Sjögren’s Syndrome: The Role of Serological Profiles Versus Minor Salivary Gland Histopathology
Abstract
1. Introduction
Aims and Goals
2. Materials and Methods
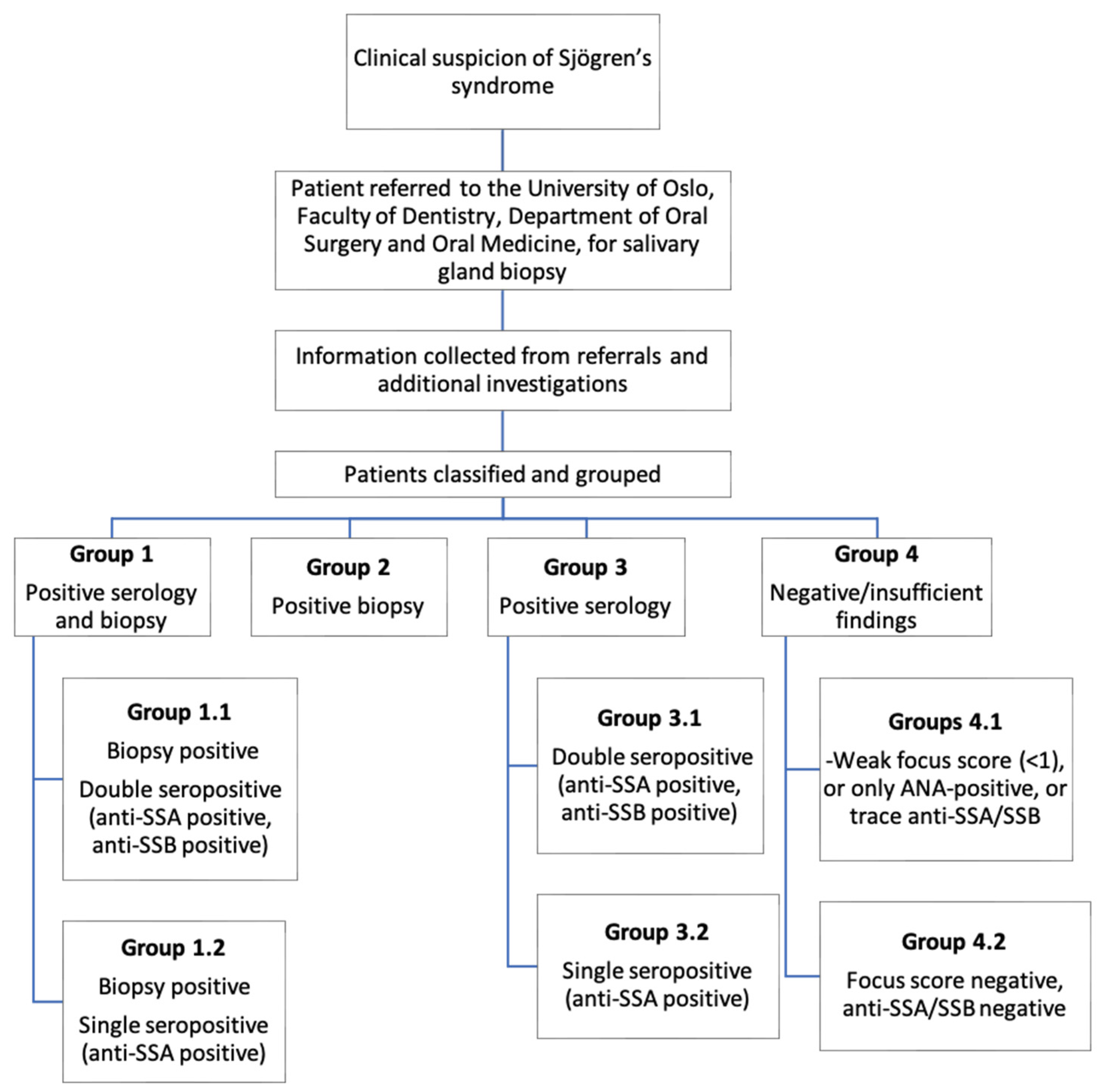
2.1. Population
2.2. Clinical Investigations
2.3. Data Analysis and Presentation
3. Results
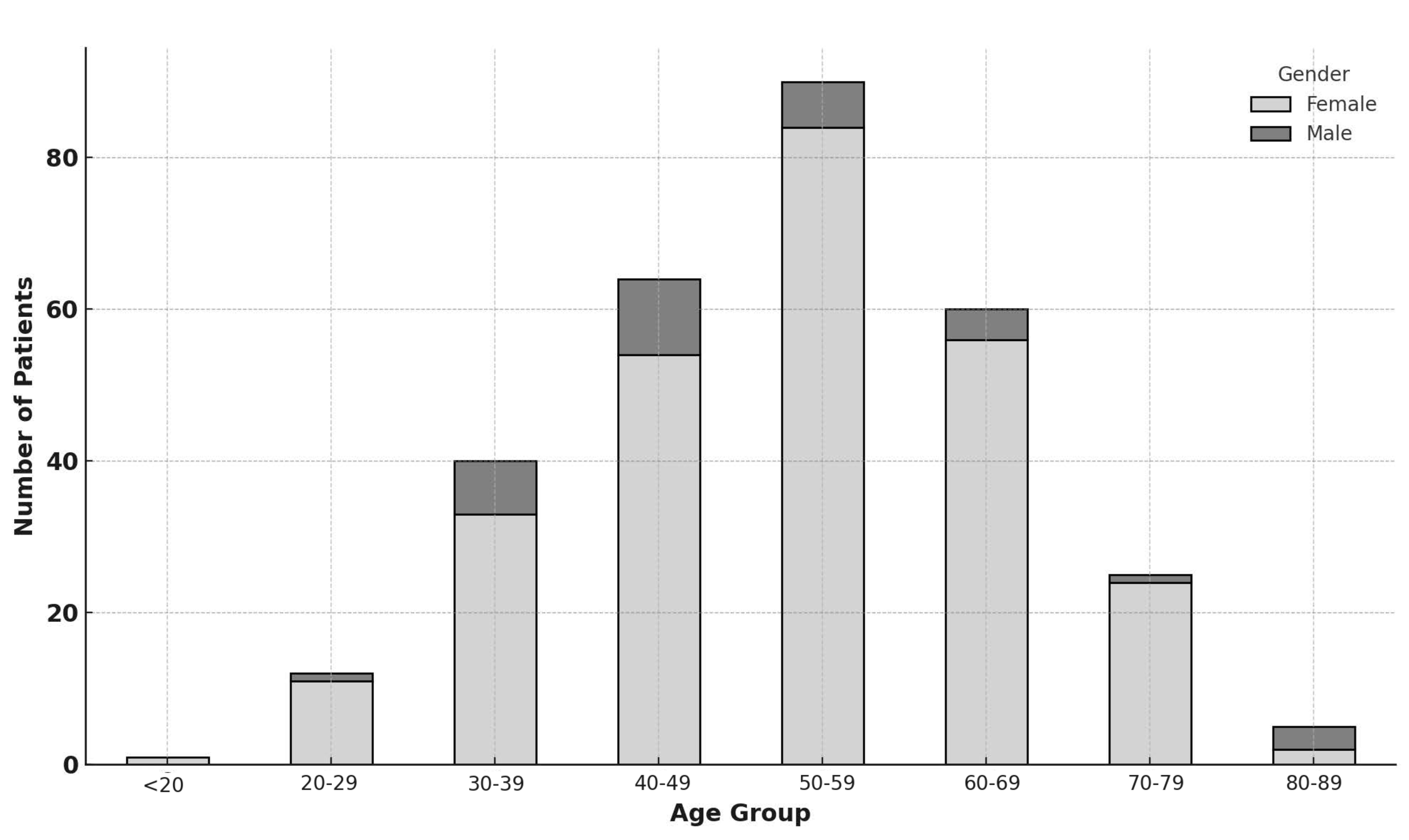
Patient Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nocturne, G.; Mariette, X. Advances in Understanding the Pathogenesis of Primary Sjögren’s Syndrome. Nat. Rev. Rheumatol. 2013, 9, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zerón, P.; Baldini, C.; Bootsma, H.; Bowman, S.J.; Jonsson, R.; Mariette, X.; Sivils, K.; Theander, E.; Tzioufas, A.; Ramos-Casals, M. Sjögren Syndrome. Nat. Rev. Dis. Primers 2016, 2, 16047. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.A.; Jonsson, R.; Daniels, T.; Bombardieri, M.; Brown, R.M.; Morgan, P.; Bombardieri, S.; Ng, W.F.; Tzioufas, A.G.; Vitali, C.; et al. Standardisation of Labial Salivary Gland Histopathology in Clinical Trials in Primary Sjögren’s Syndrome. Ann. Rheum Dis. 2017, 76, 1161–1168. [Google Scholar] [CrossRef]
- Vitali, C.; Moutsopoulos, H.M.; Bombardieri, S. The European Community Study Group on Diagnostic Criteria for Sjögren’s Syndrome. Sensitivity and Specificity of Tests for Ocular and Oral Involvement in Sjögren’s Syndrome. Ann. Rheum Dis. 1994, 53, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kawakami, A.; Eguchi, K. Mechanisms of Autoantibody Production and the Relationship between Autoantibodies and the Clinical Manifestations in Sjögren’s Syndrome. Transl. Res. J. Lab. Clin. Med. 2006, 148, 281–288. [Google Scholar] [CrossRef]
