Exploration of the Anti-Photoaging Mechanisms of Lactiplantibacillus plantarum TWK10 in a UVB-Induced Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Animals
2.3. UVB Induction
2.4. Preparation of Serum and Skin Tissue Extracts
2.5. Measurement of Inflammatory Cytokine Levels in Skin Tissue
2.6. Measurement of Collagen-Related Indicators
2.7. Histopathology and Immunohistochemical Staining of Skin Sections
2.8. Statistical Analyses
3. Results and Discussion
3.1. Wrinkles on the Dorsal Skin of Nude Mice
3.2. Dorsal Skin Water Loss in Nude Mice
3.3. SC Hydration of the Dorsal Skin of Nude Mice
3.4. Erythema, Melanin, and Lightness of the Dorsal Skin of Nude Mice
3.5. Pro-Inflammatory Cytokines in the Dorsal Skin of Nude Mice
3.6. Determination of Collagen-Related Proteins in the Dorsal Skin of Nude Mice
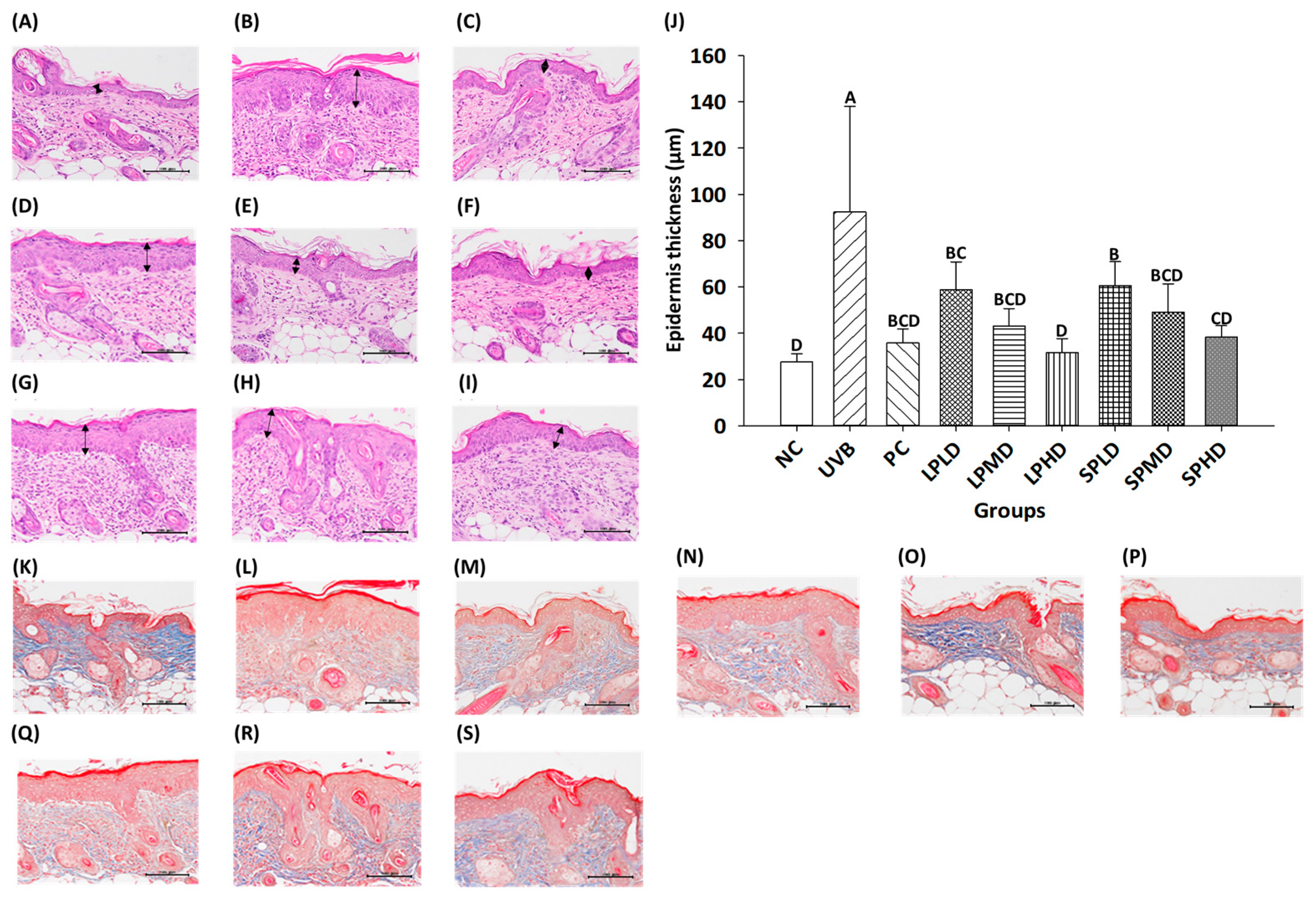
3.7. Dorsal Skin Tissue Sections of Nude Mice
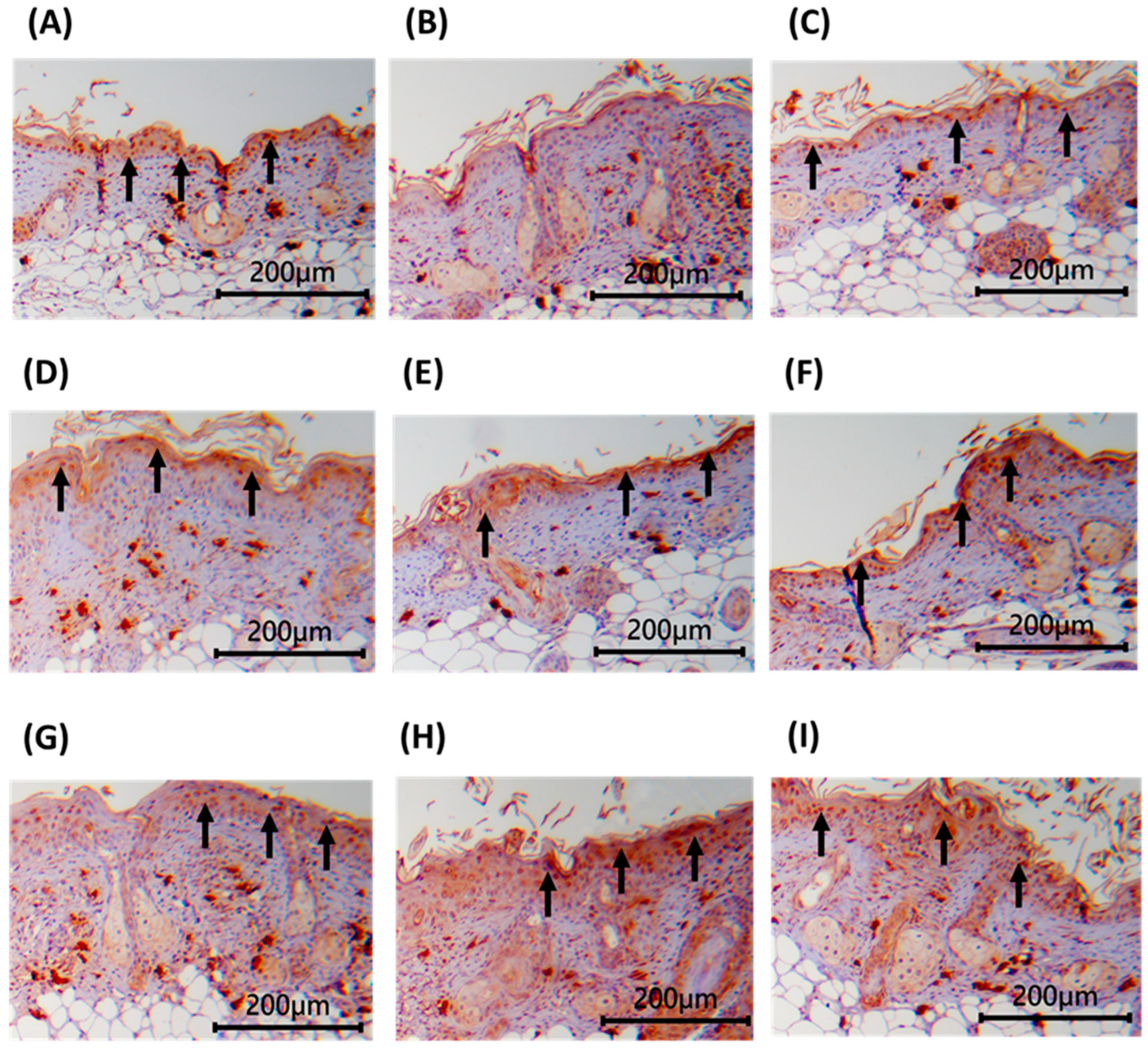
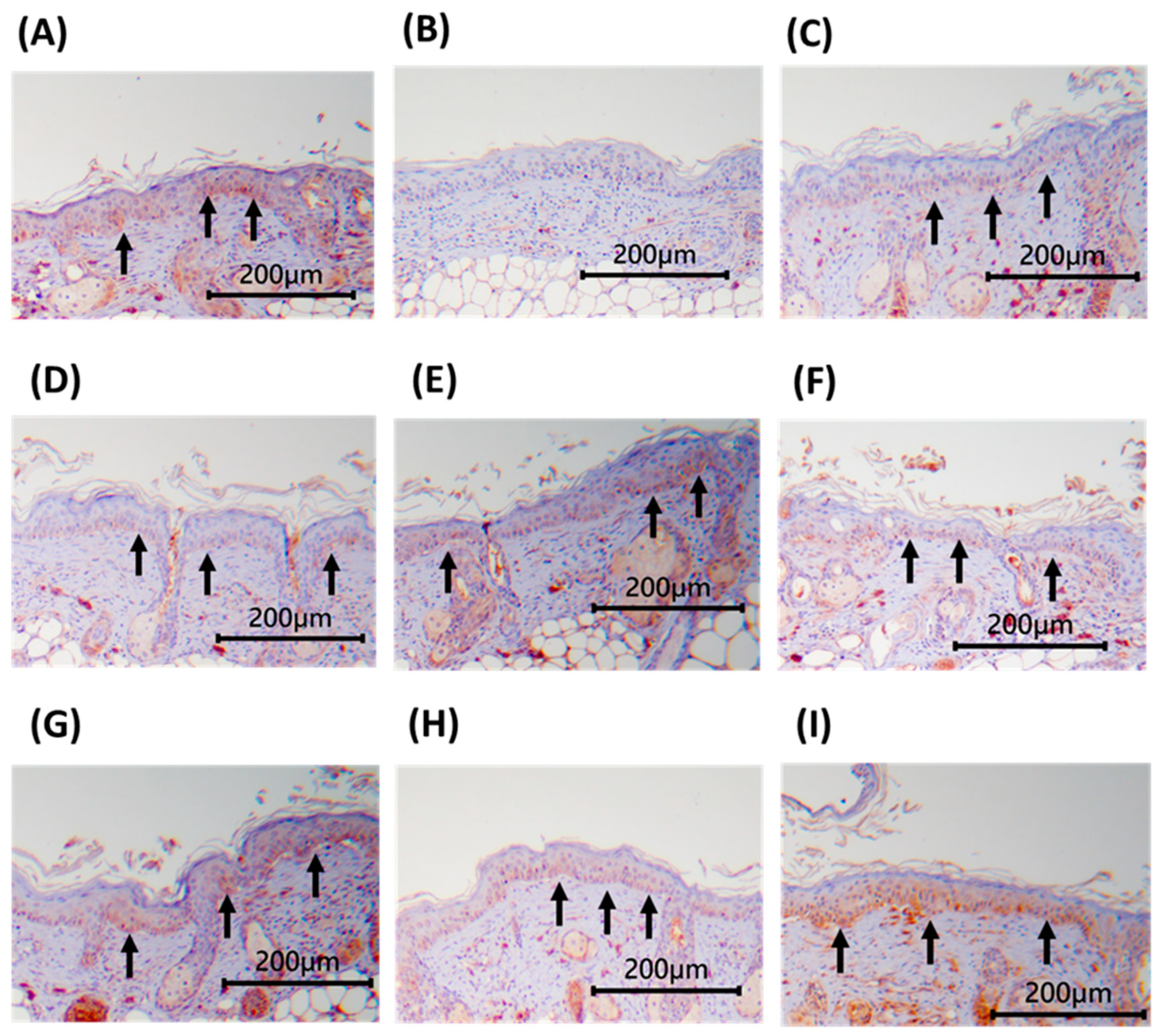
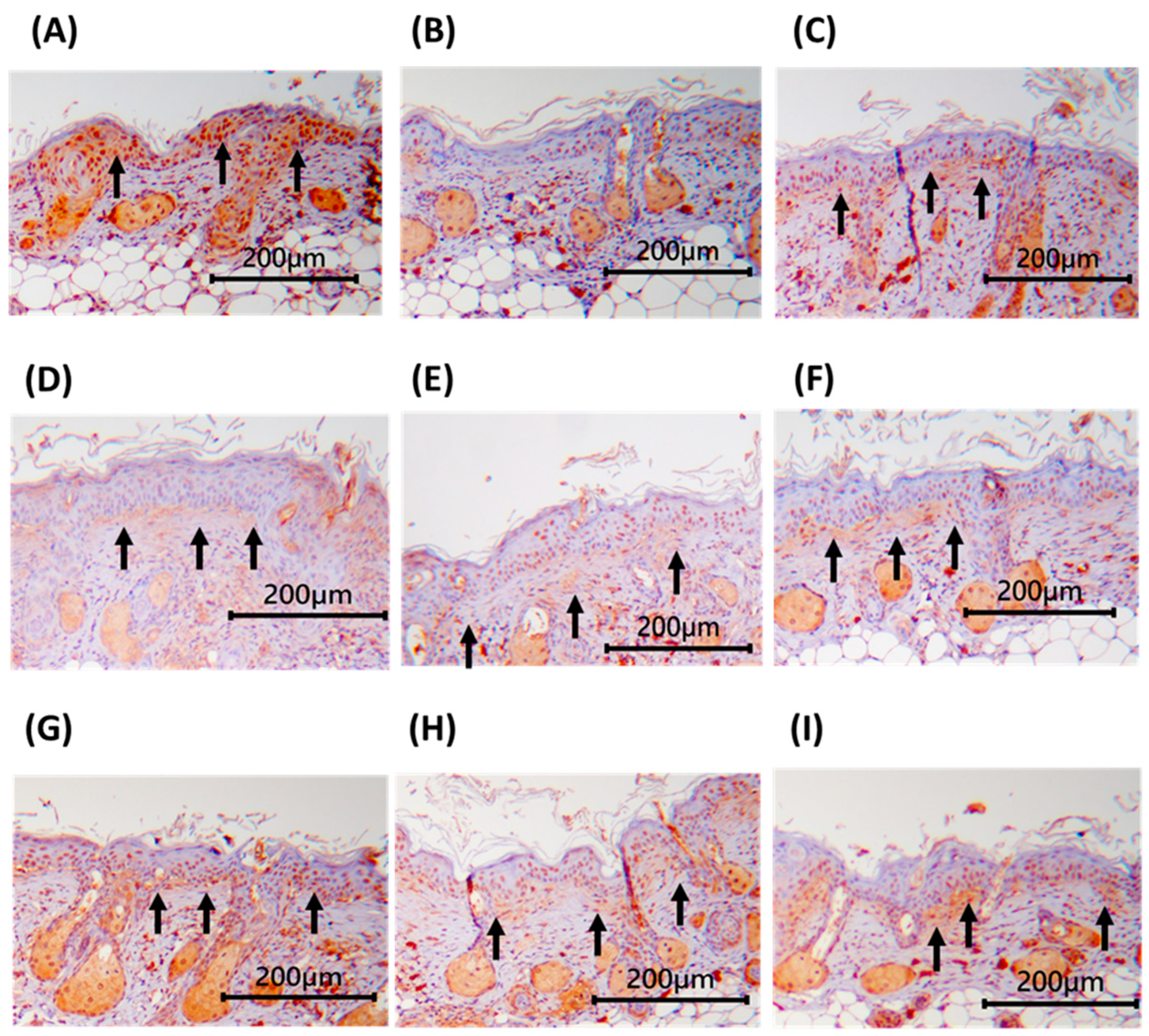
3.8. Immunohistochemical Staining of Dorsal Skin Tissue in Nude Mice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montagna, W.; Parakkal, P.F. 5—Blood Supply. In The Structure & Function of Skin, 3rd ed.; Montagna, W., Parakkal, P.F., Eds.; Academic Press: Cambridge, MA, USA, 1974; pp. 142–156. [Google Scholar] [CrossRef]
- Yi, R.; Zhang, J.; Sun, P.; Qian, Y.; Zhao, X. Protective Effects of Kuding Tea (Ilex kudingcha C. J. Tseng) Polyphenols on UVB-Induced Skin Aging in SKH1 Hairless Mice. Molecules 2019, 24, 1016. [Google Scholar] [CrossRef]
- Lawton, S. Skin 1: The Structure and Functions of the Skin. Nurs. Times 2019, 115, 30–33. [Google Scholar] [CrossRef]
- Miyamae, Y.; Yamakawa, Y.; Kawabata, M.; Ozaki, Y. A Combined Near-Infrared Diffuse Reflectance Spectroscopy and Principal Component Analysis Method of Assessment for the Degree of Photoaging and Physiological Aging of Human Skin. Anal. Sci. 2012, 28, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Rijken, F.; Bruijnzeel-Koomen, C.A.F.M. Photoaged Skin: The Role of Neutrophils, Preventive Measures, and Potential Pharmacological Targets. Clin. Pharmacoloy Ther. 2011, 89, 120–124. [Google Scholar] [CrossRef]
