Abstract
Fruit mousses, as low-processed products, are highly susceptible to external conditions, and storage leads to the degradation of bioactive compounds, particularly phenolic compounds and vitamins, as well as promoting the growth of yeasts and molds. This study investigated the impact of storage conditions on the microbiological quality and degradation of selected bioactive compounds in fruit mousses from various producers (from apples, pears, and multi-components). Total phenolic (TPC) and total flavonoid (TFC) contents, vitamin C level, antioxidant capacity (AC, measured by the DPPH assay), and concentrations of macro- and microminerals were evaluated in fresh mousses and those stored for 48 h at 23 °C and 4 °C. Changes in total aerobic mesophilic bacteria (TAMB), yeast and mold counts, and selected microbial groups were also checked. It was found that the analyzed compounds varied depending on the components of the mousses. Multi-component mousses contained the highest levels of TPC, TFC, and vitamin C, and had 2–5 times higher AC values compared to apple and pear mousses. Storage at room temperature resulted in TFC lowering of up to 25% in apple mousses and vitamin C reductions of up to 22% in multi-component mousses. During refrigerated storage, the highest losses were observed in pear mousses, with TPC decreasing by up to 13% and vitamin C by up to 11%. Among the minerals, magnesium and zinc levels decreased most significantly in apple mousses stored at 23 °C (up to 33% and up to 29%, respectively). Microbiological analysis revealed variability in TAMB, yeast, and mold counts, with refrigeration (4 °C) generally limiting microbial growth compared to room temperature (23 °C). Notably, no pathogenic bacteria were detected under any storage conditions, and the mousses retained a high microbiological quality even after room-temperature storage.
1. Introduction
The fruit mousse market has evolved over the years. They have become increasingly popular in recent years as a better alternative to traditional fruit preparations, such as jams [1]. While the per capita consumption of fruit mousses was relatively low in 2016 (49 g per person per year), recent data indicate that approximately 30% of adults consume them several times a week and 37% of children daily [2]. The products are common, especially in the nutrition of infants and young children. Mousses dedicated to this age group are usually based on mild, naturally sweet fruits and vegetables (apple, pear, apricot, banana, and carrot). Spinach and broccoli are much less common as components of mousses due to their lower consumer acceptability. The production process for fruit mousses involves the use of fresh, frozen, or stored fruits, which are subjected to grinding and grating to remove inedible parts such as seeds and cores [2]. Due to their composition, based on fruits and vegetables that are a source of numerous antioxidants, vitamins, and minerals as well as a clean label composition with no added sugar or food additives, selected fruit and vegetable mousses are dedicated to infants as young as 4 months of age [3,4]. Their immune system is not fully mature; therefore, the products they consume must come from a reliable source, and the technology of their production is more restrictive than in the case of conventional products. These products, most often preserved by the pasteurization process, are of high quality and safe to eat, but it is important to store them properly after opening [5]. Due to the lack of added artificial food additives, fruit and vegetable mousses are more susceptible to external factors such as access to oxygen, temperature differences, or light [6]. To maintain their sensory, nutritional, and microbiological qualities, it is crucial to adhere to storage guidelines provided by manufacturers—typically, unopened mousses should be stored in a dry place at room temperature, while open containers should be refrigerated and consumed within 48 h. The consequence of improper storage of fruits, vegetables, and their preserves is the loss of antioxidants, including phenolic compounds or carotenoids, and antioxidant vitamins C and E. The reasons for the loss of antioxidants are enzymatic and microbial processes, high temperatures, as well as exposure to oxygen and light [7,8,9]. Advancements in research on the storage of fruit-based products provide critical insights into preserving their sensory and nutritional qualities. The storage of processed fruit products, such as mousses or purees, is associated with several physicochemical changes, including non-enzymatic browning, vitamin loss, and alterations in texture and flavor. For instance, in fruit purees, non-enzymatic browning primarily results from vitamin C degradation, and the use of specific sugars like sucrose and maltose can help minimize this process, preserving sensory quality [3]. Studies on nectarines revealed that cold storage influences sugar and organic acid profiles, helping to maintain flavor and enhance antioxidant properties during storage [10]. Innovative technologies, like high-pressure processing (HPP), effectively extend the shelf life of fruit-based products while preserving sensory and microbiological stability. For example, research on aronia berry puree demonstrated that HPP significantly reduces microbial growth with minimal impact on phenolic content and antioxidant properties, enabling up to 8 weeks of refrigerated storage [5]. These findings underscore the crucial role of optimized storage conditions and advanced preservation technologies in ensuring the quality of fruit-based products.
According to Commission Regulation (EC) No. 2073/2005 [11], fruit mousses, as ready-to-eat products, must meet certain microbiological criteria. For Listeria monocytogenes, ready-to-eat products supporting its growth must not exceed 100 CFU/g at the marketing stage or during shelf life. Products that do not support its growth maintain levels below this threshold throughout the shelf life. Furthermore, Salmonella spp. cannot be detected in 25 g samples, whether for ready-to-eat products or other foods. In addition, fruit mousses should meet different requirements for levels of Escherichia coli and other pathogens depending on product type and composition [2].
Minerals are important in infant nutrition for their functions, such as proper development and the formation of the immune system. Due to the presence of a wide variety of fruits and vegetables in infant mousses, these products are naturally rich in minerals, particularly zinc, copper, or selenium. It is assumed that minerals are not particularly sensitive to external factors; hence, the study of their content in ready-to-eat products is limited [4].
The level of bioactive compounds in fruit and vegetable mousses are determined by the diversity of their ingredients. Mousses with a more diverse composition, consisting of more fruits or vegetables, are richer in bioactive compounds and have higher antioxidant properties [12]. Bioactive compounds are present in small amounts in foods and contribute to health improvement or exhibit preventive effects against various diseases [13]. The most popular in this group are phenolic compounds, including phenolic acids, flavonoids, tannins, stilbenes, and lignans [14]. These compounds not only exhibit potent antioxidant activity but also have antimicrobial, anti-inflammatory, and antiproliferative functions [15]. Despite their significance, only a few authors have studied the loss of bioactive compounds in fruit mousses under varying storage conditions. Ferrari et al. [16] studied the effect of high-pressure technology on changes in polyphenol and anthocyanin contents in fruit mousses during storage under refrigerated conditions (4 °C). The authors demonstrated an 11% reduction in anthocyanins in the mousses. Also, Grobelna et al. [17] observed a smaller reduction in the total phenolic content compared to the total anthocyanin content in blue honeysuckle berry puree during storage at 20 °C without light exposure. Additionally, they noted that vitamin C losses were influenced by the specific variety of blue honeysuckle and the duration of storage. Saarniit et al. [18] found the greatest decrease in the total phenolic content in a four-grain puree with banana and blueberry, characterized by a high pH and lower initial bioactive compound levels. In contrast, minimal changes were observed in mango–carrot–sea buckthorn puree and fruit-yogurt puree with biscuit, which had lower pH values and higher phenol content. In addition, no study has been conducted that also shows the microbiological properties of the mousses after storage. Therefore, the present study aimed to investigate the effect of the storage conditions on the content of selected bioactive compounds and the microbiological properties of fruit mousses for infant feeding. The study evaluated the content of selected bioactive compounds, minerals, and the microbiological quality of fruit mousses for infants and small children.
