Natural Inhibitors of the Polyphenol Oxidase Activity Isolated from Shredded Stored Iceberg Lettuce (Lactuca sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Partial Purification of PPOs
2.3.1. Preparation of Crude Extracts
2.3.2. Salting Out
2.3.3. Gel Filtration Chromatography Using Sephadex G-75
2.4. PPO Assay
2.5. Protein Assay
2.6. Characterization of PPO
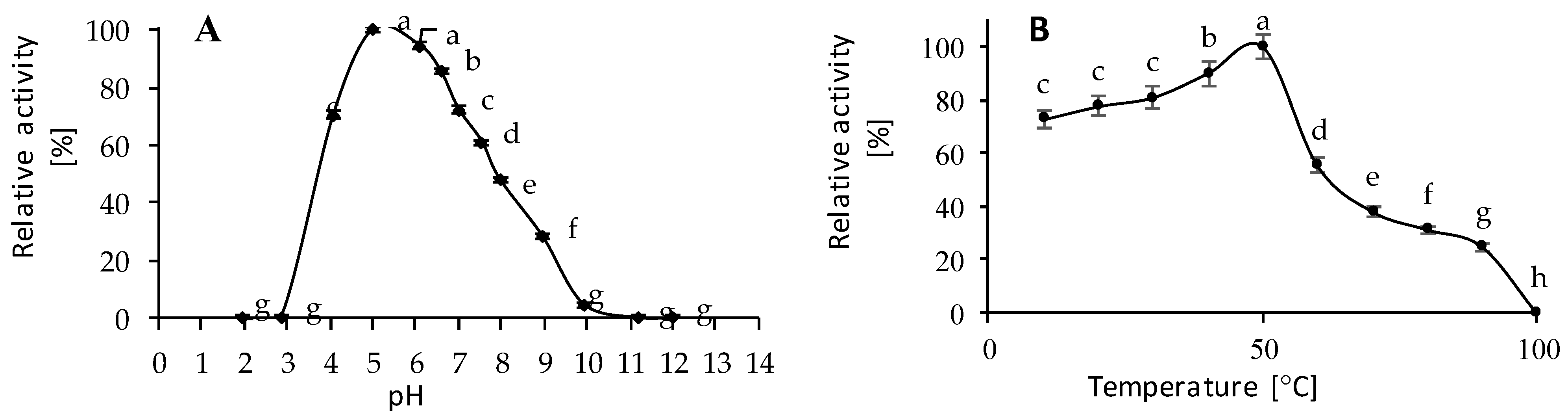
2.6.1. Effect of Temperature on Enzyme Activity
2.6.2. Effect of pH on Enzyme Activity
2.6.3. Inhibition of PPO Activity with Catechol as Substrate
Preparation of Inhibitory Solutions
Inhibition of PPO Activity (Exogenous Substrate)
Model of Inhibition and Kinetic Constants
2.6.4. Inhibition of PPO with Endogenous Substrate
Preparation of Endogenous Substrate
Inhibition of PPO Activity (Endogenous Substrate)
Mode of Inhibition and Inhibition Kinetics Parameters (Endogenous Substrate)
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, P.R.; Omeera, A.; Suradkar, P.P.; Dar, M.A. Effect of combination treatment of gamma irradiation and ascorbic acid on physicochemical and microbial quality of minimally processed eggplant (Solanum melongena L.). Radiat. Phys. Chem. 2014, 103, 131–141. [Google Scholar] [CrossRef]
- Gong, Z.; Li, D.; Liu, C.; Cheng, A.; Wang, W. Food Science and Technology Partial purification and characterization of polyphenol oxidase and peroxidase from chestnut kernel. LWT-Food Sci. Technol. 2015, 60, 1095–1099. [Google Scholar] [CrossRef]
- Xiong, Z.; Liu, W.; Zhou, L.; Zou, L.; Chen, J. Mushroom (Agaricus bisporus) polyphenoloxidase inhibited by apigenin: Multi-spectroscopic analyses and computational docking simulation. Food Chem. 2016, 203, 430–439. [Google Scholar] [CrossRef]
- De Rosa, L.A.; Alvarez-Parrilla, E.; Moyers-Montoya, E.; Villegas-Ochoa, M.; Ayala-Zavala, J.F. Mechanism for the inhibition of apple juice enzymatic browning by Palo Fierro (desert ironweed) honey extract and other natural compounds. LWT-Food Sci. Technol. 2011, 44, 269–276. [Google Scholar] [CrossRef]
- Sikora, M.; Złotek, U.; Świeca, M. Effect of basil leaves and wheat bran water extracts on enzymatic browning of shredded storage iceberg lettuce. Int. J. Food Sci. Technol. 2020, 55, 1318–1325. [Google Scholar] [CrossRef]
- Theerakulkait, C.; Sukhonthara, S. Effect of pineapple juice, pineapple shell extract and rice bran extract on browning in banana [Musa (AAA Group) ‘Gros Michel’] slices and puree. In Proceedings of the 46th Kasetsart University Annual Conference, Kasetsart, Thailand, 29 January–1 February 2008; Volume 155, pp. 150–155. [Google Scholar]
- Loizzo, M.R.; Tundis, R.; Menichini, F. Natural and Synthetic Tyrosinase Inhibitors as Antibrowning Agents: An Update. Compr. Rev. Food Sci. Food Saf. 2012, 11, 378–398. [Google Scholar] [CrossRef]
- Sikora, M.; Złotek, U.; Kordowska-Wiater, M.; Świeca, M. Spicy Herb Extracts as a Potential Improver of the Antioxidant Properties and Inhibitor of Enzymatic Browning and Endogenous Microbiota Growth in Stored Mung Bean Sprouts. Antioxidants 2021, 10, 425. [Google Scholar] [CrossRef] [PubMed]
- Martín-Diana, A.B.; Rico, D.; Barry-Ryan, C. Green tea extract as a natural antioxidant to extend the shelf-life of fresh-cut lettuce. Innov. Food Sci. Emerg. Technol. 2008, 9, 593–603. [Google Scholar] [CrossRef]