- Zhang, J.A.; Yin, Z.; Ma, L.W.; Yin, Z.Q.; Hu, Y.Y.; Xu, Y.; Wu, D.; Permatasari, F.; Luo, D.; Zhou, B.R. The Protective Effect of Baicalin against UVB Irradiation Induced Photoaging: An In Vitro and In Vivo Study. PLoS ONE 2014, 9, e99703. [Google Scholar] [CrossRef]
- Plastow, S.R.; Harrison, J.A.; Young, A.R. Early Changes in Dermal Collagen of Mice Exposed to Chronic UVB Irradiation and the Effects of a UVB Sunscreen. J. Investig. Dermatol. 1988, 91, 590–592. [Google Scholar] [CrossRef]
- Lee, D.H.; Oh, J.H.; Chung, J.H. Glycosaminoglycan and Proteoglycan in Skin Aging. J. Dermatol. Sci. 2016, 83, 174–181. [Google Scholar] [CrossRef]
- Wulf, H.C.; Sandby-Møller, J.; Kobayasi, T.; Gniadecki, R. Skin Aging and Natural Photoprotection. Micron 2004, 35, 185–191. [Google Scholar] [CrossRef]
- Avila Acevedo, J.G.; Espinosa González, A.M.; De Maria y Campos, D.M.; Benitez Flores Jdel, C.; Hernández Delgado, T.; Flores Maya, S.; Campos Contreras, J.; Muñoz López, J.L.; García Bores, A.M. Photoprotection of Buddleja cordata Extract against UVB-Induced Skin Damage in SKH-1 Hairless Mice. BMC Complement. Altern. Med. 2014, 14, 281. [Google Scholar] [CrossRef][Green Version]
- García-Bores, A.M.; Espinosa-González, A.M.; Reyna-Campos, A.; Cruz-Toscano, S.; Benítez-Flores, J.C.; Hernández-Delgado, C.T.; Flores-Maya, S.; Urzúa-Meza, M.; Peñalosa-Castro, I.; Céspedes-Acuña, C.L.; et al. Lippia graveolens Photochemopreventive Effect against UVB Radiation-Induced Skin Carcinogenesis. J. Photochem. Photobiol. B Biol. 2017, 167, 72–81. [Google Scholar] [CrossRef]
- Halliday, G.M. Inflammation, Gene Mutation and Photoimmunosuppression in Response to UV-Induced Oxidative Damage Contributes to Photocarcinogenesis. Mutat. Res. 2005, 571, 107–120. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Matsui, M.S.; Mukhtar, H. Kinetics of UV Light-Induced Cyclobutane Pyrimidine Dimers in Human Skin in Vivo: An Immunohistochemical Analysis of Both Epidermis and Dermis. Photochem. Photobiol. 2000, 72, 788–793. [Google Scholar] [CrossRef]
