Abstract
Urinary tract infections (UTIs) affect nearly 50% of women in their lifetime. Uropathogenic Escherichia coli (UPEC) expresses F9/Fml pili tipped with the protein FmlH that specifically bind to terminal galactoside and galactosaminoside units in glycoproteins on kidney and bladder cells and colonize host tissues. The traditional UTI treatment using excessive antibiotics has led to the rise in various UPEC antibiotic-resistant strains. An alternative therapeutic approach prevents the initial bacterial attachment on the host cells using competitive FmlH-binding inhibitors. In this study, we used computer-aided drug design techniques to identify novel glycomimetics that are predicted to bind strongly to and inhibit the UPEC FmlH. We performed in silico receptor-based and ligand-based scaffold hopping, and molecular docking to predict novel FmlH-binding glycomimetics with high chemical synthesizability. We replaced the two major scaffolds of the most potent known FmlH-binding ligand to obtain novel compounds. Additionally, we applied global machine-learning models to predict the ADMET properties of the molecules. Compounds with low ADMET risks were subjected to molecular dynamics simulations and a detailed investigation of the FmlH–glycomimetic interactions was performed. We have prepared and supplied a library of 58 novel glycomimetics that can be subjected to further biological activity studies.
1. Introduction
Urinary tract infections (UTIs) are among the most prevalent infectious diseases, affecting millions of people annually [1]. An acute or chronic UTI can lead to a high chance of recurrence within a period of approximately 6 months [1]. Additionally, the increasing antibiotic resistance among uropathogens imposes a significant economic burden leading to a high rise in the annual global healthcare cost of UTI treatment [1]. According to a report by Yang et al., in 2019, ~404 million cases of UTIs were estimated globally [2]. Hence there is an urgent need to design better therapeutics for UTI treatment and/or prevention. Gram-positive and Gram-negative bacteria and fungi are the primary microbial agents that cause UTIs [1,3,4,5,6,7,8], among which uropathogenic Escherichia coli (UPEC) is the most common [9,10,11]. UPEC expresses fibers known as chaperone–usher pathway (CUP) pili that are tipped with adhesins, which specifically bind to host receptors to colonize and invade various host tissues [1,12,13,14]. UPEC uses F9 pili and their adhesin protein FmlH to bind to glycoproteins present on the host cell surfaces that are heavily glycosylated with terminal galactosides. Thereby, the bacteria are able to avoid being washed out by the urinary flow and can have a long residence time in the urinary tract, leading to chronic inflammation [15,16,17,18,19,20]. The lectin domain (LD) of a type 1 pilus adhesin protein, FimH, binds to mannosylated glycoproteins on the epithelial cell surfaces in the kidney and bladder, and has been targeted by rationally developing high-affinity aryl mannosides [21,22,23,24,25,26,27,28,29] as an antivirulence strategy in UTI prevention. Another highly homologous pilus, F9/Fml, is one of the most common CUP pili in the E. coli pan genome and is involved in UTIs [17,19]. Previous work has demonstrated that UPEC upregulates F9 pili expression in response to bladder inflammation [17,20]. The F9 pilus contains a FimH-like adhesin protein, known as FmlH, which binds to glycoproteins with terminal galactosides on kidneys and inflamed bladders. Kalas et al. observed that the deletion of FmlH from clinical UPEC isolates resulted in a competitive defect in the bacterial strain to maintain infection in the UTI mouse models. Since FmlH serves an important role in the UPEC pathogenesis cascade, it represents a promising target for antivirulence strategies for UTI prevention. Very little is known regarding compounds targeting FmlH [14,17,18], hence the use of cutting-edge computational modeling techniques can be an excellent starting point in the process of discovering novel chemical matter targeting FmlH.
Traditional antibiotic treatment has led to the rise in various antibiotic-resistant strains of UPEC. The increasing antimicrobial resistance has rendered the antibiotic therapeutic strategy less effective, thus creating a greater need for alternate therapeutic strategies. One such alternative therapeutic approach is the anti-adhesion strategy [30]. This strategy aims at early-stage prevention of the adhesion of an infectious agent, thus preventing bacterial survival and pathogenesis. The underlying concept is that the anti-adhesive agents competitively block bacterial adhesion [30]. The F9 pilus is a rod-like structure that is produced on the surface of the UPEC, formed by multiple protein domains non-covalently interacting with each other. The pilus consists of a tip adhesin lectin, FmlH, which specifically binds to glycoproteins that are glycosylated with galactose-containing structures and mediates adhesion to host cell surfaces initiating infections [19]. The tip adhesin FmlH forms the end of the pili containing two domains: the C-terminal domain (FmlHPD), which contains an immunoglobulin-like fold; and the N-terminal lectin domain (FmlHLD), which recognizes the galactose units on the target receptors present on the urinary tract epithelial cells [17,18].
Recent efforts have been made to design and optimize glycomimetic small molecules that can bind strongly to FmlHLD and inhibit the adhesion of UPEC to host cell surfaces and potentially prevent infections [14,17,18]. Biphenyl derivatives containing N-acetylgalactosaminosides (GalNAc) showed strong binding to FmlHLD and also exhibited significant inhibitory potency [14,17,18]. In a rational drug design study by Maddirala et al., several GalNAc-containing biphenyl compounds showed activity against FmlH in the nanomolar range. Two of the most potent compounds showed good metabolic stability but poor oral bioavailability [18]. In that work, X-ray co-crystal structures of the potent ligands with FmlH were obtained. The structures can serve as starting points for the design of improved FmlH-binding glycomimetics, using both receptor-based and ligand-based approaches. In our recent work, we designed and implemented a state-of-the-art virtual screening method using a hybrid fragment-based approach [14]. In that work, novel hit glycomimetics that exhibited improved FmlH binding were proposed, along with a database of small molecule aglycone fragments that can be utilized in the identification of FmlH-binding molecules [14].
In this study, we used in silico ligand-based and receptor-based scaffold hopping techniques to obtain novel GalNAc-containing matched molecular pairs (MMPs) that are predicted to exhibit a high binding affinity for FmlH (Figure 1). Scaffold hopping has tremendous potential for generating multiple novel scaffolds that can be screened as part of a search for new lead compounds with improved properties that still preserve key FmlH–ligand interactions. In addition to its role in lead optimization, scaffold hopping is valuable in idea generation on the path to creating new intellectual property. In this study, we considered hits that have high synthesizable accessibility after scaffold hopping. Additional molecular modeling approaches, including molecular docking, molecular dynamics (MD) simulations, and binding free energy calculations, were conducted to further investigate and validate the FmlH–hit binding. We have designed a total of 58 glycomimetics with high synthesizable accessibility scores and have predicted their pharmacokinetic profiles using machine-learning models. Several of the hits exhibited improved predicted ADMET properties relative to the glycomimetic that has been reported as the most potent against FmlH. Our results show promise as a starting point for a novel class of small molecules to serve as potential antivirulence therapies in the treatment of UTIs. The library of molecules provided in this study can serve as an excellent resource for further investigation using in vitro and in vivo studies. Additionally, this study can serve as an attractive area for next-generation research using computer-aided drug design techniques for FmlH-binding glycomimetics discovery. Using the molecular modeling approaches that are adopted in this work can help to expedite the drug design process by reducing the chances of failure of late-stage drug candidates targeting FmlH in the prevention of UTIs.
