Featured Application
The developed HPTLC method was able to detect and quantify four targeted fat-soluble vitamins in commercial pharmaceutical preparations.
Abstract
A simple, cost-effective, and efficient novel high-performance thin-layer chromatography (HPTLC) tool was developed and validated in accordance with International Conference on Harmonisation (ICH) guidelines for the detection and quantification of four fat-soluble vitamins, D2, D3, E, and K1, using chloroform: cyclohexane (55:45, v/v) as mobile phase. The detection and quantification limits were found to be 30.86 and 93.52 ng/band for vitamin D2, 19.44 and 58.92 ng/band for vitamin D3, 14.17 and 42.95 ng/band for vitamin E, and 0.86 and 2.61 ng/band for vitamin K1, which were similar or lower than those reported in previous methods. The advantage of the developed method is that it uses a simple mobile phase in a single development step and has low detection and quantification limits. The application of the developed HPTLC method was successfully demonstrated with the quantitative analysis of these vitamins in some commercially available pharmaceutical preparations.
1. Introduction
Vitamins can be classified as fat-soluble and water-soluble vitamins based on their respective solubility profiles. Fat-soluble vitamins include vitamins A, D, E, and K whereas water-soluble vitamins include members of the vitamin B-complex (vitamins B1, B2, B3, B5, B6, B7, B9, and B12) as well as vitamin C. Vitamins are essential for cellular development and growth and also play a crucial role in metabolic processes and cellular regulation, thus they are important for maintaining health [1,2]. When vitamin levels are insufficient to meet physiological requirements, vitamin intake can be increased either through diet, nutraceutical supplements, or pharmaceutical preparations [2]. Commonly fat-soluble vitamins are incorporated in these formulations as acetate in the case of vitamin A, as α-tocopherol or tocopheryl acetate for vitamin E, in the form of either cholecalciferol (vitamin D3) or ergocalciferol (vitamin D2) and as phylloquinone (vitamin K1) or menaquinones (vitamin K2) [3,4]. As both deficiency and excess intake of vitamins can cause health problems [5], it is crucial to establish analytical methods that enable the evaluation of the quality of the nutraceutical and pharmaceutical preparations in terms of meeting their stated vitamin content.
In earlier studies, a wide range of analytical methods has been employed, including spectrophotometry, high-performance liquid chromatography (HPLC), reverse phase high-performance liquid chromatography (RP-HPLC), liquid-chromatography/mass spectrometry (LC-MS), liquid-chromatography/UV-spectrometry (LC-UV), liquid chromatography coupled with diode array/mass spectrometry (LC-DAD-MC) and gas-liquid chromatography (GLC), for the analysis of fat-soluble vitamins in complex matrices like human plasma and serum, pharmaceutical preparations and food items such as fruits and vegetables [2,6,7,8,9,10,11]. In recent years, High-performance thin-layer chromatography (HPTLC) has also been developed into a popular analytical tool based on its simple, multifunctional, highly adaptable and economically efficient analysis process [5]. Moreover, high sample throughput coupled with minimal sample preparation steps facilitate a highly efficient and rapid analysis using HPTLC [4].
In light of the above, HPTLC has become widely popular for the analysis of targeted molecules in various complex matrices like food items (e.g., honey), pharmaceutical formulations, and also nutraceutical preparations [12,13,14]. Specifically in the context of fat-soluble vitamins, previous studies found that HPTLC analysis is a “green” technique to analyze vitamin D3 in commercial pharmaceutical products [15]. Another study revealed that using a two-step development coupled with densitometric detection, vitamins A and E as well as some water-soluble vitamins can be determined simultaneously by HPTLC [16] whereas other studies found that fat-soluble vitamins can be effectively analyzed in human plasma and food items using HPTLC [4,17,18]. Table 1 provides an overview of previously published HPTLC-based methods for the analysis of fat-soluble vitamins. Reviewing these studies, it can be concluded that there are still some limitations to the quantitative analysis of fat-soluble vitamins either as pure substances or in biological, pharmaceuticals, or food items using existing HPTLC-based methods. For example, they were found to be unable to adequately separate more than two fat-soluble vitamins simultaneously using a single development step.