- Li, G.; Fang, T.; Zhang, Q.; Tan, A.; Cheng, Y.; Zhou, Q.; Liu, M.; Ta, X.; Huang, L.H.; Rouseff, R.; et al. Protective Effects of Polymethoxyflavone-Rich Cold-Pressed Orange Peel Oil against Ultraviolet B-Induced Photoaging on Mouse Skin. J. Funct. Foods. 2020, 67, 103834. [Google Scholar] [CrossRef]
- Robinson, E.S.; Werth, V.P. The Role of Cytokines in the Pathogenesis of Cutaneous Lupus Erythematosus. Cytokine 2015, 73, 326–334. [Google Scholar] [CrossRef]
- Park, B.; Hwang, E.; Seo, S.A.; Cho, J.G.; Yang, J.E.; Yi, T.H. Eucalyptus Globulus Extract Protects against UVB-Induced Photoaging by Enhancing Collagen Synthesis via Regulation of TGF-β/Smad Signals and Attenuation of AP-1. Arch. Biochem. Biophys. 2018, 637, 31–39. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zeng, S.Y.; Leu, Y.L.; Tsai, T.Y. Antihypertensive Effect of a Combination of Uracil and Glycerol Derived from Lactobacillus plantarum Strain TWK10-Fermented Soy Milk. J. Agric. Food Chem. 2015, 63, 7333–7342. [Google Scholar] [CrossRef]
- Liu, T.H.; Chiou, J.; Tsai, T.Y. Effects of Lactobacillus plantarum TWK10-Fermented Soymilk on Deoxycorticosterone Acetate-Salt-Induced Hypertension and Associated Dementia in Rats. Nutrients 2016, 8, 260. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef]
- Chang, C.J.; Dai, R.Y.; Leu, Y.L.; Tsai, T.Y. Effects of the Melanogenic Inhibitor, Uracil, Derived from Lactobacillus plantarum TWK10-Fermented Soy Milk on Anti-Melanogenesis in B16f0 Mouse Melanoma Cells. J. Funct. Foods 2015, 17, 314–327. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Cheng, M.C.; Lee, C.C.; Chiou, T.Y.; Tsai, T.Y. Effect of Ethanol Extract from Lactobacillus plantarum TWK10-Fermented Soymilk on Wound Healing in Streptozotocin-Induced Diabetic Rat. AMB Express 2019, 9, 163. [Google Scholar] [CrossRef]