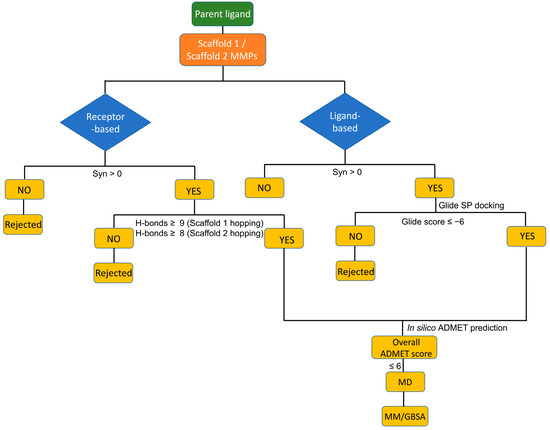
Figure 1.
Molecular modeling decision tree adopted in this study.
2. Methods and Materials
2.1. Generation of the Protein 3D Structure
The prepared X-ray crystal structure of the complex of an ortho-GalNAc biphenyl antagonist and FmlH (PDB: 6MAW) used in our previously published study [14] was used as a starting point of this work.
2.2. Scaffold Hopping
The hit molecule is a combination of a glycone (GalNAc) and an aglycone fragment. The aglycone fragment can further be considered to be composed of two scaffolds (Figure 2A). In this work, we performed scaffold hopping for each of the scaffolds, the central moiety (Scaffold 1), and the terminal moiety (Scaffold 2). Scaffold hopping is an extensively used computer-aided approach to search for active compounds that contain different core structural features. It is utilized in the design of compounds that are structurally significantly different than the parent compound but that are similar enough to exhibit similar or improved biological properties relative to those of the parent compound [31,32].
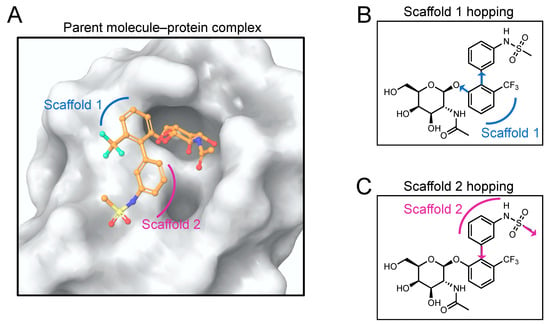
Figure 2.
Definition of the scaffolds used in this study. (A) The most potent co-crystallized ligand (considered as the reference ligand) is characterized by two scaffolds (indicated in blue and violet), other than the sugar. The glycomimetic binding pocket in FmlH is shown in surface representation. (B) The two scaffold growing points (shown in blue) used to replace Scaffold 1 in this study. (C) The two scaffold growing points (shown in violet) used to replace Scaffold 2 in this study.
2.2.1. Scaffold 1 Hopping
The Scaffold 1 growing points for hopping are shown in Figure 2B. Ligand-based and receptor-based scaffold hopping were performed on the parent molecule. To enhance the interactions exhibited in the protein–hit complexes, the minimum cut-off for the number of hydrogen bonds was set to eight for the receptor-based scaffold hopping. Heavy-atom steric clashes with the receptor were prevented by setting the maximum number of clashing ligand atoms to two and the clash criterion to <2.2 Å. The Schrödinger core hopping library (named core_library_2014.1-86640.sqlite) was used for the scaffold searching. The Schrödinger scaffold library used in this study contains 86,640 cores that have been prepared from a compound database of ~6 million drug-like compounds. This core library is highly diverse and follows most of the fragment selection rules of drug discovery. We profiled the diversity of the library with molecular descriptors and included the analysis in the Supporting Information (Figure S1). For this, we used physicochemical properties that are commonly used to describe chemical libraries, such as the molecular weight, number of rotatable bonds, hydrogen bond donors, and hydrogen bond acceptors. The hits obtained from scaffold hopping are ordinarily grouped based on three measures: overlap score, side chain root-mean-square deviations (RMSDs), and synthesizability scores. A high overlap score and a low RMSD score would indicate highly homologous compound structures. It is crucial to obtain significantly diverse chemical matter from the first step of the screening process. The more structurally diverse the compounds that are found, the higher the chance of finding novel hits. We, therefore, did not consider the overlap score or the RMSD score in the selection of compounds. It is possible that compounds with good synthetic accessibility could still have high overlap and low RMSD scores relative to the parent compound. However, if most short-listed compounds were highly homologous, that would indicate the tendency of FmlH to bind to a particular class of chemical matter. We have not observed that in this study. Our obtained hits were structurally diverse and good starting points to be considered for the subsequent steps in the screening process. So, to enable the design of chemical species with novel structures, the overlap scores and RMSD values were not considered in this study, but instead only the synthesizability scores were used for the compound ranking. For the ligand-based scaffold hopping, a synthesizability score of >0 was considered as a criterion to collect hits. The Schrödinger core hopping capability calculates the synthesizability score based on whether a particular substitution pattern has been observed in drug-like molecules, and on the number of attachment points that are substituted in the new core molecule—the higher the synthesizability score, the better [33]. A study by Pasala et al. used similar criteria for the synthesizability scores for selecting the hit compounds as our study [34]. To maximize our hit-finding chances, we needed to ensure that we had enough compounds from the first few steps of the screening funnel. Hence, we chose all the compounds with a positive synthesizability score. These hit compounds were subjected to molecular docking studies. The known most potent glycomimetic exhibited a Glide score of −5.71 kcal·mol−1, as reported in our preceding study [14]. Therefore, a Glide score of <−6 kcal·mol−1 was used as a criterion to determine which molecules showed enhanced binding to FmlH compared with the known most potent ligand. Previously published work by Samanta et al. also used a cut-off of −6.0 kcal·mol−1 to identify the compounds targeting FmlH [14]. For the receptor-based scaffold hopping, compounds with a synthesizability score >0 and the number of hydrogen bonding interactions >8 were selected as our potential hit compounds (Figure 1).
2.2.2. Scaffold 2 Hopping
The Scaffold 2 growing points for hopping are shown in Figure 2C. Scaffold hopping of Scaffold 2 was performed using the same method as described above.
2.3. Molecular Docking
The hits from the ligand-based scaffold hopping were docked into the binding pocket of FmlH using the previously published docking protocol for GalNAc-containing glycomimetics [14]. In short, molecular docking was performed using the Glide Standard Precision docking protocol [35,36,37,38,39,40,41] in Schrödinger, using the wild-type FmlH 3D crystal structure (PDB: 6MAW) [18]. The OPLS4 force field was used for all the docking calculations. A docking grid with an outer box of 25 × 25 × 25 Å3 and an inner box of 20 × 20 × 20 Å3 was used. The center of mass of the previously identified interacting protein residues was used as the center of the docking boxes [18]. Ten poses per ligand were generated for each docking run. In this work, we reported and analyzed the best Glide-scored pose as the best pose.