Table 1.
Published studies using HPTLC for the analysis of fat-soluble vitamins.
Addressing those limitations, the aim of the current study was to develop and validate a HPTLC-based method for the simultaneous quantification of four fat-soluble vitamins including D2, D3, E, and K1 (Figure 1) in pharmaceutical preparations using a simple mobile phase and a single development step.
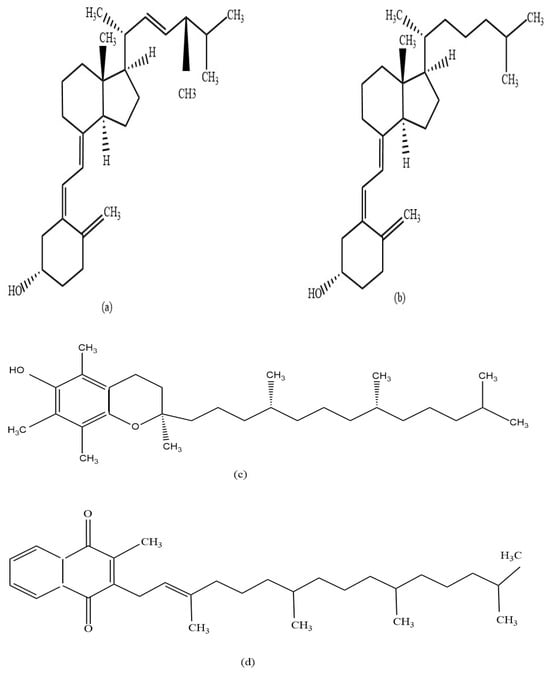
Figure 1.
Chemical structures of the analyzed fat-soluble vitamins; vitamin D2 (a), vitamin D3 (b), vitamin E (c), and vitamin K1 (d).
2. Materials and Methods
2.1. Chemicals and Reagents
All chemicals and reagents used in this study were of analytical grade. The vitamin standards (Ergocalciferol—vitamin D2, cholecalciferol—vitamin D3, d-alpha-tocopherol—vitamin E, and phytomenadione—vitamin K1) as well as chloroform were sourced from Sigma-Aldrich (Bayswater, VIC, Australia). Cyclohexane and ethanol were bought from Chem-Supply (Gillman, Adelaide, South Australia) and methanol from Scharlau (Barcelona, Spain). Silica gel 60 F254 HPTLC glass plates (20 cm × 10 cm) were purchased from Merck KGaA (Darmstadt, Germany).
2.2. Commercial Samples
Three different dosage forms (i.e., hard gelatine capsules, soft gelatine capsules, IV solution) of four commercially available pharmaceutical preparations were sourced from Chemist Warehouse (Australia). The samples were labelled S1–S4 (Table 2) and stored at 4 °C before analysis.

Table 2.
Analyzed pharmaceutical preparations.
2.3. Sample Preparation
2.3.1. Preparation of Stock Solutions and Mobile Phase
Ethanolic solutions were prepared for the four fat-soluble vitamin standards at different concentration ranges: 100–300 µg/mL for vitamin D2, D3, and E and 20–60 µg/mL for vitamin K1. Due to the vitamins’ inherent instability, the stock solutions were prepared freshly before every analysis. Different mobile phases were trialled (Table 3) and a mobile phase comprising chloroform and cyclohexane at a ratio of 55:45 (v/v) was found to be best for the analysis of the four fat-soluble vitamins.

Table 3.
Trial experiments with different mobile phase compositions.