- Altunkaya, A. Effect of grape seed extract on phenolic profile and browning of fresh-cut lettuce (L. sativia). J. Food Biochem. 2012, 36, 268–274. [Google Scholar] [CrossRef]
- Yuzugullu, Y.; Yildirim, B.; Acemi, A. Characterization of polyphenol oxidase from fennel (Foeniculum vulgare Mill.) seeds as a promising source. Int. J. Biol. Macromol. 2021, 170, 261–271. [Google Scholar] [CrossRef]
- Benaceur, F.; Chaibi, R.; Berrabah, F.; Neifar, A.; Leboukh, M.; Benaceur, K. Purifi ation and characterization of latent polyphenol oxidase from truffles (Terfezia arenaria). Int. J. Biol. Macromol. 2020, 145, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Iimure, K.; Okamoto, S.; Saito, A. Expression analysis of polyphenol oxidase isozymes by active staining method and tissue browning of head lettuce (Lactuca sativa L.). Biosci. Biotechnol. Biochem. 2017, 81, 1484–1488. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cantos, E.; Espín, J.C.; Tomás-Barberán, F.A. Effect of wounding on phenolic enzymes in six minimally processed lettuce cultivars upon storage. J. Agric. Food Chem. 2001, 49, 322–330. [Google Scholar] [CrossRef]
- Terefe, N.S.; Delon, A.; Buckow, R.; Versteeg, C. Blueberry polyphenol oxidase: Characterization and the kinetics of thermal and high pressure activation and inactivation. Food Chem. 2015, 188, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Galeazzi, M.A.M.; Sgarbieri, V.C.; Constantinides, S.M. Isolation, purification and physicochemical characterization of polyphenoloxidases (PPO) from a dwarf variety of banana (Musa cavendishii L.). J. Food Sci. 1981, 46, 150–155. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Gawlik-Dziki, U. Characterization of polyphenol oxidase from butter lettuce (Lactuca sativa var. capitata L.). Food Chem. 2008, 107, 129–135. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, X. Characterisation of Polyphenol Oxidase and Peroxidase and the Role in Browning of Loquat Fruit. Czech J. Food Sci. 2015, 33, 109–117. [Google Scholar] [CrossRef]
- Gonzales, E.M.; De Ancos, B.; Cano, M.P. Partial Characterization of Peroxidase and Polyphenol Oxidase Activities in Blackberry Fruits. J. Agric. Food Chem. 2000, 48, 5459–5464. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonzalez, E.M.; De Ancos, B.; Cano, M.P. Partial Characterization of Polyphenol Oxidase Activity in Raspberry Fruits. J. Agric. Food Chem. 1999, 47, 4068–4072. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cao, S.; Qi, X.; Yang, Z. The effect of pH on the activity, thermokinetics and inhibition of polyphenol oxidase from peach. J. Food Sci. Technol. 2015, 52, 7465–7471. [Google Scholar] [CrossRef]
- Zhang, S. Recent Advances of Polyphenol Oxidases in Plants. Molecules 2023, 28, 2158. [Google Scholar] [CrossRef] [PubMed]
- Serradell, D.L.A.; Rozenfeld, P.A.; Civello, P.M.; Chaves, A.R. Polyphenoloxidase activity from strawberry fruit (Fragaria × ananassa, Duch., cv Selva): Characterisation and partial purification. J. Sci. Food Agric. 2000, 1427, 1421–1427. [Google Scholar] [CrossRef]