- van der Reijden, W.A.; Vissink, A.; Veerman, E.C.; Amerongen, A.V. Treatment of Oral Dryness Related Complaints (Xerostomia) in Sjögren’s Syndrome. Ann. Rheum Dis. 1999, 58, 465–474. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; Bombardieri, S.; Bootsma, H.; De Vita, S.; Dörner, T.; Fisher, B.A.; Gottenberg, J.E.; Hernandez-Molina, G.; Kocher, A.; et al. Eular Recommendations for the Management of Sjögren’s Syndrome with Topical and Systemic Therapies. Ann. Rheum Dis. 2020, 79, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Tsifetaki, N.; Kitsos, G.; Paschides, C.A.; Alamanos, Y.; Eftaxias, V.; Voulgari, P.V.; Psilas, K.; Drosos, A.A. Oral Pilocarpine for the Treatment of Ocular Symptoms in Patients with Sjögren’s Syndrome: A Randomised 12 Week Controlled Study. Ann. Rheum Dis. 2003, 62, 1204–1207. [Google Scholar] [CrossRef]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. Tfos Dews Ii Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef]
- Ramia, T.; Daada, S.; Chaabene, I.; Klii, R.; Hammami, S.; Ines, K.; Kechida, M. Ab0539 Depression, Self-Esteem and Quality of Life in Sjögren’s Syndrome. Ann. Rheum. Dis. 2022, 81 (Suppl. S1), 1397. [Google Scholar] [CrossRef]
- Carubbi, F.; Alunno, A.; Cipriani, P.; Bartoloni, E.; Baldini, C.; Quartuccio, L.; Priori, R.; Valesini, G.; De Vita, S.; Bombardieri, S.; et al. A Retrospective, Multicenter Study Evaluating the Prognostic Value of Minor Salivary Gland Histology in a Large Cohort of Patients with Primary Sjögren’s Syndrome. Lupus 2015, 24, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Dal Pozzolo, R.; Cafaro, G.; Perricone, C.; Calvacchi, S.; Bruno, L.; Colangelo, A.; Tromby, F.; Gerli, R.; Bartoloni, E. Salivary Gland Biopsy as a Prognostic Tool in Sjögren’s Syndrome. Expert Rev. Clin. Immunol. 2024, 20, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Tashbayev, B.; Garen, T.; Palm, O.; Chen, X.; Herlofson, B.B.; Young, A.; Hove, L.H.; Rykke, M.; Singh, P.B.; Aqrawi, L.A.; et al. Patients with Non-Sjogren’s Sicca Report Poorer General and Oral Health-Related Quality of Life Than Patients with Sjogren’s Syndrome: A Cross-Sectional Study. Sci. Rep. 2020, 10, 2063. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification Criteria for Sjögren’s Syndrome: A Revised Version of the European Criteria Proposed by the American-European Consensus Group. Ann. Rheum Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- Berry, J.S.; Tarn, J.; Casement, J.; Duret, P.M.; Scott, L.; Wood, K.; Johnsen, S.J.; Nordmark, G.; Devauchelle-Pensec, V.; Seror, R.; et al. Examining the Biological Pathways Underlying Clinical Heterogeneity in Sjogren’s Syndrome: Proteomic and Network Analysis. Ann. Rheum Dis. 2024, 83, 88–95. [Google Scholar] [CrossRef]
- Daniels, T.E.; Cox, D.; Shiboski, C.H.; Schiødt, M.; Wu, A.; Lanfranchi, H.; Umehara, H.; Zhao, Y.; Challacombe, S.; Lam, M.Y.; et al. Associations between Salivary Gland Histopathologic Diagnoses and Phenotypic Features of Sjögren’s Syndrome among 1726 Registry Participants. Arthritis Rheum. 2011, 63, 2021–2030. [Google Scholar] [CrossRef]
- Risselada, A.P.; Looije, M.F.; Kruize, A.A.; Bijlsma, J.W.; van Roon, J.A. The Role of Ectopic Germinal Centers in the Immunopathology of Primary Sjögren’s Syndrome: A Systematic Review. Semin Arthritis Rheum 2013, 42, 368–376. [Google Scholar] [CrossRef]
- Skarstein, K.; Aqrawi, L.A.; Oijordsbakken, G.; Jonsson, R.; Jensen, J.L. Adipose Tissue Is Prominent in Salivary Glands of Sjogren’s Syndrome Patients and Appears to Influence the Microenvironment in These Organs. Autoimmunity 2016, 49, 338–346. [Google Scholar] [CrossRef]
- Fonzetti, S.; Fulvio, G.; La Rocca, G.; García, I.C.N.; Governato, G.; Izzetti, R.; Donati, V.; Ferro, F.; Mosca, M.; Baldini, C. Pos1459 the Weight of Anti-La/Ssb Antibodies in Ro/Ssa-Positive Patients with a Newly Diagnosed Sjögren’s Syndrome: A Phenotype Characterized by a More Severe Major and Minor Glandular Involvement and a Higher Biological Activity. Ann. Rheum. Dis. 2023, 82 (Suppl. S1), 1083–1084. [Google Scholar]