2.4. ADMET Prediction
The co-crystalized ligand and the hits from the molecular docking and the receptor-based hopping were subjected to ADMET calculations using ADMET PredictorTM 10.3.0.7 machine-learning models [42]. The ADMET risk scores were predicted for the compounds. The ADMET risk score is a combination of three risks (Absn_Risk, CYP_Risk, and Tox_Risk) as well as two more that touch on potential pharmacokinetic problems: hum_fup% (fraction unbound) and Vd (steady-state volume of distribution). There are 20 rules that contribute to the three risk models [42]. Simulations Plus considered a World Drug Index (WDI) reference set that contained 2260 commercial drugs. Only 7% of those commercial drugs violated more than 8 out of these 20 rules [42]. Therefore, a threshold value of concern of 8 for the overall ADMET risk score was considered. All the short-listed compounds from the Scaffold 1 hopping showed an ADMET risk score of ≤8, hence exhibiting a significantly low predicted ADMET risk. The study of protein–ligand complex dynamics can be an expensive and time-consuming process [43]. To maximize efficiency and conduct dynamics simulations on a reasonable number of compounds, the protein–ligand complexes formed between FmlH and the compounds that exhibited an ADMET risk of ≤ 6 were then subjected to MD simulations. A detailed toxicity property prediction, such as of the compounds’ cardiotoxicity, hepatotoxicity, acute toxicity in rats, carcinogenicity in mice, and mutagenicity, was also performed using the ADMET PredictorTM 10.3.0.7 Tox Module. The ADMET models that were used in this study are explained in-depth in the ADMET Predictor modules (https://www.simulations-plus.com/software/admetpredictor/toxicity/ (accessed on 3 October 2024), https://www.simulations-plus.com/wp-content/uploads/ADMETPredictor9.5_5-16-19.pdf (accessed on 3 October 2024)). Briefly, the cardiotoxicity of our compounds was assessed by their predicted affinity for hERG-encoded K+ channels. The Simulations Plus ADMET Predictor hERG prediction model is an artificial neural network ensemble model trained on a diverse set of 582 small molecules, and tested on 164 other molecules (https://www.simulations-plus.com/software/admetpredictor/toxicity/ (accessed on 3 October 2024)). The model showed a root-mean-square error (RMSE) of ~0.55 M (https://www.simulations-plus.com/software/admetpredictor/toxicity/ (accessed on 3 October 2024)). The adverse effects of the compounds on the human liver were assessed by the prediction of the elevation of certain enzymes in the blood that are used in hepatotoxicity diagnostics (alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, and gamma-glutamyl transferase). The neural network ensemble hepatotoxicity models were trained on a subset of 490 compounds from a publicly available Spontaneous Reporting System database. The models showed an average sensitivity value of ~90% and an average specificity value of ~76%. The compounds’ carcinogenicity was predicted from models based on Ames mutagenicity data from five individual strains of Salmonella or E. coli (https://www.simulations-plus.com/software/admetpredictor/toxicity/ (accessed on 3 October 2024)), (https://www.simulations-plus.com/resource/qsar-based-prediction-ames-mutagenicity-ich-m7-submissions/ (accessed on 3 October 2024)). The artificial neural network ensemble models were built using the Carcinogenic Potency Database. Data from the Registry of Toxic Effects of Chemical Substances and the ChemIDplus database [44] were used to develop models that predicted the lethality in 50% of the rats that was produced by the compounds (also known as acute rate toxicity). These models were trained on 5718 compounds and 1430 different compounds were used as a test set, and the models showed an RMSE of ~0.59 mol/kg.
2.5. Molecular Dynamics Simulations
The protein–ligand complexes formed between FmlH and the compounds that exhibited an ADMET risk ≤6 were subjected to MD simulations, using the OPLS4 force field in Desmond, Schrödinger [45,46]. For the docked ligands at the end of ligand-based scaffold hopping, the best Glide-scored docking pose was used as the starting structure. A 12 Å TIP3P water buffer was used to solvate each protein–ligand complex. Each system was neutralized with Na+ and Cl− ions. The systems were equilibrated using a previously published multistep protocol for the equilibration of FmlH–glycomimetic complexes [14], followed by a 100 ns production run in the NPT ensemble at 298 K. The non-bonded interactions were cut off at 9 Å. The Nosé–Hoover chain thermostat and Martyna–Tobias–Klein barostat were used for the production run. A timestep of 2 fs was used for each MD simulation.
2.6. Prime MM-GBSA Calculations
Binding free energy calculations were performed using the Prime Molecular Mechanics Generalized Born/Surface Area (MM-GBSA) method on 200 snapshots per MD simulation of the FmlH–hit complex, using the OPLS4 force field. The MM-GBSA calculations were performed using the previously published settings [14]. Briefly, the thermal_mmgbsa script by Prime Schrödinger was used following the MD simulations of the FmlH–hit complexes to compute the binding free energies of the ligands on frames extracted from the trajectory at an interval of 5 ns. Using this script, hetgroups (ions and water atoms) in the frames were removed and the VSGB continuum solvation model for water was used [47]. The dielectric constant was set to 80. The binding free energies of the hits were calculated using the equation in our previously published report [14].
3. Results and Discussion
3.1. Scaffold 1
The FmlH–parent reference molecule complex is shown in Figure 2A. Two scaffold growing points were selected on the parent reference molecule to replace the central scaffold (Scaffold 1) of the molecule (Figure 2B). Both in silico ligand-based and in silico receptor-based scaffold hopping were performed on Scaffold 1.
Numerous existing X-ray structures of FmlH–ligand complexes show the inhibitor to be in the binding site investigated in this work (PDB IDs: 6MAQ, 6MAP, 6MAW, 6ARM, 6AOW, 6MOY, 6ARN, 5LNE, 6ARO) [14,17,18]. Additionally, existing mutagenesis evidence for a highly homologous type 1 pilus, FimH, further supports our choice of binding site [18,48,49,50]. Several existing X-ray structures of this highly homologous type 1 pilus, FimH, with the ligand bound in the same binding site that we have studied, further validate our site (PDB IDs: 4BUQ, 3MCY, 5MUC, 5JCQ, 4X5P, 4X5Q, 4X50, 4LOV, 5L4V, 5L4W, 4X5R, 6G2S, 5L4T, 5L4U) [24,51,52,53,54]. We performed a SiteMap analysis using Maestro to identify all the possible binding sites of FmlH. SiteMap conducts a search to determine which regions of the protein are suitable for interaction with a binding partner and then generates contour maps to produce hydrophobic and hydrophilic maps. Finally, it conducts an evaluation step where the program assesses each identified potential binding site by calculating a variety of proteins and provides a SiteScore at the end of the calculation. The various calculated properties that are accounted for in the SiteScore are the number of site points, size of the binding site, exposure and enclosure (indicates solvent accessibility), contact (indicates how strongly the site point interacts with the surrounding receptor using van der Waals interactions), as well as the site’s donor and acceptor properties [55,56,57,58]. SiteScore assesses a site’s propensity for ligand binding and is used to rank all the possible binding sites with respect to their relevance. SiteMap is a widely used method to identify ligand binding sites and protein–protein interfaces with high accuracy [56,57,58]. For FmlH, two sites were identified (Figure S2). The top scored site corresponds to the small molecule docking site that we have investigated in this work. The second site is close to the protein–protein interface at which FmlH is known to interact with the neighboring FmlH pili domain (FmlHP) to form the F9/Fml pili [18,59,60].