- Gao, Z.J.; Liu, J.B.; Xiao, X.G. Purification and characterisation of polyphenol oxidase from leaves of Cleome gynandra L. Food Chem. 2011, 129, 1012–1018. [Google Scholar] [CrossRef]
- Navarro, J.L.; Tárrega, A.; Sentandreu, M.A.; Sentandreu, E. Partial purification and characterization of polyphenol oxidase from persimmon. Food Chem. 2014, 157, 283–289. [Google Scholar] [CrossRef]
- Altunkaya, A.; Gökmen, V. Effect of various inhibitors on enzymatic browning, antioxidant activity and total phenol content of fresh lettuce (Lactuca sativa). Food Chem. 2008, 4, 1173–1179. [Google Scholar] [CrossRef]
- Ali, H.M.; El-Gizawy, A.M.; El-Bassiouny, R.E.I.; Saleh, M.A. Browning inhibition mechanisms by cysteine, ascorbic acid and citric acid, and identifying PPO-catechol-cysteine reaction products. J. Food Sci. Technol. 2014, 52, 3651–3659. [Google Scholar] [CrossRef]
- Yu, K.; Zhou, L.; Sun, Y.; Zeng, Z.; Chen, H.; Liu, J. Anti-browning effect of Rosa roxburghii on apple juice and identification of polyphenol oxidase inhibitors. Food Chem. 2021, 359, 129855. [Google Scholar] [CrossRef]
- Yee, W.; Chen, L.; Wong, W. Inhibitory effect of chemical and natural anti-browning agents on polyphenol oxidase from ginger (Zingiber officinale Roscoe). J. Food Sci. Technol. 2018, 55, 3001–3007. [Google Scholar]
- Gawlik-Dziki, U.; Szymanowska, U.; Baraniak, B. Characterization of polyphenol oxidase from broccoli (Brassica oleracea var. botrytis italica) florets. Food Chem. 2007, 105, 1047–1053. [Google Scholar] [CrossRef]
- Aydemir, T. Partial purification and characterization of polyphenol oxidase from artichoke (Cynara scolymus L.) heads. Food Chem. 2004, 87, 59–67. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.; Liu, P.; Meng, A.; Deng, L.; Xue, W.; Chen, F.; Che, Z. Comparative study of the biochemical properties of membrane-bound and soluble polyphenol oxidase from Prunus mume. LWT 2022, 171, 114156. [Google Scholar] [CrossRef]
- Sun, J.; You, Y.; García-García, E.; Long, X.; Wang, J. Biochemical properties and potential endogenous substrates of polyphenoloxidase from chufa (Eleocharis tuberosa) corms. Food Chem. 2010, 118, 799–803. [Google Scholar] [CrossRef]
- Benaceur, F.; Gouzi, H.; Meddah, B.; Neifar, A.; Guergouri, A. Purification and characterization of catechol oxidase from Tadela (Phoenix dactylifera L.) date fruit. Int. J. Biol. Macromol. 2019, 125, 1248–1256. [Google Scholar] [CrossRef]

| Step | Total Activity [U] | Total Protein [mg] | Specific Activity [mU/mg] | Yield [%] | Purification Fold |
|---|---|---|---|---|---|
| Crude extract | 0.35 | 65.79 | 5.29 | 100 | 1 |
| Salting out | 0.17 | 25.8 | 6.43 | 47.65 | 1.21 |
| Sephadex G-75 | 0.05 | 0.65 | 75.65 | 14.21 | 14.3 |
| Inhibitor | IC50 | |
|---|---|---|
| Pyrocatechol | Endogenous Substrate | |
| Chemical compounds | [%] | |
| L-cysteine | 0.00085 ± 0.00002 k | 0.34 ± 0.01 c |
| kojic acid | 0.00043 ± 0.00001 k | 0.0067 ± 0.0002 k |
| ascorbic acid | 0.00053 ± 0.00001 k | 0.026 ± 0.007 jk |
| Plant infusions | [%] | |
| basil (Ocimum basilicum L.) | 0.22 ± 0.01 de | 0.21 ± 0.01 e |
| lovage (Levisticum officinale) | 0.09 ± 0.003 hi | 0.09 ± 0.004 i |
| oregano (Origanum vulgare) | 0.15 ± 0.007 fg | 0.04 ± 0.002 j |