2.3.2. Preparation of Pharmaceutical Samples for HPTLC Analysis
Ten capsules were randomly sampled to determine the percentage of the stated content of the vitamins in commercial hard gelatine capsule dosage form (S1). Each hard gelatine capsule was carefully opened, and the powder content was collected and weighed. For analysis, 1200 mg powder, equivalent to four capsules, was weighed into a 25-mL volumetric flask. Absolute ethanol was added, and extraction was aided with vortexing (MX-S, China) for 5 min before the flasks were made up to volume. The suspension was filtered and immediately used for HPTLC analysis. For products S2 and S3, which were soft gelatine capsule formulations, five capsules were randomly selected, the content of the capsules was carefully squeezed out, and a total of 750 mg (S2) and 30 mg (S3) equivalent to a single capsule were sampled respectively into separate 100 mL volumetric flasks before the content was made up to volume with ethanol. For product S4, 0.2 mL of the injection solution was collected and mixed in 100 mL ethanol. The prepared solutions were immediately used for HPTLC analysis. All analyses were carried out in three replications.
2.4. HPTLC Method and Instruments
2.4.1. Application of Standards and Samples
A semi-automated HPTLC applicator (Linomat 5, CAMAG, Muttenz, Switzerland) was employed to apply the standard solutions onto the HPTLC plates. Application volumes ranged from 1–3 μL for vitamins D2, D3, and E and 2–6 μL for vitamin K1. The standard solutions were applied at a rate of 30 nL/s with the application points located 20 mm from the side edges and 8.0 mm from the lower edges of the HPTLC plates, resulting in 8.0 mm long bands spaced 11.4 mm apart. Calibration curves were constructed over a concentration range of 100–300 ng/μL for vitamins D2, D3, and E and 20–60 ng/μL for vitamin K1.
2.4.2. Sample Development
The chromatographic separation was carried out using silica gel 60 F254 HPTLC plates (glass plates 20 × 10 cm) as stationary phase. Chloroform: cyclohexane (55:45, v/v) was used as a mobile phase at ambient temperature in an activated (33% relative humidity) and saturated development chamber (ADC2, CAMAG). The plates were pre-conditioned with the mobile phase for 5 min and the development chamber was saturated for 45 min before the plates were automatically developed with 10 mL of the mobile phase to a migration distance of 75 mm prior to being dried for 5 min. A specialized HPTLC software (visionCATS v3.1, CAMAG) was used to operate the HPTLC instrument modules. The documentation of the chromatographic results at 254 nm was carried out by an HPTLC imaging device (TLC Visualiser 2, CAMAG). After imaging, a TLC Scanner 4 (CAMAG) was used to analyze the developed plates with a scanning speed of 20 mm/s at 263 nm (vitamin D2), 270 nm (vitamin D3), 272 nm (vitamin E) and 255 nm (vitamin K1), which correspond to the respective absorbance maxima (λmax) of the investigated fat-soluble vitamins.
2.5. Method Validation
The developed HPTLC method for the quantification of fat-soluble vitamins D2, D3, E and K1, was validated for specificity, linearity, sensitivity, precision, accuracy, repeatability, and robustness in accordance with the International Conference on Harmonisation (ICH) guidelines Q2 (R1) [20,21].
2.5.1. Specificity
Specificity assesses the capability of the chosen method to detect the analyte of interest in the presence of other substances or closely related compounds. In HPTLC analysis specificity is ensured through the complete separation of the peak(s) of the analyte(s) from other peaks as well as by their distinct retardation factors (RF). In this study, specificity focused on the adequate separation of the four investigated fat-soluble vitamins, D2, D3, E, and K1.
2.5.2. Linearity
The linearity of an analytical procedure defines the ability to generate data that are directly proportional to the analyte’s concentration over a specified range. To evaluate the linearity of the proposed method, three replicate calibration curves with five data points for each fat-soluble vitamin were constructed in a concentration range of 100–300 ng/band for the standard solution of vitamins D2, D3, and E and 20–60 ng/band for vitamin K1 standard. Peak areas versus the respective concentrations of vitamin D2 and D3 standards and peak height versus the respective concentrations of vitamin E and K1 standards were evaluated by linear regression analysis and the coefficient of determination (r2), slope (m), y-intercept (c), and standard deviation (SD) of the calibration curves calculated. Calculations were done by using Microsoft Excel.