3.1.1. Ligand-Based Scaffold Hopping
A total of 10,000 core-hopping structures were obtained. Of those, 1115 hits were obtained that exhibited a synthesizability score >0. We docked these compounds into the binding pocket of FmlH. Nine compounds showed a Glide score of <–6 kcal/mol (Figure 3; Table 1). The three-dimensional structures of the hits from the molecular docking are shown in Figure 3. The binding poses of these compounds are shown in Figure 3 (bottom panel). We observed three distinct binding modes, in which the ligand’s aglycone end occupied three different sub-pockets of the main FmlH binding pocket. This suggests that there can be three exit vectors, which can be utilized in the design of novel compounds by Scaffold 1 replacement. For all the ligands, the GalNAc moiety occupied the same part of the main binding pocket (Figure 3). The synthesizability scores for all the molecular docking hits are summarized in Table 1.
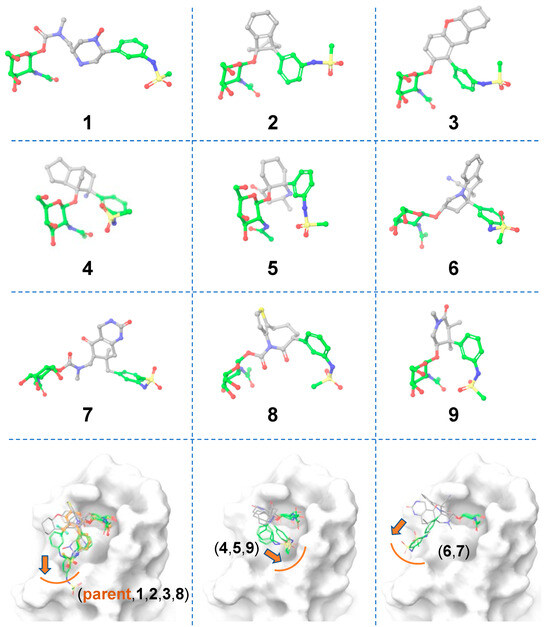
Figure 3.
Matched molecular pairs obtained from in silico ligand-based Scaffold 1 hopping. Three-dimensional structures of the obtained hits. The unreplaced scaffolds are shown with green carbons and the novel scaffolds after replacement are shown with gray carbons. The three distinct binding sub-pockets of the main FmlH binding pocket that are occupied by the aglycone of the hits are shown in the bottom row panels. The orange arrows point to adjacent sub-pockets, which highlight possible exit vectors to facilitate the ligand design. The FmlH binding pocket is shown in the surface representation.

Table 1.
Glide scores (in kcal·mol−1) and synthesizability scores. Glide scores are only listed for the ligand-based Scaffold 1 hopping hits that were subjected to molecular docking, whereas the other data are available for both the ligand-based and receptor-based hits. The ligands that exhibited at least one interaction between the novel Scaffold 1 and FmlH residues are highlighted. Ligands 01, 02, 03, and 04 are from Samanta et al. [14] and were included in the table for comparison. Not applicable is denoted as n/a.
3.1.2. Receptor-Based Scaffold Hopping
A total of 1684 core-hopping structures were obtained. Of those, 177 hits were obtained that exhibited a synthesizability score >0. We chose only those compounds that had a synthesizability score >0 and that exhibited ≥9 hydrogen bonding interactions with FmlH, which yielded 29 compounds (Table 1). The molecules that showed at least one interaction with the novel central scaffold obtained after replacement are shown in Figure 4. The FmlH–hit interactions as obtained from in silico receptor-based scaffold hopping are shown in Figure 4. For compound (a), the π–π interaction that was observed between Y46 and the most potent co-crystallized ligand [18], as well as the ligands identified by Samanta et. al. [14], was preserved (Figure 4). This π–π interaction was replaced by a cation–π interaction in ligand 16 (Figure 4). Additionally, an electrostatic interaction was observed between ligand 16 and D45 (Figure 4). In all cases, the GalNAc moiety exhibited the same complex network of hydrogen bonding interactions with FmlH residues as observed for the parent reference molecules [14]. For compounds 10, 20, 25, and 33, the GalNAc and Scaffold 1 linker moiety exhibited interactions with the FmlH binding pocket (Figure 4). The novel Scaffold 1 in ligand 38 showed a halogen bonding interaction with K97, which was not observed for the other ligands (not shown in the figure). The hits from both ligand-based and receptor-based scaffold hopping were subjected to ADMET prediction calculations. The synthesizability scores for all the hits obtained from receptor-based scaffold hopping of Scaffold 1 are summarized in Table 1.
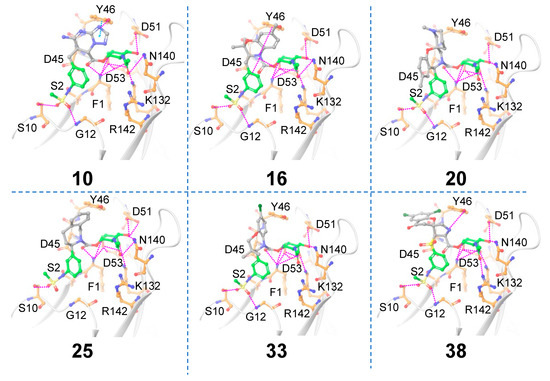
Figure 4.
Matched molecular pairs obtained from in silico receptor-based Scaffold 1 hopping. Only those molecules are shown here that exhibited interactions between the novel Scaffold 1 and FmlH residues. The unreplaced scaffolds are shown with green carbons and the novel scaffolds after replacement are shown with gray carbons. Pink dashed lines indicate FmlH–ligand polar interactions. Cyan dashed lines represent π–π interactions. The key residues in the FmlH binding pocket are shown (with orange carbons). The key interacting residues are labeled in the figure.
3.1.3. ADMET Predictions
The overall pharmacokinetic risks of the hits obtained after Scaffold 1 replacement were calculated (Figure 5). An ADMET Predictor risk score ranges between 0–22, and indicates the number of potential ADMET problems that a compound might have: Absorption contributes up to 8 points; Distribution up to 2 points; CYP metabolism up to 6 points; and Toxicity up to 6 points. A cut-off of the ADMET risk score of ≤6 was used to select the hit compounds with a significantly low ADMET risk (01, 03, 2, 5, 9, and 24). 01 and 03 are the most potent co-crystallized ligand [18] and a hit molecule identified in our previously reported in silico study [14], respectively. The selected hits from Scaffold 1 hopping that exhibited improved ADMET scores were subjected to all-atom MD simulations and binding free energy calculations. The molecule-level intrinsic clearance (uL/min/mg) for human (HLM) and rat (RLM) liver microsomes were predicted and are shown in Figure S3.
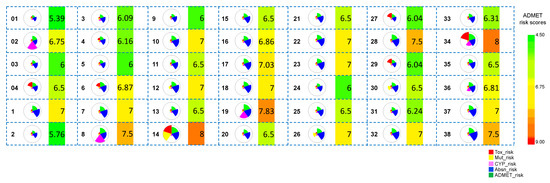
Figure 5.
Overall risks and ADMET risk scores of all the hit molecules obtained from Scaffold 1 hopping. The wedge plots represent the predicted overall risks, which are comprised of five different scores, distinctly shown in five colors (Tox_risk, Mut_risk, CYP_risk, Absn_risk, and ADMET_risk). Cross-hatched wedges indicate predictions for out-of-scope molecules. The heat maps next to the wedge plots represent the ADMET_risk score. Green indicates low ADMET_risk and red indicates high ADMET_risk.