2. Materials and Methods
2.1. Chemicals and Reagents
2,2-diphenyl-1-picrylhydrazyl (DPPH) and 6-hydrozy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Sodium thiosulfate (Na2S2O3) and sodium hydroxide (NaOH) were obtained from Chempur (Piekary Śląskie, Poland). Methanol, Folin–Ciocalteu reagent, oxalic acid, gallic acid, catechin, sodium nitrite (NaNO2), aluminum chloride hydrate (AlCl3H2O), and 2,6-dichloroindophenol were purchased from Sigma Aldrich (St. Louis, MO, USA).
2.2. Research Material
The research material consisted of 12 fruit mousses intended for feeding infants and small children, including 8 one-component samples (apple or pear) and 4 multi-component samples containing apples. The mousses were purchased from a local market in Olsztyn (Poland). Each sample was from a different company. This study used samples that had a shelf life of at least two months. The composition of the mousses is detailed in Table 1.

Table 1.
Percentage composition of fruit mousses used in the study.
For the analysis, 5 g of the research materials were diluted five times with distilled water and then centrifuged in an Eppendorf-type centrifuge (Eppendorf AG, Hamburg, Germany) (4000 rpm/10 min). The extracts were prepared from fresh mousses (immediately after opening) as well as from those stored for 48 h at room temperature (23 °C ± 2 °C) and at refrigerated temperature (4 °C ± 0.5 °C). The prepared aqueous extracts were subsequently used to determine the total phenolic content (TPC), total flavonoid content (TFC), and antioxidant capacity (AC).
2.3. Microbiological Analysis
The microbiological quality of the mousses was evaluated immediately after opening under conditions simulating consumer behavior (48 h of storage at room temperature after opening) and according to the manufacturers’ recommendations (after 48 h of refrigerated storage (at 4 °C)). Microbiological analyses were carried out using pre-prepared weighing (10 g) of individual mousse samples. Samples were obtained directly from the packages, aseptically weighed, transferred to 90 mL of sterile saline solution (0.85% NaCl), and mixed by shaking. After shaking, serial 10-fold dilutions (1:10 (v/v)) of the sample were made using the same diluent. Standard plate methods were used for microbiological analyses. Total aerobic mesophilic bacteria count (TAMB), total mold and yeast count, the total number of coliform bacteria, the number of spores of Bacillus sp., total Enterobacteriaceae, total Enterococcus sp., total Staphylococcus sp., and total Listeria monocytogenes were determined in the mousse samples. Data on microorganisms are presented as the logarithm of the number of colony-forming units per gram (CFU/g). The type of medium, the method of culture, and the incubation conditions for the tested groups of microorganisms are shown in Table 2.

Table 2.
Methods for the determination of the tested groups of microorganisms.
2.4. Determination of Mineral Content
The mousses (5 g) were weighed into quartz crucibles, dried at 60 °C for 8–12 h, and then carbonized for 8 h on an electric cooker and placed in a muffle furnace, as described by Dobrowolska-Iwanek et al. [19] and Żmudzińska et al. [4]. The contents of copper (Cu), manganese (Mn), iron (Fe), zinc (Zn), calcium (Ca), and magnesium (Mg) were determined using flame atomic absorption spectroscopy (AAS) with an acetylene–air flame, using an iCE 3000 SERIES-THERMO atomic absorption spectrometer (Thermo-Scientific, Hemel Hempstead, Hertfordshire, UK) with a GLITE data station, deuterium background correction, and cathode ray tubes. Sodium (Na) and potassium (K) were determined using the emission technique with an acetylene–air flame, using the same spectrometer in emission mode. Calibration curves for minerals were based on average absorbance values.
2.5. Determination of Total Phenolic Content
The total phenolic content (TPC) was determined according to the method described by Zakrzewski et al. [20] To perform the test, Folin–Ciocalteu reagent (diluted 1:2) and a 14% sodium carbonate solution were used. The solutions were left for 60 min in the dark. Absorbance was measured at 720 nm using a FLUOstar OME-GA BGM LAB Tech spectrophotometer, with the reagent sample as a blank. The TPC was expressed as mg catechin equivalent (CE)/100 g, based on a standard catechin curve.
2.6. Determination of Total Flavonoid Content
The total flavonoid content (TFC) was determined according to the method developed by Zakrzewski et al. [20]. NaNO2, AlCl3·6H2O, and NaOH were added to the extracts, which were then centrifuged at 4000 rpm for 10 min. Absorbance was measured at 415 nm using a FLUOstar OMEGA BGM LAB Tech spectrophotometer, with distilled water as a blank. TFC was expressed in mg CE/100 g based on a catechin standard curve.
2.7. Determination of Vitamin C Content
The content of vitamin C was determined by the redox titration method using 2,6-dichloroindophenol, described by Dioha et al. [21]. A 2% oxalic acid solution was added to a sample of the mousse (5 g), mixed, and left in the dark for 15 min. After precipitation, 10 mL of the supernatant was taken and titrated with 2,6-dichloroindophenol until the color changed. The content of ascorbic acid was expressed in mg/100 g of mousse in relation to the prepared standard curve.
2.8. Determination of Antioxidant Capacity
The antioxidant capacity (AC) was determined using the DPPH assay according to the procedure developed by Yang et al. [22]. DPPH was dissolved in 80% methanol, and absorbance at 515 nm was measured using a FLUOstar OMEGA BGM spectrophotometer (LAB Tech, Ortenberg, Germany), with distilled water as a blank. The AC was expressed in µM Trolox equivalent (TE)/100 g based on a Trolox standard curve.
2.9. Statistical Analysis
The values were expressed as the mean ± standard deviation (SD). The results were subjected to a one-way analysis of variance (ANOVA) using Tukey’s test. Linear Pearson’s correlation coefficients were calculated to show relationships between bioactive compounds and AC for fresh, as well as stored, mousses, and p ≤ 0.05 was considered significant. A principal component analysis (PCA) was also carried out to show differences between samples. The statistical analysis was performed using Statistica 13.1 (Statsoft Inc., Kraków, Poland).
3. Results
3.1. Microbiological Quality of Fresh and Stored Fruit Mousses
The results of the microbiological analyses of the products during storage are shown in Table 3. In general, differences were noted in the mousse samples regarding total aerobic mesophilic bacteria and mold and yeast counts. The studied fruit mousses were characterized by total viable counts, ranging from <10 to log 5.97. The highest TAMB values were found in a mousse sample obtained from pears (P3), both on the day of testing (log 1.90) and after 48 h of storage, at 23 °C (log 5.97) and at 4 °C (log 5.59). The absence of TAMB was observed in five samples (41.7%) regardless of the storage conditions. No TAMB was observed in all three samples (25.0%) from different raw materials (multi-component samples). Two mousse samples showed an increase in TAMB after storage under both storage conditions. Also in the two samples, in the initial absence of TAMB, an increase was found after storage.

Table 3.
Microbiological characteristics of the studied fruit mousses.
The occurrence of mold and yeast was observed in four mousse samples (33.3%). There was also an increase in mold and yeast after storage in four samples (33.3%)—in sample P3 (after storage at 4 °C) and samples A3, P2, P3, and M1 (after storage at 23 °C). Only one sample (8.3%; P3, pear sample) showed growth after 48 h at 4 °C. Although, in some cases, the storage of the mousses under refrigeration (4 °C) resulted in a small increase in TAMB (average differences of one order of magnitude, compared to the day of testing), this storage resulted in a smaller increase in TAMB and mold and yeast count than storage at 23 °C, where average differences of about 4 orders of magnitude were found. Coliforms, spores of Bacillus sp., Enterobacteriaceae, Enterococcus sp., Staphylococcus sp., and L. monocytogenes were not found in the mousse samples analyzed, regardless of storage conditions.