2.5.3. Sensitivity
The ability to detect and quantify the analyte in a sample matrix is defined as the sensitivity of an analytical method. It is commonly expressed as limit of detection (LOD) and the limit of quantification (LOQ), which refer in the case of LOD to the minimum amount of the sample that can be detected but not necessarily quantified and in the case of LOQ to the lowest concentration of the analyte that can be quantified with acceptable accuracy, precision, and reproducibility under the specific experimental conditions. In this study, based on the evaluation of three calibration curves, the LOD and LOQ values for each fat-soluble vitamin standard were determined using the formulas stipulated in the ICH guidelines [20]:
where σ is the regression line’s standard deviation (SD) and S is the average slope value of triplicate calibration curves.
LOD = 3.3 σ/S and
LOQ = 10 σ/S
2.5.4. Precision
The degree of consistency among individual test results when the method is repeatedly applied to multiple samples under the same prescribed conditions but at different times, on different instruments, and/or by different laboratories is defined as its precision. In this study intra-day and inter-day precision were assessed to validate the method. For this, triplicate analyses of three different concentrations of the respective fat-soluble vitamin samples were carried out either on the same day or over three days using 150, 200, and 250 ng/band for vitamin D2, D3, and E and 30, 40, and 50 ng/band for vitamin K1. Precision was expressed as the percent relative standard deviation (%RSD) of the results obtained for peak areas for vitamin D2 and D3 as well as peak heights for vitamin E and K1.
2.5.5. Accuracy
The accuracy of the method expresses the closeness of agreement between the value, which is accepted either as a conventional true value or an accepted reference value, and the value found. The accuracy of the method, which reflects the closeness of agreement between experimental and theoretical analysis results, was tested by calculating the percentage recovery of each vitamin at three different concentration levels, 150, 200, and 250 ng/band for vitamins D2, D3 and E, and 30, 40, and 50 ng/band for vitamin K1. Each experiment was performed in triplicate, and the % recovery and %RSD of the vitamin standards were used to determine the accuracy of the method.
2.5.6. Repeatability (System Precision)
Obtaining precise results under the same operating conditions over a short time interval reflects the repeatability of an analytical method. In this study, repeatability was assured by analyzing standard solutions five times at a concentration of 200 ng/band for vitamin D2, D3, and E, and 40 ng/band for vitamin K1. Results were expressed as %RSD of standards.
2.5.7. Robustness
The robustness of a method is the ability to produce reliable and reproducible results following some small changes to the experimental conditions. In this study, robustness was measured by analysing three different concentrations of vitamin standards: 200 ng/band for vitamin D2, D3, and E, and 40 ng/band for vitamin K1 after a slight change in the mobile phase volume (±2 mL), mobile phase composition (chloroform: cyclohexane, 55:47 and 57:45, v/v), and saturation time of the development chamber (±5 min). The results were tested for robustness regarding % recovery and RF values for the individual vitamins.
3. Results and Discussion
3.1. Mobile Phase Selection
The objective of the study was to develop a simple and suitable HPTLC method for quantifying four fat-soluble vitamins in complex matrices like pharmaceutical preparations in a single development step. During the method development stage of this study, various mobile phases were trialled to accomplish this goal (Table 3). Mobile phase (MP) 5 was found to be the optimum mobile phase, which ensured adequate separation of the fat-soluble vitamins D, K, and E based on their respective RF values (Figure 2). However, given their closely related structures (Figure 1), the optimised mobile phase could not separate the two forms of vitamin D, D1 and D2, as they produced the same RF value (0.20). However, since vitamin D2 is only sourced from plants and fungi and vitamin D3 is derived from animals, these two vitamins are rarely combined in a single nutraceutical preparation as the former is mainly found in vegan friendly preparations [3,22]. The developed method is thus suitable for the quantitative analysis of fat-soluble vitamins as long as only one form of vitamin D is present. The optimised mobile phase was used previously to quantify retinol (vitamin A) and a-tocopherol (vitamin E) in human serum [18], however, in this study, satisfactory analysis results for vitamin A could not be achieved.
