Abstract
This study investigated Portulaca oleracea (purslane) as a potential antioxidant supplement in cooked sausages, focusing on its effects on lipid oxidation, fatty acid composition, and antioxidant activity. The fatty acid profile of the sausages enriched with 1.2% purslane powder revealed a 1.3-fold increase in alpha-linolenic acid (ALA), an essential omega-3 fatty acid. Improved oxidative stability during refrigerated storage was observed, with peroxide values of 10.9 meq/kg in the sausages with purslane by day 10 compared with 12.5 meq/kg in the control sausages. The thiobarbituric acid (TBA) values, reflecting lipid peroxidation, were also significantly lower in the sausages with purslane. The antioxidant capacity of the sausages containing purslane was significantly enhanced, demonstrating a ferric-reducing antioxidant power (FRAP) of 13.5 mg GAE/g, whereas the control sausages showed undetectable FRAP levels. Additionally, the DPPH radical-scavenging activity in the sausages with purslane was 21.70% compared with 13.73% in the control. These findings suggest that purslane improves the nutritional profile of meat products by increasing beneficial fatty acids while providing substantial protection against oxidative spoilage. Purslane offers a promising natural alternative to synthetic antioxidants, enhancing the shelf life and quality of processed meats.
1. Introduction
Cardiovascular diseases (CVDs) remain one of the predominant causes of death worldwide, emphasizing the urgent need for effective preventive measures within contemporary healthcare systems. According to the World Health Organization, CVDs are responsible for approximately 17.9 million deaths annually, representing approximately 32% of global mortality rates [1]. Hyperlipidemia, marked by elevated concentrations of lipids in the bloodstream, is widely acknowledged as a major contributor to the development of cardiovascular complications [2]. Targeting serum cholesterol reduction, a critical element of blood lipids, is a well-established method for minimizing cardiovascular risk and managing related metabolic disorders such as diabetes and obesity [3]. Extensive clinical trials and observational studies have repeatedly confirmed that lifestyle modifications, notably the integration of regular physical activity and tailored dietary practices, play a pivotal role in optimizing lipid profiles. These strategies are associated with decreased levels of total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglycerides (TGs) and elevated levels of high-density lipoprotein cholesterol (HDL-C) [4].
Approximately a decade ago, the primary interventions for reducing plasma LDL-C levels were predominantly limited to dietary adjustments and pharmacological treatments, with statins being the cornerstone of lipid-lowering therapy [5]. Although statins have proven highly effective in lowering LDL-C levels and reducing cardiovascular events, their long-term use has been linked to adverse effects, including myopathy and a heightened risk of diabetes [6]. Consequently, there has been a growing interest within the scientific community in exploring nutraceuticals and functional foods as alternative approaches for managing hyperlipidemia with a better safety profile [7]. This shift has been particularly noticeable in Mediterranean regions, where research into plant-derived bioactive compounds has gained momentum due to their potential cardiovascular benefits [8]. Functional foods, which offer health-promoting properties beyond basic nutritional value, have thus garnered significant attention for their potential to support both preventive and therapeutic strategies in cardiovascular care.
The rising incidence of non-communicable diseases (NCDs), particularly cardiovascular disorders, has significantly amplified the need for natural and sustainable alternatives to traditional pharmacotherapies. Among these, omega-3 fatty acids have garnered considerable scientific attention due to their well-documented cardioprotective effects. Extensive epidemiological research has revealed that omega-3 supplementation can lead to a notable decrease in cardiovascular events such as mortality, non-fatal myocardial infarction, and non-fatal stroke [9]. Specifically, daily consumption of 1.5 to 3 g of alpha-linolenic acid (ALA) has been associated with a marked reduction in cardiovascular-related mortality [10]. While dietary intake of omega-3 remains the ideal approach, supplementation serves as a viable solution for populations with limited access to these crucial fatty acids via their regular diet [11].
In this regard, Portulaca oleracea (commonly referred to as purslane) has attracted significant attention as a potential functional food. Belonging to the Portulacaceae family, which includes over 120 species predominantly composed of succulent herbs and shrubs, Portulaca oleracea stands out for its remarkable adaptability and resilience in adverse environmental conditions, such as saline and nutrient-deficient soils. This makes it a promising and sustainable crop, particularly in regions grappling with the effects of climate change and soil degradation [12]. Additionally, its high seed viability and competitive growth advantage enhance its suitability as a functional plant with substantial nutritional and therapeutic potential [13].
Historically, Portulaca oleracea has been utilized as a dietary component and in traditional medicinal practices, primarily due to its abundant profile of bioactive compounds [14]. These bioactives include a wide array of flavonoids, alkaloids, organic acids, vitamins, minerals, sterols, and essential fatty acids, contributing to Portulaca oleracea’s considerable nutritional and medicinal value [15]. Its high concentration of alpha-linolenic acid (ALA), an essential omega-3 fatty acid comprising an eighteen-carbon chain with three cis-double bonds, is of particular interest. Alpha-linolenic acid (ALA) is essential for maintaining human health, as the body cannot produce it naturally. Therefore, it must be sourced from dietary intake. ALA is fundamental to processes related to human development, growth, and the prevention of various diseases [16]. Furthermore, ALA is a precursor to longer-chain omega-3 fatty acids such as eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA), although the conversion rate is limited and inefficient [17]. Nevertheless, these long-chain omega-3 fatty acids are renowned for their antioxidant and anti-inflammatory properties, conferring numerous health benefits [18].
The abundant concentration of long-chain polyunsaturated fatty acids (PUFAs) in purslane positions it as a noteworthy alternative source of these critical nutrients, with promising implications for managing hyperlipidemia [19]. An increasing body of research has validated the effectiveness of purslane supplementation in modulating lipid profiles, showing marked reductions in total cholesterol, LDL-C, and triglyceride levels, alongside elevations in HDL-C levels [20,21]. Furthermore, the bioactive constituents of this plant have exhibited beneficial influences on glucose regulation, highlighting its potential therapeutic value for individuals managing diabetes [22].