3.2. Content of Selected Macro- and Microminerals of Fresh and Stored Fruit Mousses
Table 4 shows selected minerals from the macromeneral group (Na, K, Mg, and Ca) in the fresh and stored studied mousses. The proportion of macromineral varied depending on the type of mousse. Multi-component ones contained the most Na (10.80–15.61 mg/100 g) and Mg (5.81–9.82 mg/100 g). On the other hand, apple mousses contained the most K (34.62–70.49 mg/100 g), and pear ones contained the most Ca (7.94–9.95 mg/100 g). The content of the studied macrominerals differed within the same mousse in terms of composition, depending on the producer. In A1 mousse, there was more than twice the amount of Na as in A2, A3, and A4 mousses. In the case of K, Mg, and Ca contents, the values also varied within the same type of mousse.

Table 4.
The content of selected macrominerals (Na, K, Mg, and Ca) in the studied fruit mousses (fresh and stored at room temperature and refrigerated temperature).
It was noted a decrease in the content of the tested macrominerals depending on the storage conditions of the mousses after opening. Fresh mousses contained the most macrominerals, while those stored at room temperature contained the least. A decrease in the content of selected minerals was observed in each case, although it was not statistically significant in all samples of each type. The most notable storage-induced differences in macrominerals were observed for Mg (loss of up to 33% in apple mousses stored for 48 h at 23 °C) and Ca (loss of up to 11% in multi-components mousses stored for 48 h at 23 °C).
Table 5 summarizes selected microminerals (Mn, Cu, Fe, and Zn) determined in the fruit mousses. Microminerals were present in much smaller amounts than macrominerals. From the four determined trace minerals, the mousses showed the highest Fe content. The content of individual microminerals varied among mousse types.

Table 5.
The content of selected microminerals (Mn, Cu, Fe, and Zn) in the studied fruit mousses (fresh and stored at room temperature and refrigerated temperature).
The effect of storage had little effect on losses of microminerals. In the cases of Mn and Cu, no changes were noted in their content after the storage of the mousses. Significant changes in content were noted only for Zn in one of the apple mousses (A4) during storage at room temperature (loss of up to 29%).
3.3. Content of Bioactive Compounds of Fresh and Stored Fruit Mousses
The results of individual bioactive compounds in fruit mousses are summarized in Table 6. Among apple mousses, the highest TPC was recorded in the A1 mouse (p ≤ 0.05). This content was almost 4, 1.5, and almost 3 times higher relative to other apple mousses (A2, A3, and A4, respectively). In pear mousses, P4 mouse was the richest in TPC (p ≤ 0.05), whose content exceeded the level of these compounds in P1, P2, and P3 mousses by 3, 4.5, and 3 times, respectively. In multi-component ones, the highest TPC value was recorded in M2 mousse and comparatively the least in M1 and M3 mousses (p ≤ 0.05). Variations were also noted in the TFC values of the products studied. It was found that A1, A3, and A4 mousses contained similar flavonoid amounts (p > 0.05). Among the pear mousses, P1 mousse contained the lowest amount of flavonoids, and among the multi-component mousses, M1 and M3 mousses had the least amount of these compounds. In each type of mousse, the lowest concentration of vitamin C was recorded in A1, P1, and M1 mousses. It was noted that A1 mousse had 1.5, 1.2, and 1.8 times less vitamin C than A2, A3, and A4 mousses, whereas P1 mousse had 1.3, 1.3, and 2.3 times less than P2, P3, and P4 mousses. Among multi-component mousses, the M1 mousse had 1.3, 1.3, and 1.4 times less than M2, M3, and M4 mousses, respectively.

Table 6.
The content of selected antioxidants of the studied fruit mousses.
The content of selected bioactive compounds differed significantly according to the storage conditions. In each type of mousse, the highest antioxidant contents were recorded in fresh mousses, while the lowest were in samples stored for 48 h at room temperature. There was a decrease in the TPC, TFC, and vitamin C in mousses stored at refrigerated temperature, but the decrease was not always statistically significant. In the case of phenolics, the greatest losses after storage in refrigeration were recorded in apple mousse A4 (9%), pear mousses P1 and P2 (13% and 6%, respectively), and multi-component mousse M3 (10%). In the case of flavonoids, the highest losses were in all apple mousses (8–11%), pear mousse P4 (13%) and multi-component mousse M3 (10%). Noticeably higher losses in phenolic and flavonoid contents were recorded after storage at room temperature for phenolics in P2, P4, M3, and M4 mousses (10–11%) and for flavonoids in apple mousses (13–25%). There were large differences in the loss of vitamin C in relation to the storage conditions. During refrigerated storage, the following losses were recorded: apple mousses (2–6%), pear mousses (3–11%), and multi-component mousses (2–7%), while at room temperature, apple mousses (12–15%), pear mousses (12–18%), and multi-component mousses (5–22%).
3.4. Antioxidant Capacity of Fresh and Stored Fruit Mousses
Figure 1 shows the AC values of the studied fruit mousses, both fresh and stored. The parameter measured by the DPPH assay was expressed in uM Trolox/100 g of mousse. The values of AC differed depending on the type of mousse. In apple ones, the significantly highest AC was recorded in A1 mousse, while the significantly lowest was in A2 mousse (three times lower than A1) (p ≤ 0.05). Among pear mousses, the P4 mousse had the highest ability to capture oxygen free radicals. The AC values was three, two, and more than one times higher than mousses P1, P2, and P3, respectively. The multi-component mousses had significantly higher AC (80.37–110.31 µM TE/100 g) (p ≤ 0.05). There was a decrease in AC during the storage of the mousses, both at room temperature and at refrigerated temperature, but losses during storage at room temperature were higher. In apple and multi-component mousses, losses of 5–8% were recorded during storage at refrigerated temperature and 10–17% during storage at room temperature. On the other hand, in pear mousses, the losses were at a level of 1–6% after storage at refrigerated temperature and 12–18% after storage at room temperature.
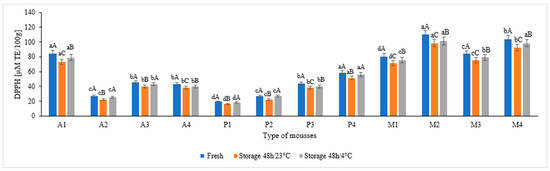
Figure 1.
Antioxidant capacity (DPPH assay values) of the studied fruit mousses (fresh and stored at room temperature and refrigerated temperature). Different lowercase letters (a,b,c,d) placed above the bars for mousses of the same type, separate for fresh, stored at 23 °C, and stored at 4 °C, indicate significant differences (p ≤ 0.05), whereas different uppercase letters (A,B,C) placed above the bars for various storage conditions, separate for each mousse sample, indicate significant differences (p ≤ 0.05).
