Abstract
This study investigates the cytotoxicity of various orthodontic archwires, which are essential in directing tooth movement through biomechanical forces. With advancements in material science, different archwire materials have been developed to balance mechanical performance with aesthetic and biological considerations. The study focuses on evaluating the biocompatibility and mechanical properties of stainless steel, nickel–titanium, and chromium–cobalt archwires, particularly their cytotoxic effects on oral cavity cells. In vitro cell culture experiments with fibroblasts, combined with scanning electron microscopy (SEM) analysis, were conducted to assess cell viability and morphology. The results revealed significant differences in cytotoxicity, with copper wires showing high toxicity and causing extensive cell death, while nickel–titanium and chromium–cobalt wires supported better cell viability and healthier cell morphology. These findings highlight the importance of selecting archwire materials that ensure mechanical efficiency without compromising cellular health, emphasizing the need for ongoing assessment of material biocompatibility in the oral environment.
1. Introduction
Rapid developments in orthodontics are revolutionary factors in diagnostic processes, treatment execution, and long-term care management. The mainstay of these advancements is the archwire, an essential component in orthodontic therapy. Archwires within fixed orthodontic appliances play a pivotal role in delivering targeted forces to the teeth, using stored energy to induct biomechanical movement and produce torque as they seek to return to their original form. It is widely recognized in the orthodontic field that gentler forces are preferable to achieve superior treatment results compared to stronger ones [1].
Several key attributes, such as malleability, flexibility, durability, ease of welding, and affordability, determine the performance of archwires. Table 1 [2] shows the characteristics of the individual materials used to make archwires. The complex demands placed on wires mean that no single material can meet all requirements, leading to the development of a variety of archwire materials tailored to specific treatment objectives. The significance of aesthetics in treatment has grown, resulting in archwires that are effective and aesthetically appealing. Therefore, orthodontic specialists face the challenge of selecting the most suitable wire, taking into account a spectrum of choices, from aesthetically pleasing and coated wires to those fabricated from advanced materials such as super engineering plastics, nanocoated substances, and medical grade titanium [3,4].

Table 1.
Features of selected orthodontic archwires.
The key to successful orthodontic tooth movement lies in the application of uniform, mild forces. Insufficient force does not adequately stress the periodontal ligament (PDL) to initiate movement, while excessive force can slow progress and cause tissue damage. It is widely recognized in the orthodontic field that gentler forces are preferable to achieve superior treatment results compared to stronger ones. Identifying an optimal force range is crucial to facilitate tooth movement through bone remodeling, involving growth, resorption, and renewal. The challenge of overcoming bone resorption at the PDL interface is a significant challenge. Advancements in archwire technology aim to produce materials that deliver these optimal forces consistently, reducing the need for frequent adjustments. According to the principles of Hooke’s law and Burstone’s load deflection rate, an ideal archwire is characterized by a low load deflection rate over a wide operating range, aligning with the orthodontic principle of applying ideal forces for treatment [5].
Initially, dentistry used noble metals, combining gold with platinum or copper, until the 1930s, when these were replaced by stainless steel. Today, orthodontics employs several alloys to create metal archwires: stainless steel (SS), chromium–cobalt steel (CrCo), nickel–titanium alloys, and titanium–molybdenum (Table 2).

Table 2.
Percent composition of different types of orthodontic alloys.
Stainless steel (SS), specifically the austenitic stainless steel chromium–nickel blend (Vipla 18/8) blend, is frequently chosen to make orthodontic archwires. This blend, consisting of carbon, iron, 18% chromium, 8% nickel and small amounts of manganese, silicon, phosphorus, and sulphur, offers enhanced durability and ductility thanks to nickel, while its rigidity can be adjusted through the annealing process. Chromium contributes to its corrosion resistance by creating a protective oxide coating, though the gradual release of nickel could impact biocompatibility. Compared to nickel–titanium variants, stainless steel wires are less likely to trigger allergies and can be easily manipulated and connected using welding or soldering techniques. They offer an economical solution but are characterized by their rigidity and limited flexibility, which requires more regular adjustment in treatment [1,2].
Elgiloy, a chromium–cobalt steel (CrCo) alloy, is known for its enhanced elasticity and corrosion resistance, attributed to the addition of 40% cobalt and 20% chromium to its base composition. This alloy, which can be modified by heat treatment to become malleable and subsequently hard and resilient, has found specific applications in orthodontics, such as palatal transpositions, despite a decline in popularity due to the emergence of newer alloys [2]. Material science advancements have introduced Elgiloy among various alloys crafted for orthodontic wires. Favored by orthodontists, especially in its blue form for its formability and heat treatment-enhanced durability, Elgiloy faces challenges in clinical use due to its increased deformation after loading, as revealed by three-point bending tests. This deformation might limit its application, yet Elgiloy’s mechanical properties remain consistent across manufacturers. Compared to stainless steel (SS) and titanium–molybdenum alloy (TMA), Elgiloy demonstrates force levels similar to those of SS at lower loadings but shows a decrease at higher loadings. Despite its beneficial flexibility, the tendency of the alloy to deform poses potential challenges in orthodontic practice [6].
Nickel–Titanium Alloy (NiTi): Originating in the 1960s in the United States, the nickel–titanium alloy (NiTi) was introduced to the field of orthodontics by Andreasen. This alloy is known for its remarkable elasticity, resistance to corrosion, and the ability to recall its shape. Such features enable it to be flexible at cooler temperatures and revert to its initial form when warmed. To tailor its characteristics specifically for orthodontic use, this group of materials has undergone a series of refinements [2,7]. NiTi wires exhibit notable shifts in mechanical behavior and the force they apply in response to temperature variations, which are applicable to both conventional and thermally activated wires. Although exposure to high temperatures may cause irreversible deformation, any temporary strain experienced at cooler temperatures can be reversed as warmth is reintroduced [8].
Allergic reactions, particularly to nickel, are a significant concern in orthodontics due to the widespread use of nickel-containing alloys in braces and other appliances. Nickel allergy, which affects up to 30% of women and 3% of men, can cause various oral and dermal symptoms in orthodontic patients. Although the risk of developing nickel sensitivity from orthodontic treatment is low, a history of exposure to nickel, such as through ear piercing, can increase susceptibility. In general, with adverse reactions in orthodontic practices estimated at 0.3–0.4%, understanding and managing potential allergies is crucial to safe and effective treatment [9].
The aim of this study was to evaluate the biocompatibility of selected orthodontic archwires. To achieve this overarching goal, specific objectives were set, including the assessment of the surface structure of the archwires using SEM, and the evaluation of cytotoxicity through proliferation tests and cell viability assessments.
2. Materials and Methods
2.1. Experimental Design
This study focused on in vitro cell culture experiments using fibroblasts to assess the impact of orthodontic archwires on oral cavity cells. Furthermore, the surface characteristics of the orthodontic wires were analyzed using scanning electron microscopy (SEM).
2.2. Selection of Orthodontic Archwires
The following orthodontic wires were used during the treatment:
- Orthos Copper NiTi with a temperature of 27 °C and a size of 0.018 upper/small (Ormco Cooperation, Glendora, CA, USA) with part number 219-0204, which is made from NiTiCu.
- Orthos Copper NiTi with a temperature of 40 °C and a size of 0.016 × 0.022 upper/small (Ormco, part number 219-5208) was utilized, also made from NiTiCu.
- TMA wire with dimensions of 0.016 × 0.022 in rectangular form from Ormco was also used, though the part number is unspecified.
- Remanium® rectangular wire of size 0.41 × 0.56 mm (16 × 22), federhart, from Dentaurum GmbH, Ispringen, Germany, (REF: 766-602-00) was employed, consisting of stainless steel.
- Remaloy®, also rectangular, sized 0.41 × 0.56 mm (16 × 22), hart plus UK (Dentaurum GmbH; REF: 537-510-00), made from a CoCr alloy.
- Pure brass wire, with a diameter of 0.60 mm/23 (Dentaurum GmbH; REF: 572-060-00), was part of the treatment, along with
- Rematitan® Lite, sized 0.41 × 0.41 mm (16 × 16), made from pure NiTi, from Dentaurum GmbH (REF: 766-069-00).
All of the archwires used in the research were sterilized.
2.3. SEM Analysis
Before and after cell colonization, the surfaces of the orthodontic archwires were observed using an SEM XL30 ESEM (Philips, Hamburg, Germany), equipped with a large field detector. The SEM analysis allowed for the examination of the wire surfaces under high water vapor pressure conditions (1.3 kPa). Further evidence of cell adhesion was performed by light microscopy and prior staining of cells with hematoxylin/eosin. With this staining, cell nuclei are blue and the cytoplasm of the cells is stained red.
2.4. Cell Culture Experiments
Different types of cells may exhibit varying responses to exposure to a given metal, as shown in Huang’s experiment (2005) [10], where four types of metal brackets were found to be compatible with human fibroblasts. Human cell cultures are often chosen for research in dentistry; however, they are expensive to maintain, have a long life cycle, and are difficult to analyze quantitatively [11]. The experiments involved mouse fibroblasts (strain L929), a subclone of the parental strain established by W.A. Earle in 1940. These fibroblasts were derived from the normal, vascularized, subcutaneous, and defatted tissue of 100-day-old male C3H/An mice, noted for their functional similarity to human fibroblasts. Archwires were incubated with fibroblasts for three weeks. The control group in this study consisted of fibroblast cultures maintained under identical conditions but without exposure to the examined factor (archwire).
2.5. Cell Culture Conditions
The fibroblasts were seeded at a density of 1 × 105 cells per plate and cultured in DMEM supplemented with 10% fetal bovine serum and 1% streptomycin. The cultures were maintained in a 37 °C incubator with an atmosphere of 95% air and 5% CO2. The medium was replaced every three days, after aspiration of the spent medium, PBS rinsing, trypsin-EDTA digestion, and centrifugation.
2.6. Viability and Proliferation Assays
Cell viability was assessed using the Trypan Blue exclusion method and the CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Madison, WI, USA), which measures ATP as an indicator of metabolically active cells. Cytotoxicity was determined using the CytoTox-Glo® Assay based on the release of proteases from dead cells. The statistical power of the study was calculated separately for the cell viability and cytotoxicity assays based on sample sizes for each material group. For the cell viability assay, the sample sizes were as follows: Brass (n = 12), TMA (n = 12), CrCo (n = 12), SS (n = 12), NiTi 27 (n = 8), NiTi 40 (n = 8), and NiTi (n = 8), with a total sample size of n = 72. For the cytotoxicity assay, the sample sizes were Brass (n = 12), TMA (n = 12), CrCo (n = 12), SS (n = 6), NiTi 27 (n = 8), NiTi 40 (n = 8), and NiTi (n = 8), resulting in a total sample size of n = 66.
2.7. Statistical Analysis
Data were analyzed using SigmaStat software (version 3.5, Systat Software, San Jose, CA, USA). Student’s t-test was applied for normally distributed data, while the Mann–Whitney U test was used for nonnormally distributed data. Results are presented as mean ± standard deviation.