In light of the growing consumer interest in functional foods and dietary supplements enriched with omega-3 fatty acids, Portulaca oleracea emerges as a viable option for addressing both nutrient insufficiencies and chronic diseases. Its integration into processed foods, particularly sausages, presents an opportunity to significantly elevate the nutritional profile of such products while providing cardiovascular health benefits [23]. This approach is in line with the modern shift toward healthier, more functional food alternatives, as consumers increasingly gravitate away from traditional processed foods that are often criticized for their high levels of saturated fats and sodium.
Zidan et al.’s study (2014) investigated the impact of lyophilized aqueous extracts from purslane on serum HDL and lecithin levels using a rat model subjected to a cholesterol-enriched diet [14]. The results demonstrated that supplementation with purslane alleviated triglyceridemia and cholesterolemia while enhancing reverse cholesterol transport, indicating its potential anti-atherogenic properties [24]. Integrating Portulaca oleracea into common dietary staples presents a novel approach for boosting the intake of cholesterol-lowering bioactive compounds, thereby supporting healthier lipid profiles and promoting cardiovascular health [25].
Purslane is also a rich source of bioactive compounds including alkaloids, catecholamines, organic acids, anthocyanins, and flavonoids [26,27]. These compounds possess significant antioxidant, anti-inflammatory, and antimicrobial activities, which offer health benefits while improving food preservation [28]. The antioxidant capabilities of these compounds may also be crucial in reducing oxidative stress within processed foods, thereby prolonging shelf life and improving product safety.
Consequently, this study aimed to assess the chemical composition of meat products enriched with Portulaca and investigate its viability as an alternative or complementary ingredient for the food industry, especially in creating functional foods. This research’s outcomes could facilitate the broader incorporation of Portulaca into contemporary dietary approaches, meeting both nutritional needs and health-related challenges.
2. Materials and Methods
2.1. Materials
The sausages were produced using standard methods and processes, such as grinding the meat, mixing it with spices and additives, stuffing it into casings, and cooking. The sausages were produced from premium and first-grade beef. The spices used were cardamom, pepper, salt, and purslane powder. The purslane extract powder was added during the grinding process in an amount of 1.2% of the total sausage mixture mass. To obtain the extract, an alcoholic extraction from Portulaca oleracea L. was used. Five grams of crushed raw material, with particle sizes passing through a 1 mm sieve, were placed in a 100 mL ground-glass flask, and 50 mL of solvent (96% ethanol) was added. After cooling the extraction to room temperature, it was filtered through cotton and a folded paper filter.
The stock solution for the extract was prepared by dissolving 500 mg of dry extract in 1 mL of ethanol to obtain an alcoholic extract, resulting in a final concentration of 500 mg/mL. After lyophilization, the extract was milled to achieve particle sizes capable of passing through a sieve with mesh openings of 100–150 microns (µm). This particle size provided sufficient powder dispersion, enabling uniform integration with other components of the sausage matrix and ensuring active interaction within the final product. This amount was selected because our pilot study showed that a higher amount of purslane powder negatively affects the organoleptic properties of the final product (imparting a bitter taste). Thermal processing was carried out until the internal temperature of the sausages reached 72 °C (Figure 1). The samples were produced at the experimental facility of Saken Seifullin Kazakh Agro Technical Research University.
Figure 1.
The sausage with purslane (a) and without purslane (b).
The sensory evaluation of the sausages was conducted according to a standard sensory evaluation methodology. The intensity of 20 descriptors was evaluated using a 10-point linear structured scale by 15 trained panelists. Based on the obtained data, the results demonstrated that sausages supplemented with purslane exhibited a fresh taste and a slightly darker color, with the hue more closely resembling that of natural cooked meat.
2.2. Determination of Fatty Acid Composition
The methyl esters of fatty acids were analyzed using an Agilent 7890 gas chromatograph (Agilent Technologies, Andover, MA, USA) equipped with a flame ionization detector and an HP-Innowax capillary column (60 m × 0.32 mm × 0.5 µm) under nitrogen flow. The temperature gradient was set from 100 to 260 °C at a rate of 10 °C/min. A 1 µL sample was injected, with a split ratio of 1:100, and the detector temperature was maintained between 250 and 300 °C. A Supelco No. 47885U standard mixture of fatty acid methyl esters (C6–C24) was used as a reference, with automatic data processing. Quantitative analysis of the fatty acids was conducted using the internal standard method.
2.3. Determination of Color Characteristics
The samples’ color properties were analyzed with a Konica Minolta CM-2300d spectrophotometer, following the methodology described in the study by Iftikhar et al. [29], which evaluated antioxidant potential in minced beef meat. Before the measurements, the spectrophotometer underwent both zero and white calibration according to the manufacturer’s guidelines. The final result for each sample was derived as the arithmetic mean of the five measurements.
2.4. Analysis of Molecular Weight Distribution of Protein Fractions in Samples Carried Out Using One-Dimensional Electrophoresis
A 100 mg sample was mixed with 500 μL of a lysis buffer (4.5 M urea, 2.5% β-mercaptoethanol, 1% Triton X-100, and 1% ampholytes, with pH 3–10) and centrifuged at 14,000 rpm for 20 min. The supernatant was carefully separated, and an equal volume of protein buffer was added to the mixture. The protein buffer was prepared by combining 1 mL of 10% sodium dodecyl sulfate (SDS), 250 μL of concentrated β-mercaptoethanol, 625 μL of 0.5 M Tris-HCl, 1.5 g of urea, and bromophenol blue as a dark stain. The total volume of the solution was adjusted to 5 mL with distilled water. Following this, the samples were heated in a boiling water bath for 5 min. Protein visualization was achieved using Coomassie G-250 staining. The staining solution consisted of 10% acetic acid, 25% isopropanol, and 0.05% Coomassie G-250. To eliminate excess unbound dye, 10% acetic acid was used as a washing solution. One-dimensional electrophoresis gels in their wet state were utilized for densitometric analysis. High-resolution digital images were captured using a Bio-5000 Plus scanner (Serva, Heidelberg, Germany) at 600 ppi in 2D-RGB mode. The obtained digital images were subsequently refined using graphic editing software for further analysis. The version number of ImageJ was 1.53t, and it was accessed via the official website (https://imagej.net/ij/) on 20 August 2024.