3.5. Relationships Between Studied Mousse Samples
3.5.1. Linear Correlation
Table 7 presents the results of the linear correlation between the tested antioxidant parameters. Based on the data, a very strong positive correlation between AC and TPC was indicated (r = 0.96, r = 0.95, and r = 0.97 for fresh, stored at room temperature, and stored at refrigerated temperature, respectively). There was also a positive correlation between AC and TFC (r = 0.86, r = 0.81, and r = 0.75 for fresh, stored at room temperature, and stored at refrigerated temperature, respectively), and a slightly smaller positive correlation between AC and vitamin C (r = 0.76, r = 0.71, and r = 0.73 for fresh, stored at room temperature, and stored at refrigerated temperature, respectively). There was a very strong positive correlation between DPPH and TPC for apple, pear, and multi-component mousses (r = 0.92, r = 0.86, and r = 0.85, respectively). It was also noted a strong positive correlation between AC and TFC for multi-component mousses (r = 0.77). In addition, a weak positive correlation was noted between DPPH and vitamin C in pear mousses and multi-component ones (r = 0.46 and r = 0.53, respectively).

Table 7.
Correlation coefficients (r) calculated for the relationships between the antioxidant capacity (DPPH assay) and content of bioactive compounds.
3.5.2. Principal Component Analysis (PCA)
The structure of variation in the obtained results was explained using a principal component analysis (PCA). PCA was carried out among all tested samples and variables (TPC, TFC, vitamin C, AC, sum of macrominerals, and sum of microminerals). The principal component analysis for the tested mousses is shown in Figure 2. The first two principal components (PC) explained 76.70%, 81.26%, and 86.12% of total data variance for apple, pear, and multi-components mousses, respectively. Figure 2a,c,e show a plot of points in the plane of the principal components that illustrates the similarity between the content of analyzed compounds of the studied mousses. The distribution of the analyzed groups of mousses in relation to each other indicates the different content of selected bioactive compounds and antioxidant properties in them. The greatest variation in the content of the studied compounds in relation to storage conditions was observed for apple mousses (Figure 2a), particularly for mousses stored at room temperature (RT) and refrigeration temperature (CT).
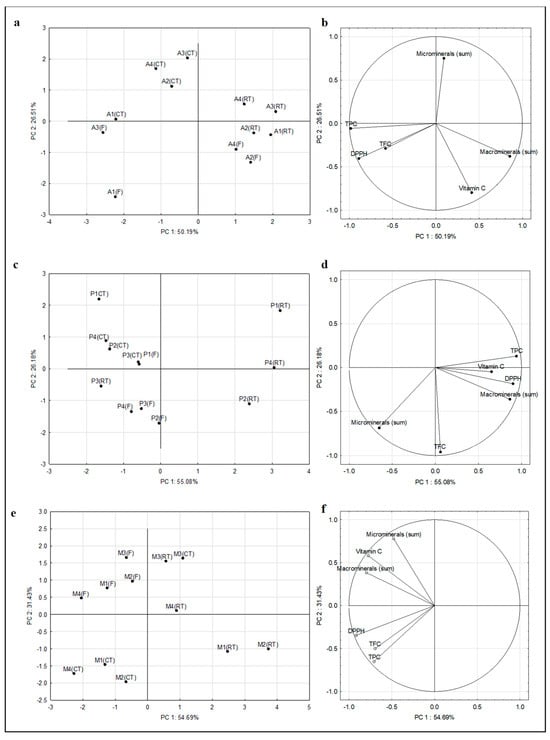
Figure 2.
Principal component analysis (PCA) results presented as score plots of the analyzed fresh fruit mousses (a,c,e) and loading plots of the analyzed parameters (TPC, TFC, DPPH, sum of macrominerals, and sum of microminerals) in the analyzed fresh fruit mousses (b,d,f). Explanations: A1, A2, A3, A4—apple mousses; P1, P2, P3, P4—pear mousses; M1, M2, M3, M4—multi-component mousses; F—fresh mousses; CT—mousses stored at refrigerated temperature (48 h/4 °C); RT—mousses stored at room temperature (48 h/23 °C).
Figure 2b,d,f show the intercorrelations between the variables and the resulting principal components. The direction and length of the vectors indicate how much the variables affect the principal components. Figure 2b shows that the variables TPC, DPPH, and sum of macrominerals are located near the circle, which means that the information contained in them is carried by the principal components. In contrast, the information contained in the other variables (TFC, vitamin C, and sum of microminerals) is carried to a lesser extent by the principal components. In Figure 2d,e, all variables, except vitamin C (Figure 2d) and TFC (Figure 2f), are located near the circle, which means that the information they contain is carried by the principal components. There was a strong positive correlation between DPPH and TPC and DPPH and TFC, and a negative correlation between vitamin C and sum of microminerals for fresh mousses (Figure 2b). For mousses stored at refrigerated temperature (Figure 2d), there was a strong positive correlation between DPPH and vitamin C, DPPH and TPC, DPPH and sum of microminerals, and DPPH and TFC. In mousses stored at room temperature (Figure 2f), a strong positive correlation was also noted between DPPH and TPC and DPPH and TFC.
4. Discussion
Fruits and vegetables are highly valued for their high content of valuable compounds, including phenolics, carotenoids, vitamins, minerals, and dietary fiber. Fruit-based products such as jams, pulps, purees, and mousses are widespread in the food market, allowing access to fruits and vegetables throughout the year, regardless of the season. Modern food processing technologies, including high-pressure processing, pulsed electric field (PEF) technology [23], ultrasound-assisted processing [24], infrared heating [25], and modified atmosphere packaging (MAP) [26], allow food manufacturers to meet the growing demands of consumers and create fruit and vegetable products that are characterized by a good composition (clean label), have a high nutritional value, and are sensorially acceptable to consumers [27]. However, bioactive compounds, especially the group of phenolics, carotenoids, and vitamins, are unstable compounds that, during fruit processing, can be reduced or completely degraded. In particular, they are sensitive to light, temperature, and storage [28]. This is the first study that focuses on evaluating the content of selected bioactive compounds, minerals, and microbiological quality during the storage of fruit mousses.
Testing the microbiological quality of products is an essential step to ensure control of microbial development, increase product shelf life, and thus improve product quality and safety [29]. In fruit mousses, parameters such as pH, water activity (aw), and sugar concentration are key to preventing recontamination and microbial growth. Studies show that bacteria, such as L. monocytogenes, thrive in environments with pH ≥ 5.6, while pH ≤ 3.8 effectively inhibits their growth, even with a high sugar content [30]. Low water activity (aw < 0.7) in mousses significantly reduces the growth of microorganisms, which improves their stability during storage [31]. A high sugar concentration has an osmotic effect, inhibiting microbial growth, but it must be fine-tuned to maintain the product’s flavor and nutritional value [32]. Considering these properties in fruit mousses will provide a better understanding of the factors affecting their microbiological safety and shelf life. The variation in microbial development during post-opening storage of products is related to the phases of microbial growth [2]. Raw fruits used for ready-to-eat foods are more susceptible to contamination from bacteria, mold, and yeast due to their high concentrations of water, sugars, and other nutrients [33]. In our study, we only observed an increase in the total aerobic mesophilic bacteria count and mold and yeast count during storage, with the increase being more intense at the higher temperature (23 °C) of storage. This is due to the change in the storage conditions of the samples (increase in temperature and storage time) or the loss of sterility of the product, which consequently leads to fluctuations in the rate of change in the population of microorganisms [2]. Despite a significant increase in the identified microorganisms in some mousse samples, none of the samples analyzed showed signs of spoilage. Stankiewicz and Płotka [2] demonstrated the presence of total aerobic mesophilic bacteria and populations of mold and yeasts in the fruit mousse samples analyzed. Their levels of occurrence on the day of analysis were similar to our results (average values within log 0.46–log 2.01 CFU/g); however, samples with a higher presence were also reported, at levels as high as log 4.86 CFU/g. As for the results obtained after the storage of the mousse samples, they were significantly lower than our results (average values within log 0.82–log 1.64 CFU/g). This fact may be due to the differences in storage conditions used (higher refrigerated temperature (6 °C) and shorter storage time (24 h)). Sequino et al. [34] suggest a relationship between the internal factors of fruit and vegetable raw materials (shape, surface) and the microbial count, which can directly affect the microbiological quality of the products made from them. Researchers report that the Enterobacteriaceae family is the dominant microflora of vegetables. Their count in fruits is significantly lower [34], which, in addition to the use of heat treatment to fix the mousses, may indirectly confirm the absence of this group of microorganisms in the fruit mousse samples we studied. Current regulations in the EU [11] define criteria (food safety and process hygiene) that indicate limits related to the presence of pathogens and other undesirable microorganisms. There are no specified limits for fruit mousses in the regulations; however, fruit mousses intended for children and adults are classified as ready-to-eat food. Two variants of sample storage did not affect the development of pathogenic microflora or other microbiological indicators determined in this study. The requirements were met, even with different storage conditions. Although the microbiological quality of these products is high, longer storage at room temperature can lead to an increase in the number of aerobic mesophilic bacteria, as well as mold and yeast, which can affect the flavor and safety of the product with prolonged storage. Although the product remains safe to eat and does not show visible quality changes, storage conditions have a significant impact on its nutritional value and microbiological stability.