3. Results
3.1. Scanning Electron Microscopy (SEM)
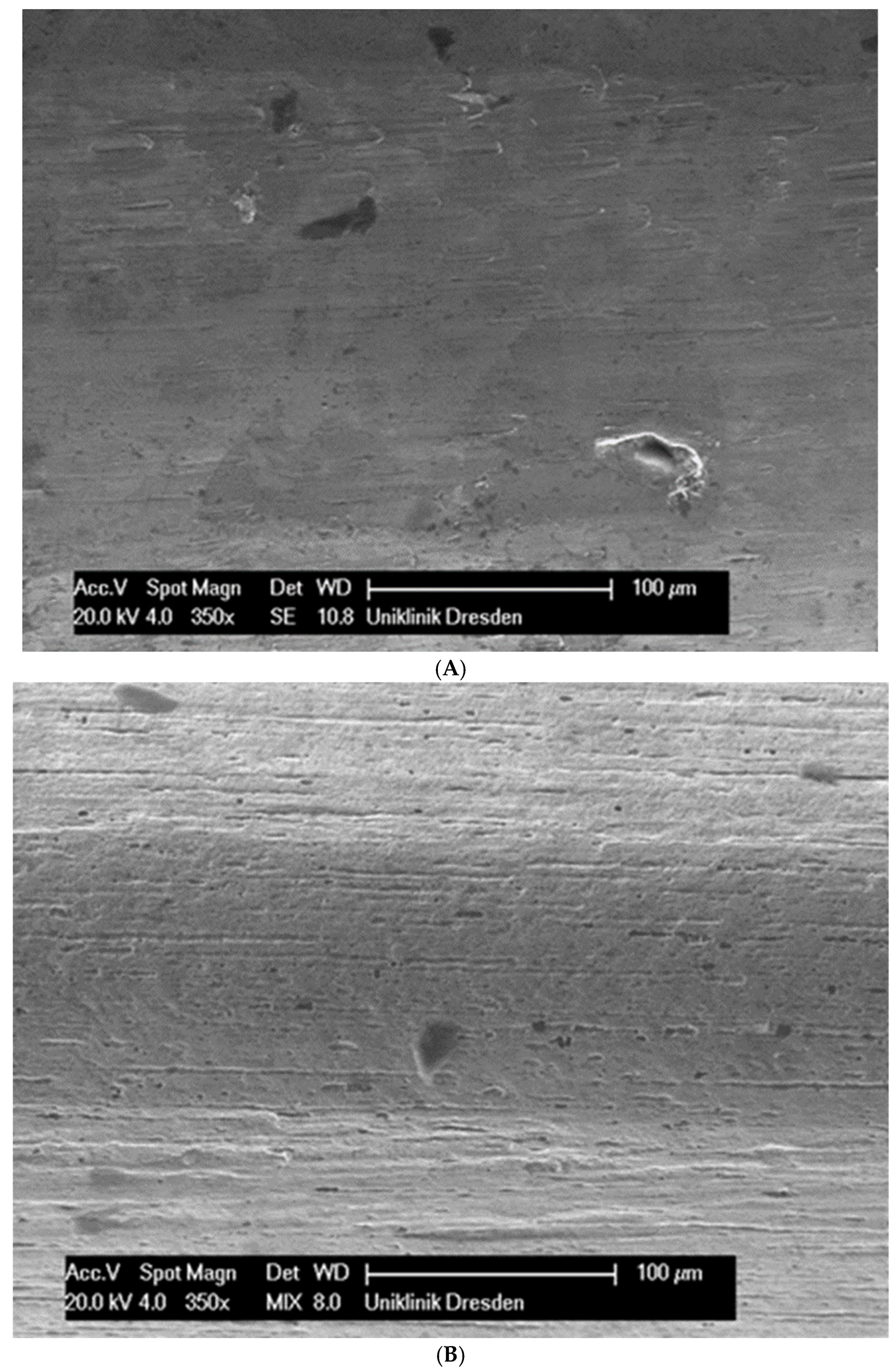
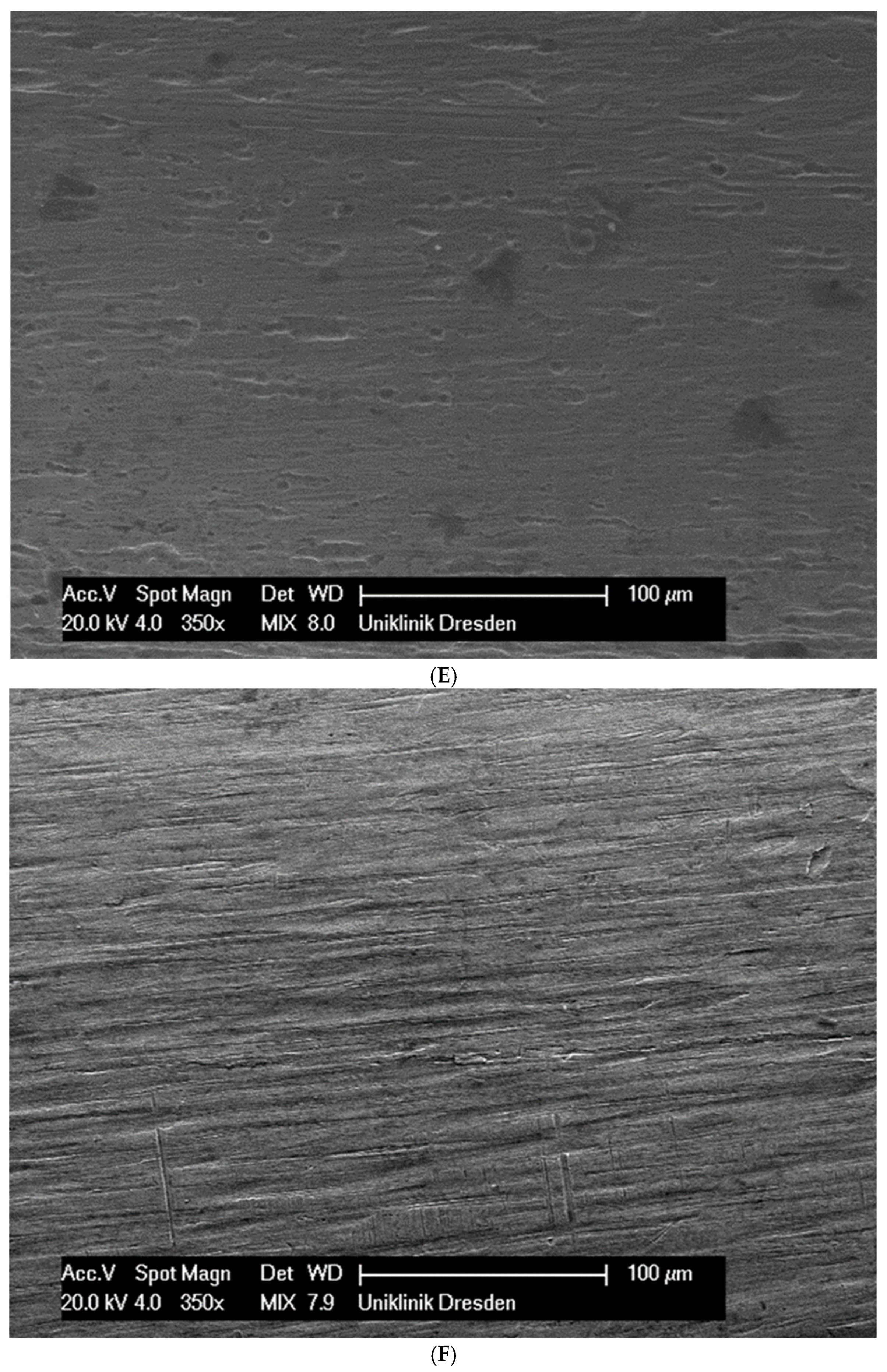
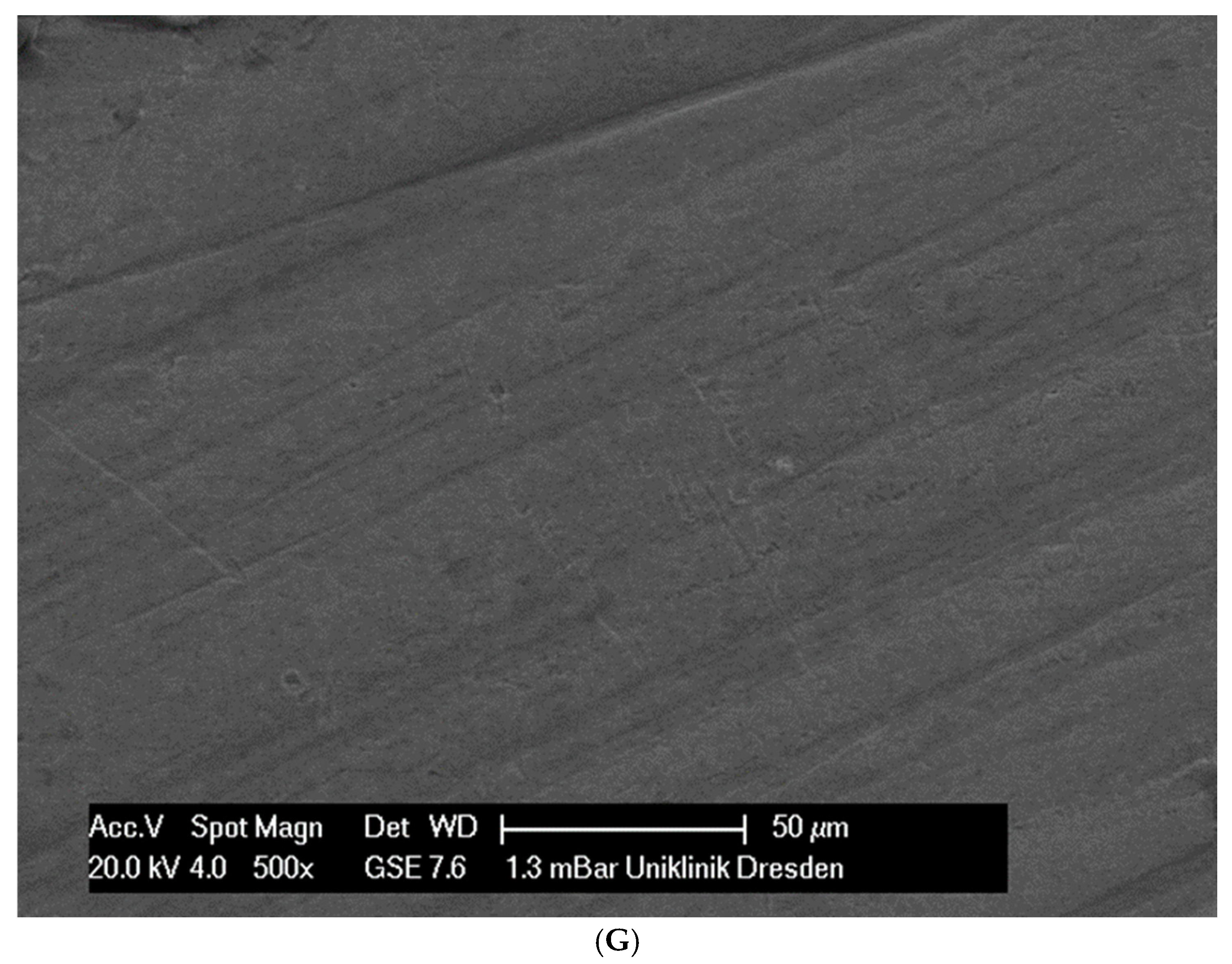
Although the surface of the wires is the first component to interact with the cells and is very important for cell attachments, wire surfaces were investigated by SEM before and after cell attachment. The surface of the arches before the colonization in SEM is visualized in Figure 1A–G.