- Ishii, Y.; Sugimoto, S.; Izawa, N.; Sone, T.; Chiba, K.; Miyazaki, K. Oral Administration of Bifidobacterium breve Attenuates UV-Induced Barrier Perturbation and Oxidative Stress in Hairless Mice Skin. Arch. Dermatol. Res. 2014, 306, 467–473. [Google Scholar] [CrossRef]
- Satoh, T.; Murata, M.; Iwabuchi, N.; Odamaki, T.; Wakabayashi, H.; Yamauchi, K.; Abe, F.; Xiao, J.Z. Effect of Bifidobacterium breve B-3 on Skin Photoaging Induced by Chronic UV Irradiation in Mice. Benef. Microbes 2015, 6, 497–504. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, D.E.; Park, S.D.; Kim, Y.T.; Kim, Y.J.; Jeong, J.W.; Jang, S.S.; Ahn, Y.T.; Sim, J.H.; Huh, C.S.; et al. Oral Administration of Lactobacillus plantarum HY7714 Protects Hairless Mouse against Ultraviolet B-Induced Photoaging. J. Microbiol. Biotechnol. 2014, 24, 1583–1591. [Google Scholar] [CrossRef]
- Zhou, Y.; Bai, R.; Huang, Y.; Li, W.; Chen, J.; Cheng, Z.; Wu, X.; Diao, Y. The Anti-Photoaging Effect of C-phycocyanin on Ultraviolet B-Irradiated Balb/c-nu Mouse Skin. Front. Bioeng. Biotechnol. 2023, 11, 1229387. [Google Scholar] [CrossRef]
- Gilhar, A.; Etzioni, A. The Nude Mouse Model for the Study of Human Skin Disorders. Dermatology 1994, 189, 5–8. [Google Scholar] [CrossRef]
- Fan, Y.; Jeong, J.H.; You, G.Y.; Park, J.U.; Choi, T.H.; Kim, S. An Experimental Model Design for Photoaging. J. Craniofac. Surg. 2015, 26, e467–e471. [Google Scholar] [CrossRef]
- Gilchrest, B.A. Photoaging. J. Investig. Dermatol. 2013, 133, E2–E6. [Google Scholar] [CrossRef]
- Hara, Y.; Hirao, T.; Iwai, I. Facial Expression under Stiff Stratum Corneum Leads to Strain Concentrations, Followed by Residual Wrinkle Formation. Int. J. Cosmet. Sci. 2017, 39, 66–71. [Google Scholar] [CrossRef]
- Kong, Y.H.; Xu, S.P. Juglanin Administration Protects Skin against UVB-Induced Injury by Reducing Nrf2-Dependent ROS Generation. Int. J. Mol. Med. 2020, 46, 67–82. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Lin, T.Y.; You, Y.J.; Wen, K.C.; Sung, P.J.; Chiang, H.M. Antiinflammatory and Antiphotodamaging Effects of Ergostatrien-3β-ol, Isolated from Antrodia camphorata, on Hairless Mouse Skin. Molecules 2016, 21, 1213. [Google Scholar] [CrossRef]