2.5. Determination of Ferric-Reducing Antioxidant Power (FRAP) and Antioxidant Activity Using 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
The ferric-reducing antioxidant power (FRAP) assay was conducted using BHT and α-tocopherol as standard antioxidants. To perform the assay, 1 mL of the extract at varying dilutions was combined with 2.5 mL of phosphate buffer (0.1 M; pH 6.6) and 2.5 mL of potassium ferricyanide solution (1%, w/v). The mixture was incubated at 50 °C for 20 min. Following incubation, 2.5 mL of trichloroacetic acid solution (10%, w/v) was added. From this mixture, 2.5 mL was withdrawn and combined with 2.5 mL of deionized water and 0.5 mL of ferric chloride solution (0.1%, w/v). The solution was allowed to stand for 30 min before the absorbance was measured at 700 nm. The FRAP values were expressed as milligrams of gallic acid equivalents (GAEs) per gram of dry extract (mg GAE/g).
For the DPPH radical-scavenging activity assay analysis, 2 mL of a DPPH solution (0.1 mg/mL in methanol) was mixed with 2 mL of the sample solutions at a concentration of 200 μg/mL. All analyses were performed in triplicate. The reaction mixture was shaken and incubated in the dark at room temperature for 30 min. After incubation, the absorbance was measured at 517 nm against a blank. Ascorbic acid was prepared as a positive control in a similar manner, with the antioxidant solution being replaced accordingly.
2.6. Statistical Analyses
The effects of the purslane addition and storage time were assessed using general linear model (GLM) (SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA). Tukey’s multiple comparison test was used in GLM analyses to compare the means between different storage times.
3. Results and Discussion
3.1. Analysis of Fatty Acid Composition Results
Lipid oxidation in meat products frequently results in a decline in consumer quality, affecting key attributes such as flavor, aroma, color, texture, and both nutritional and biological value while also reducing the shelf life. Furthermore, lipid oxidation products pose significant health risks due to their mutagenic, carcinogenic, and cytotoxic effects on humans [30]. The progression of oxidative processes in fats, influenced by the type of fat and storage conditions, can lead to a noticeable degradation in the organoleptic properties of the product. This deterioration is further exacerbated by alterations in the fats during storage, which subsequently diminishes the meat’s nutritional value. The formation of carbonyl compounds during fat oxidation also promotes carbonyl–amine reactions, altering the color of dried meat and adversely impacting overall quality. These oxidative reactions are accelerated by increased temperature, light exposure, and the presence of catalysts such as meat pigments. Lipid oxidation can proceed via both enzymatic and non-enzymatic pathways, with the oxidation rate largely governed by the degree of unsaturation in fatty acids. This rate escalates with the number of double bonds in the fatty acid structure, making polyunsaturated fats particularly susceptible to oxidative degradation. Given this, this study’s primary interest was to evaluate the total amount of fatty acids in sausages with purslane to understand the oxidation processes while considering the quantity of unsaturated fatty acids. Table 1 presents the main fatty acid compositions of the studied samples.

Table 1.
Fatty acid compositions of sausage samples with addition of purslane.
Only 22 of the 36 fatty acids analyzed could be quantified. The sausages with purslane exhibited a slightly higher proportion of saturated fatty acids than did the control sample (29.0% versus 28.6%). Although the difference in the saturated fatty acid content was marginal, at just 0.4%, it is noteworthy that the control sample contained two additional fatty acids that were absent in the sausages with purslane. Similarly, there was virtually no difference in the levels of monounsaturated fatty acids between the samples. Both the control and sausages with purslane contained nearly identical amounts, at 38.4% and 38.3%, respectively. No significant variations in individual monounsaturated fatty acids were observed, except for gondoic acid, which was present at 0.5% in the sausages with purslane and 1% in the control sample, and erucic acid, which was detected at 0.3% in the control but was not detected in the sausages with purslane. The omega-3 fatty acid content exhibited the most pronounced difference between the samples. The incorporation of purslane into the product led to a nearly 1.3-fold increase in linolenic acid levels. The levels of omega-6 fatty acids, like the monounsaturated fatty acids, showed minimal variation between the control and experimental samples, at 31.6% and 30.8%, respectively. Furthermore, no trans fatty acid isomers (such as elaidic and linolelaidic acids) were detected in either sample.
The overall level of the total unsaturated fatty acid content in the control sausage sample was marginally, yet noticeably, higher compared with the sausages containing Portulaca oleracea (purslane). This disparity could have implications for the shelf life and the susceptibility of fats to oxidative spoilage during storage, as unsaturated fatty acids, characterized by double or triple bonds between adjacent carbon atoms, are more prone to oxidation. In contrast, the inclusion of purslane in the sausage formulation exerted a stabilizing effect on the oxidation process, as evidenced by the evaluation of oxidative spoilage indices, which are detailed later in this report. Additionally, the sausages with purslane exhibited higher levels of stearic acid, a saturated fatty acid. Synthetic antioxidants are extensively employed in the meat industry to prevent the oxidation of lipids and proteins. However, oxidative reactions during the production, retail, and storage phases of meat and meat products induce undesirable physicochemical alterations and off-flavors, detrimentally impacting product quality. These changes often lead to consumer dissatisfaction and potential economic losses. One common strategy to address this issue has been the use of synthetic antioxidants. However, with rising consumer awareness of health concerns and a growing preference for natural additives, the demand for natural alternatives to synthetic antioxidants has intensified [31]. This study aimed to assess the protective effects of Portulaca oleracea extract against lipid and protein oxidation in meat during refrigerated storage over 10 days. The progression of oxidative spoilage in cooked products is presented in Table 2, Table 3 and Table 4.