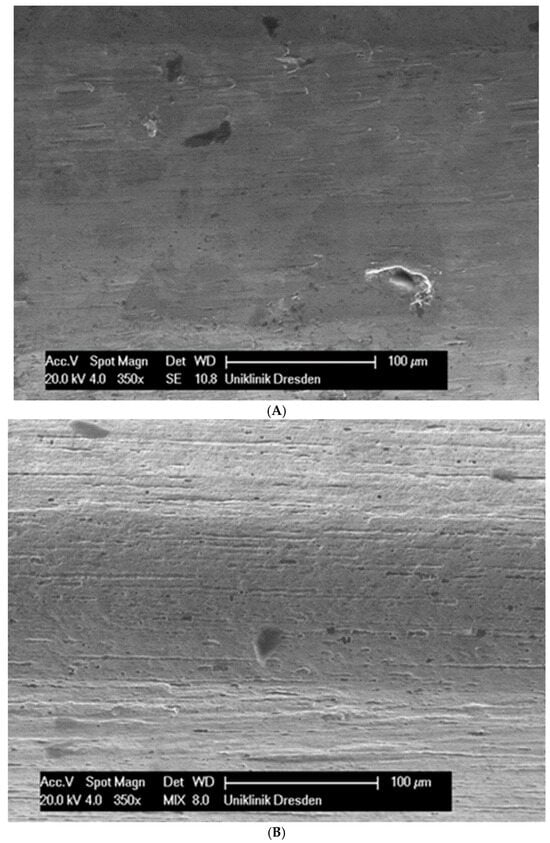

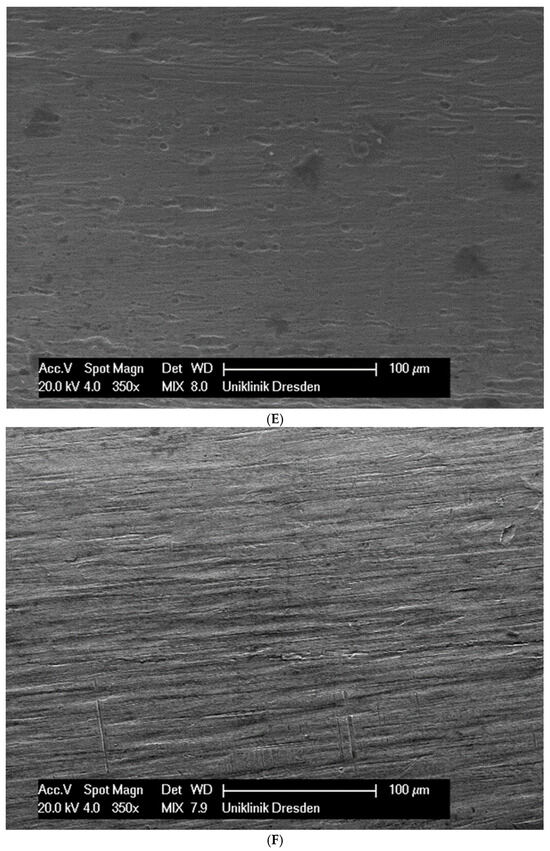
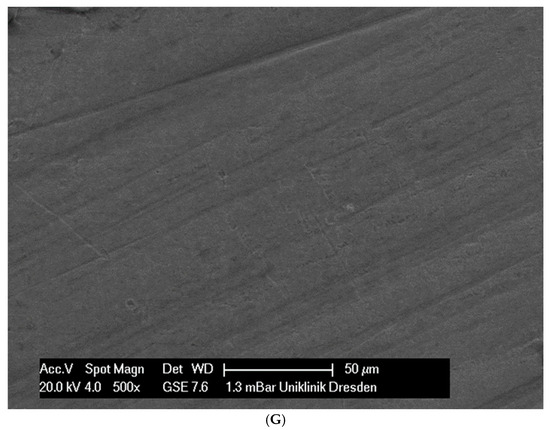
Figure 1.
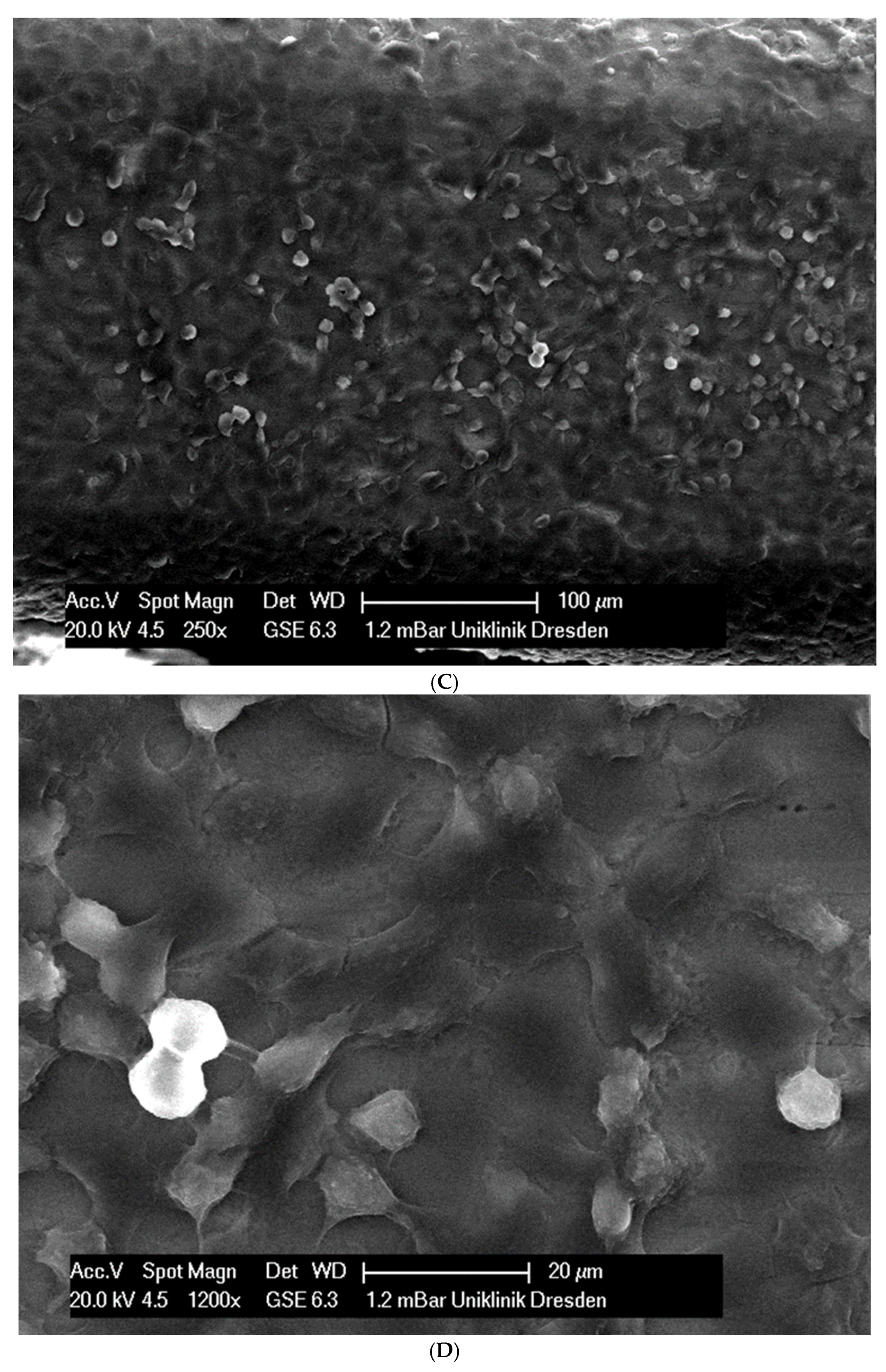
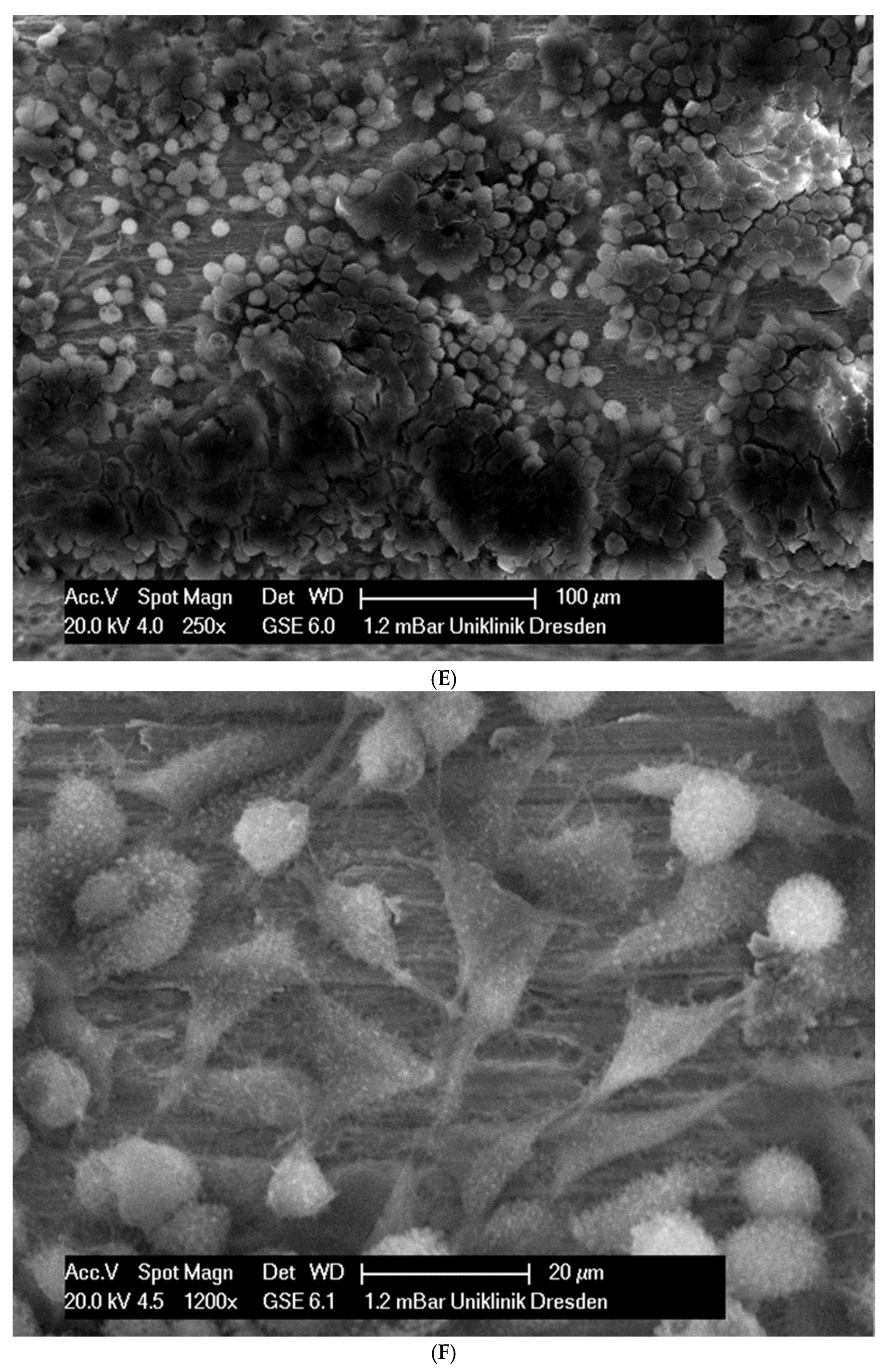
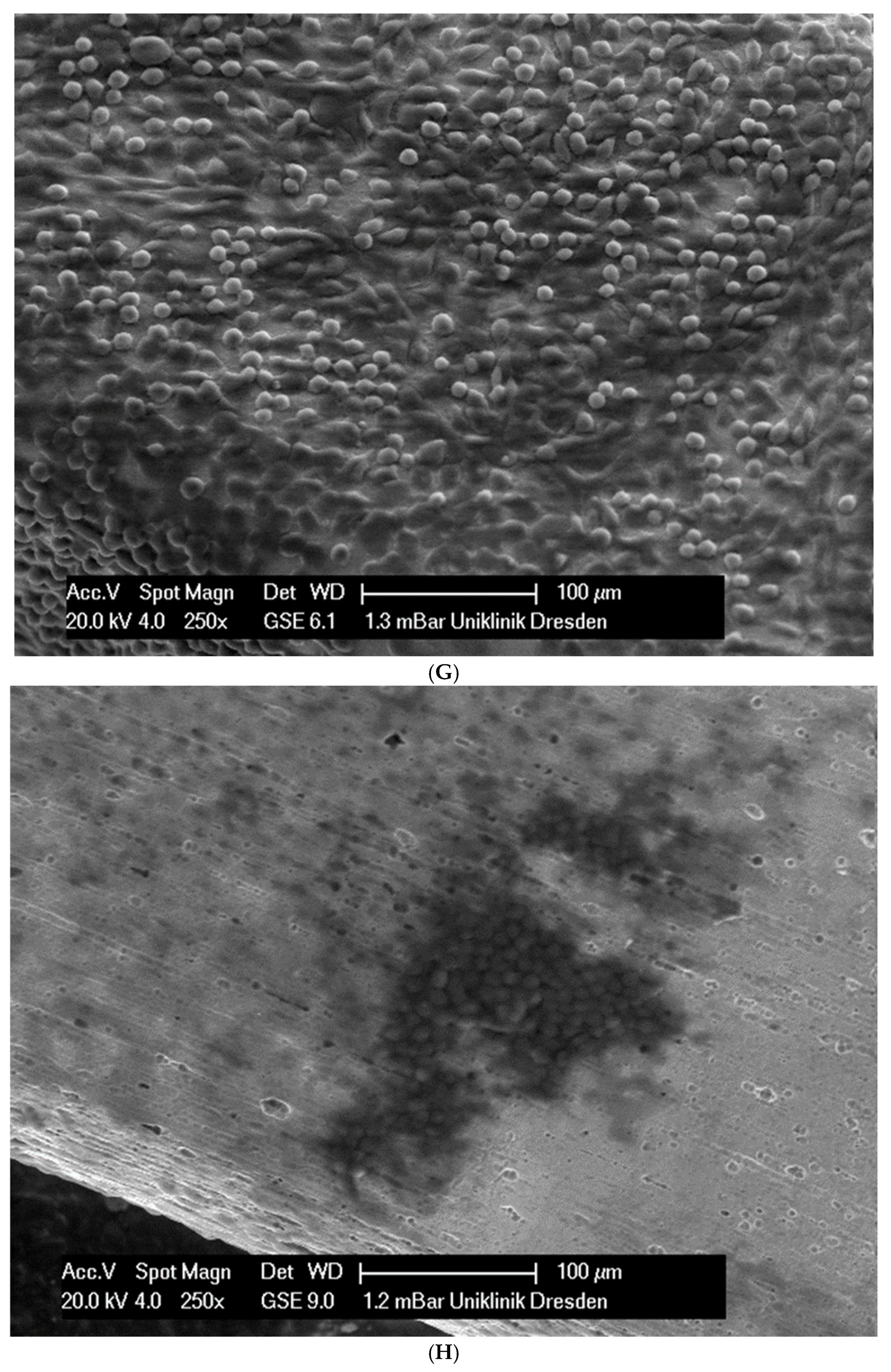
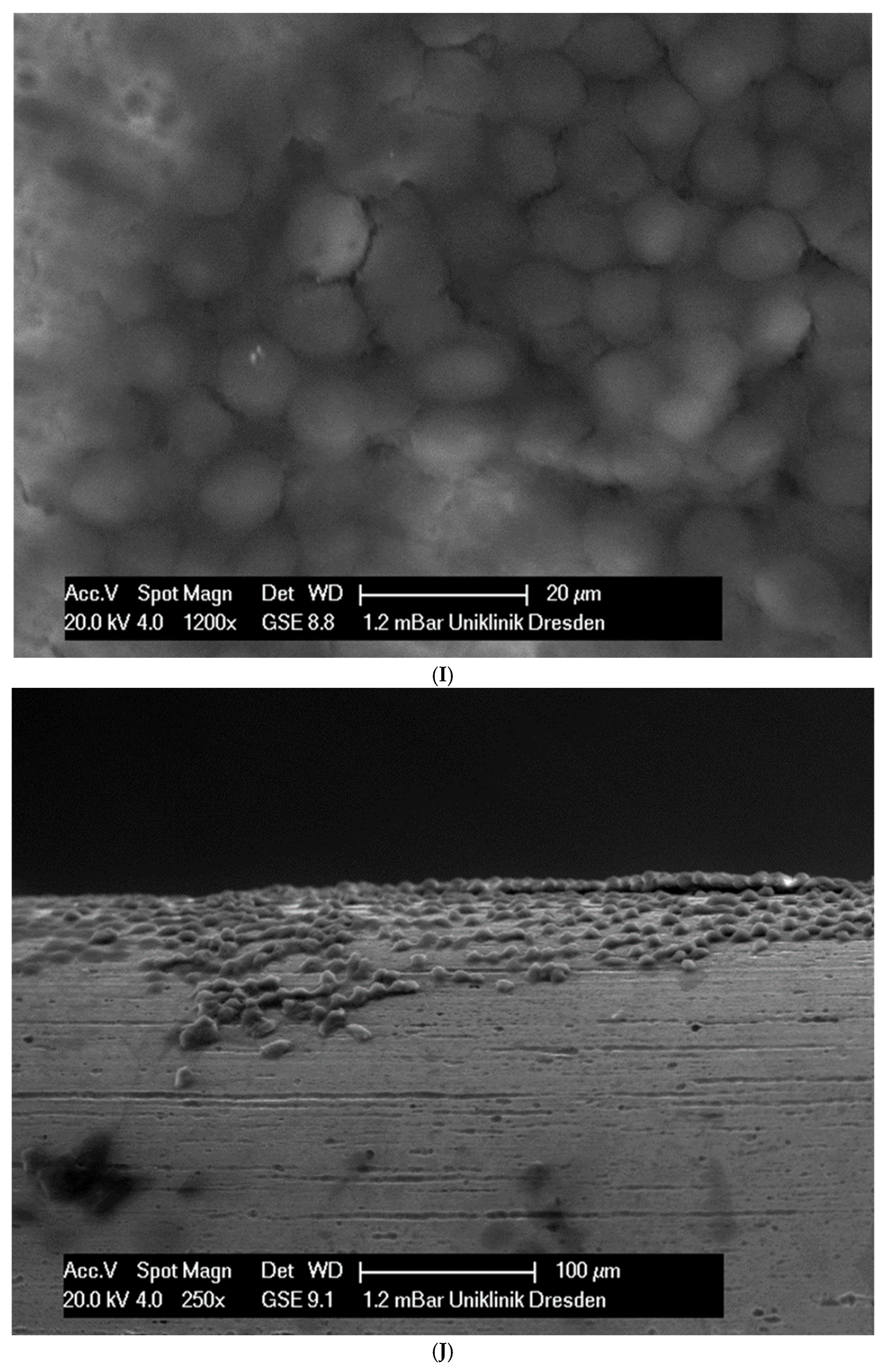
(A): Surface image of a Remanium steel arch. (B): Surface image of a thermal NiTi arch at 40 °C. (C): Surface image of a thermal NiTi arch at 27 °C. (D): Surface image of a copper separation wire (Brass). (E): Surface image of a titanium–molybdenum arch (Rematitan special). (F): Surface image of a chromium–cobalt arch (Remaloy). (G): Surface image of a nickel–titanium arch (Rematitan lite).
The brass and pure NiTi wires showed a very smooth homogenous surface with few small holes, whereas the TMA and stainless steel wires showed a slightly roughened heterogeneous surface also with few small wells. Using the same magnification, CoCr alloy and NiTiCu wires showed a surface with high roughness and heterogeneity such as that formed by etching.