The fruit mousses studied were characterized by different TPC and TFC, and the differentiating factors were the type of producer and the percentage of each component in the mousses. Although the composition of each mousse was the same (100% apple), the A1 mousse had significantly more TPC relative to the A2, A3, and A4 mousses. There was a similar trend for flavonoids; the A1 mousse had the most of these compounds. In pear mousses, significant differences in the content of phenolic compounds were noted in the P4 mousse. The reason for this may be the use of different varieties of apples to make mousses, the country of origin of the apples, and the storage conditions of the fruit before thermal processing [35]. Improper fruit storage conditions lead to the loss of polyphenol compounds and antioxidant vitamins [36]. Of the three types of mousses, the multi-component ones (M1, M2, M3, and M4) had the highest phenolic and flavonoid contents. The reason for this is the almost 30% share of strawberry pure in the composition of the mousse. According to the literature data, berries contain the highest levels of phenolic compounds [37], which resulted in this study in higher levels in mousses containing strawberries in the composition. Strawberries are classified as fruits with a high content of nutrients and phytochemicals, including phenolics such as anthocyanins, proanthocyanidins, and ellagitannins [38]. In a study by Čížkova et al. [39], the content of phenolic compounds in a mousse with strawberries was 48.5 mg/100 g, while that with blueberries was 77 mg/100 g. In addition, mousses containing more than one fruit in the composition may have a higher content of phenolic compounds due to a more diverse composition. Carbonell-Capella et al. [40] studied 23 commercial fruit-based baby foods, of which the product containing four fruits in the formulation and the one containing berries (blueberries) had the highest content of phenolic compounds. In each of the three flavor variants of the mousses, the contents of phenolic compounds and flavonoids were correlated with AC (determined by the DPPH assay). It was found that the M2 mousse had the highest of the three determined parameters (TFC, TPC, and AC).
Based on this study, it is indicated that mousses are a rich source of vitamin C. Its content significantly affects the measurement of phenolic compounds, as the Folin–Ciocalteau reagent reacts with ascorbic and dehydroascorbic acid (DHA) and reducing sugars, often providing overestimated results [41]. The vitamin C content of fresh fruit mousses ranged from 5.88 mg/100 g to 16.66 mg/100 g, while in the study by Carbonell-Capella et al. [40] the range of ascorbic acid content was much wider (1.9–71.5 mg/100 g). However, in these authors’ study, some of the mousses contained synthetically added vitamin C. Analyzing the content of vitamin C from fruit only, it is indicated that the highest level is similar to the vitamin C content obtained in our study for multi-components mousses. Because of the most varied ingredients, the multi-components mousses (M1, M2, M3, and M4) contained the highest amounts of vitamin C. Multi-components mousses contained 30% strawberries, which are an excellent source of ascorbic acid [42]. In the single-component mousses (apple and pear mousses), the level of vitamin C varied among producers, which may be due to the type of fruit, its origin, and the state in which the fruit was before thermal processing [43]. In fresh mousses, vitamin C levels were positively correlated with TPC, TFC, and AC.
The studied mousses were analyzed for AC by the DPPH assay. The multi-component mousses (M1, M2, M3, and M4) had the highest ability to capture oxygen free radicals, due to their diverse composition. It should be noted that they were also characterized by the highest TPC. These mousses have a more varied composition relative to apple and pear mousses, which may result in a higher concentration of antioxidants [12,44]. Filipiak-Florkiewicz and Dereń [45] indicated a significant relationship between the antioxidant properties and the species of fruits and vegetables completing the composition of mousses. Purkiewicz and Pietrzak-Fiećko [12] indicate higher values of antioxidant properties in mousses with a more diverse composition than in those with a lower variety. It should also be noted that, in this study, only DPPH, an organic solvent-soluble radical, was used to determine antioxidant properties. Because some of the antioxidants will not be detected by the DPPH assay, the result of the total AC may be underestimated [46,47]. The correlation results show a strongly positive correlation between the TFC and DPPH, and a positive correlation between DPPH and TFC and DPPH and vitamin C.
Because fruit and vegetable mousses are dedicated especially to infants and young children, it is important to store them properly according to the manufacturers’ storage instructions on the product label [2,4,48]. Losses of vitamin C in fruit products can cause deficiencies and their resulting health consequences. An important aspect is to provide adequate vitamin C to the diet to ensure optimal health and support the immune system [49]. In general, ascorbic acid deficiencies are relatively rare in children due to its prevalence in the diet, especially its presence in fruits and vegetables [50]. In addition to supporting immune function, vitamin C is also a strong antioxidant and aids in iron absorption. Symptoms of vitamin C deficiency include a weakened immune system, skin problems, and scurvy in cases of severe deficiency [51]. The results of the studies have shown a significant decrease in compounds from the antioxidant group—phenolics, flavonoids, and vitamin C, as well as AC. Fruit mousses are preserved by pasteurization, which effectively ensures the safety of the product. However, the manufacturer recommends storing the mousses after opening for a maximum of 48 h at refrigerated temperature [2]. After this period of refrigerated storage, there was a significantly lower loss of bioactive compounds in the fruit mousses compared to storage at room temperature. The relatively high losses of bioactive compounds in the mousses could also be caused by the lack of added saccharose in the products. Saccharose, in addition to shaping flavor, is a preservative, effectively reducing water activity levels [52]. A significant decrease in selected antioxidant substances and AC was recorded during storage of the mousses, both at room and refrigerated temperatures. However, significantly higher losses were recorded in mousses stored at room temperature. This is related to the intensification of transformations occurring in the fruit (oxidation) under the influence of external factors such as light and temperature. Refrigerated temperature (4–5 °C) is the temperature recommended by manufacturers for storing fruit and vegetable preserves. This temperature ensures the longest possible preservation of sensory characteristics and nutritional value in these products [53,54]. In the studies conducted, losses of compounds from the group of phenolics and flavonoids during storage at room temperature reached up to 26%, which significantly reduced the nutritional value of the product. Significantly lower losses, up to approx. 13%, were recorded during the storage of the mousses at refrigerated temperature. Storing food products at a refrigerated temperature protects the valuable bioactive ingredients, allows for longer preservation of freshness, and ensures product safety [55]. Although appropriate reference intake values for polyphenolic compounds have not been established, their consumption is indicated to have a significant impact on human health in the prevention of certain noncommunicable diseases (NCDs) [56]. Due to their ring structure, phenolic compounds are characterized by a variety of bioactivities, while providing the potential to prevent and help in the treatment of various diseases [57]. Phenolic compounds are considered essential components of the human diet, exhibiting strong antioxidant, anti-inflammatory, anti-atherosclerotic, anti-cancer, or anti-diabetic effects [58]. The adequate intake of phenolic compounds is also crucial among children; they can promote the development of cognitive function and improve memory and concentration. There are studies available showing positive effects on school-aged children’s ability to learn and concentrate with a diet higher in polyphenols [59].