Table 2.
Fat and protein oxidation dynamics (baseline) in products during storage, with accumulation of peroxide value.

Table 3.
Fat oxidation dynamics, with accumulation of thiobarbituric number in sausages during storage.

Table 4.
Fat oxidation dynamics, with accumulation of acid number in sausages during storage.
The accumulation of peroxide values occurred at a relatively fast rate, likely driven by autoxidation. Autoxidation refers to the reaction between oxygen and unsaturated lipids, leading to the formation of hydroperoxides, which subsequently undergo further transformations. Since the accumulation of hydroperoxides is measured according to the peroxide value, their levels initially rise and then begin to decrease, as was observed in the sausages containing Portulaca oleracea starting from day 10 of storage. The peroxide value in the sausages with purslane reached 10.1 meq/kg on day 8 and had only slightly increased to 10.9 meq/kg by day 10. In contrast, the oxidation and accumulation of peroxides were more pronounced in the control samples, with values rising from 8.6 meq/kg on day 8 to 12.5 meq/kg on day 10. This difference may be attributed to the antioxidant properties of purslane, which appeared to have a stabilizing effect, or to a reduction in oxidation-initiating compounds during the first six days of storage. The rate of peroxide accumulation significantly slowed after day 6.
The extent of oxidative degradation in the fat fraction of the sausages during refrigerated storage was further assessed via the thiobarbituric acid (TBA) value. The progression and severity of oxidative changes in the fat phase of meat products are positively correlated with the TBA value, which quantifies the presence of malondialdehyde. The relative stability of malondialdehyde allows the accumulation of secondary oxidation products to be accurately evaluated. Malondialdehyde formation is regarded as an adverse outcome of lipid peroxidation, stemming from the breakdown of polyunsaturated fatty acids under the influence of free radicals, a process often associated with the development of undesirable off-flavors [32]. The data on the malondialdehyde contents in the studied sausage formulations are presented in Table 3.
A gradual increase in TBA values was observed across all the tested sausage formulations during sausage storage. The accumulation of malondialdehyde, relative to its initial concentration, resulted in a 4-fold increase in the control sausages by the 10th day of storage, while the sausages containing Portulaca oleracea (purslane) showed a 3.7-fold increase over the same period. Despite this rise, the absolute TBA values for all tested products remained below 0.5 mg/kg, indicating that the lipid fraction remained relatively stable throughout the storage period. Additionally, the differences between TBA values in control sausages and sausages with purslane were not significant (p > 0.05). TBA concentrations exceeding 0.5 mg/kg indicate the onset of oxidation, while values surpassing 1.0 mg/kg indicate significant oxidative degradation [32]. The accumulation dynamics of the acid value (AV) in fats demonstrated signs of oxidative changes only after 8 days of storage. However, the rate of AV increase in the experimental sausages containing purslane was lower than in the control samples. The AV in the sausages with purslane was slightly higher than in the control until the 6th day of storage, after which the rate of fat spoilage significantly accelerated in the samples without purslane. Based on this, it can be concluded that the inclusion of purslane extract in sausage formulations notably impacted the rate and extent of lipid oxidation, demonstrating its potential as an effective antioxidant in meat products.
In addition to lipid oxidation, proteins in meat products are also vulnerable to oxidative processes. One of the most commonly employed methods for assessing protein oxidation is the measurement of carbonyl derivatives. The total content of carbonyl compounds extracted from the protein matrices of the control sausages and the sausages with purslane was evaluated in this study. This assessment was conducted only during the initial days of storage. Unfortunately, due to the lack of extended storage studies, it was not possible to monitor the accumulation of carbonyl compounds over time to fully examine purslane’s role as an antioxidant and stabilizer in mitigating protein oxidation. However, the control sausages displayed higher levels of carbonyl compounds than those with purslane supplementation even in the early stages of storage. The presence of a higher proportion of unsaturated fatty acids and sulfur-containing amino acids in animal muscles leads to an elevated susceptibility of both lipids and proteins to oxidative processes. Lipid oxidation, in particular, is a critical factor impacting the quality of meat products, as it contributes to color changes, the development of off-flavors and odors, and the formation of toxic compounds, all of which pose potential risks to human health [33]. Moreover, the oxidation of both lipids and proteins can lead to undesirable changes in meat products from both a technological and sensory standpoint, including discoloration, defects in texture, diminished nutritional value, and negative health implications [34].
Various essential oils are being investigated for their strong antioxidant properties as natural alternatives to synthetic antioxidants used in the meat industry. Many of these oils are classified as generally recognized as safe (GRAS) and have demonstrated beneficial effects on meat products when used alone or in combination with other essential oils, ingredients, or preservation technologies. These oils’ effectiveness depends on various factors, such as the concentration, potential synergistic effects, and extraction method. While steam distillation remains the most widely used method for extracting essential oils industrially, new extraction technologies have been developed to overcome the limitations of traditional methods, enabling the production of higher-quality essential oils [35]. Portulaca oleracea extract is known for its potent antioxidant activity, attributed to its high concentration of polyphenols, short-chain organic acids, and saccharides. As a natural antioxidant, purslane offers several advantages over synthetic antioxidants, including superior stability, non-toxicity, and medicinal properties. Additionally, it is a low-calorie food with numerous therapeutic benefits [36,37,38]. Using dried purslane further enhances its shelf life and maintains its usability, as this form retains a greater proportion of its beneficial properties compared with extracts, whether water- or solvent-based. This makes dried purslane a valuable ingredient for improving the nutritional and functional properties of meat products.
3.2. Analysis of Color Characteristic Results
The following values for the main color characteristics were obtained in the study on stability, before and after light exposure, based on the indicators of cooked sausages with purslane: L—lightness, a—redness, and b—yellowness. The data are presented in Table 5.

Table 5.