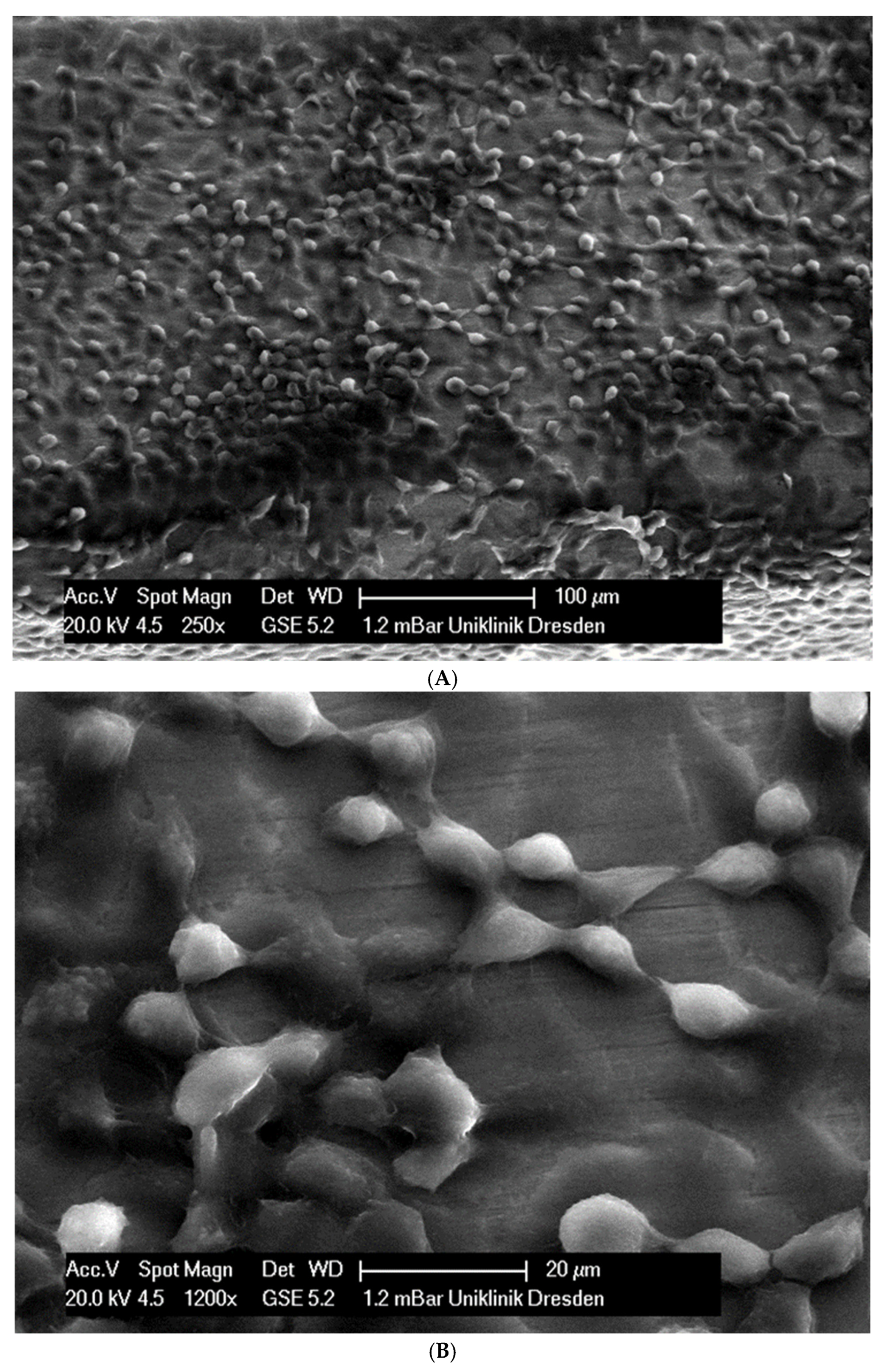
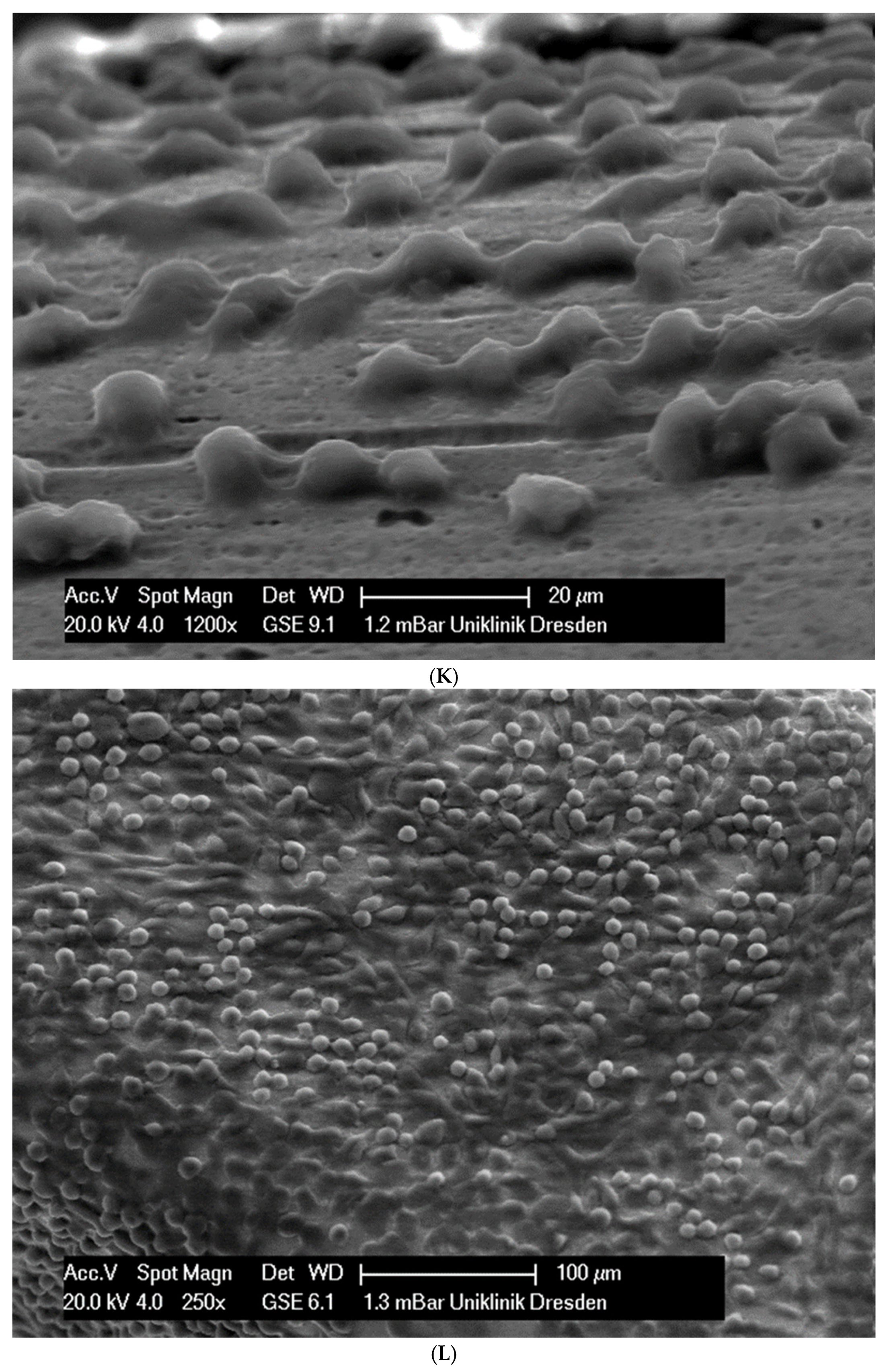
In total, 21 days after cell seeding, a confluent cell layer was detected on the following wires: CoCr alloy, stainless steel, TMA, pure NiTi and both NiTiCu. On TMA, CoCr alloy, pure NiTi and stainless steel, the cells showed a typical cell structure of healthy cells with many cell processes. A special feature of TMA was that the cells grew in large round clusters, which was not detectable by the other three wires. In the case of both NiTiCu wires, cell growth was also detectable. But this was not confluent over the entire wire. The existing cells were rounded, but still in contact with each other. The cells were still alive but exposed to stressors. In contrast to all other wires, no cells were detected on brass. The surface of the arches before the colonization in SEM at 250× and 1200× magnification is visualized in Figure 2A–M.