Compared to storage-sensitive antioxidant compounds and water- and fat-soluble vitamins, minerals are much more stable [60]. The greatest loss of mineral components, especially Ca, Cu, Fe, Mg, P, K, or Zn, occurs during technological processing, especially heating. It should be noted that some mineral components are more stable after heat treatment (e.g., Ca), and some are more sensitive to external factors (e.g., K) [61,62]. In our study, only slight losses of minerals were noted, both during storage at room temperature and at refrigerated temperature. The least stable macrominerals were magnesium and calcium, the contents of which, in apple and multi-component mousses after storage at room temperature was significantly lower than in fresh mousses. It is generally recognized that minerals are constant and their levels in food are hardly variable, regardless of external factors. The study showed that the storage method of the mousses affected the loss of macrominerals. Significant losses in the calcium and magnesium contents were shown in mousses stored at room temperature (48 h/23 °C). The greater loss of minerals in mousses stored at room temperature was a result of their greater exposure to oxygen and light. Alshallash et al. [62] reported that the loss of minerals in fruit products, in addition to pretreatment procedures, is strongly influenced by access to light. As fruit mousses are increasingly consumed, especially by children, the loss of minerals from storage can significantly reduce their contribution to the diet. Of minerals, children are particularly vulnerable to iron deficiencies. It is estimated that about 40% of children worldwide suffer from anemia resulting from deficiencies of this micromineral [63]. For children, Fe deficiency can also lead to concentration disorders and problems with attention and learning [64].
The results of our research show that fruit mousses are products with high nutritional value, rich in bioactive components such as phenolic compounds, flavonoids, and vitamin C, which have beneficial effects on consumer health. These products are characterized by their high initial quality, especially in terms of antioxidant content and microbiological purity. This means that consumers are buying a product with good health benefits and microbiologically safe, thanks to the minimal processing and heat treatment used. However, the quality and nutritional value of fruit mousses can change depending on the storage conditions. Storage at room temperature leads to greater losses of bioactive compounds. Under refrigerated conditions, these losses are significantly lower, which means that consumers can retain more of their nutritional value when storing mousses in the refrigerator. To maximize the health-promoting properties of fruit mousses, consumers should store them in the refrigerator and consume them within 48 h of opening, according to the manufacturer’s recommendations.
5. Limitations of This Study and Future Prospects
This study highlights the impact of storage conditions on the microbiological quality and degradation of bioactive compound in fruit mousses, but it also revealed several limitations that should be addressed in future research. One key limitation is the lack of pH and acidity measurements, which are crucial for understanding the chemical factors that may influence both microbial growth and the stability of bioactive compounds during storage. The absence of these measurements limits the ability to fully assess the interactions between the chemical composition and microbiological characteristics of the fruit mousses. Another limitation lies in the relatively small sample size and limited diversity of fruit mousse types analyzed, which may restrict the generalizability of the findings. Including a broader range of formulations, brands, and production methods in future studies would allow for a more comprehensive evaluation. Furthermore, the impact of specific production processes on the preservation of bioactive compounds and microbial stability was not explored, which could be another area for further research.
In future studies, the authors plan to incorporate measurements of pH and acidity to better understand the chemical dynamics affecting both bioactive compound retention and microbiological quality during storage. The inclusion of these parameters will help to correlate the chemical composition with microbial growth patterns more effectively. Additionally, expanding the study to include a larger variety of fruit mousse products and exploring different storage scenarios will enhance the applicability of the findings to a broader range of products and conditions. Moreover, evaluating the influence of manufacturing processes on product stability could yield valuable insights for optimizing the shelf life and enhancing the nutritional value of fruit mousses. By addressing these areas, future research can offer more targeted and practical recommendations for improving these products.
6. Conclusions
The diversity in fruit composition enhances the antioxidant capacity, with more varied formulations generally showing higher levels of antioxidants. However, storage conditions have a substantial impact on the retention of these bioactive compounds. Refrigerated storage (4–5 °C) significantly reduces their losses, while storage at room temperature leads to the highest degradation, particularly affecting flavonoids (up to 25% in apple mousses) and vitamin C (up to 22% in multi-component mousses). In addition, there were reported losses of phenolic compounds, flavonoids, and vitamin C of 11–13% in pear mousses during refrigeration storage. The microbiological analysis confirms the high quality of these low-processed, heat-treated mousses, which remain microbiologically safe even under less ideal storage conditions (23 °C), although this can increase the total aerobic mesophilic bacteria and mold and yeast counts. Despite these increases, the products do not show visible quality changes or pathogenic microflora growth.
Author Contributions
Conceptualization, A.P. and P.W.; methodology, A.P. and P.W.; validation, M.T., G.G. and R.P.-F.; formal analysis, A.P. and P.W.; investigation, A.P. and P.W.; resources, A.P.; data curation, A.P. and P.W.; writing—original draft preparation, A.P. and P.W.; writing—review and editing, M.T., G.G. and R.P.-F.; visualization, A.P.; supervision, M.T. and R.P.-F.; funding acquisition, R.P.-F. All authors have read and agreed to the published version of the manuscript.
Funding
APC funded by the Minister of Science under the Regional Initiative of Excellence Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kaczmarek, D.; Wójcik, M.; Kapuśniak, K. Assessment of Functional Properties of Vegetableand Fruit Mousses Enriched with a Fiber Preparationfrom Potato Starch. Zywn. Nauk. Technol. Ja 2023, 30, 284–299. [Google Scholar] [CrossRef]
- Stankiewicz, J.; Płotka, P. Evaluation of Selected Microbiological Quality Parameters of Fruit Mousses. Sci. J. Gdyn. Marit. Univ. 2019, 110, 32–39. [Google Scholar] [CrossRef]
- Westland, S.; Crawley, H. Fruit and Vegetable Based Purées in Pouches for Infants and Young Children. First Steps Nutrition Trust. 2018. Available online: https://www.firststepsnutrition.org/babyfood-composition (accessed on 7 November 2024).