The dynamics of fat oxidation, with the accumulation of acid number in sausages during storage.
Generally, the addition of purslane to the sausages did not alter lightness or yellowness (p > 0.05). The lower redness observed in sausages with purslane could be due to the presence of chlorophyll, which may interact with meat pigments. The color characteristics of the meat products undergoing thermal processing (heating to 70–72 °C), the addition of chemical agents (as per the standard recipe), and storage were assessed to determine their overall color stability. The results indicated no significant changes in lightness, redness, and yellowness values (p > 0.05 for all). The stable color formation in the sausages with purslane, which possesses potent antioxidant properties, effectively prevent the oxidation of structural protein components that would otherwise contribute to color degradation.
3.3. Analysis of Molecular Weight Distribution of Protein Fractions in Samples Using One-Dimensional Electrophoresis
The analysis of the molecular weight distribution of protein fractions in the samples was performed via one-dimensional electrophoresis in polyacrylamide gel, which yielded the results shown in Figure 2.
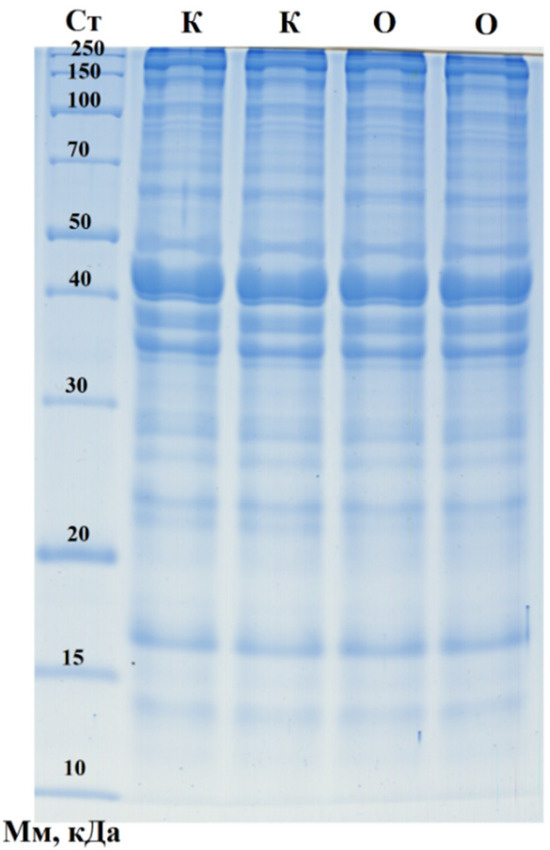
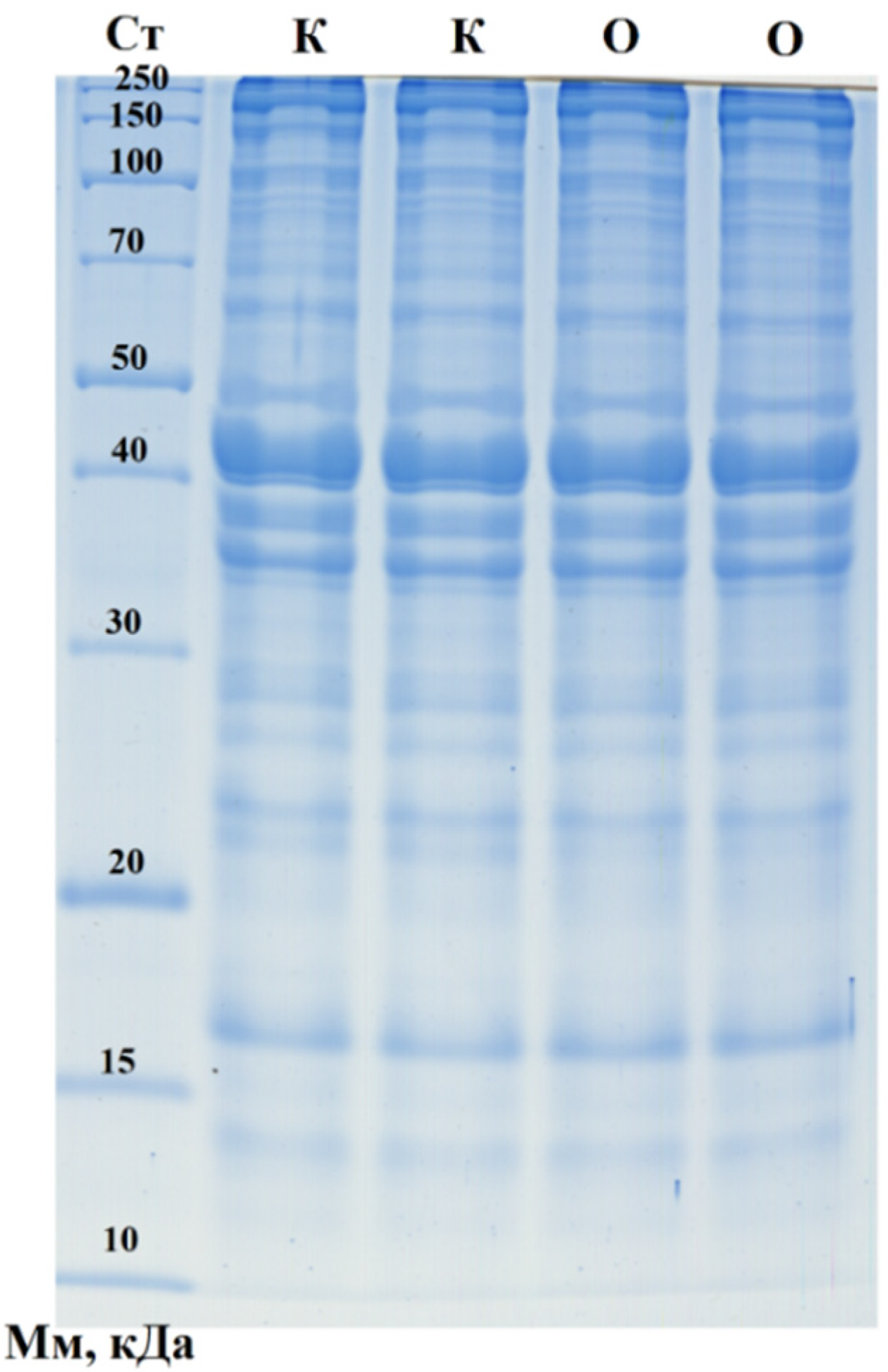
Figure 2.