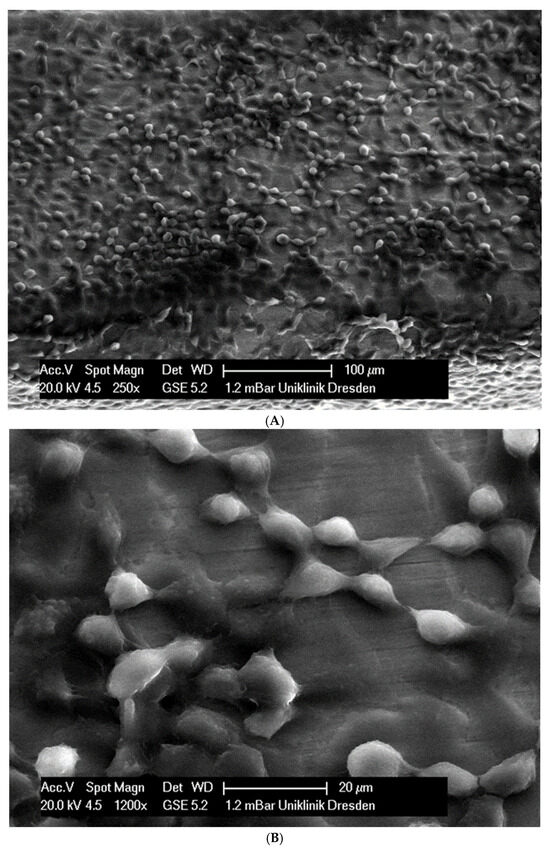
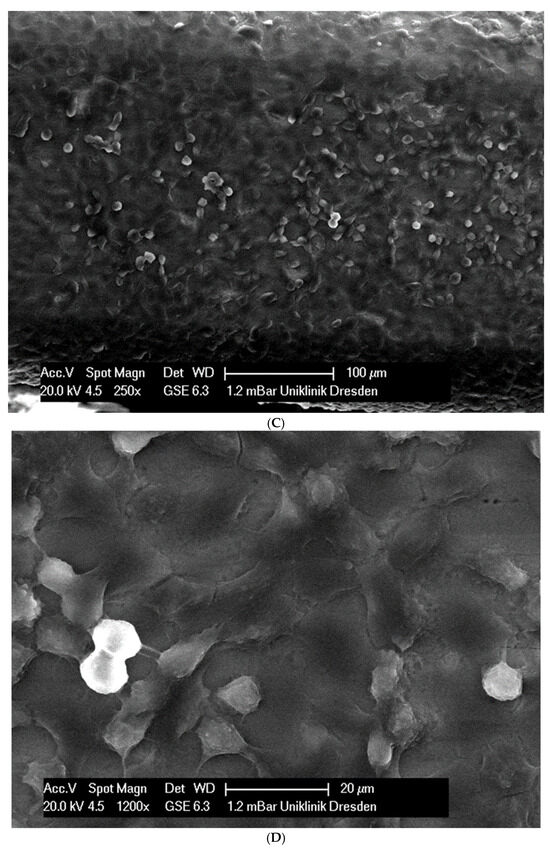
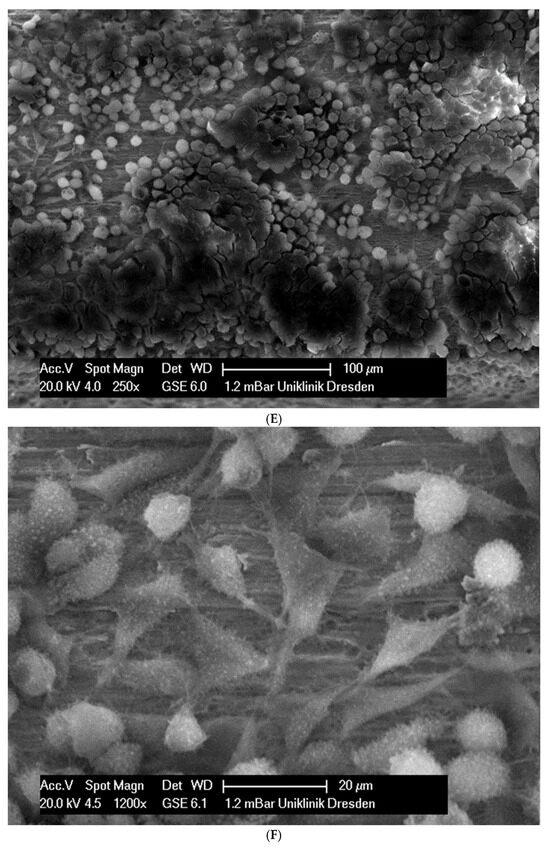
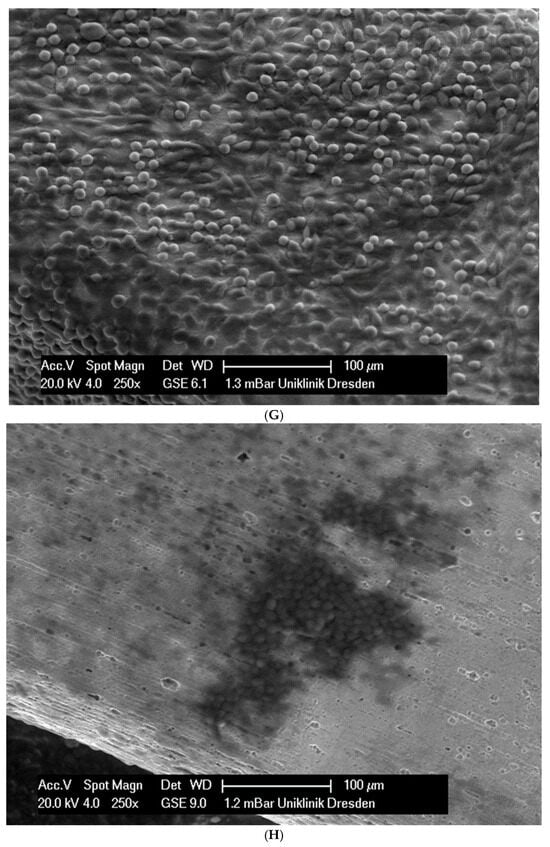
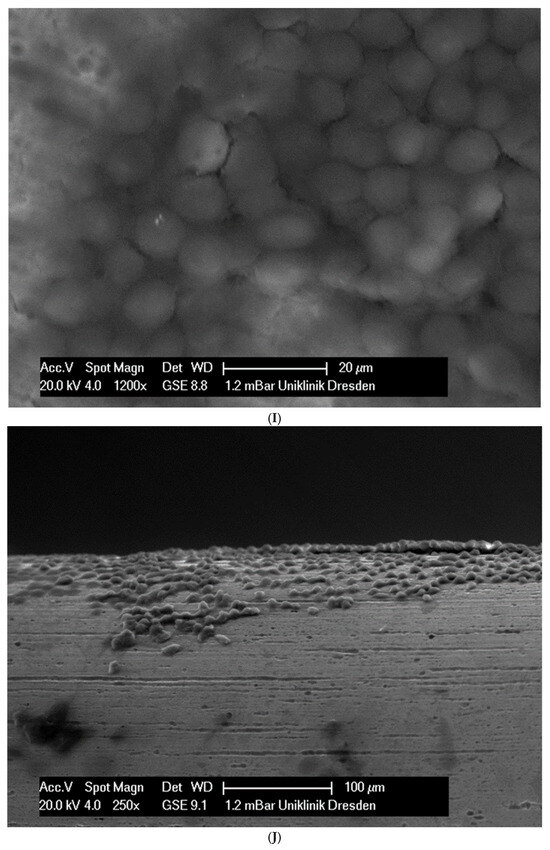
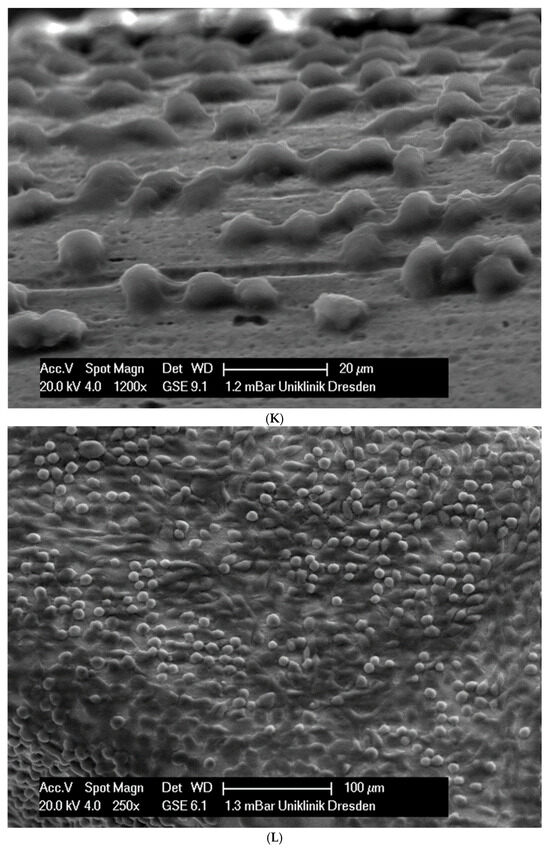

Figure 2.
(A): Remaloy (250×). (B): Remaloy (1200×). (C): Remanium (250×). (D): Remanium (1200×). (E): TMA (250×). (F): TMA (1200×). (G): Brass (250×). (H): NiTi 40 °C (250×). (I): NiTi 40 °C (1200×). (J): NiTi 27 °C (250×). (K): NiTi 27 °C (1200×). (L): NiTi (250×). (M): NiTi (1200×).
3.2. Detection of Cell Adhesion by Light Microscopy
The untreated cells formed a confluent monolayer, where the blue nuclei are mainly recognized. The same picture emerges also for pure NiTi, CoCr alloy and TMA wires. In contrast, the cell layer at stainless steel and NiTiCu is loosened so that more red color is visible. As seen in the scanning electron microscope, no cells grow on brass. The wires cause beyond that almost all cells die. Only isolated cells are stained without any contact with the wire.
3.3. Cell Viability
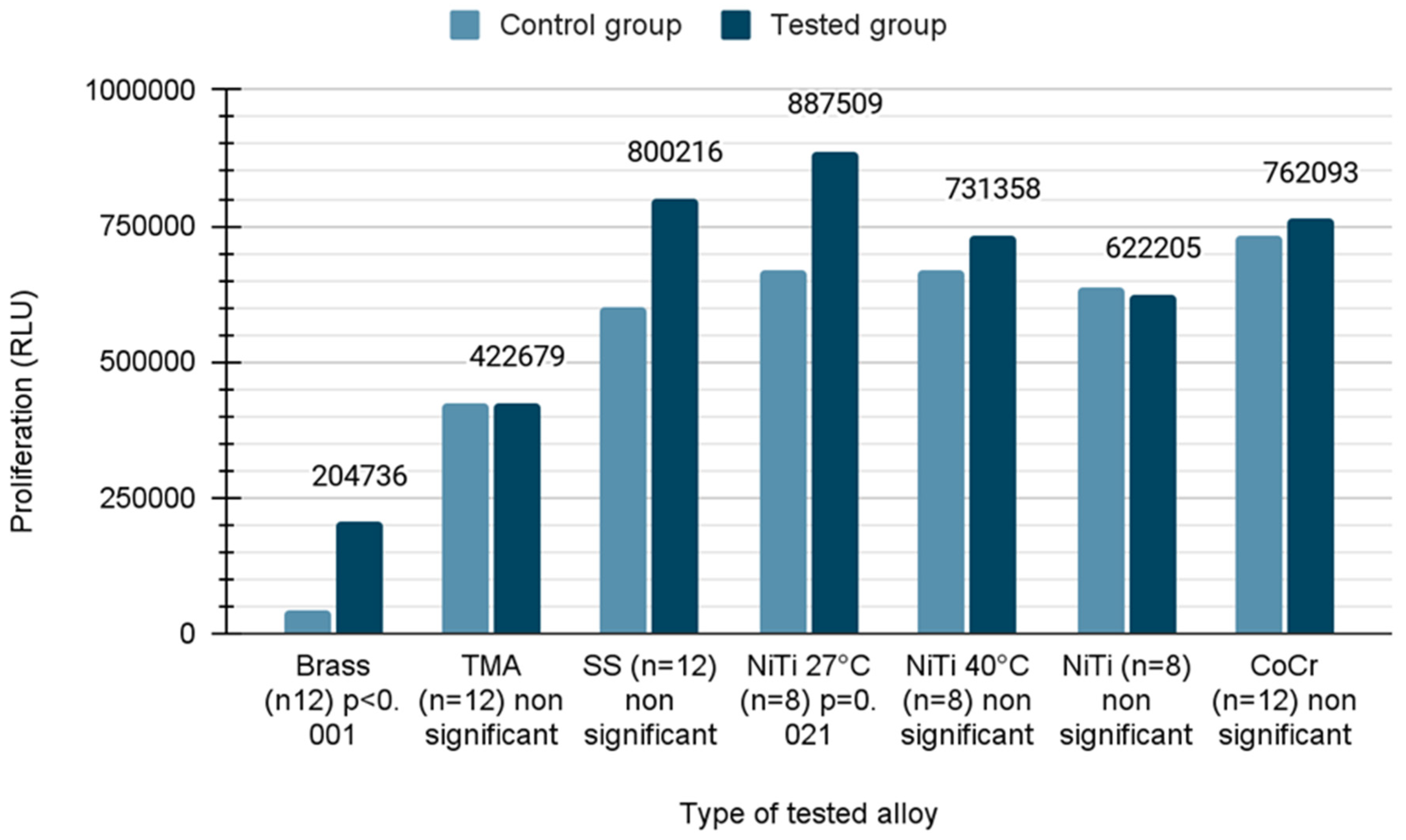
To quantitatively examine the influence of the wires, the mouse fibroblast cells were co-cultured with different wires over a period of 21 days and their viability was analyzed using CellTiter-Glo® assay. Due to the fact that the distribution was normal in the case of TMA, SS, NiTi 40, NiTi and CoCr samples, Student’s t-test was applied. In the remaining cases, the Mann–Whitney U test was used due to the rejection of the hypothesis of normal distribution. In the presence of brass wires, the cell viability was reduced by 51% compared to untreated cells (mean value ± standard error; control versus wire, 419,640 ± 50,455 versus 204,736 ± 116,265, p < 0.001), whereas the cell viability was increased 1.3 fold in the presence of NiTiCu 27 °C wires (668,941 ± 61,633 versus 887,509 ± 57,933, p = 0.021). The growth of cells on different types of wires described in the Relative Light Unit (RLU) is shown in Figure 3.