- Żmudzińska, A.; Puścion-Jakubik, A.; Soroczyńska, J.; Socha, K. Evaluation of Selected Antioxidant Parameters in Ready-to-Eat Food for Infants and Young Children. Nutrients 2023, 15, 3160. [Google Scholar] [CrossRef]
- Mielech, A.; Puścion-Jakubik, A.; Socha, K. Assessment of the Risk of Contamination of Food for Infants and Toddlers. Nutrients 2021, 13, 2358. [Google Scholar] [CrossRef]
- Qadri, O.S.; Yousuf, B.; Srivastava, F.Y. Fresh-Cut Fruits and Vegetables: Critical Factors Influencing Microbiology and Novel Approaches to Prevent Microbial Risks—A Review. Cogent Food Agric. 2015, 1, 1121606. [Google Scholar] [CrossRef]
- Toydemir, G.; Subasi, B.G.; Hall, R.D.; Beekwilder, J.; Boyacioglu, D.; Capanoglu, E. Effect of Food Processing on Antioxidants, Their Bioavailability and Potential Relevance to Human Health. Food Chem. X 2022, 14, 100334. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, I.; Chekir, L.; Ghoul, M. Effect of Heat Treatment and Light Exposure on the Antioxidant Activity of Flavonoids. Processes 2020, 8, 1078. [Google Scholar] [CrossRef]
- ElGamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal Degradation of Bioactive Compounds during Drying Process of Horticultural and Agronomic Products: A Comprehensive Overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Wu, J.; Li, Y.; Deng, W.; Cao, K.; Li, Z.; Wang, L. Effect of Postharvest Cold Storage and Subsequent Shelf-Life on Fruit Quality and Endogenous Phytohormones in Nectarine Fruit. Postharvest Biol. Technol. 2024, 218, 113197. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02005R2073-20200308&from=EN (accessed on 25 November 2024).
- Purkiewicz, A.; Pietrzak-Fiećko, R. Antioxidant Properties of Fruit and Vegetable Whey Beverages and Fruit and Vegetable Mousses. Molecules 2021, 26, 3126. [Google Scholar] [CrossRef]
- Bansal, M.; Poonia, A.; Kolluri, S.R.P.; Bansal, M.; Kolluri, S.R.P. Introduction on Bioactive Compounds, Sources and their Potential Applications 1. In Bioactivecomponents: A Sustainable System for Good Health and Well-Being; Springer: Berlin/Heidelberg, Germany, 2022; pp. 3–26. [Google Scholar]
- Zhang, Y.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic Compounds: Current Industrial Applications, Limitations and Future Challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Maresca, P.; Ciccarone, R. The Effects of High Hydrostatic Pressure on the Polyphenols and Anthocyanins in Red Fruit Products. Procedia Food Sci. 2011, 1, 847–853. [Google Scholar] [CrossRef][Green Version]
- Grobelna, A.; Kalisz, S.; Kieliszek, M. Effect of Processing Methods and Storage Time on the Content of Bioactive Compounds in Blue Honeysuckle Berry Purees. Agronomy 2019, 9, 860. [Google Scholar] [CrossRef]
- Saarniit, K.; Lang, H.; Kuldjärv, R.; Laaksonen, O.; Rosenvald, S. The Stability of Phenolic Compounds in Fruit, Berry, and Vegetable Purees Based on Accelerated Shelf-Life Testing Methodology. Foods 2023, 12, 1777. [Google Scholar] [CrossRef]
- Dobrowolska-Iwanek, J.; Zagrodzki, P.; Galanty, A.; Fołta, M.; Kryczyk-Kozioł, J.; Szlósarczyk, M.; Rubio, P.S.; Saraiva de Carvalho, I.; Pasko, P. Determination of Essential Minerals and Trace Elements in Edible Sprouts from Different Botanical Families—Application of Chemometric Analysis. Foods 2022, 11, 371. [Google Scholar] [CrossRef]
- Zakrzewski, A.; Purkiewicz, A.; Jakuć, P.; Wiśniewski, P.; Sawicki, T.; Chajęcka-Wierzchowska, W.; Tańska, M. Effectiveness of Various Solvent-Produced Thyme (Thymus vulgaris) Extracts in Inhibiting the Growth of Listeria monocytogenes in Frozen Vegetables. NFS J. 2022, 29, 26–34. [Google Scholar] [CrossRef]
- Dioha, I.J.; Olugbemi, O.; Onuegbu, T.U.; Shahru, Z. Determination of Ascorbic Acid Content of Some Tropical Fruits by Iodometric Titration. Int. J. Biol. Chem. Sci. 2011, 5, 2180–2184. [Google Scholar] [CrossRef][Green Version]
- Yang, L.; Zhang, H.; Cheng, L.; Gu, Z.; Hua, D.; Qi, X.; Qian, H.; Wang, L. Effect of Extrusion on the Hydrophilic Antioxidant Capacity of Four Whole Grains. J. Food Nutr. Res. 2014, 2, 80–87. [Google Scholar] [CrossRef][Green Version]
- Kempkes, M.; Simpson, R. Pulsed Electric Field (PEF) Processing of Fruit and Vegetables. In Proceedings of the 2018 IEEE International Power Modulator and High Voltage Conference (IPMHVC), Jackson, WY, USA, 3–7 June 2018; pp. 499–503. [Google Scholar]
- Zhou, W.; Sarpong, F.; Zhou, C. Use of Ultrasonic Cleaning Technology in the Whole Process of Fruit and Vegetable Processing. Foods 2022, 11, 2874. [Google Scholar] [CrossRef]
- Pan, Z. Innovative Infrared Heating Technologies for Food and Agricultural Processing. Technol. Innov. 2020, 21, 1–16. [Google Scholar] [CrossRef]
- Oliveira, M.; Abadias, M.; Usall, J.; Torres, R.; Teixidó, N.; Viñas, I. Application of Modified Atmosphere Packaging as a Safety Approach to Fresh-Cut Fruits and Vegetables—A Review. Trends Food Sci. Technol. 2015, 46, 13–26. [Google Scholar] [CrossRef]
- Tobal, T.M.; Rodrigues, L.V. Effect of Storage on the Bioactive Compounds, Nutritional Composition and Sensory Acceptability of Pitanga Jams. Food Sci. Technol. 2019, 39, 581–587. [Google Scholar] [CrossRef]
- Kopjar, M.; Pichler, A.; Turi, J.; Pilizota, V. Influence of Trehalose Addition on Antioxidant Activity, Colour and Texture of Orange Jelly During Storage. Int. J. Food Sci. 2016, 51, 2640–2646. [Google Scholar] [CrossRef]
- Montero-Prado, P.; Ruiz Morales, G.A. Recent Advances in Research and Development to Increase Shelf Life and Safety of Packaged Foods: New Technologies in Food Packaging. Agron. Mesoam. 2022, 33, 48389. [Google Scholar] [CrossRef]
- Redding, M.; Bolten, S.; Gu, G.; Luo, Y.; Micallef, S.A.; Millner, P.; Nou, X. Growth and Inactivation of Listeria monocytogenes in Sterile Extracts of Fruits and Vegetables: Impact of the Intrinsic Factors pH, Sugar and Organic Acid Content. Int. J. Food Microbiol. 2023, 386, 110043. [Google Scholar] [CrossRef]
- Taştan, Ö. Effect of Dietary Fiber Enrichment on Quality Characteristics and Consumer Acceptance of Fruit Snacks. Akad. Gıda 2023, 21, 343–352. [Google Scholar] [CrossRef]
- Pandanwangi, A.A.; Rosida, R.; Sudiana, I.M.; Napitupulu, T.P.; Kanti, A. Effect of Sucrose Concentration on Microbiological, Physicochemical, Antioxidant Activity, and Organoleptic Characteristics of Salak Fruit Juice Beverage Fermented with Lactobacillus plantarum InaCC B153. AJARCDE 2024, 2024, 169–174. [Google Scholar] [CrossRef]