One-dimensional electropherogram of sausage samples. CT—molecular weight standards: 250, 150, 100, 70, 50, 40, 30, 20, 15, and 10 kDa (from top to bottom); K—control sausages; O—sausages with purslane.
A broad range of protein fractions was identified, including key structural proteins, with no significant differences observed between the control sausages and those containing Portulaca oleracea (purslane). Several protein bands likely corresponded to fragments of major proteins, such as NADH dehydrogenase (21.7 kDa), frataxin (23.5 kDa), NADH dehydrogenase subunit K (31.1 kDa), myozenin-1 (31.6 kDa), and alpha-1D tubulin chain (50.2 kDa), which were more prominently expressed in the control sample. Additionally, other protein fractions were well represented, including fragments of cofilin (18.5 kDa), cathepsin B (36.6 kDa), cysteine protease (39.9 kDa), RNA-binding protein (40.0 kDa), cyclin-dependent kinase (42.7 kDa), actin (42.0 kDa), serine/threonine protein kinase (49.0 kDa), ribonuclease (64.2 kDa), heat-shock protein (70.2 kDa), catenin beta-1 (85.5 kDa), spondin-1 (90.9 kDa), desmocollin 2 (100.4 kDa), histidine-rich calcium-binding protein (104.6 kDa), and supervillin (244.8 kDa). Moreover, the densitometric analysis revealed two distinct proteins unique to the purslane family, identified in the sausages containing purslane (Table 6). These findings suggest purslane’s potential influence on the product’s protein composition, although the overall protein profile between the two sausage types remained largely similar.

Table 6.
Proteins identified as belonging to Portulaca.
Two plant-associated proteins (enzymes) were identified in the sausages containing Portulaca oleracea—namely 4,5-DOPA dioxygenase extradiol and maturase K—using computer-assisted densitometry.
4,5-DOPA dioxygenase extradiol plays a crucial role in the formation of betalamic acid, which functions similarly to anthocyanins in other plants, serving as a structural, chromophoric, and bioactive component of plant betalain pigments [39]. This enzyme catalyzes the oxidative cleavage of DOPA, producing 4,5-secodopa, which spontaneously cyclizes to form betalamic acid. Betalains serve as physiological pigments while also possessing strong radical-scavenging activity [40]. These water-soluble pigments are unique to plants in the Caryophyllaceae family and are simpler to synthesize and regulate than anthocyanins and carotenoids, beginning from tyrosine and regulated primarily by the MYB transcription factor [41]. Functionally, betalains are analogous to anthocyanins, attracting animal pollinators and exhibiting high antioxidant and free radical-scavenging properties [42]. In addition, betalains are used commercially as food colorants and additives, further highlighting their practical value [43].
Maturase K (matK) is a plastid gene found in plants and is considered one of the most variable coding genes in angiosperms. It has been proposed as a molecular “barcode” for identifying terrestrial plants [44]. Given its variability, matK could potentially serve as a marker to identify the addition of Portulaca oleracea in food products, though further research is necessary to confirm this hypothesis [45]. While plant mitochondrial genes evolve slowly and are less effective in distinguishing between plant species, matK offers a more promising alternative for plant identification [46].
The inclusion of purslane in meat products is thus a functionally justified approach, as it helps reduce oxidative lipid spoilage under various environmental conditions. This natural additive provides antioxidant protection without the need for synthetic stabilizers or chemical antioxidants in the product formulation, making it a valuable and sustainable ingredient.
3.4. Evaluation of Antioxidant Capacity: Ferric-Reducing Antioxidant Power (FRAP) and 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay Results
Exploring the antioxidant potential of plant extracts holds considerable relevance for the advancement of novel, safer natural antioxidants in the food industry. Within this framework, the subsequent phase of this study concentrated on evaluating the ferric-reducing antioxidant power (FRAP) and DPPH radical-scavenging activity, as presented in Table 7. This analysis provides critical insights into the efficacy of the extracts in neutralizing oxidative processes, thus offering valuable contributions to the development of functional food ingredients.

Table 7.
Ferric-reducing antioxidant power (FRAP) and antioxidant activity (DPPH).
This study demonstrated substantial levels of ferric-reducing antioxidant power (FRAP) in the experimental sausage samples. The incorporation of purslane into the sausage formulations enhanced the antioxidant properties of the final product. As evidenced by the data in Table 7, the sausages with purslane exhibited a significant FRAP value of 13.5 mg GAE/g, while no measurable FRAP activity was detected in the control samples. This highlights purslane’s ability to markedly improve the product’s capacity for ferric ion reduction, an important indicator of antioxidant potential. Moreover, the antioxidant activity, as evaluated by DPPH radical-scavenging, showed a pronounced improvement in the experimental sample compared with the control. The DPPH radical-scavenging activity in the sausages with purslane reached 21.70%, considerably higher than the 13.73% observed in the control, confirming the purslane-enriched sausages’ enhanced ability to neutralize free radicals. Additionally, the IC50 value for DPPH radical-scavenging activity was greater in the sausages with purslane (116.55 µg/mL) compared with the control (76.12 µg/mL), indicating that a higher concentration of the purslane-enriched sample was required to achieve 50% inhibition of DPPH radicals. This further underscores that the addition of purslane significantly elevated the antioxidant profile of the sausages.