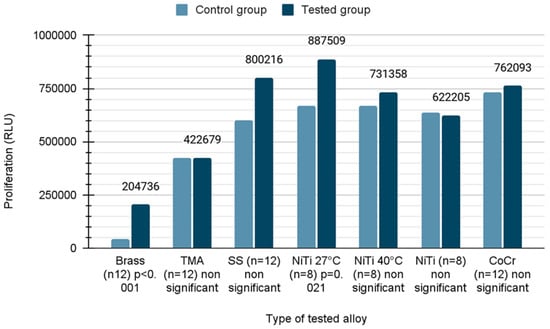
Figure 3.
Proliferation of cells on orthodontic wires.
3.4. Cytotoxicity
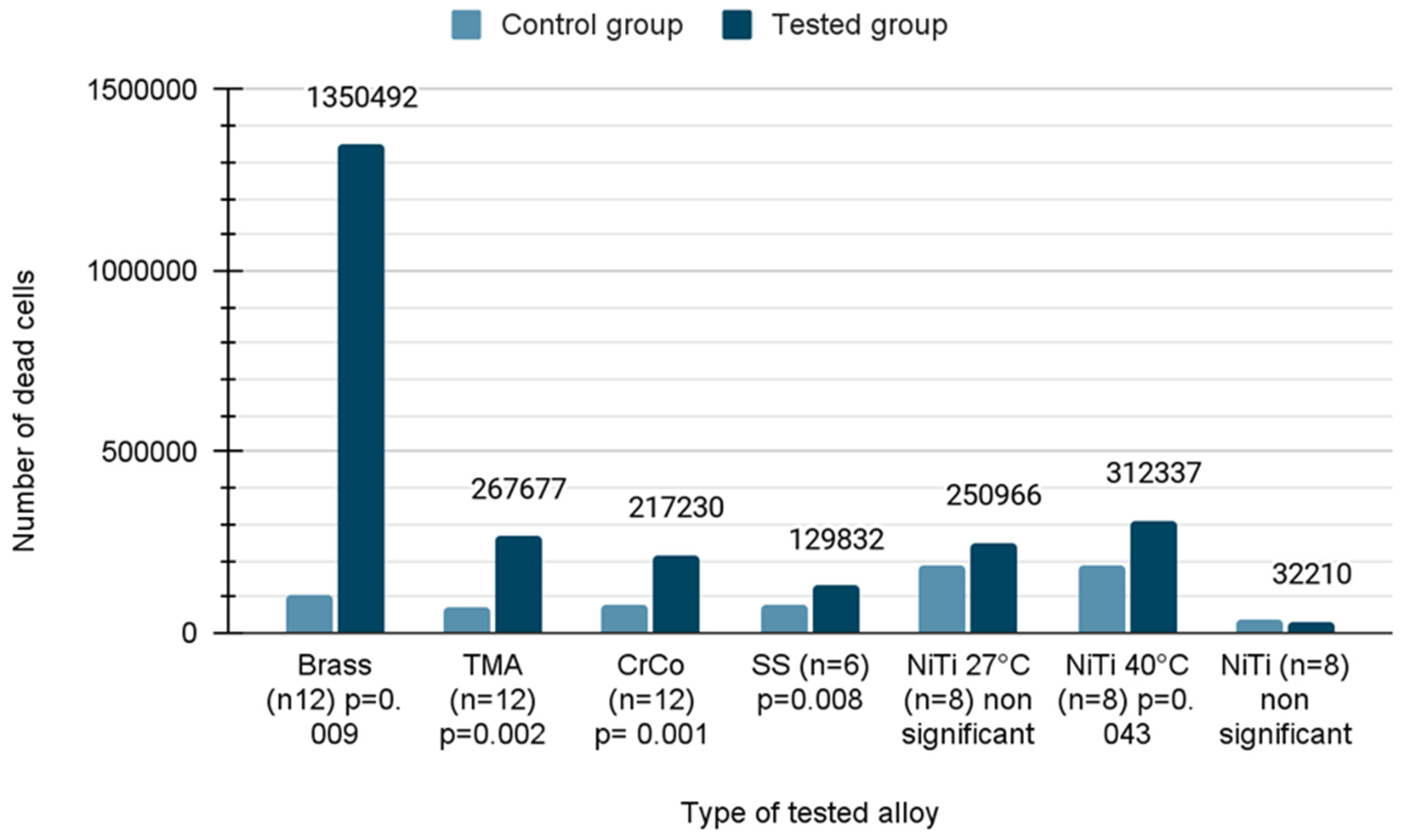
The number of dead cells caused by loss of membrane integrity was calculated using the CytoTox-Glo assay after 21 days of incubation. Due to the fact that the distribution was normal in the case of NiTi 27 and NiTi, the Student’s t-test was applied. In the remaining cases, the Mann–Whitney U test was used due to the rejection of the hypothesis of normal distribution. As seen in the figure, the number of dead cells was significantly increased when using almost all wires with the exception of NiTiCu 27 °C and pure NiTi. The increase in the cytotoxicity ranged between 1.7 fold and 12 fold compared to untreated cells, respectively. The brass wires were the worst (12 fold; p = 0.009), followed by TMA (3.6 fold; p = 0.002), CoCr alloy (2.7 fold; p < 0.001), stainless steel (1.7 fold; p = 0.008) and NiTiCu 40 °C (1.7 fold; p = 0.043) (Figure 4).
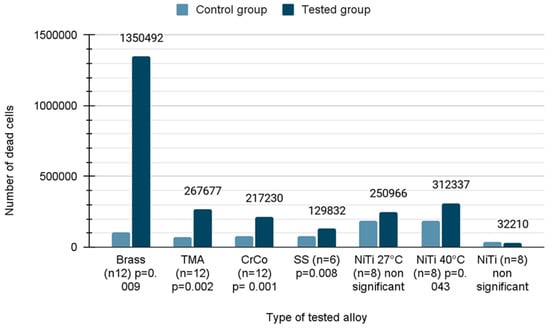
Figure 4.
Number of dead cells on orthodontic wires after incubation.
4. Discussion
4.1. Review of the Literature
As mentioned in Section 1, S. Kapila and K. Sachdeva [1] emphasize that an ideal orthodontic wire should possess not only optimal physical characteristics such as elasticity, stiffness, malleability, and low friction but also essential chemical traits, particularly biocompatibility and stability, to function effectively in fluctuating conditions of the oral cavity.
The biocompatibility of components used in fixed thin-arch orthodontic devices is determined both by their composition and resistance to corrosion, that is, by their ability to release metal ions. It must be emphasized that the specific, “aggressive” conditions prevailing in the oral cavity (such as variable pH, temperature, microbial colonization) create favorable conditions for various types of corrosion, which occurs in all metal alloys used in orthodontics, as noted by G. Schmalz [12].
The principles for the biological evaluation of medical devices are specified in ISO 10993 [13]; however, ISO 15841 [14] and the ADA specification No. 32, relating to orthodontic wires, do not contain clear guidelines for the evaluation of ion release, which are pointed out by T. Eliades and W. Brantley [7], leading to a diversity of methods for testing arches both in vitro and in vivo.
Common methods to assess the biocompatibility of metal alloys in orthodontics include examining the impact of metals on cell cultures and analyzing ion release in solutions such as artificial saliva or NaCl. Due to potential allergenic, cytotoxic, and mutagenic properties, the effects of nickel and chromium ions are most frequently assessed in biocompatibility studies. Nickel, being a component of alloys, is considered potentially toxic and is capable of causing gum inflammation even in non-allergic individuals with good hygiene, as stated by G. Schmalz [10]. However, opinions on NiTi arches in the literature are divided, being described as both toxic [15] and non-toxic [16,17,18].
The results of the in vitro study on the cytotoxic action of released metal ions are varied. Woody and associates found that nickel–chromium alloys were nontoxic in the agar diffusion test, and microscopic observation of cells exposed to nickel-containing alloys showed no differences compared to those of the control group. In contrast, Bumgardner and Lucas [19] observed toxic cellular reactions in the form of reduced proliferation of gingival fibroblasts, similarly as reported by Brantley et al. [20] and A. Berstein et al. [21]. Meanwhile, studies by O. Mockers et al. [22] and H.S. Hafez et al. [23] present diverse results regarding the impact of orthodontic arches on cells. Based on research by M. Mikulewicz [24], the results of cell viability and proliferation tests showed the following order of biocompatibility of alloys: steel > nickel–titanium alloys > titanium. The most favorable ratio of living cells to dead cells was found in nickel–titanium alloys > titanium > steel > nickel.
Several modern studies have explored the cytotoxic effects of orthodontic archwires. Miodrag Čolić et al. [25] found that while NiTi archwires generally show minimal direct cytotoxicity, their physical and chemical properties, such as surface microstructures and corrosion resistance, can influence cellular responses. Lina M. Escobar et al. [26] observed minimal cytotoxic effects in most orthodontic wires after use, except Polytetrafluoroethylene PTFE-coated copper–nickel–titanium wires, which demonstrated significant reductions in cell viability and changes in chemical composition and surface wear. Pervin Imirzalioglu et al. [27] highlighted that recasting dental alloys affects cytotoxicity levels and metal ion release, impacting the biocompatibility of Co-Cr, Ni-Cr, and Au-Pt materials. Roberto Rongo [28] noted that the cytotoxicity of nickel–titanium orthodontic wires vary over time, increasing in cytotoxicity after 30 days, contrasting with the stable biocompatibility of others. These findings underscore the varying impacts of material properties and usage duration on the safety and effectiveness of orthodontic wires.
The variety of study results highlights the complexity of assessing the biocompatibility of orthodontic materials and the need for more research in this area. Locci et al. [29] indicate good biocompatibility of stainless steel; however, they note the cytotoxic effect of the soldering alloy, attributed to the release of silver and palladium ions. Conclusions from studies on various types of cells, including fibroblasts and osteoblasts, illustrate the variability of cellular responses to different metal alloys, as illustrated by different authors, such as E.C. Rose et al. [30] and T. Eliades and associates [31].
4.2. Limitations
One of the key limitations is the variability in the manufacturing processes of orthodontic wires. Differences in alloy composition, surface treatment, and production techniques can lead to variations in surface structure, corrosion resistance, and overall biocompatibility of the wires. These inconsistencies in the manufacturing process can result in differences in how various wires interact with cells.