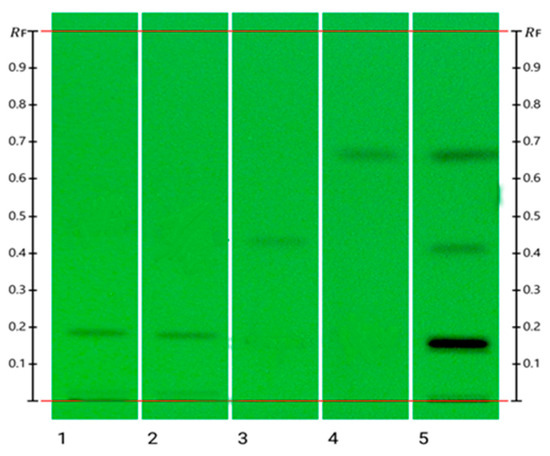
Figure 2.
HPTLC image visualized at 254 nm: track 1: Vit D2; track 2: Vit D3; track 3: Vit E; track 4: Vit K1; track 5: Over spotting of vitamins D2, D3, E, and K1.
3.2. Evaluation of Visual Images and Selection of Quantification Method
A set of images at 254 nm were obtained and visionCATS (CAMAG) software was used to interpret each of the images. Percent (%) recovery and correlation coefficient (r2) values were found to be consistent for all four fat-soluble vitamins at 254 nm over the entire concentration ranges of their respective calibration curves. For the quantification of vitamins D2 and D3, absorbance peak area versus concentration of their calibration curves were found to produce the best results with high levels of reproducibility, whereas standard curves using peak height versus concentration were found to be most suited to quantify vitamins E and K1 (Figure S1).
3.3. Chromatographic Results and UV–Vis Spectra
Vitamins D2, D3, E and K1 were well separated using the optimized chromatographic system and produced distinct RF values: RF 0.20 for vitamins D2 and D3, 0.46 for vitamin E, and 0.68 for vitamin K1. To determine their absorbance maxima (λmax) for optimal detection, the UV–Vis spectrum of each fat-soluble vitamin was recorded. The λmax values for vitamin D2, D3, E and K1 were found to be 263 nm, 270 nm, 272 nm and 255 nm, respectively (Figure S2). Although their RF values were identical, the λmax values of vitamin D2 and D3 differed slightly with 263 nm for vitamin D2 and 270 nm for vitamin D3, however, this difference was not sufficient to quantify them accurately individually at their respective absorbance maxima.
3.4. Method Validation
3.4.1. Specificity
Respective volumes of the four fat-soluble vitamin standards (2 µL for vitamin D2, D3, and E; 4 µL for vitamin K1) were applied and the HPTLC plates developed with the optimized mobile phase chloroform: cyclohexane (55:45, v/v). The specificity of the developed method was confirmed by distinctly different RF values for the four vitamins (Figure 2), 0.20 for vitamins D2 and D3, 0.46 for vitamin E, and 0.68 for vitamin K1.
3.4.2. Linearity
The linearity of the method was validated rigorously through linear regression analysis, with the correlation coefficients (r2) derived from the five-point calibration curves for the respective fat-soluble vitamins. The resulting linearity ranges were 100–300 ng/band for vitamin D2, D3 and E and 20–60 ng/band for vitamin K1. The r2 values of three replicate experiments for all analyzed fat-soluble vitamins were found to be more than 0.98, confirming adequate linearity of the method in the defined concentration range (Table 4).

Table 4.
Linear regression analysis for the calibration curves of vitamins D2, D3, E, and K1.