4. Conclusions
Our results conclusively demonstrate that Portulaca oleracea (purslane) is an effective natural additive for enhancing both the antioxidant properties and oxidative stability of cooked sausage products. The inclusion of purslane led to a 1.3-fold increase in omega-3 fatty acids, particularly alpha-linolenic acid (ALA). Moreover, lipid oxidation was significantly mitigated, as evidenced by a lower peroxide value of 10.9 meq/kg versus 12.5 meq/kg in the control by day 10 and a 3.7-fold rise in TBA values compared to a 4-fold increase in the control. The antioxidant capacity of purslane-enriched sausages was further highlighted by a 13.5 mg GAE/g ferric-reducing antioxidant power (FRAP), while the control sausages showed no detectable FRAP activity. Additionally, DPPH radical-scavenging activity improved to 21.70% compared to 13.73% in the control. These findings underscore purslane’s dual role in both nutritionally enriching meat products and reducing oxidative spoilage, presenting a natural alternative to synthetic additives for developing functional foods, maintaining product quality, and extending shelf life.
Author Contributions
Conceptualization, K.M. and T.T.; methodology, G.T.; validation, G.O.; formal analysis, G.Z. (Gulmira Zhakupova); investigation, G.T. and G.Z. (Gulmira Zhakupova); resources, K.M.; data curation, S.A. and A.E.; writing—original draft preparation, K.M.; writing—review and editing, G.Z. (Galia Zamaratskaia); visualization, K.M.; supervision, K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Republic of Kazakhstan (AP14871765).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Cardiovascular Diseases (CVDs) Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 30 September 2024).
- Jain, K.S.; Kathiravan, M.K.; Somani, R.S.; Shishoo, C.J. The biology and chemistry of hyperlipidemia. Bioorg. Med. Chem. 2007, 15, 4674–4699. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.H.; Ballestros, K.; Echko, M.; Glenn, S.; Olsen, H.E.; Mullany, E.; Lee, A.; Khan, A.R.; Ahmadi, A.; Ferrari, A.J. The state of US health, 1990–2016: Burden of diseases, injuries, and risk factors among US states. JAMA 2018, 319, 1444–1472. [Google Scholar] [CrossRef]
- Poli, A.; Visioli, F.; Bianchi, A.; Romeo, F.; Cimaglia, P.; Mambelli, G.; Bernini, W. Nutraceuticals and functional foods for the control of plasma cholesterol levels. Pharmacol. Res. 2018, 134, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Panza, G.; Zaleski, A.; Taylor, B. Statin-associated side effects. J. Am. Coll. Cardiol. 2016, 67, 2395–2410. [Google Scholar] [CrossRef]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and cardiovascular disease: Evidence explained and mechanisms explored. Clin. Sci. 2020, 134, 277–300. [Google Scholar]
- Simopoulos, A.P. Omega-3 fatty acids and cardiovascular disease: The epidemiological evidence. Environ. Health Prev. Med. 2004, 9, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Lemaitre, R.N.; King, I.B.; Song, X.; Huang, H.; Sacks, F.M.; Rimm, E.B.; Wang, M.; Siscovick, D.S. Plasma phospholipid long-chain ω-3 fatty acids and total and cause-specific mortality in older adults: A cohort study. Ann. Intern. Med. 2013, 158, 515–525. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Arampatzis, D.A.; Tsiropoulos, N.G.; Petrović, J.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Seed oil and seed oil byproducts of common purslane (Portulaca oleracea L.): A new insight to plant-based sources rich in omega-3 fatty acids. LWT 2020, 123, 109099. [Google Scholar] [CrossRef]
- Liu, L.; Howe, P.; Zhou, Y.-F.; Hocart, C.; Zhang, R. Fatty acids and β-carotene in Australian purslane (Portulaca oleracea) varieties. J. Chromatogr. A 2000, 893, 207–213. [Google Scholar] [CrossRef]
- Bhuiyan, N.H.; Murakami, K.; Adachi, T. Variation in betalain content and factors affecting the biosynthesis in Portulaca sp. “Jewel” cell cultures. Plant Biotechnol. 2002, 19, 369–376. [Google Scholar] [CrossRef]
- Gu, Y.; Leng, A.; Zhang, W.; Ying, X.; Stien, D. A novel alkaloid from Portulaca oleracea L. and its anti-inflammatory activity. Nat. Prod. Res. 2020, 36, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Zidan, Y.; Bouderbala, S.; Djellouli, F.; Lacaille-Dubois, M.A.; Bouchenak, M. Lyophilized aqueous extract of Portulaca oleracea improves serum high-density lipoproteins and lecithin levels in cholesterol-enriched diet-fed rats. Phytomedicine 2014, 21, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P.; Norman, H.A.; Gillaspy, G.E.; Duke, J.A. Common purslane: A source of omega-3 fatty acids and antioxidants. J. Am. Coll. Nutr. 1992, 11, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Dubois, V.; Breton, S.; Linder, M.; Fanni, J.; Parmentier, M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur. J. Lipid Sci. Technol. 2007, 109, 710–732. [Google Scholar] [CrossRef]
- Hadi, A.; Mohammadi, H.; Hadi, Z.; Roshanravan, N.; Kafeshani, M. Cumin (Cuminum cyminum L.) is a safe approach for management of lipid parameters: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2018, 32, 9–16. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Yalcin, H.; Elibol, M.; Sevim, P.; Bakkal, G. The effects of purslane (Portulaca oleracea L.) on serum lipid profiles and oxidative stress in atherogenic rats. Food Res. Int. 2020, 136, 109558. [Google Scholar] [CrossRef]