- Son, D.J.; Jung, J.C.; Choi, Y.M.; Ryu, H.Y.; Lee, S.; Davis, B.A. Wheat Extract Oil (Weo) Attenuates UVB-Induced Photoaging Via Collagen Synthesis in Human Keratinocytes and Hairless Mice. Nutrients 2020, 12, 300. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, R.; Wang, M.; Zhai, L.; Liu, J.; Xu, X.; Sun, L.; Zhao, D. Ginsenosides Repair UVB-Induced Skin Barrier Damage in Balb/c Hairless Mice and HaCat Keratinocytes. J. Ginseng. Res. 2022, 46, 115–125. [Google Scholar] [CrossRef]
- Costin, G.E.; Hearing, V.J. Human Skin Pigmentation: Melanocytes Modulate Skin Color in Response to Stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Liu, T.H.; Chiang, W.T.; Cheng, M.C.; Tsai, T.Y. Effects of Germination Black Soy Milk Fermented with Lactobacillus plantarum TWK10 on Anti-Oxidative and Anti-Melanogenesis. Appl. Sci. 2022, 12, 277. [Google Scholar] [CrossRef]
- Aoki, R.; Aoki-Yoshida, A.; Suzuki, C.; Takayama, Y. Protective Effect of Indole-3-Pyruvate against Ultraviolet B-Induced Damage to Cultured HaCat Keratinocytes and the Skin of Hairless Mice. PLoS ONE 2014, 9, e96804. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet Radiation and Skin Aging: Roles of Reactive Oxygen Species, Inflammation and Protease Activation, and Strategies for Prevention of Inflammation-Induced Matrix Degradation—A Review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Wang, X.P.; Schunck, M.; Kallen, K.J.; Neumann, C.; Trautwein, C.; Rose-John, S.; Proksch, E. The Interleukin-6 Cytokine System Regulates Epidermal Permeability Barrier Homeostasis. J. Investig. Dermatol. 2004, 123, 124–131. [Google Scholar] [CrossRef]
- Fan, J.; Zhuang, Y.; Li, B. Effects of Collagen and Collagen Hydrolysate from Jellyfish Umbrella on Histological and Immunity Changes of Mice Photoaging. Nutrients 2013, 5, 223–233. [Google Scholar] [CrossRef]