3.4.3. Sensitivity (LOD and LOQ)
The average slope values and y-intercepts obtained from linear regression analysis from the respective fat-soluble vitamins’ standard curves were used to calculate the LOD and LOQ of each vitamin (Table 4). The LOD for vitamin D2, D3, E, and K1 was 30.86 ng/band, 19.44 ng/band, 14.17 ng/band, and 0.86 ng/band, respectively, and the LOQ was 93.52 ng/band, 58.92 ng/band, 42.95 ng/band and 2.61 ng/band, respectively (Table 4). In comparison with previously developed HPTLC methods, with a LOD of 17.54 and 0.19 ng/band and a LOQ of 52.62 and 2.50 ng/band respectively we were able to determine and quantify vitamin D3 and K1 with similar limits [4,15]. Moreover, the newly developed method can also detect and quantify two other fat-soluble vitamins, D2 and E, with a low LOD and LOQ as seen in Table 4 whereas a previous study was only able to determine and quantify vitamin E (LOD and LOQ 0.89 and 5.71 ng/band respectively [18]). However, no other HPTLC-based study was found that allows for the quantification of vitamin D2 in complex matrices.
3.4.4. Accuracy
Based on sample recovery, the method’s accuracy and level of conformity were assessed. The standard addition method was used to calculate sample recovery by taking the percentage mean recovery of each vitamin sample. The findings of this analysis demonstrate that the accuracy level of the developed method was high with percent mean recovery ranging from 99.37% to 101.53% for vitamin D2, 100.89% to 101.51% for vitamin D3, 99.67% to 102.51% for vitamin E, and 97.64% to 102.18% for vitamin K1. According to ICH guidelines, all results fall within the acceptable limit (Table 5).

Table 5.
Recovery of vitamins D2, D3, E, and K1.
3.4.5. Precision
The study’s intra-day and intermediate (inter-day) precision were calculated based on %RSD values, which ranged from 0.92% to 4.11%, and thus were found to be within acceptable limits as per ICH guidelines (Table 6 and Table 7), indicating a high degree of precision of the developed method.

Table 6.
Precision study of vitamins D2, D3, E, and K1 (Intra-Day).

Table 7.
Precision study of vitamin D2, D3, E, and K1 (Inter-Day).
3.4.6. Repeatability
The method’s repeatability was expressed as SD and %RSD. The findings demonstrate that with 1.58-2.76%, the %RSD was within the acceptable limits of the ICH guidelines (Table 8), which confirmed the high confidence level in the methods’ repeatability.

Table 8.
Repeatability (System precision).
3.4.7. Robustness
Small changes in mobile phase volume, development chamber saturation time, and composition of the mobile phase were intentionally made to assess the method’s robustness. The findings of the robustness study are shown in Table 9, Table 10 and Table 11. As can be seen from the data presented in these tables, small changes in the respective parameters had minimal effects on the RF values of the investigated vitamins and did not affect their accurate quantification based on mean % recovery calculations.

Table 9.
Robustness: Change in mobile phase volume.

Table 10.
Robustness: Change in saturation time.

Table 11.
Robustness: Change in mobile phase composition.
4. Application of the Method
This study aimed to develop and validate a HPTLC method suitable for the analysis of fat-soluble vitamins in various pharmaceutical preparations. Three different types of pharmaceutical dosage forms (hard gelatine capsules, soft gelatine capsules, and injection-Table 2) were selected to demonstrate the applicability of the method. The results of the quantification study are shown in Table 12. The findings revealed that 97.24% of the label claimed for vitamin D2 in the S1 sample could be recovered based on three replicate analyses. For samples S2, S3, and S4, the vitamin content analyzed expressed as a percentage of the labelled content was 99.42% for vitamin D3, 103.55% for vitamin E, and 94.42% for vitamin K1, respectively (Table 12). All the samples contained different excipients, as listed in Table 2, but none was found to interfere with the quantification, demonstrating the broad applicability of the method.

Table 12.
Determined the stated content of vitamins D2, D3, E and K1 in commercial samples.