- Samuelsen, T.; Aqrawi, L.; Skarstein, K.; Jensen, J. Evaluering Av Spyttkjertelbiopsier Ved Utredning Av Sjögrens Syndrom. Nor. Tann. Tid. 2021, 131, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. Tfos Dews Ii Diagnostic Methodology Report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Baldini, C.; Pepe, P.; Quartuccio, L.; Priori, R.; Bartoloni, E.; Alunno, A.; Gattamelata, A.; Maset, M.; Modesti, M.; Tavoni, A.; et al. Primary Sjogren’s Syndrome as a Multi-Organ Disease: Impact of the Serological Profile on the Clinical Presentation of the Disease in a Large Cohort of Italian Patients. Rheumatology 2014, 53, 839–844. [Google Scholar] [CrossRef]
- Liao, R.; Yang, H.T.; Li, H.; Liu, L.X.; Li, K.; Li, J.J.; Liang, J.; Hong, X.P.; Chen, Y.L.; Liu, D.Z. Recent Advances of Salivary Gland Biopsy in Sjögren’s Syndrome. Front. Med. 2021, 8, 792593. [Google Scholar] [CrossRef]
- Baer, A.N.; DeMarco, M.M.; Shiboski, S.C.; Lam, M.Y.; Challacombe, S.; Daniels, T.E.; Dong, Y.; Greenspan, J.S.; Kirkham, B.W.; Lanfranchi, H.E.; et al. The Ssb-Positive/Ssa-Negative Antibody Profile Is Not Associated with Key Phenotypic Features of Sjögren’s Syndrome. Ann. Rheum Dis. 2015, 74, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Conticini, E.; Bardelli, M.; Vitale, A.; De Stefano, R.; Falsetti, P.; Selvi, E.; Bacarelli, M.R.; D’Alessandro, R.; Cantarini, L.; Frediani, B.; et al. Diagnostic Role of Minor Salivary Glands Biopsy in Sjögren’s Syndrome: Correlations between Histology and Autoimmunity in a Large, Monocentric Cohort. Reumatologia 2023, 61, 109–115. [Google Scholar] [CrossRef]
- Chatzis, L.; Vlachoyiannopoulos, P.G.; Tzioufas, A.G.; Goules, A.V. New Frontiers in Precision Medicine for Sjogren’s Syndrome. Expert Rev. Clin. Immunol. 2021, 17, 127–141. [Google Scholar] [CrossRef]