- Kim, H.R.; Jeong, D.H.; Kim, S.; Lee, S.W.; Sin, H.S.; Yu, K.Y.; Jeong, S.I.; Kim, S.Y. Fermentation of Blackberry with L. plantarum JBMI F5 Enhance the Protection Effect on UVB-Mediated Photoaging in Human Foreskin Fibroblast and Hairless Mice through Regulation of MAPK/NF-κB Signaling. Nutrients 2019, 11, 2429. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix Metalloproteinases (MMPs), the Main Extracellular Matrix (ECM) Enzymes in Collagen Degradation, as a Target for Anticancer Drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Gupta, A.; Kaur, C.D.; Jangdey, M.; Saraf, S. Matrix Metalloproteinase Enzymes and Their Naturally Derived Inhibitors: Novel Targets in Photocarcinoma Therapy. Ageing Res. Rev. 2014, 13, 65–74. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, P.; Wang, X. Skin Ageing and Cancer. In The Role of Matrix Metalloproteinase in Human Body Pathologies; Francesco, T., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 6. [Google Scholar] [CrossRef]
- Feng, X.X.; Yu, X.T.; Li, W.J.; Kong, S.Z.; Liu, Y.H.; Zhang, X.; Xian, Y.F.; Zhang, X.J.; Su, Z.R.; Lin, Z.X. Effects of Topical Application of Patchouli Alcohol on the UV-Induced Skin Photoaging in Mice. Eur. J. Pharm. Sci. 2014, 63, 113–123. [Google Scholar] [CrossRef]
- Freitas-Rodríguez, S.; Folgueras, A.R.; López-Otín, C. The Role of Matrix Metalloproteinases in Aging: Tissue Remodeling and Beyond. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef]
- Seo, E.; Song, H.H.; Kim, H.; Kim, B.-Y.; Park, S.; Suh, H.J.; Ahn, Y. Oral Administration of Mixed Probiotics Improves Photoaging by Modulating the Cecal Microbiome and MAPK Pathway in UVB-Irradiated Hairless Mice. Mol. Nutr. Food Res. 2023, 67, 2200841. [Google Scholar] [CrossRef]
- Im, A.R.; Kim, H.S.; Hyun, J.W.; Chae, S. Potential for Tyndalized Lactobacillus acidophilus as an Effective Component in Moisturizing Skin and Anti-Wrinkle Products. Exp. Ther. Med. 2016, 12, 759–764. [Google Scholar] [CrossRef]