- Krahulcová, M.; Micajová, B.; Olejníková, P.; Cverenkárová, K.; Bírošová, L. Microbial Safety of Smoothie Drinks from Fresh Bars Collected in Slovakia. Foods 2021, 10, 551. [Google Scholar] [CrossRef]
- Sequino, G.; Valentino, V.; Torrieri, E.; De Filippis, F. Specific Microbial Communities are Selected in Minimally-Processed Fruit and Vegetables According to the Type of Product. Foods 2022, 11, 2164. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gławdel, E.; Cebulak, T.; Ochmian, I. Determination of Phytochemical Composition and Antioxidant Capacity of 22 Old Apple Cultivars Grown in Poland. Eur. Food Res. Technol. 2018, 244, 647–662. [Google Scholar] [CrossRef]
- Salazar-Orbea, G.L.; Garcia-Villalba, R.; Bernal, M.J.; Hernandez-Jimenez, A.; Egea, J.A.; Tomas-Barberan, F.A.; Sanchez-Siles, L.M. Effect of Storage Conditions on the Stability of Polyphenols of Apple and Strawberry Purees Produced at Industrial Scale by Different Processing Techniques. J. Agric. Food Chem. 2023, 71, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.; Lahlou, R.A.; Silva, L.R. Phenolic Compounds from Cherries and Berries for Chronic Disease Management and Cardiovascular Risk Reduction. Nutrients 2024, 16, 1597. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Miller, M.G.; Thamgthaeng, N.; Sott, T.M.; Shukitt, B.H.; Edirisinghe, I.; Burton-Freeman, B. Metabolic Fate of Strawberry Polyphenols After Chronic Intake in Healthy Older Adults. Food Funct. J. 2018, 9, 96–106. [Google Scholar] [CrossRef]
- Čížková, H.; ŠevČík, R.; Rajchl, A.; Voldřich, M. Nutritional Quality of Commercial Fruit Baby Food. Czech J. Food Sci. 2009, 27, 134–137. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Quality Parameters, Bioactive Compounds and Their Correlation with Antioxidant Capacity of Commercial Fruit-Based Baby Foods. Food Sci. Technol. Int. 2013, 20, 479–487. [Google Scholar] [CrossRef]
- Sanchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. The Folin-Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Anal. Methods 2013, 3, 5990–5999. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.-K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef] [PubMed]
- Akpomie, T.; Augustine, A.; Anwani, S.; Aandaka, B.I. Comparative Analysis of Ascorbic Acid Content of Three Varieties of Apple Fruit Sold in Lafia Open Markets, North Central Nigeria. Int. J. Adv. Chem. 2021, 9, 8–11. [Google Scholar] [CrossRef]
- Ravimannan, N.; Nisansala, A. Study on Antioxidant Activity in Fruits and Vegetables—A Review. Int. J. Adv. Res. Biol. Sci. 2017, 4, 93–101. [Google Scholar] [CrossRef]
- Filipiak-Florkiewicz, A.; Dereń, K. The Content of Phenolic Compounds and Antioxidant Activity Ready to Eat Desserts for Infants. Rocz. Panstw. Zakl. Hig. 2011, 62, 383–388. [Google Scholar] [PubMed]
- Gu, C.; Howell, K.; Dushea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol-Rich Fruits and Vegetables and their Potential Antioxidant Activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of the Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Żmudzińska, A.; Puścion-Jakubik, A.; Bielecka, J.; Grabia, M.; Soroczyńska, J.; Mielcarek, K.; Socha, K. Health Safety Assessment of Ready-to-Eat Products Consumed by Children Aged 0.5–3 Years on the Polish Market. Nutrients 2022, 14, 2325. [Google Scholar] [CrossRef]
- Feszterová, M.; Kowalska, M.; Mišiaková, M. Stability of Vitamin C Content in Plant and Vegetable Juices under Different Storing Conditions. Appl. Sci. 2023, 13, 10640. [Google Scholar] [CrossRef]
- Cloney, K.; Ramsey, S.; Burns, E. Vitamin C deficiency in a 12-year-old male presenting with knee pain: A case report. Clin. Corresp. 2022, 24, 544–546. [Google Scholar] [CrossRef]
- Carr, A.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Khan, A.; Shamrez, B.; Lital, U.; Zeb, A.; Rehman, Z.; Naz, R.; Khan, S.H.; Shah, A.S. Effect of Sucrose Solution and Chemical Preservatives on Overall Quality of Strawberry Fruit. Food Process. Technol. 2014, 6, 2. [Google Scholar] [CrossRef]
- Patthamakanokporn, O.; Puwastien, P.; Nitithamyong, A.; Sirichakwal, P.P. Changes of antioxidant activity and total phenolic compounds during storage of selected fruits. J. Food Compos. Anal. 2008, 21, 241–248. [Google Scholar] [CrossRef]
- Makule, E.; Dimoso, N.; Tassou, S.A. Precooling and Cold Storage Methods for Fruits and Vegetables in Sub-Saharan Africa—A Review. Horticulturae 2022, 8, 776. [Google Scholar] [CrossRef]
- Paulsen, E.; Moreno, D.A.; Martinez-Romero, D.; Garcia-Viguera, C. Bioactive Compounds of Broccoli Florets as Affected by Packing Micro-perforations and Storage Temperature. Coatings 2023, 13, 568. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Kim, Y.H.; Won, Y.-S.; Yang, X.; Kumazoe, M.; Yamashita, S.; Hara, A.; Takagaki, A.; Goto, K.; Nanjo, F.; Tachibana, H. Green Tea Catechin Metabolites Exert Immunoregulatory Effects on CD4+ T Cell and Natural Killer Cell Activities. J. Agric. Food Chem. 2016, 64, 3591–3597. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Darzi, M.; Abbasi, K.; Ghiasvand, R.; Tabib, M.A.; Rouhani, M.H. The Association Between Dietary Polyphenol Intake and Attention-deficit Hyperactivity Disorder: A Case-Control Study. BMC Pediatr. 2022, 22, 700. [Google Scholar] [CrossRef]
- Bouzari, A.; Holstege, D.; Barrett, D.M. Mineral, Fiber, and Total Phenolic Retention in Eight Fruits and Vegetables: A Comparison of Refrigerated and Frozen Storage. J. Agric. Food Chem. 2015, 63, 951–956. [Google Scholar] [CrossRef]
- Yadav, S.; Arora, S.K.; Vats, S. Vitamins and Minerals: A Review on Processing Losses and Strategies to Control It. Mod. Conc. Dev. Agron. 2023, 12, 1178–1182. [Google Scholar] [CrossRef]
- Alshallash, K.S.; Shahat, M.; Ibrahim, M.I.; Hegazy, A.I.; Hamdy, A.E.; Elnaggar, I.A.; El-Wahend, N.A.; Taha, I.M. The Effect of Different Processing Methods on the Behavior of Minerals Content in Food Products. J. Ecol. Eng. 2023, 24, 263–275. [Google Scholar] [CrossRef]
- Yue, T.; Zhang, Q.; Li, G.; Qin, H. Global Burden of Nutritional Deficiencies among Children under 5 Years of Age from 2010 to 2019. Nutrients 2022, 14, 2685. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron Deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).