Riccaboni, M.T.; Banfi, S.; Biagetti, R.; Pozzi, O.; Magnani, M.; Dorigotti, L. Synthesis and pharmacological activity of a series of dihydro-1H-pyrrolo [1, 2-a] imidazole-2, 5 (3H, 6H)-diones, a novel class of potent cognition enhancers. J. Med. Chem. 1993, 36, 4214. [Google Scholar] [CrossRef]
- Hashizume, K.; Kunimoto, M.; Maeda, T.; Tanaka, T. Antiepileptic effect of nefiracetam on kainic acid-induced limbic seizure in rats. Epilepsy Res. 2000, 39, 221–228. [Google Scholar] [CrossRef]
- Langarizadeh, M.A.; Ameri, A.; Tavakoli, M.R.; Abiri, A.; Forootanfar, H. The trimethoxyphenyl (TMP) functional group: A versatile pharmacophore. Med. Chem. Res. 2023, 32, 2473–2500. [Google Scholar] [CrossRef]
- Patel, T.M.; Patel, A.M. Synthesis of 5-arylidine-2-(3,4,5-trimethoxyphenyl)-3-(4-phenylthiazol-2-yl)Thiazolidin-4-one Derivatives as a Novel Class of Antimicrobial Agents. Orient. J. Chem. 2012, 28, 471–478. [Google Scholar] [CrossRef]
- Ayrim, N.B.; Balakitb, A.A.; Laftaa, S.J. Synthesis, Characterization, Molecular Docking and Biological Activity Studies of Hydrazones with 3,4,5-Trimethoxyphenyl Moiety. Egypt. J. Chem. 2022, 65, 159–169. [Google Scholar] [CrossRef]
- Araniciu, C.; Oniga, S.; Ovidiu, O.; Palage, M. Antimicrobial and anti-pathogenic activity evaluation of some 2-(Trimethoxyphenyl)-4-AR1-5-R2-thiazoles. Farmacia 2015, 63, 40. [Google Scholar]
- Xing, L.; Mao, P.; He, B.; Qin, Y.; Meng, K.; Zeng, W.; Sun, Z.; Xue, W. Design, synthesis, and antiviral activities of myricetin derivatives containing phenoxypyridine. J. Saudi Chem. Soc. 2023, 27, 101721. [Google Scholar] [CrossRef]
- El-Damasy, A.K.; Jin, H.; Sabry, M.A.; Kim, H.J.; Alanazi, M.M.; Seo, S.H.; Bang, E.-K.; Keum, G. Design and Synthesis of New 4-(3,4,5-Trimethoxyphenyl)Thiazole–Pyrimidine Derivatives as Potential Antiproliferative Agents. Medicina 2023, 59, 1076. [Google Scholar] [CrossRef]
- Chen, J.; Liu, T.; Dong, X.W.; Hu, Y.Z. Recent development and SAR analysis of colchicine binding site inhibitors. Mini-Rev. Med. Chem. 2009, 9, 1174–1190. [Google Scholar] [CrossRef]
- Wang, Q.; Arnst, K.E.; Wang, Y.; Kumar, G.; Chen, D.M.H.; Wu, Z.; Yang, J.; White, S.D.; Miller, D.D.; Li, M.W. Structural Modification of the 3,4,5-Trimethoxyphenyl Moiety in the Tubulin Inhibitor VERU-111 Leads to Improved Antiproliferative Activities. J. Med. Chem. 2018, 61, 7877–7891. [Google Scholar] [CrossRef]
- Sakamoto, T.; Koga, Y.; Hikota, M.; Matsuki, K.; Murakami, M.; Kikkawa, K.; Fujishige, K.; Kotera, J.; Omori, K.; Morimoto, H.; et al. Design and synthesis of novel 5-(3,4,5-trimethoxybenzoyl)-4-aminopyrimidine derivatives as potent and selective phosphodiesterase 5 inhibitors: Scaffold hopping using a pseudo-ring by intramolecular hydrogen bond formation. Bioorganic Med. Chem. Lett. 2014, 24, 5175–5180. [Google Scholar] [CrossRef]
- Bertašiūtė, M.; Kavaliauskas, P.; Vaickelionienė, R.; Grybaitė, B.; Petraitis, V.; Petraitienė, R.; Naing, E.; Garcia, A.; Šiugždaitė, J.; Lelešius, R.; et al. Synthesis of 1-(2-Hydroxyphenyl)- and (3,5-Dichloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic Acid Derivatives as Promising Scaffolds for the Development of Novel Antimicrobial and Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 7966. [Google Scholar] [CrossRef]