- Kim, H.; Oh, I.; Park, K.H.; Kim, N.M.; Do, J.H.; Cho, Y. Stimulatory Effect of Dietary Red Ginseng on Epidermal Hydration and Ceramide Levels in Ultraviolet-Irradiated Hairless Mice. J. Med. Food 2009, 12, 746–754. [Google Scholar] [CrossRef]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, Prevention and Therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shu, S.; Marunaka, K.; Matsunaga, T.; Ikari, A. Weak Ultraviolet B Enhances the Mislocalization of Claudin-1 Mediated by Nitric Oxide and Peroxynitrite Production in Human Keratinocyte-Derived HaCat Cells. Int. J. Mol. Sci. 2020, 21, 7138. [Google Scholar] [CrossRef]
- Yokouchi, M.; Kubo, A. Maintenance of Tight Junction Barrier Integrity in Cell Turnover and Skin Diseases. Exp. Dermatol. 2018, 27, 876–883. [Google Scholar] [CrossRef]
- Sugawara, T.; Iwamoto, N.; Akashi, M.; Kojima, T.; Hisatsune, J.; Sugai, M.; Furuse, M. Tight Junction Dysfunction in the Stratum Granulosum Leads to Aberrant Stratum Corneum Barrier Function in Claudin-1-Deficient Mice. J. Dermatol. Sci. 2013, 70, 12–18. [Google Scholar] [CrossRef]
- Park, E.K.; Lee, H.J.; Lee, H.; Kim, J.H.; Hwang, J.; Koo, J.I.; Kim, S.H. The Anti-Wrinkle Mechanism of Melatonin in UVB Treated HaCat Keratinocytes and Hairless Mice via Inhibition of ROS and Sonic Hedgehog Mediated Inflammatory Proteins. Int. J. Mol. Sci. 2018, 19, 1995. [Google Scholar] [CrossRef]
- Tamaru, E.; Watanabe, M.; Nomura, Y. Dietary Immature Citrus unshiu Alleviates UVB- Induced Photoaging by Suppressing Degradation of Basement Membrane in Hairless Mice. Heliyon 2020, 6, e04218. [Google Scholar] [CrossRef]
- Sinha, S.; Lin, G.; Ferenczi, K. The Skin Microbiome and the Gut-Skin Axis. Clin. Dermatol. 2021, 39, 829–839. [Google Scholar] [CrossRef]
- Teng, Y.; Huang, Y.; Danfeng, X.; Tao, X.; Fan, Y. The Role of Probiotics in Skin Photoaging and Related Mechanisms: A Review. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2455–2464. [Google Scholar] [CrossRef]
- Im, A.R.; Lee, B.; Kang, D.J.; Chae, S. Protective Effects of Tyndallized Lactobacillus acidophilus IDCC 3302 Against UVB-induced Photodamage to Epidermal Keratinocytes Cells. Int. J. Mol. Med. 2019, 43, 2499–2506. [Google Scholar] [CrossRef]