To investigate the toxicity profiles of the hit compounds, we performed a thorough and detailed toxicity profile prediction study (Figure 6). Most hits obtained from Scaffold 1 replacement showed lower predicted acute hepatoxicity through the liver enzyme alanine aminotransferase (SER_ALT) than the known most potent co-crystallized ligand (01) (cyan). However, most compounds showed similar predicted hepatotoxicity through the liver enzyme aspartate aminotransferase (SER_AST) to that of 01 (red). Almost all the hits had a low predicted mutagenicity risk (gray), similar to 01. Nineteen of the hit compounds (03, 04, 5, 7, 9, 10, 13, 14, 18, 19, 23, 26, 27, 28, 29, 30, 31, 34, and 35) showed lower hERG_pIC50 than 01 (green).
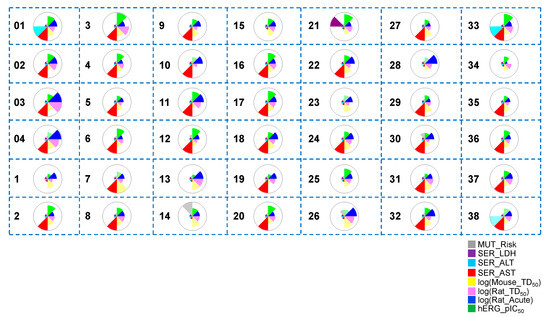
Figure 6.
Toxicity property predictions for all the hit compounds obtained from Scaffold 1 hopping. The wedge plots show properties for the molecules calculated using machine-learning ADMET PredictorTM10.3.0.7 models. Cross-hatched wedges indicate predictions for out-of-scope molecules. The different colored wedges represent the various toxicity properties, which are indicated in the figure labels.
3.2. Scaffold 2
Two scaffold growing points were selected on the parent reference molecule to replace the aglycone terminal scaffold (Scaffold 2) of the molecule (Figure 2C).
3.2.1. Ligand-Based Scaffold Hopping
A total of 10,000 core hopping structures were obtained. Of those, 1109 hits were obtained with a synthesizability score >0. We docked these compounds into the binding pocket of FmlH. Twelve compounds showed a Glide score of <–6 kcal/mol (Table 2). The three-dimensional structures of the hits from the molecular docking are shown in Figure 7. The binding poses of these compounds are also shown in Figure 7. In this case, only two of the sub-pockets in the binding region were seen to be occupied by the hits. The synthesizability scores for all the hits after the molecular docking are summarized in Table 2.

Table 2.
Glide scores (in kcal·mol−1) and synthesizability scores. Glide scores are only listed for the ligand-based Scaffold 2 hopping hits that were subjected to molecular docking, whereas the other data are available for both ligand-based and receptor-based hits. The ligands that exhibited at least one interaction with the novel Scaffold 2 and FmlH residues are highlighted. Ligand 01 was reported by Samanta et al. and is included in the table for comparison [14]. n/a denotes not applicable.
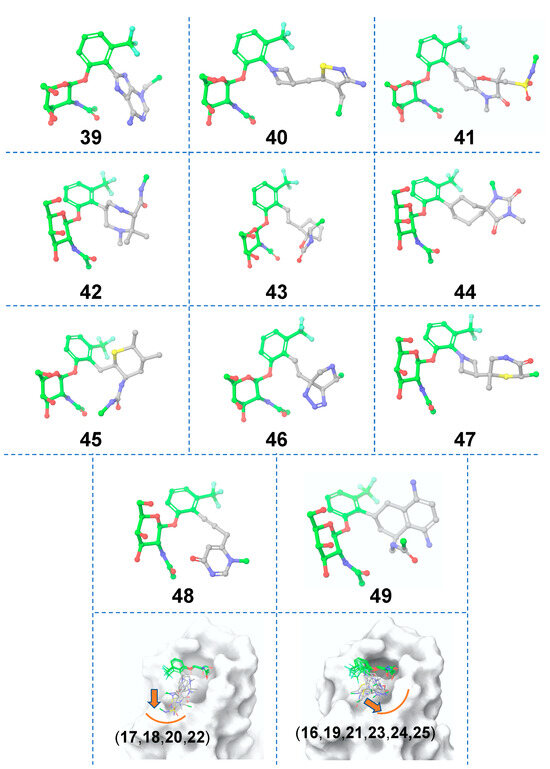
Figure 7.
Matched molecular pairs obtained from in silico ligand-based Scaffold 2 hopping. Three-dimensional structures of the obtained hits. The unreplaced scaffolds are shown with green carbons and the novel scaffolds after replacement are shown with gray carbons. The two distinct binding sub-pockets in the FmlH binding region that are occupied by the hits are shown in the right bottom row panels. The orange arrows point to adjacent sub-pockets, which highlight possible exit vectors to facilitate the ligand design. The FmlH binding pocket is shown in surface representation.
3.2.2. Receptor-Based Scaffold Hopping
A total of 65 core hopping structures were obtained, out of which 9 hits were obtained with a synthesizability score >0, which also exhibited ≥8 hydrogen bonding interactions with FmlH (Table 2). The molecules that showed at least one interaction with the novel central scaffold were considered. The FmlH–hit interactions as obtained from in silico receptor-based scaffold hopping are shown in Figure 8. In all cases, the GalNAc moiety exhibited a complex network of hydrogen bonding interactions with FmlH residues, as observed for the parent reference molecules [14]. Hydrogen bonding interactions were observed between the FmlH binding pocket residues and the side-chain functional groups of the novel terminal scaffold. Additionally, electrostatic interactions were observed between ligand 52 and K132/R142. For ligands 50, 52, 53, 54, and 55 the linker moiety between Scaffold 1 and Scaffold 2 exhibited interactions with the FmlH binding pocket. The synthesizability scores for all the hits obtained from receptor-based scaffold hopping of Scaffold 2 are summarized in Table 2.
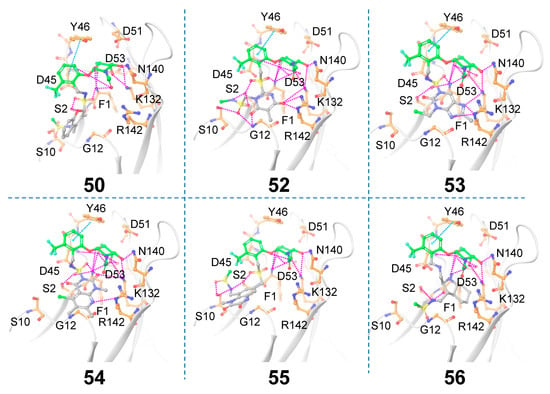
Figure 8.
Matched molecular pairs obtained from in silico receptor-based Scaffold 2 hopping. Only those molecules that exhibited interactions between the novel Scaffold 2 and FmlH residues are shown here. The unreplaced scaffolds are shown in green and the novel scaffolds after replacement are shown in gray. Pink dashed lines indicate FmlH–ligand polar interactions. Cyan dashed lines represent π–π interactions. The key residues in the FmlH binding pocket are shown (C orange). The key interacting residues are labeled in the figure. Blue N, red O, yellow S.