- Risselada, A.P.; Kruize, A.A.; Goldschmeding, R.; Lafeber, F.P.J.G.; Bijlsma, J.W.J.; van Roon, J.A.G. The Prognostic Value of Routinely Performed Minor Salivary Gland Assessments in Primary Sjögren’s Syndrome. Ann. Rheum. Dis. 2014, 73, 1537–1540. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Ice, J.A.; Li, H.; Grundahl, K.; Kelly, J.A.; Radfar, L.; Stone, D.U.; Hefner, K.S.; Anaya, J.M.; Rohrer, M.; et al. Comparison of the American-European Consensus Group Sjogren’s Syndrome Classification Criteria to Newly Proposed American College of Rheumatology Criteria in a Large, Carefully Characterised Sicca Cohort. Ann. Rheum Dis. 2014, 73, 31–38. [Google Scholar] [CrossRef]
- Tsuboi, H.; Hagiwara, S.; Asashima, H.; Takahashi, H.; Hirota, T.; Noma, H.; Umehara, H.; Kawakami, A.; Nakamura, H.; Sano, H.; et al. Comparison of Performance of the 2016 Acr-Eular Classification Criteria for Primary Sjögren’s Syndrome with Other Sets of Criteria in Japanese Patients. Ann. Rheum Dis. 2017, 76, 1980–1985. [Google Scholar] [CrossRef]
- Le Goff, M.; Cornec, D.; Jousse-Joulin, S.; Guellec, D.; Costa, S.; Marhadour, T.; Le Berre, R.; Genestet, S.; Cochener, B.; Boisrame-Gastrin, S.; et al. Comparison of 2002 Aecg and 2016 Acr/Eular Classification Criteria and Added Value of Salivary Gland Ultrasonography in a Patient Cohort with Suspected Primary Sjögren’s Syndrome. Arthritis Res. Ther. 2017, 19, 269. [Google Scholar] [CrossRef] [PubMed]
- Ono, J.; Toya, S.; Ogura, I.; Okada, Y. Study of Clinical Factors, Focus Score, Lymphocyte Type and Nf-Κb Pathway in Sjögren’s Syndrome. Odontology 2023, 111, 207–216. [Google Scholar] [CrossRef] [PubMed]


| Group 1.1 (Biopsy-Positive and Double-Seropositive) | Group 1.2 (Biopsy-Positive and Single-Seropositive) | Group 2 (Biopsy-Positive) | Group 3.1 (Double-Seropositive) | Group 3.2 (Single-Seropositive) | Group 4.1 (FS < 1 and/or Trace ANA and/or Trace SSA/SSB) | Group 4.2 (Biopsy-Negative and Seronegative) | Total | |
|---|---|---|---|---|---|---|---|---|