Additionally, environmental factors within the oral cavity, such as fluctuations in pH, temperature changes, and the presence of bacterial colonization, can significantly influence cellular processes. These factors, often difficult to accurately replicate in in vitro conditions, may lead to discrepancies between laboratory results and clinical outcomes.
Finally, the reliance on specific cell lines in the study presents another limitation. Different cell types, such as fibroblasts, osteoblasts, and epithelial cells, may respond differently to the same material. The variability in cellular reactions to orthodontic materials may mean that results obtained from one cell line are not fully applicable to others. A broader examination of various cell lines in future studies could provide a more comprehensive understanding of biocompatibility.
Due to the fact that the studies were conducted using high-quality materials, which eliminated inaccuracies related to poor-quality orthodontic archwires, the results suggest that orthodontists should pay attention to the origin of a given archwire and consider that the properties described in the article may not apply to cheaper alternatives available on the market. The application of these findings in daily clinical practice may involve the use of these archwires in patients with nickel allergy, compromised immunity or those at risk of systemic diseases. Satisfactory results for each archwire can reinforce the belief that their use in such cases is safe.
4.3. Suggestions for Future Research
Future studies should focus on long-term in vitro and in vivo research to assess the extended effects of orthodontic materials in the oral environment. Monitoring biocompatibility over time could reveal potential changes in toxicity after prolonged use. Research should also prioritize the impact of mechanical stress on cellular responses, as forces like chewing and adjustments may affect cell interactions with wire surfaces. Simulating these conditions could provide more realistic insights. Finally, developing and testing new materials or coatings that minimize cytotoxicity and corrosion, while maintaining mechanical properties, is a promising area for further investigation.
5. Conclusions
In the evaluation of wire surfaces using SEM, it was demonstrated that NiTi wires presented a smooth surface with a few small indentations, while TMA and SS wires exhibited slight surface roughness and heterogeneity with small defects. The CoCr and NiTiCu alloys were characterized by a heterogeneous and porous surface. In the proliferation tests, it was found that no changes in the number of proliferating cells were observed on the Rematitan special (TMA), Remaloy (CrCo), Copper NiTi 40 °C, and Rematitan lite (NiTi) wires, while on the Remanium (SS) and Copper NiTi 27° wires, an increase in the number of proliferating cells was noted. Regarding cell viability assessment, the highest number of dead cells was observed in the presence of Rematitan special and Remaloy, while the lowest number was in the presence of Copper NiTi 27 °C and Rematitan. In conclusion, there is significant need to highlight the critical importance of material selection due to their varying impacts on cellular health.
These results underscore the need to balance mechanical properties with biological safety in the selection of orthodontic wires to ensure effective and safe patient treatment. Research supports the need for an ongoing evaluation of the biocompatibility of orthodontic materials, particularly in the dynamic oral environment, to guide clinical decisions and material advancements in the field of orthodontics.
Author Contributions
Conceptualization, B.T. and M.M.; Methodology, B.T. and M.M.; Validation, M.M.; Formal analysis, B.T.; Investigation, O.T.; Resources, P.S.; Data curation, B.T.; Writing—original draft, O.T. and P.S.; Writing—review & editing, O.T. and P.S.; Supervision, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research eceived no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kapila, S.; Sachdeva, K. Mechanical properties and clinical applications of orthodontic wires. Am. J. Orthod. Dentofac. Orthop. 1989, 96, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Anny, K.; Warych, B.; Deregowska-Nosowicz, P.; Schow, H. (Eds.) Materiały i Techniki Ortodontyczne; PTO: Lublin, Poland, 2009; pp. 75–132. ISBN 978-83-928880-0-0. [Google Scholar]
- Roberts, W.E.; Sarandeep, S.H. Bone physiology, metabolism, and biomechanics in orthodontic practice. In Orthodontics: Current Principles and Techniques, 6th ed.; Graber, L.W., Vanarsdall, R.L., Katherine, E.L., Vig, G., Huang, G.J., Eds.; Elsevier Health Sciences: Oxford, UK, 2016; pp. 99–152. [Google Scholar]
- Proffit, W.R.; Fields, H.W.; Larson, B.E.; Sarver, D.M. Contemporary Orthodontics, 5th ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2019; Volume 213, p. 258. [Google Scholar]
- Kuntz, M.L.; Vadori, R.; Khan, M.I. Review of Superelastic Differential Force Archwires for Producing Ideal Orthodontic Forces: An Advanced Technology Potentially Applicable to Orthognathic Surgery and Orthopedics. Curr. Osteoporos. Rep. 2018, 16, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Alobeid, A.; Hasan, M.; Al-Suleiman, M.; El-Bialy, T. Mechanical properties of cobalt-chromium wires compared to stainless steel and β-titanium wires. J. Orthod. Sci. 2014, 3, 137–141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eliades, T.; Brantley, W.A. Orthodontic Applications of Biomaterials a Clinical Guide; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-08-100383-1. [Google Scholar]
- Lombardo, L.; Toni, G.; Stefanoni, F.; Mollica, F.; Guarneri, M.P.; Siciliani, G. The effect of temperature on the mechanical behavior of nickel-titanium orthodontic initial archwires. Angle Orthod. 2013, 83, 298–305. [Google Scholar] [CrossRef]
- Chakravarthi, S.; Padmanabhan, S.; Chitharanjan, A.B. Allergy and orthodontics. J. Orthod. Sci. 2012, 1, 83–87. [Google Scholar] [CrossRef]
- Huang, H.-H. Surface characterizations and corrosion resistance of nickel-titanium orthodontic archwires in artificial saliva of various degrees of acidity. J. Biomed. Mater. Res. Part A 2005, 44, 629–639. [Google Scholar] [CrossRef]
- Limberger, K.M.; Westphalen, G.H.; Menezes, L.M.; Medina-Silva, R. Cytotoxicity of orthodontic materials assessed by syrvival test Saccharomyces cerevisiae. Dent. Mater. 2011, 27, 81–86. [Google Scholar] [CrossRef]
- Schmalz, G.; Garhammer, P. Biological interactions of dental cast alloys with oral tissues. Dent. Mater. 2002, 18, 396–406. [Google Scholar] [CrossRef]
- ISO 10993; Biological Evaluation of Medical devices—Part 1: Evaluation and Testing Within a Risk Management Process. ISO: Geneva, Switzerland, 2018.
- ISO 15841; Dentistry—Wires for use in Orthodontics. ISO: Geneva, Switzerland, 2014.
- Shih, C.C.; Shih, C.M.; Chen, Y.L.; Su, Y.Y.; Shih, J.S.; Kwok, C.F.; Lin, S.J. Growth inhibition of cultured smooth muscle cells by corrosion products of 316 L stainless steel wire. J. Biomed. Mater. Res. 2001, 57, 200–207. [Google Scholar] [CrossRef]
- Ryhänen, J.; Niemi, E.; Serlo, W.; Niemelä, E.; Sandvik, P.; Pernu, H.; Salo, T. Biocompatibility of nickel-titanium shape memory metal and its corrosion behavior in human cell cultures. J. Biomed. Mater. Res. 1997, 35, 451–457. [Google Scholar] [CrossRef]
- Wever, D.J.; Veldhuizen, A.G.; Sanders, M.M.; Schakenraad, J.M.; van Horn, J.R. Cytotoxic, allergic and genotoxic activity of a nickel-titanium alloy. Biomaterials 1997, 18, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Lobner, D. In vitro cytotoxicity of orthodontic archwires in cortical cell cultures. Eur. J. Orthod. 2004, 26, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Bumgardner, J.D.; Lucas, L.C. Cellular response to metallic ions released from nickel-chromium dental alloys. J. Dent. Res. 1995, 74, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Brantley, W.A.; Eliades, T. Materiały Ortodontyczne w Ujęciu Naukowym i Klinicznym; Czelej: Lublin, Poland, 2003; ISBN 83-89309-20-3. [Google Scholar]
- Berstein, A.; Bernauer, I.; Marx, R.; Geurtsen, W. Human cell culture studies with dental metallic materials. Biomaterials 1992, 13, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Mockers, O.; Deroze, D.; Camps, J. Cytotoxicity of orthodontic bands, brackets and archwires in vitro. Dent. Mater. 2002, 18, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.S.; Selim, E.M.; Kamel Eid, F.H.; Tawfik, W.A.; Al-Ashkar, E.A.; Mostafa, Y.A. Cytotoxicity, genotoxicity, and metal release in patients with fixed orthodontic appliances: A longitudinal in-vivo study. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 298–308. [Google Scholar] [CrossRef]
- Mikulewicz, M.; Chojnacka, K. Cytocompatibility of medical biomaterials containing nickel by osteoblasts: A systematic literature review. Biol. Trace Elem. Res. 2011, 142, 865–889. [Google Scholar] [CrossRef]
- Čolić, M.; Tomić, S.; Rudolf, R.; Marković, E.; Šćepan, I. Differences in cytocompatibility, dynamics of the oxide layers’ formation, and nickel release between superelastic and thermo-activated nickel-titanium archwires. J. Mater. Sci. Mater. Med. 2016, 27, 128. [Google Scholar] [CrossRef]
- Escobar, L.M.; Rivera, J.R.; Arbelaez, E.; Torres, L.F.; Villafañe, A.; Díaz-Báez, D.; Mora, I.; Lafaurie, G.I.; Tanaka, M. Comparison of Cell Viability and Chemical Composition of Six Latest Generation Orthodontic Wires. Int. J. Biomater. 2021, 2021, 8885290. [Google Scholar] [CrossRef]
- Imirzalioglu, P.; Alaaddinoglu, E.; Yilmaz, Z.; Oduncuoglu, B.; Yilmaz, B.; Rosenstiel, S. Influence of recasting different types of dental alloys on gingival fibroblast cytotoxicity. J. Prosthet. Dent. 2012, 107, 24–33. [Google Scholar] [CrossRef]
- Rongo, R.; Valletta, R.; Bucci, R.; Rivieccio, V.; Galeotti, A.; Michelotti, A.; D’Antò, V. In vitro biocompatibility of nickel-titanium esthetic orthodontic archwires. Angle Orthod. 2016, 86, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Locci, P.; Marinucci, L.; Lilli, C.; Belcastro, S.; Staffolani, N.; Bellocchio, S.; Damiani, F.; Becchetti, E. Biocompatibility of alloys used in orthodontics evaluated by cell culture tests. J. Biomed. Mater. Res. 2000, 51, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.C.; Jonas, I.E.; Kappert, H.F. In vitro investigation into the biological assessment of orthodontic wires. J. Orofac. Orthop. 1998, 59, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Eliades, T.; Pratsinis, H.; Kletsas, D.; Eliades, G.; Makou, M. Characterization and cytotoxicity of ions released from stainless steel and nickel-titanium orthodontic alloys. Am. J. Orthod. Dentofac. Orthop. 2004, 125, 24–29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).