3.2.3. ADMET Predictions
The overall pharmacokinetic risks of the hits obtained after Scaffold 2 replacement were calculated (Figure 9). The hit molecule 48 obtained from Scaffold 2 hopping showed an improved ADMET score relative to that of the known potent co-crystallized ligand (01). This selected hit was subjected to all-atom MD simulations and binding free energy calculations. The molecule-level intrinsic clearance (uL/min/mg) in rat or human liver microsomes was also predicted (cf. Figure S4). Several of the compounds obtained from Scaffold 2 hopping exhibited a low ADMET risk score (≤8), out of which compound 48 (score of ≤6) was subjected to MD simulations. However, from the set of compounds from Scaffold 2 hopping, five compounds (40, 49, 50, 55, and 58) exhibited a high predicted overall ADMET risk.
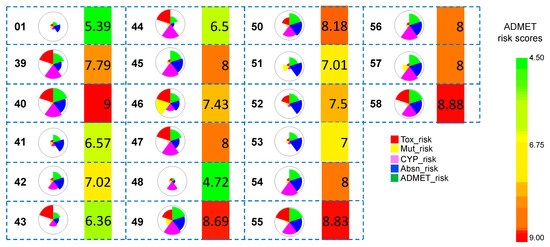
Figure 9.
Overall risks and ADMET risk scores of all the hit molecules obtained from Scaffold 2 hopping. The wedge plots represent the predicted overall risks, which comprise five different scores, distinctly shown in five colors (Tox_risk, Mut_risk, CYP_risk, Absn_risk, and ADMET_risk). Cross-hatched wedges indicate predictions for the out-of-scope molecules. The heat maps next to the wedge plots represent the ADMET_risk score. Green indicates low ADMET_risk and red indicates high ADMET_risk.
A thorough and detailed toxicity profile prediction study was conducted to investigate the toxicity profiles of the hit molecules after Scaffold 2 hopping (Figure 10). Several of the hits (50, 53, 54, 55, 56, and 58) obtained from Scaffold 2 replacement showed lower predicted acute hepatoxicity through the liver enzyme alanine aminotransferase (SER_ALT) than the known most potent co-crystallized ligand (01) (cyan). Only one hit from Scaffold 2 hopping (54) exhibited reduced predicted hepatotoxicity through the liver enzyme aspartate aminotransferase (SER_AST) relative to that of the known most potent molecule (01) (red). Almost all hits had low predicted mutagenicity risk (gray), except for 48. Several of the hit molecules (41, 42, 46, 50, 51, 52, 53, 54, 55, and 56) showed lower hERG_pIC50 than the known most potent (01) (green).
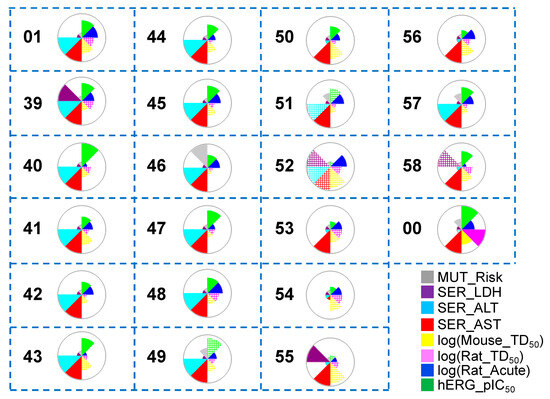
Figure 10.
Toxicity property predictions for all the hit compounds obtained from Scaffold 2 hopping. The wedge plots show the properties for the molecules calculated using machine-learning ADMET PredictorTM10.3.0.7 models. Cross-hatched wedges indicate the predictions for out-of-score molecules. 00 is the negative control used in this work.
Figure 11 shows the predicted hERG toxicity, mutagenicity risk, acute rat toxicity, and Rat TD50 and Mouse TD50 values for the hits.
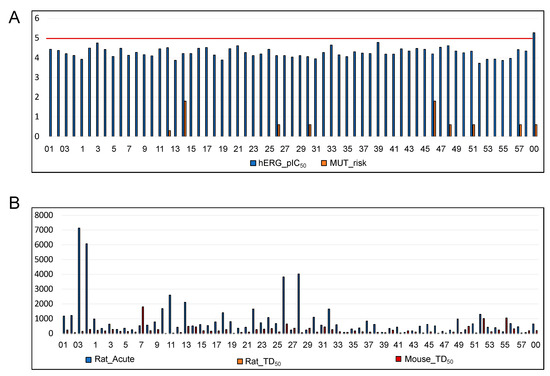
Figure 11.
Numerical values of the predicted (A) hERG toxicity, mutagenicity risk, (B) acute rat toxicity (LD50), and Rat TD50 and Mouse TD50 values for the hits. The red line in (A) represents all the hits that exhibited hERG toxicity lower than the cut-off. The negative control (00) used in this study exhibited higher hERG toxicity. hERG_pIC50 is given in mol/L. LD50 for rat acute toxicity = mg/kg in the oral dose that would be lethal to 50% of the rats. Rat_TD50 = mg/kg/day in the oral dose. Mouse_TD50 = mg/kg/day in the oral dose.
3.3. Molecular Dynamics Simulations
The molecules that exhibited an ADMET risk ≤6 (01, 03, 2, 5, 9, 24, and 48) were subjected to MD simulations. Molecules 01 and 03 are matched molecular pairs of Scaffold 1 identified using our previously designed virtual screening workflow. The MD simulation results for 01 and 03 were reported in our previously published work [14].
For the top hits from this work (2, 5, 9, 24, and 48), the interactions that were maintained during the 100 ns MD simulations between the ligands and FmlH are given in Figure 12. It was observed in the previous work by Samanta et al. that the most potent co-crystallized ligand did not show a π–π interaction with Y46 [14]. The novel scaffold in 2 exhibited π–π stacking with Y46, which was maintained for 63% of the simulation time (Figure 12A), a significant improvement over the most potent co-crystallized ligand [14]. In addition, a hydrogen bonding interaction was observed with the neighboring S10 residue. One of the sulfonamide oxygens of the terminal aglycone fragment interacted with the sidechain of the S10 residue in the previously reported research on FmlH-binding small molecule glycomimetics [14,18]. In molecule 5, the central novel scaffold is a non-aromatic moiety; therefore, a π–π stacking interaction with Y46 was not possible, but instead, there was hydrogen bonding of the carbonyl group with Y46. (Figure 12B). In molecule 9, a water-mediated hydrogen bonding interaction was observed between the glycosidic linkage connecting the GalNAc and the novel Scaffold 1 moiety, which was maintained for 52% of the simulation time (not shown for clarity). A hydrogen bonding interaction was found between the primary amine group in the central scaffold in molecule 24 and residue D45 (Figure 12D). D45 was previously identified as an important residue involved in polar interactions with strong FmlH-binding glycomimetics [14,18]. Finally, for molecule 48 (obtained after replacing Scaffold 2), the novel aglycone terminal scaffold exhibited a hydrogen bonding interaction with the neighboring R142 residue, which was maintained for 43% of the simulation time (Figure 12E). In all cases, a complex network of hydrogen bonding interactions between the GalNAc moiety and FmlH residues were maintained. During the molecular dynamics (MD) simulations, the top-ranked compounds did not dissociate from the binding site. The root-mean-square deviations (RMSD) of the five hits are shown in Figure S5. Compounds 5 and 9 exhibited lower RMSD variations than 2, 24, and 48 (Figure S5). All the ligands maintained low RMSD values throughout the MD simulation trajectories, indicating that they were stably bound in the FmlH binding pocket.