- Lin, S.; Huang, X.; Cheng, H.; Yang, L.; Zhang, D.; Zhang, Q.; Shi, J.; Zhang, M. Extraction and purification of alpha-linolenic acid from Portulaca oleracea L. and its effects on lipid profiles and oxidative stress in high-fat diet rats. Nutrients 2020, 12, 1704. [Google Scholar] [CrossRef]
- González, M.; Zarzuelo, A.; Guerra, J.; Menéndez, R.; Burrell, M.A.; Risco, S. Hypoglycemic activity of Portulaca oleracea in normal and diabetic rats. J. Ethnopharmacol. 2014, 93, 461–466. [Google Scholar] [CrossRef]
- Dubois, V.; Parmentier, M.; Baudet, D.; Collinet, A.; Breton, S. Fatty acid content and antioxidant activity of Portulaca oleracea extracts. Food Chem. 2007, 104, 1801–1810. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef]
- Chan, E.; Bailey, D.; Busta, F.; Cohen, R.; Schwarzschild, M. Clinical effects of plant-based lipids on plasma lipoproteins. Clin. Nutr. 2019, 38, 2349–2357. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Peng, D.; Zhang, M.; Zhang, H. Antioxidant activity of Portulaca oleracea polysaccharides and their influence on rice starch digestion. Antioxidants 2020, 9, 1075. [Google Scholar] [CrossRef]
- Rydlewski, B.; Nowakowski, A.; Lewandowska, K.; Kwiatkowska, M. Bioactive compounds in Portulaca oleracea seeds and their effects on oxidative stress in a food matrix. J. Agric. Food Chem. 2017, 65, 177–185. [Google Scholar] [CrossRef]
- Voulgaridou, G.P.; Anestopoulos, I.; Franco, R.; Panayiotidis, M.I.; Pappa, A. Antioxidant and anticancer properties of polyphenolic compounds in foods. Antioxid. Redox Signal. 2011, 15, 2027–2041. [Google Scholar] [CrossRef]
- Iftikhar, A.; Dupas-Farrugia, C.; De Leonardis, A.; Macciola, V.; Moiz, A.; Martin, D. Antioxidant potential of olive leaf (Olea europaea L.) sustainable extracts evaluated in vitro and minced beef meat. Ital. J. Food Sci. 2024, 36, 305–316. [Google Scholar] [CrossRef]
- Cheng, J.H. Lipid Oxidation in Meat. J. Nutr. Food Sci. 2016, 6, 494. [Google Scholar] [CrossRef]
- Pateiro, M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Gurinovich, G.V.; Patrakova, I.S.; Kudryashov, L.S. Study of the effect of curing mixture composition on the lipid oxidation process in meat systems. Meat Syst. 2018, 48, 31–40. [Google Scholar]
- Fan, X.; Liu, S.; Li, H.; He, J.; Feng, J.; Zhang, X.; Yan, H. Effects of Portulaca oleracea L. extract on lipid oxidation and color of pork meat during refrigerated storage. Meat Sci. 2019, 147, 82–90. [Google Scholar] [CrossRef]
- Estévez, M.; Luna, C. Dietary protein oxidation: A silent threat to human health? Crit. Rev. Food Sci. 2017, 57, 3781–3793. [Google Scholar] [CrossRef]
- Šojić, B. Isolation, Bioactive Potential, and Application of Essential Oils and Terpenoid-Rich Extracts as Effective Antioxidant and Antimicrobial Agents in Meat and Meat Products. Molecules 2023, 28, 2293. [Google Scholar] [CrossRef] [PubMed]
- Gatea, F.; Dumitra-Teodor, E.; Maria-Seciu, A.; Nagodă, E.; Lucian-Radu, G. Chemical constituents and bioactive potential of Portulaca pilosa L. vs. Portulaca oleracea L. Med. Chem. Res. 2017, 26, 1516–1527. [Google Scholar] [CrossRef]
- Zhao, R.; Meng, X.; Jia, G.; Yu, Y.; Song, B. Oral pre-administration of Purslane polysaccharides enhance immune responses to inactivated foot-and-mouth disease vaccine in mice. BMC Vet. Res. 2019, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Shi, S.; Jiang, L. Evaluation of antioxidant and immuno-enhancing activities of Purslane polysaccharides in gastric cancer rats. Int. J. Biol. Macromol. 2014, 68, 113–116. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; García-Carmona, F. Characterization of recombinant Beta vulgaris 4,5-DOPA-extradiol-dioxygenase active in the biosynthesis of betalains. Planta 2012, 236, 91–100. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Azuine, M.A.; Sridhar, R.; Okuda, Y.; Tsuruta, A.; Ichiishi, E.; Mukainake, T.; Takasaki, M.; Konoshima, T.; Nishino, H.; et al. Chemoprevention of DMBA-induced UV-B promoted, NOR-1-induced TPA promoted skin carcinogenesis, and DEN-induced phenobarbital promoted liver tumors in mice by extract of beetroot. Pharmacol. Res. 2003, 47, 141–148. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Long, T.; Wang, S.; Yang, J. Regulation Mechanism of Plant Pigments Biosynthesis: Anthocyanins, Carotenoids, and Betalains. Metabolites 2022, 12, 871. [Google Scholar] [CrossRef]
- Jain, G.; Gould, K.S. Are betalain pigments the functional homologues of anthocyanins in plants? Environ. Exp. Bot. 2015, 119, 48–53. [Google Scholar] [CrossRef]
- Christinet, L.; Burdet, F.; Zaiko, M.; Hinz, U.; Zrÿd, J. Characterization and Functional Identification of a Novel Plant 4,5-Extradiol Dioxygenase Involved in Betalain Pigment Biosynthesis in Portulaca grandiflora. Plant Physiol. 2004, 134, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Ahlert, D.; Piepenburg, K.; Kudla, J.; Bock, R. Evolutionary origin of a plant mitochondrial group II intron from a reverse transcriptase/maturase-encoding ancestor. J. Plant Res. 2006, 119, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Jian-Hua, X.; Shi-Liang, C. New universal matK primers for DNA barcoding of angiosperms. J. Syst. Evol. 2011, 49, 176–181. [Google Scholar] [CrossRef]
- Chase, M.W.; Salamin, N.; Wilkinson, M.; Dunwell, J.M.; Kesanakurthi, R.P.; Haidar, N.; Savolainen, V. Land plants and DNA barcodes: Short-term and long-term goals. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1889–1895. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).