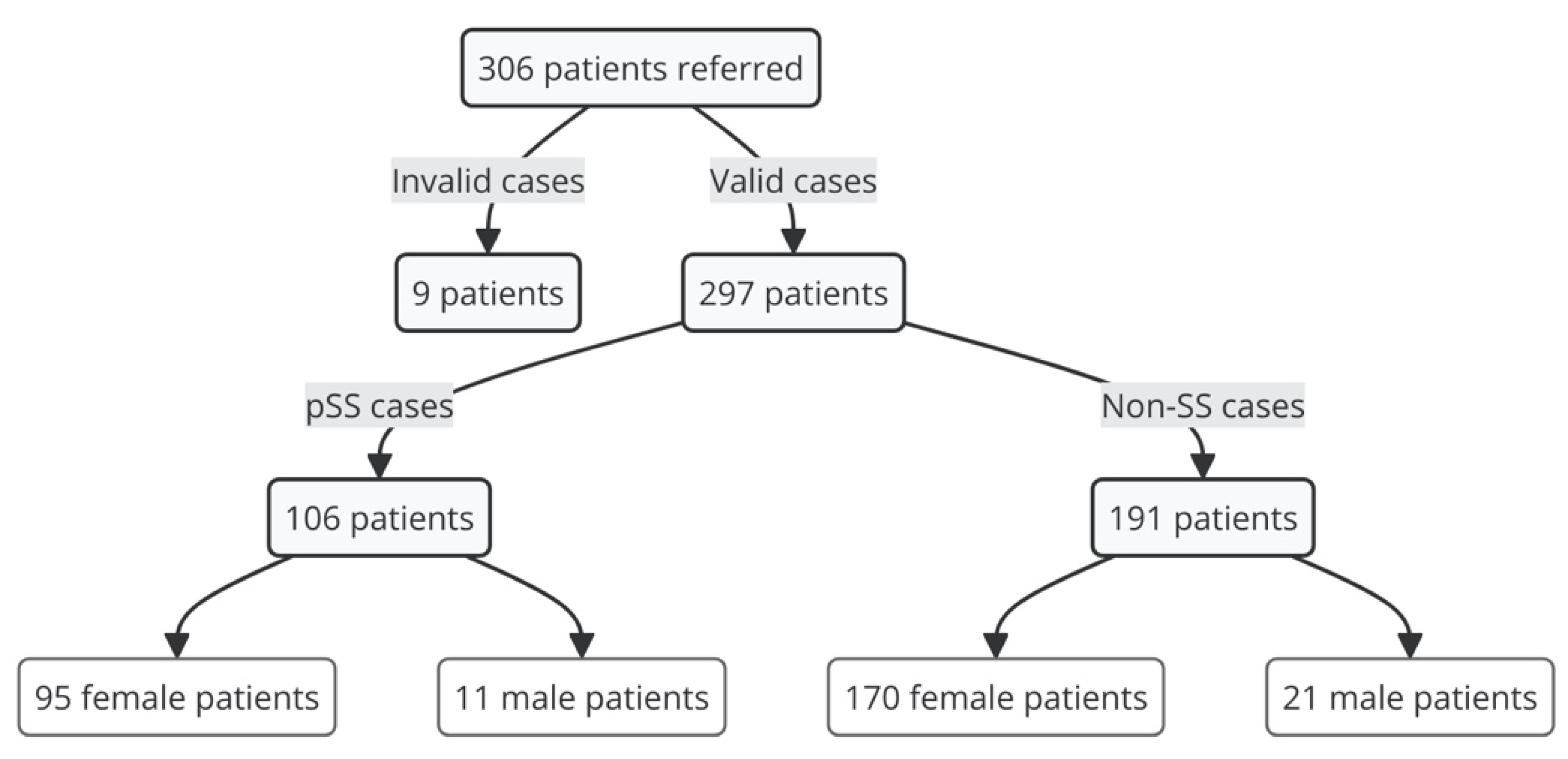
| Number of patients | 23 | 18 | 30 | 5 | 45 | 83 | 93 | 297 |
| Percentage of referred patients | 7.7% | 6.1% | 10.1% | 1.7% | 15.2% | 27.9% | 31.3% | 100.0% |
| Gender | ||||||||
| Male | ||||||||
| Frequency | 3 | 1 | 3 | 1 | 8 | 7 | 9 | 32 |
| Min. age | 30 | 47 | 43 | 82 | 33 | 28 | 36 | |
| Max. age | 80 | 47 | 61 | 82 | 69 | 79 | 85 | |
| Mean age | 48.7 | 47.0 | 52.7 | 82.0 | 45.9 | 50.7 | 53.1 | |
| Female | ||||||||
| Frequency | 20 | 17 | 27 | 4 | 37 | 76 | 84 | 265 |
| Min. age | 26 | 18 | 39 | 21 | 24 | 32 | 25 | |
| Max. age | 84 | 74 | 76 | 68 | 77 | 76 | 81 | |
| Mean age | 48.5 | 48.4 | 55.8 | 51.0 | 50.7 | 52.8 | 54.8 |
| Group 1.1 (Biopsy-Positive and Double-Seropositive) | Group 1.2 (Biopsy-Positive and Single-Seropositive) | Group 2 (Biopsy-Positive) | Group 3.1 (Double-Seropositive) | Group 3.2 (Single-Seropositive) | Group 4.1 (FS < 1 and/or Trace ANA and/or Trace SSA/SSB) | Group 4.2 (Biopsy-Negative and Seronegative) | Total | |
|---|---|---|---|---|---|---|---|---|
| Number of patients | 23 | 18 | 29 | 3 | 33 | 0 | 0 | 106 |
| Percentage of total pSS classifications | 21.7% | 17.0% | 27.4% | 2.8% | 31.1% | 0.0% | 0.0% | 100% |
| Gender | ||||||||
| Male | ||||||||
| Frequency | 3 | 1 | 3 | 0 | 4 | 0 | 0 | 11 |
| Min. age | 30 | 47 | 43 | 33 | ||||
| Max. age | 80 | 47 | 61 | 52 | ||||
| Mean age | 48.7 | 47.0 | 52.7 | 41.3 | ||||
| Female | ||||||||
| Frequency | 20 | 17 | 26 | 3 | 29 | 0 | 0 | 95 |
| Min. age | 26 | 18 | 39 | 54 | 24 | |||
| Max. age | 84 | 74 | 76 | 68 | 77 | |||
| Mean age | 48.5 | 48.4 | 55.7 | 61.0 | 50.5 | |||
| Focus Score | ||||||||
| Lowest FS | 1.0 | 1.0 | 1.0 | 0.0 | 0.0 | |||
| Highest FS | 10.0 | 3.3 | 3.7 | 0.0 | 0.9 | |||
| Mean FS | 3.3 | 1.6 | 1.7 | 0.0 | 0.04 | |||
| Median FS | 2.0 | 1.1 | 1.5 | 0.0 | 0.0 |