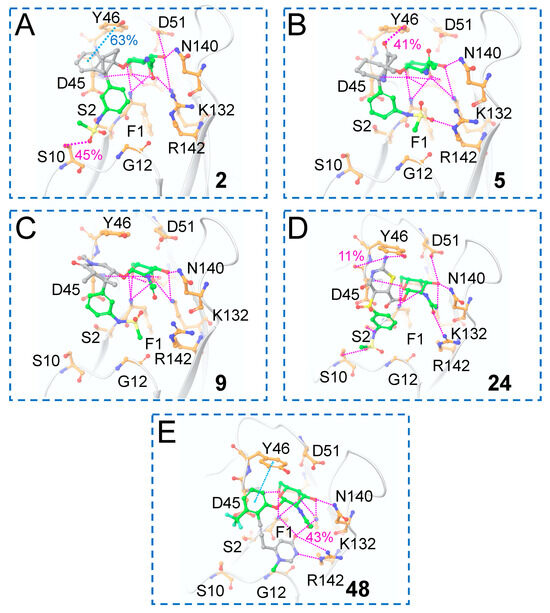
Figure 12.
MD simulation analysis of the 100 ns trajectories of the FmlH–hit complexes. (A–D) Hit glycomimetics from Scaffold 1 hopping, which exhibited improved ADMET profiles. (E) Hit glycomimetic from Scaffold 2 hopping, which showed an improved ADMET profile. The molecule number is indicated inside each box panel in bold. Blue N, red O, yellow S. Pink dashed lines indicate FmlH–ligand polar interactions, with the percent occupancy listed for one case in each image.
3.4. Binding Free Energy Calculations from the MD Simulations
Binding free energy calculations using the MM-GBSA method were performed on frames extracted from each FmlH–ligand complex MD simulation trajectory (Figure 13). The binding free energy of the known most potent co-crystallized ligand was obtained from Samanta et al. and plotted for comparison [14]. All the ligands showed average negative binding free energies, indicating favorable binding to FmlH. Hits 2, 5, 9, 24, and 48 exhibited comparable predicted binding free energies to the most potent ligand (01) and hence can be considered as strong FmlH-binding glycomimetics that merit further investigation in the design of FmlH anti-adhesives.
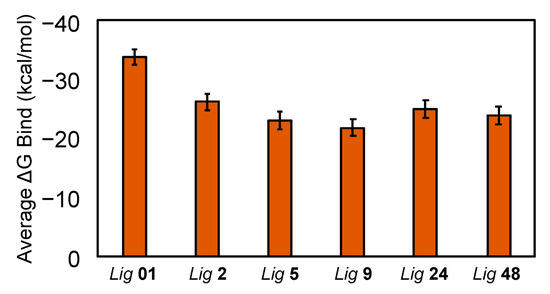
Figure 13.
Average binding free energies of the hit ligands (2, 5, 9, 24, 48) from the MD simulation trajectories, calculated using Prime MM-GBSA. The average binding free energy of the known most potent co-crystallized ligand (01), from Samanta et al. [14], was included for comparison. Error bars represent the standard error of the mean (SEM).
The previous study by Samanta et al. showed that three molecules with a novel central scaffold (Scaffold 1) exhibited enhanced FmlH binding with improved pharmacokinetic properties [14]. In addition, the study focused on the design and implementation of a novel virtual screening protocol to design FmlH-binding glycomimetics using a hybrid fragment-based e-pharmacophore modeling approach [14]. The hits identified in that study showed thiazole and modified thiazole moieties as Scaffold 1. It was also established that both aromatic and non-aromatic moieties in the central scaffold can be considered as potential approaches to the FmlH-binding glycomimetics design. The hits obtained from Scaffold 1 hopping in our study have shown two distinct kinds of hits: ones with aromatic moieties or with non-aromatic moieties as Scaffold 1 (Figure 4). The non-aromatic Scaffold 1 moieties exhibited hydrogen bonding interactions between the protein residues and the GalNAc–aglycone linker or the peripheral functional groups of the central moiety. The aromatic Scaffold 1 moieties showed a π–π interaction with residue Y46 of FmlH, which was also observed in previous FmlH-binding molecular design studies [14,18]. This residue is present at the tip of the binding pocket. Additionally, an electrostatic interaction between D45 and the charged center of Scaffold 1 (16) was also observed (Figure 4). Residue D45 was found to be important in the glycomimetics binding to FmlH [14,18].
Several of the hits (1–9 and 39–49) obtained in this study exhibited improved predicted binding compared with the previously reported most potent co-crystallized ligand (01), as is evident from their Glide scores. Several of the compounds obtained from Scaffold 1 and Scaffold 2 hopping exhibited a low ADMET risk score of ≤ 8, which was much similar to the previously reported compounds 01, 02, 03, and 04. However, from the set of compounds from Scaffold 2 hopping, five compounds (40, 49, 50, 55, and 58) exhibited a high predicted overall ADMET risk.
Our negative control compound (00) was a non-galactoside analogue of the most potent co-crystallized ligand. It has been established that the lectin domain of FmlH (FmlHLD) recognizes the galactose units on the target receptors present on the urinary tract epithelial cells. Without the galactoside moiety, the compound will not bind to FmlH or it will exhibit very little binding. The Glide score of the negative control compound was −3.935 kcal/mol. An MD simulation was performed on this negative control–FmlH complex. The calculated binding free energy was −18.25 kcal/mol, a much poorer value than for the hit compounds. Additionally, this compound exhibited several pharmacokinetic risk flags, such as that it is predicted to have a high mutagenicity risk, which was not observed for our other hits. Additionally, it is predicted to have high hERG toxicity (Figure 10, green and Figure 11A) and some mutagenicity risk (Figure 11A).
It is important to note that small molecule drug discovery is a multi-parameter optimization problem, with the aim of identifying high-quality compounds that exhibit a balance of properties, include potency as well as ADMET [61]. Even though some of our hits exhibited slightly reduced predicted binding free energies relative to the positive control, they exhibited improved predicted pharmacokinetic profiles. Compound 9 (MMGBSA value of ~−22 kcal/mol), which showed a reduced MM-GBSA energy compared with the parent compound, exhibited lower predicted toxicity than parent compound. Many of our hits exhibited comparable predicted binding free energies to that of the most potent co-crystallized ligand.
It was previously reported that co-crystallized ligands bound to a highly homologous protein, FimH, have been seen to exhibit two different binding modes: (a) an in docking mode; and (b) an out docking mode [62,63]. Hits from the Maddirala et al. and Samanta et al. studies exhibited a somewhat out docking mode [14,18]. Overall, the docking modes of the hits in this study were also bound in the out docking mode.