The developed method appears more convenient than previous HPTLC-based methods developed for the analysis of fat-soluble vitamins as it only used a simple mobile phase in a single development step with satisfactory accuracy, precision and robustness. Moreover, its sensitivity is also good with LOD and LOQ levels that were found to be similar or lower compared to previously published methods [4,15,16,17,18]. Moreover, the newly developed method can analyze four fat-soluble vitamins, vitamin D2, D3, E and K1 whereas previously developed HPTLC methods can only identify and quantify a single vitamin, like vitamin D3 in pharmaceutical preparations, vitamin A and E in human plasma and vitamin K1 in pharmaceutical preparations, fruits and vegetables [4,15,16,17,18]. This illustrates the novelty of the newly developed HPTLC method in comparison of previous methods.
Despite its advantages over previous studies, a limitation of the method is its inability to separate vitamin D2 and D3. However, this does not seem to have practical implications as formulations always tend to contain either one or the other but not both types of vitamin D at the same time [3].
5. Conclusions
Fat-soluble vitamins play an important role in various physiological processes like bone and vision health, immunity and blood coagulation. To meet the required intake levels of these vitamins, dietary supplements like nutraceuticals or pharmaceutical formulations might need to be taken to complement dietary intake. To ensure the quality of these preparations, a simple and efficient HPTLC tool was developed and validated which can assist in the simultaneous quantitative analysis of four fat-soluble vitamins, D2, D3, E and K1. The developed method appeared more convenient than previous HPTLC-based methods developed for the analysis of fat-soluble vitamins as it only used a simple mobile phase in a single development step with satisfactory accuracy, precision and robustness. Thus, the method has been demonstrated to successfully quantify a range of fat-soluble vitamins in complex pharmaceutical preparations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app142311064/s1, Figure S1. Exemplary calibration curves of fat-soluble vitamins: (a) Vit D2, (b) Vit D3, (c) Vit E and (d) Vit K1. Figure S2. The absorption maxima of fat-soluble vitamins: (a) Vit D2—263 nm, (b) Vit D3—270 nm, (c) Vit E—272 nm, and (d) Vit K1—255 nm.
Author Contributions
Conceptualization, C.L., L.Y.L. and K.M.Y.K.S.; methodology, K.M.Y.K.S., M.K.I., T.S. and C.L.; validation, K.M.Y.K.S., M.K.I., T.S. and C.L.; formal analysis, K.M.Y.K.S.; writing—original draft preparation K.M.Y.K.S.; writing—review and editing, C.L., L.Y.L., T.S. and M.K.I.; supervision, C.L., and L.Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions included in the study are presented within the article. For further inquiries can be sent to the corresponding author.
Acknowledgments
This research was conducted during the author receipt of an International Fee Scholarship and a University Postgraduate Award from the University of Western Australia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khayat, S.; Fanaei, H.; Ghanbarzehi, A. Minerals in Pregnancy and Lactation: A Review Article. J. Clin. Diagn. Res. 2017, 11, Qe1–Qe5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, W.E.; Yan, J.Q.; Liu, M.; Zhou, Y.; Shen, X.; Ma, Y.L.; Feng, X.S.; Yang, J.; Li, G.H. A Review of the Extraction and Determination Methods of Thirteen Essential Vitamins to the Human Body: An Update from 2010. Molecules 2018, 23, 1484. [Google Scholar] [CrossRef] [PubMed]
- Scientific, T.F. Determination of Water- and Fat-Soluble Vitamins by HPLC. In Knowledge Creation Diffusion Utilization; Thermo Fischer Scientific: Waltham, MA, USA, 2017; pp. 1–23. [Google Scholar]
- Atia, N.N.; Ahmed, S. A Validated High-Throughput Chromatographic Method for Simultaneous Determination of Vitamin K Homologues. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 486–498. [Google Scholar] [CrossRef]