- Gao, T.; Wang, X.; Li, Y.; Ren, F. The Role of Probiotics in Skin Health and Related Gut-Skin Axis: A Review. Nutrients 2023, 15, 3123. [Google Scholar] [CrossRef]
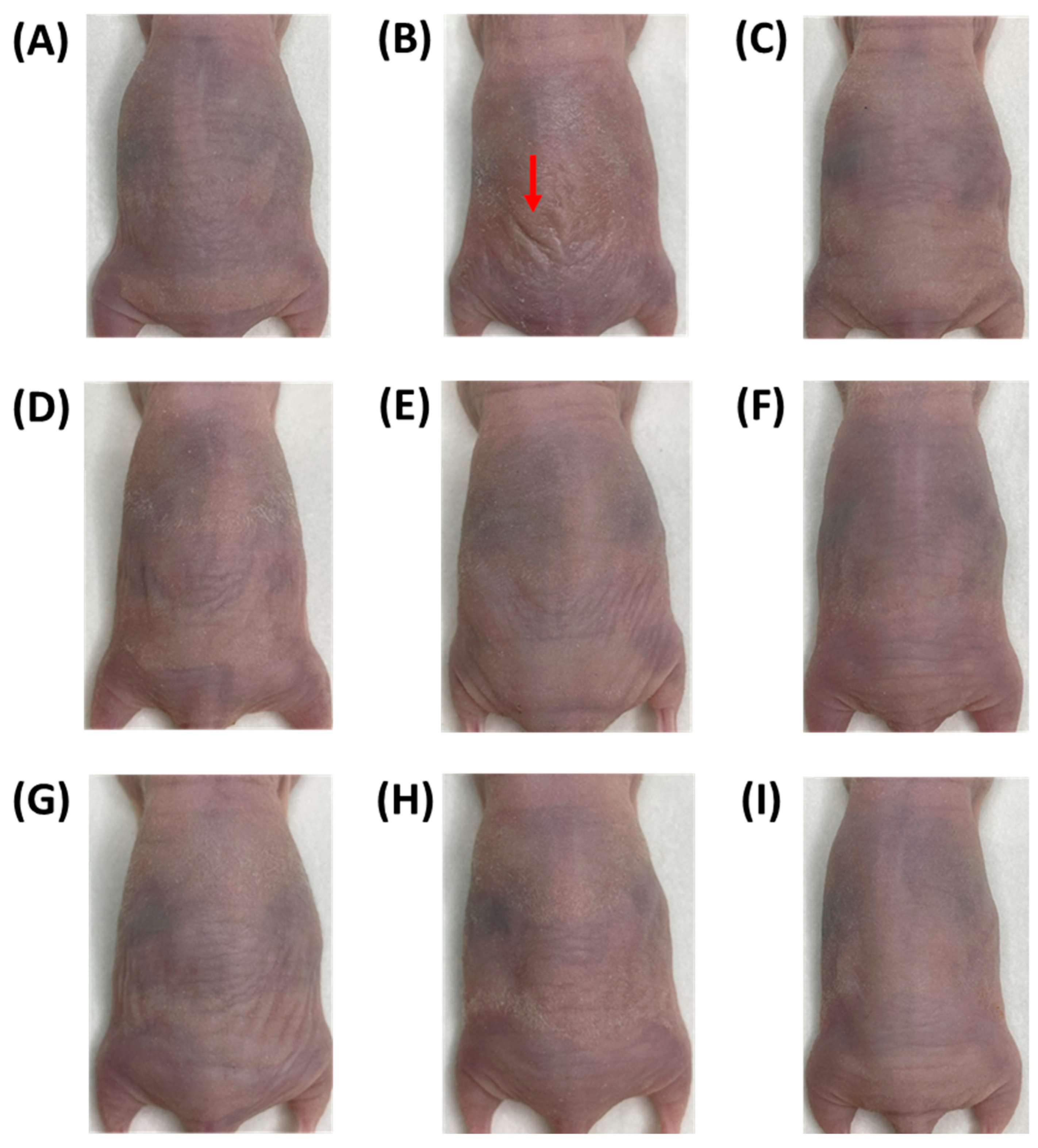

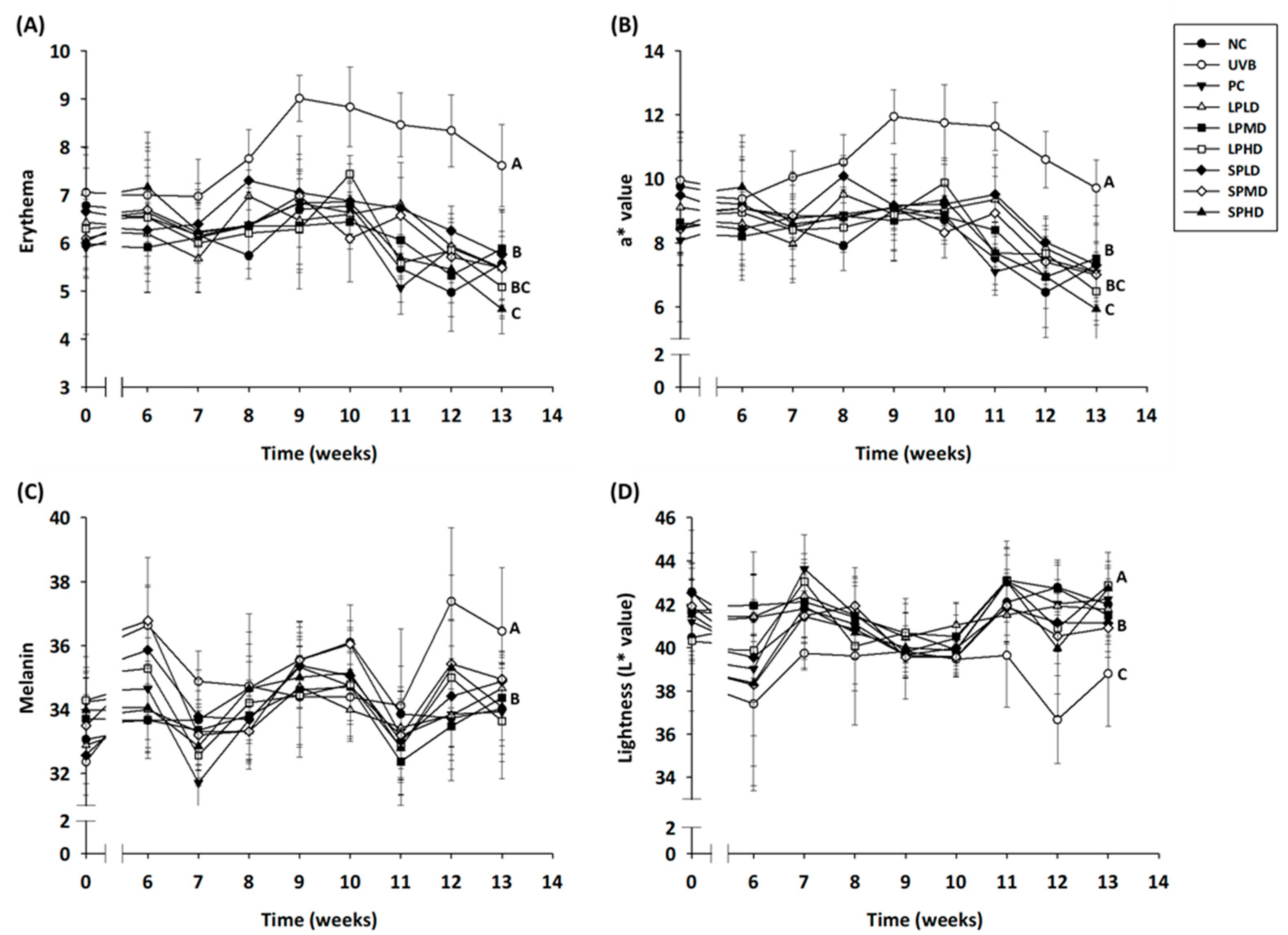
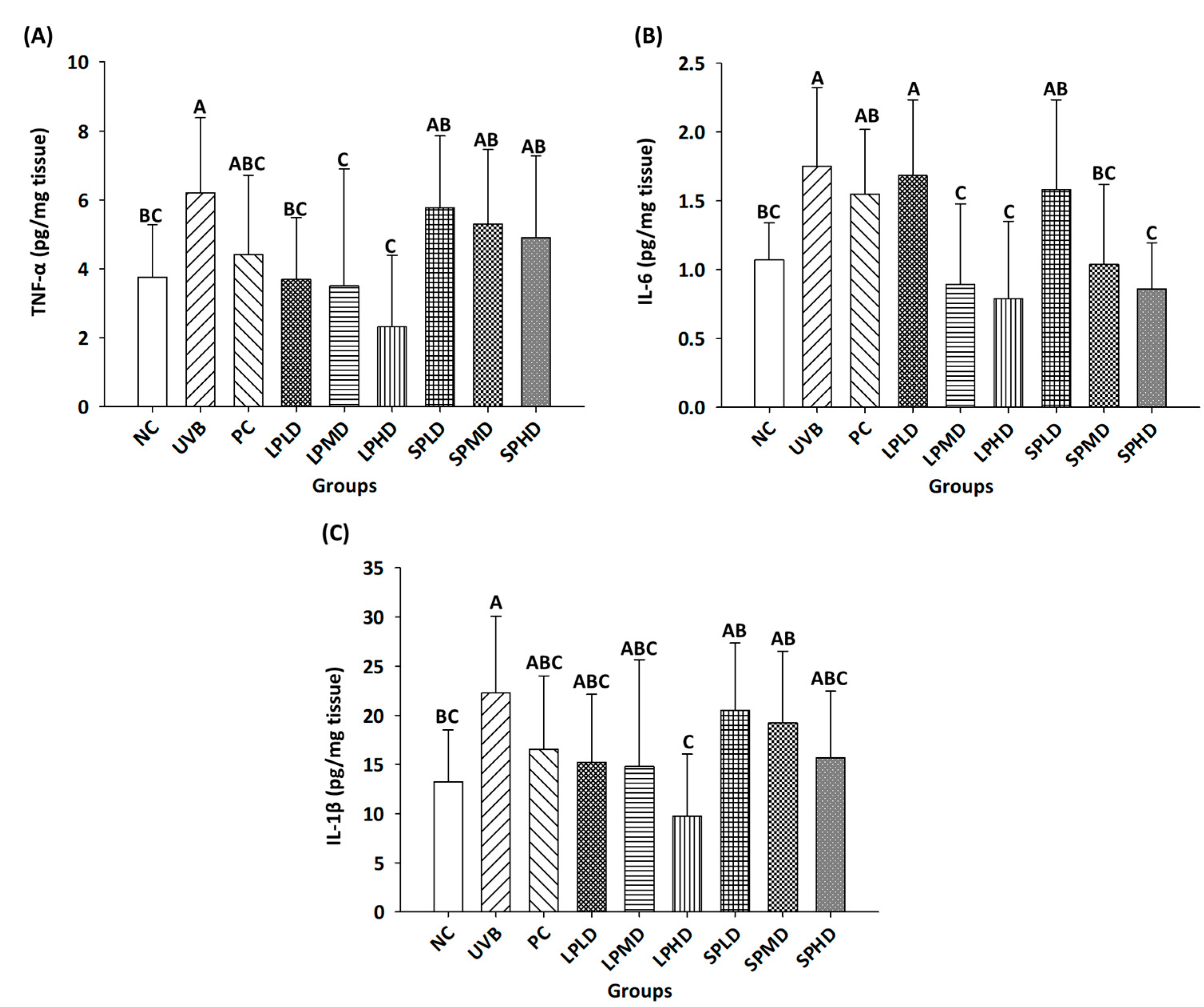
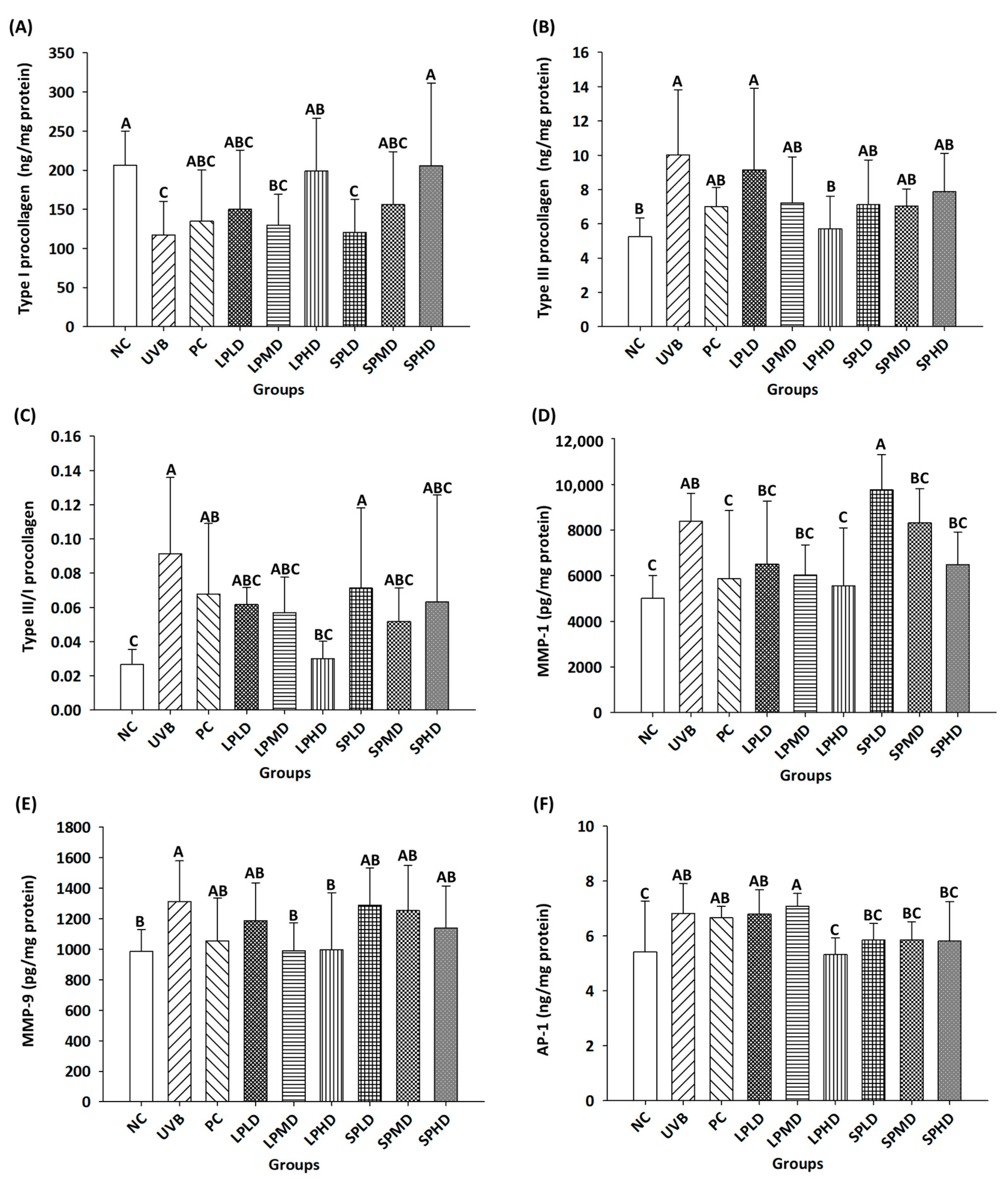
| Groups | Number * | UVB Irradiation | Samples | Dosage (/kg bw/Day) |
|---|---|---|---|---|
| NC | 7 | - | sterile water | - |
| UVB | 8 | + | sterile water | - |
| PC | 7 | + | hyaluronic acid | 60 mg |
| LPLD | 8 | + | LPTWK10 (0.1×) | 2 × 108 CFU |
| LPMD | 8 | + | LPTWK10 (1.0×) | 2 × 109 CFU |
| LPHD | 8 | + | LPTWK10 (10.0×) | 2 × 1010 CFU |
| SPLD | 8 | + | LPTWK10 spray-dried supernatant powders (0.5×) | 0.30 g |
| SPMD | 7 | + | LPTWK10 spray-dried supernatant powders (1.0×) | 0.60 g |
| SPHD | 8 | + | LPTWK10 spray-dried supernatant powders (2.0×) | 1.20 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.-H.; Lin, W.-J.; Cheng, M.-C.; Cheng, Y.-C.; Lee, C.-C.; Lin, J.-S.; Tsai, T.-Y. Exploration of the Anti-Photoaging Mechanisms of Lactiplantibacillus plantarum TWK10 in a UVB-Induced Mouse Model. Appl. Sci. 2024, 14, 9497. https://doi.org/10.3390/app14209497
Liu T-H, Lin W-J, Cheng M-C, Cheng Y-C, Lee C-C, Lin J-S, Tsai T-Y. Exploration of the Anti-Photoaging Mechanisms of Lactiplantibacillus plantarum TWK10 in a UVB-Induced Mouse Model. Applied Sciences. 2024; 14(20):9497. https://doi.org/10.3390/app14209497
Chicago/Turabian StyleLiu, Te-Hua, Wan-Jyun Lin, Meng-Chun Cheng, Yi-Chen Cheng, Chia-Chia Lee, Jin-Seng Lin, and Tsung-Yu Tsai. 2024. "Exploration of the Anti-Photoaging Mechanisms of Lactiplantibacillus plantarum TWK10 in a UVB-Induced Mouse Model" Applied Sciences 14, no. 20: 9497. https://doi.org/10.3390/app14209497
APA StyleLiu, T.-H., Lin, W.-J., Cheng, M.-C., Cheng, Y.-C., Lee, C.-C., Lin, J.-S., & Tsai, T.-Y. (2024). Exploration of the Anti-Photoaging Mechanisms of Lactiplantibacillus plantarum TWK10 in a UVB-Induced Mouse Model. Applied Sciences, 14(20), 9497. https://doi.org/10.3390/app14209497