| Group 1.1 (Biopsy-Positive and Double-Seropositive) | Group 1.2 (Biopsy-Positive and Single-Seropositive) | Group 2 (Biopsy-Positive) | Group 3.1 (Double-Seropositive) | Group 3.2 (Single-Seropositive) | Group 4.1 (FS < 1 and/or Trace ANA and/or Trace SSA/SSB) | Group 4.2 (Biopsy-Negative and Seronegative) | Total | |
|---|---|---|---|---|---|---|---|---|
| Number of patients | 0 | 0 | 1 | 2 | 12 | 83 | 93 | 191 |
| Percentage of all referrals | 0.5% | 1.0% | 6.3% | 43.5% | 48.7% | 100.0% | ||
| Gender | ||||||||
| Male | ||||||||
| Frequency | 0 | 0 | 0 | 1 | 4 | 7 | 9 | 21 |
| Min. age | 82 | 42 | 28 | 36 | ||||
| Max. age | 82 | 69 | 79 | 85 | ||||
| Mean age | 82 | 50.5 | 50.7 | 53.1 | ||||
| Female | ||||||||
| Frequency | 0 | 0 | 1 | 1 | 8 | 76 | 84 | 170 |
| Min. age | 60 | 21 | 40 | 32 | 25 | |||
| Max. age | 60 | 21 | 67 | 76 | 81 | |||
| Mean age | 60 | 21 | 51.4 | 52.8 | 54.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, S.; Holm, H.; Rahel, A.; Skarstein, K.; Jensen, J.L.; Hynne, H. Sjögren’s Syndrome: The Role of Serological Profiles Versus Minor Salivary Gland Histopathology. Appl. Sci. 2024, 14, 11482. https://doi.org/10.3390/app142411482
Uddin S, Holm H, Rahel A, Skarstein K, Jensen JL, Hynne H. Sjögren’s Syndrome: The Role of Serological Profiles Versus Minor Salivary Gland Histopathology. Applied Sciences. 2024; 14(24):11482. https://doi.org/10.3390/app142411482
Chicago/Turabian StyleUddin, Shahad, Håkon Holm, Arian Rahel, Kathrine Skarstein, Janicke Liaaen Jensen, and Håvard Hynne. 2024. "Sjögren’s Syndrome: The Role of Serological Profiles Versus Minor Salivary Gland Histopathology" Applied Sciences 14, no. 24: 11482. https://doi.org/10.3390/app142411482
APA StyleUddin, S., Holm, H., Rahel, A., Skarstein, K., Jensen, J. L., & Hynne, H. (2024). Sjögren’s Syndrome: The Role of Serological Profiles Versus Minor Salivary Gland Histopathology. Applied Sciences, 14(24), 11482. https://doi.org/10.3390/app142411482