- Ullah, Q.; Mohammad, A. Vitamins determination by TLC/HPTLC—A mini-review. JPC-J. Planar. Chromat. 2020, 33, 429–437. [Google Scholar] [CrossRef]
- De Leenheer, A.P.; Lambert, W. Modern Chromatographic Analysis of Vitamins: Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2000; Volume 84. [Google Scholar]
- Dialameh, G.H.; Olson, R.E. Gas-Liquid Chromatography of Phytyl Ubiquinone, Vitamin-E, Vitamin-K1, and Homologs of Vitamin-K2. Anal. Biochem. 1969, 32, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Po, E.S.M.; Ho, J.W.; Gong, B.Y. Simultaneous chromatographic analysis of eight fat-soluble vitamins in plasma. J. Biochem. Bioph. Meth. 1997, 34, 99–106. [Google Scholar]
- Santos, J.; Mendiola, J.A.; Oliveira, M.B.; Ibáñez, E.; Herrero, M. Sequential determination of fat-and water-soluble vitamins in green leafy vegetables during storage. J. Chromatogr. A 2012, 1261, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Phinney, K.W.; Rimmer, C.A.; Thomas, J.B.; Sander, L.C.; Sharpless, K.E.; Wise, S.A. Isotope dilution liquid chromatography—Mass spectrometry methods for fat- and water-soluble vitamins in nutritional formulations. Anal. Chem. 2011, 83, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Monakhova, Y.B.; Mushtakova, S.P.; Kolesnikova, S.S.; Astakhov, S.A. Chemometrics-assisted spectrophotometric method for simultaneous determination of vitamins in complex mixtures. Anal. Bioanal. Chem. 2010, 397, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. A validated method for the quantitative determination of sugars in honey using high-performance thin-layer chromatography. JPC-J. Planar. Chromat. 2020, 33, 489–499. [Google Scholar] [CrossRef]
- Sikdar, K.Y.K.; Islam, M.K.; Sostaric, T.; Lim, L.Y.; Locher, C. Development and Validation of a Quantitative Analysis of Water-Soluble Vitamins Using High-Performance Thin-Layer Chromatography and its Application to the Analysis of Nutraceuticals. Separations 2024, 11, 207. [Google Scholar] [CrossRef]
- Gill, B.D.; Abernethy, G.A.; Green, R.J.; Indyk, H.E. Analysis of vitamin D2 and vitamin D3 in fortified milk powders and infant and nutritional formulas by liquid chromatography–tandem mass spectrometry: Single-laboratory validation, first action 2016.05. J. AOAC Int. 2016, 99, 1321–1330. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alqarni, M.H.; Shakeel, F.; Foudah, A.I.; Aljarba, T.M.; Alam, A.; Alshehri, S.; Alam, P. Comparison of Validation Parameters for the Determination of Vitamin D3 in Commercial Pharmaceutical Products Using Traditional and Greener HPTLC Methods. Separations 2022, 9, 301. [Google Scholar] [CrossRef]
- Kartsova, L.A.; Koroleva, O.A. Simultaneous determination of water-and fat-soluble vitamins by high-performance thin-layer chromatography using an aqueous micellar mobile phase. J. Anal. Chem. 2007, 62, 255–259. [Google Scholar] [CrossRef]
- Pyka, A.; Sliwiok, J. Chromatographic separation of tocopherols. J. Chromatogr. A 2001, 935, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Chavan, J.D.; Khatri, J.M. Determination of retinol and α-tocopherol in human plasma by HPTLC. J. Planar Chromatogr. Morden TLC 1992, 5, 280–282. [Google Scholar]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Sugar profiling of honeys for authentication and detection of adulterants using high-performance thin layer chromatography. Molecules 2020, 25, 5289. [Google Scholar] [CrossRef]
- ICH. Harmonized Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology Q2(R1). In Proceedings of the International Conference on Harmonization (ICH), Geneva, Switzerland, 10 November 2005. [Google Scholar]
- Koll, K.; Reich, E.; Blatter, A.; Veit, M. Validation of standardized high-performance thin-layer chromatographic methods for quality control and stability testing of herbals. J. AOAC Int. 2003, 86, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hypponen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).