- Vaškevičienė, I.; Paketurytė, V.; Pajanok, N.; Žukauskas, Š.; Sapijanskaitė, B.; Kantminienė, K.; Mickevičius, V.; Zubrienė, A.; Matulis, D. Pyrrolidinone-bearing methylated and halogenated benzenesulfonamides as inhibitors of carbonic anhydrases. Bioorganic Med. Chem. 2019, 27, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Marella, A.; Shaquiquzzaman, M.; Akhtar, M.; Ali, M.R.; Alam, M.M. A review exploring biological activities of hydrazones. J. Pharm. Bioallied Sci. 2014, 6, 69–80. [Google Scholar] [PubMed]
- Czelen, P.; Skotnicka, A.; Szefler, B. Designing and Synthesis of New Isatin Derivatives as Potential CDK2 Inhibitors. Int. J. Mol. Sci. 2022, 23, 8046. [Google Scholar] [CrossRef] [PubMed]
- Kavaliauskas, P.; Acevedo, W.; Garcia, A.; Naing, E.; Grybaite, B.; Sapijanskaite-Banevic, B.; Grigaleviciute, R.; Petraitiene, R.; Mickevicius, V.; Petraitis, V. Exploring the potential of bis(thiazol-5-yl)phenylmethane derivatives as novel candidates against genetically defined multidrug-resistant Staphylococcus aureus. PLoS ONE 2024, 19, e0300380. [Google Scholar] [CrossRef]
- Kavaliauskas, P.; Opazo, F.S.; Acevedo, W.; Petraitiene, R.; Grybaitė, B.; Anusevičius, K.; Mickevičius, V.; Belyakov, S.; Petraitis, V. Synthesis, Biological Activity, and Molecular Modelling Studies of Naphthoquinone Derivatives as Promising Anticancer Candidates Targeting COX-2. Pharmaceuticals 2022, 15, 541–567. [Google Scholar] [CrossRef]
- Minickaitė, R.; Grybaitė, B.; Vaickelionienė, R.; Kavaliauskas, P.; Petraitis, V.; Petraitienė, R.; Tumosienė, I.; Jonuškienė, I.; Mickevičius, V. Synthesis of Novel Aminothiazole Derivatives as Promising Antiviral, Antioxidant and Antibacterial Candidates. Int. J. Mol. Sci. 2022, 23, 7688. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-rot095505. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavaliauskas, P.; Sapijanskaitė-Banevič, B.; Grybaitė, B.; Mickevičiūtė, E.; Anusevičius, K.; Garcia, A.; Naing, E.; Petraitienė, R.; Petraitis, V.; Grigalevičiūtė, R.; et al. Synthesis and In Vitro Anticancer Activity of Pyrrolidone Derivatives Bearing 3,4,5-Trimethoxyphenyl Moiety as a Promising Anticancer Scaffold. Appl. Sci. 2024, 14, 11784. https://doi.org/10.3390/app142411784
Kavaliauskas P, Sapijanskaitė-Banevič B, Grybaitė B, Mickevičiūtė E, Anusevičius K, Garcia A, Naing E, Petraitienė R, Petraitis V, Grigalevičiūtė R, et al. Synthesis and In Vitro Anticancer Activity of Pyrrolidone Derivatives Bearing 3,4,5-Trimethoxyphenyl Moiety as a Promising Anticancer Scaffold. Applied Sciences. 2024; 14(24):11784. https://doi.org/10.3390/app142411784
Chicago/Turabian StyleKavaliauskas, Povilas, Birutė Sapijanskaitė-Banevič, Birutė Grybaitė, Eglė Mickevičiūtė, Kazimieras Anusevičius, Andrew Garcia, Ethan Naing, Rūta Petraitienė, Vidmantas Petraitis, Ramunė Grigalevičiūtė, and et al. 2024. "Synthesis and In Vitro Anticancer Activity of Pyrrolidone Derivatives Bearing 3,4,5-Trimethoxyphenyl Moiety as a Promising Anticancer Scaffold" Applied Sciences 14, no. 24: 11784. https://doi.org/10.3390/app142411784
APA StyleKavaliauskas, P., Sapijanskaitė-Banevič, B., Grybaitė, B., Mickevičiūtė, E., Anusevičius, K., Garcia, A., Naing, E., Petraitienė, R., Petraitis, V., Grigalevičiūtė, R., & Mickevičius, V. (2024). Synthesis and In Vitro Anticancer Activity of Pyrrolidone Derivatives Bearing 3,4,5-Trimethoxyphenyl Moiety as a Promising Anticancer Scaffold. Applied Sciences, 14(24), 11784. https://doi.org/10.3390/app142411784