| marjoram (Origanum majorana L.) | 0.13 ± 0.005 fg | 0.16 ± 0.07 f |
| orange peel (Citrus sinensis) | 0.14 ± 0.002 fg | 0.11 ± 0.03 gh |
| lemon peel (Citrus limon) | 0.24 ± 0.01 d | na. |
| dried parsley leaves (Petroselinum crispum) | 0.58 ± 0.02 b | na. |
| fresh parsley leaves (Petroselinum crispum) | dose-independent | na. |
| chamomile (Matricaria chamomilla) | dose-independent | na. |
| wheat bran from common wheat (Triticum aestivum L.) | 1.06 ± 0.045 a | na. |
| Pyrocatechol as a Substrate | |||||
|---|---|---|---|---|---|
| Inhibitor (IC50 Value) | Vmaxi [mU] | Kmi [mM] | Vmax [mU] | Km [mM] | Mode of Inhibition |
| L-cysteine | 0.87 | 174 | 0.91 | 21.4 | competitive |
| kojic acid | 0.38 | 20 | 0.61 | 12.5 | mixed |
| ascorbic acid | 1.37 | 202 | 1.39 | 52.4 | competitive |
| basil infusion | 1.71 | 8.72 | 2.08 | 8.16 | mixed |
| lovage infusion | 2.03 | 27.8 | 2.05 | 11.11 | mixed |
| oregano infusion | 1.77 | 8.28 | 1.74 | 5.42 | competitive |
| wheat bran infusion | 2.39 | 3.35 | 2.45 | 3.17 | competitive |
| lemon peel infusion | 2.21 | 15.96 | 2.16 | 12.77 | non-competitive |
| orange peel infusion | 625 | 3.2 | 714 | 3.6 | uncompetitive |
| marjoram infusion | 0.37 | 5.38 | 0.66 | 2.13 | mixed |
| parsley infusion | 1.12 | 9.07 | 3.93 | 25.18 | uncompetitive |
| endogenous substrate | |||||
| U | mg GAE/mL | U | mg GAE/mL | ||
| L-cysteine | 0.104 | 17.24 | 0.067 | 4.5 | competitive |
| kojic acid | 0.0067 | 1.85 | 0.067 | 4.5 | non-competitive |
| ascorbic acid | 0.034 | 2.94 | 0.067 | 4.5 | competitive |
| lovage infusion | 0.0046 | 0.57 | 0.0098 | 0.625 | non-competitive |
| oregano infusion | 0.0059 | 0.46 | 0.0098 | 0.61 | uncompetitive |
| orange peel infusion | 0.0083 | 0.59 | 0.0098 | 0.0625 | competitive |
| Inhibitor | Relative Activity [%] * | ||
|---|---|---|---|
| Metal Ions | Concentration (in the Reaction Mixture) [mM] | ||
| 0.5 | 1 | 2 | |
| MgCl2 | 72 ± 4 g | 100 ± 5 efg | 123 ± 6 defg |
| KCl | 82 ± 4 fg | 100 ± 5 fg | 130 ± 6 defg |
| ZnCl2 | 97 ± 5 fg | 123 ± 6 defg | 197 ± 10 bcd |
| FeCl2 | 73 ± 4 g | 178 ± 24 cde | 266 ± 13 ab |
| CuCl2 | 118 ± 6 defg | 210 ± 10 bc | 316 ± 16 a |
| NaCl | 83 ± 4 fg | 112 ± 6 fg | 125 ± 6 defg |
| CaCl2 | 79 ± 4 fg | 115 ± 6 efg | 161 ± 8 cdef |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierocka, M.; Świeca, M. Natural Inhibitors of the Polyphenol Oxidase Activity Isolated from Shredded Stored Iceberg Lettuce (Lactuca sativa L.). Appl. Sci. 2024, 14, 9980. https://doi.org/10.3390/app14219980
Sierocka M, Świeca M. Natural Inhibitors of the Polyphenol Oxidase Activity Isolated from Shredded Stored Iceberg Lettuce (Lactuca sativa L.). Applied Sciences. 2024; 14(21):9980. https://doi.org/10.3390/app14219980
Chicago/Turabian StyleSierocka, Małgorzata, and Michał Świeca. 2024. "Natural Inhibitors of the Polyphenol Oxidase Activity Isolated from Shredded Stored Iceberg Lettuce (Lactuca sativa L.)" Applied Sciences 14, no. 21: 9980. https://doi.org/10.3390/app14219980
APA StyleSierocka, M., & Świeca, M. (2024). Natural Inhibitors of the Polyphenol Oxidase Activity Isolated from Shredded Stored Iceberg Lettuce (Lactuca sativa L.). Applied Sciences, 14(21), 9980. https://doi.org/10.3390/app14219980