Scaffold hopping is a technique for finding isofunctional but structurally dissimilar chemical matter from libraries of diverse chemotypes that have been rationally designed [64]. In ligand-based scaffold hopping, the binding site of the protein is not taken into account during the screening step, and compounds with novel core motifs are identified that are predicted to have similar biological activities to the template. Ligand-based scaffold hopping is a widely used virtual screening technique in modern drug discovery that is usually faster than receptor-based scaffold hopping [64,65,66,67]. However, it is also crucial to understand how compounds interact with the protein of interest. When information about the binding site is available, receptor-based scaffold hopping allows the identification of novel chemotypes that can interact strongly with the protein [68,69]. Conceptually, ligand-based screening can generate a higher number of potential hits, while receptor-based screening can help to eliminate false positives. Combining both strategies can help in compound optimization with the aim of improving target binding, ensuring drug-like properties, or reducing toxicity [69,70]. In this study, we have applied the two screening approaches in parallel to maximize our chances to find hits. A larger number of potential hits having a synthesizability score >0 were obtained from ligand-based screening (1115 from Scaffold 1 hopping and 1109 from Scaffold 2 hopping) compared with receptor-based scaffold hopping (29 from Scaffold 1 hopping and 9 from Scaffold 2 hopping; these structures also satisfied a cut-off criterion of the number of hydrogen bonding interactions with the protein binding pocket). We then used global machine-learning models for pharmacokinetic property prediction, with the aim of obtaining drug-like hits.
Leveraging the power of modern-day drug discovery methods, our current study focuses on using in silico approaches to generate a database of matched molecular pairs with a strong predicted binding to FmlH. Scaffold hopping is a valuable approach to generate ideas for the design of novel derivatives and to enhance the intellectual property of drug compounds by developing a synthetically accessible new chemical space. Global machine-learning models to predict the pharmacokinetic properties of the small molecule glycomimetics allow the rapid and accurate prediction of their ADMET profiles and enable multi-parameter optimization (MPO) for hit generation.
The potential of FmlH as a drug target and designing galactoside/N-acetylgalactosamine inhibitors is a relatively new area of research, hence the atomic-level understanding provided in this study can play a crucial role in the development of potent inhibitors with suitable pharmacokinetic profiles. Future efforts following this study can be regarding the use of in silico predictive models in the compartmental and noncompartmental pharmacokinetic analysis (NCA) of hits, which can aid in efficient FmlH inhibitor discovery. Structure-based drug design can also be used to handle the MPO problem for the optimization of several ADMET properties, as well as FmlH binding.
4. Conclusions
Using in silico scaffold hopping, we have designed novel small molecule glycomimetics that showed enhanced predicted FmlH binding. We have obtained 38 and 20 matched molecular pairs after the Scaffold 1 and 2 replacement, respectively. These novel scaffolds that we selected all were predicted to be accessible to synthesis.
In this study, we performed MPO to enhance the number of polar interactions between the ligands and FmlH and to improve the pharmacokinetic properties of the ligand. The small library of 58 compounds prepared in and provided with this study could be further investigated and can be an excellent resource for identifying novel classes of compounds that could be further studied using in vitro studies and in vivo validation. Several of the hits exhibited lowered predicted cardiotoxicity through reduced hERG off-target binding relative to previously reported FmlH inhibitors. Molecules with low ADMET risks were further investigated in MD simulations and the key pairwise FmlH–ligand interactions were identified, followed by the investigation of their binding energy profiles.
The in silico technique used in this study to identify MMPs to obtain novel small molecule glycomimetics that belong to various classes of potentially potent FmlH inhibitors is the first of its kind in FmlH computer-aided inhibitor design study. Our generated library of 58 small molecule glycomimetics with novel scaffolds will likely be of use to others who are seeking to discover potent inhibitors of FmlH.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14209496/s1. Included in the Supporting Information file are the core library diversity profile, SiteMap analysis of FmlH, clearance profiles of the hits obtained from Scaffold 1 and Scaffold 2 hopping, and root-mean square deviations of the hits from MD simulations. Additional files include an Excel file containing the SMILES strings of the 58 hits and the 4 compounds from the previous study by Samanta et al. [14] and two text files containing SMILES strings of the 1115 and 1109 compounds, respectively, that exhibited a synthesizability score > 0.
Author Contributions
P.S.: Conceptualization, Methodology, Data curation, Formal analysis, Visualization, Investigation, Writing—original draft preparation, Writing—reviewing and editing, Validation. R.J.D.: Conceptualization, Methodology, Writing—reviewing and editing, Supervision, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institutes of Health [P20GM130460]. The content does not necessarily reflect the position or the policy of the sponsors, and no official endorsement should be inferred. The funders had no role in the design or writing, or in the decision to publish the work in this form. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data underlying this article are available in the article and in the online Supplementary Material.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The abbreviations used are: UTI, urinary tract infection; UPEC, uropathogenic Escherichia coli; CUP, chaperone–usher pathway; MM-GBSA, Molecular Mechanics Generalized Born/Surface Area; SP, standard precision; GalNAc, D-N-acetylgalactosamine; MD, molecular dynamics; MMP, matched molecular pair; ADMET, Adsorption, Distribution, Metabolism, Elimination, Toxicity; hERG, human ether-a-go-go related gene; SER_ALT, alanine aminotransferase; SER_AST, aspartate aminotransferase; MPO, multi-parameter optimization.
References
- Sarshar, M.; Behzadi, P.; Ambrosi, C.; Zagaglia, C.; Palamara, A.T.; Scribano, D. FimH and Anti-Adhesive Therapeutics: A Disarming Strategy Against Uropathogens. Antibiotics 2020, 9, 397. [Google Scholar] [CrossRef]
- Yang, X.; Chen, H.; Zheng, Y.; Qu, S.; Wang, H.; Yi, F. Disease burden and long-term trends of urinary tract infections: A worldwide report. Front. Public Health 2022, 10, 888205. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Behzadi, P.; Behzadi, E.; Pawlak-Adamska, E.A. Urinary tract infections (UTIs) or genital tract infections (GTIs)? It’s the diagnostics that count. GMS Hyg. Infect. Control. 2019, 14, 1–12. [Google Scholar]
- Chockalingam, A.; Stewart, S.; Xu, L.; Gandhi, A.; Matta, M.K.; Patel, V.; Sacks, L.; Rouse, R. Evaluation of Immunocompetent Urinary Tract Infected Balb/C Mouse Model For the Study of Antibiotic Resistance Development Using Escherichia coli CFT073 infecyions. Antibiotics 2019, 8, 170. [Google Scholar] [CrossRef]
- Issakhanian, L.; Behzadi, P. Antimicrobial agents and urinary tract infections. Curr. Pharm. Des. 2019, 25, 1409–1423. [Google Scholar] [CrossRef]
- Behzadi, P. Classical chaperone-usher (CU) adhesive fimbriome: Uropathogenic Escherichia coli (UPEC) and urinary tract infections (UTIs). Folia Microbiol. 2020, 65, 45–65. [Google Scholar] [CrossRef]
- Hozzari, A.; Behzadi, P.; Kerishchi Khiabani, P.; Sholeh, M.; Sabokroo, N. Clinical cases, drug resistance, and virulence genes profiling in Uropathogenic Escherichia coli. J. Appl. Genet. 2020, 61, 265–273. [Google Scholar] [CrossRef]
- Kleeb, S.; Jiang, X.; Frei, P.; Sigl, A.; Bezençon, J.; Bamberger, K.; Schwardt, O.; Ernst, B. FimH Antagonists: Phosphate Prodrugs Improve Oral Bioavailability. J. Med. Chem. 2016, 59, 3163–3182. [Google Scholar] [CrossRef]
- Hooton, T.M.; Stamm, W.E. Diagnosis and treatment of uncomplicated urinary tract infection. Infect. Dis. Clin. 1997, 11, 551–581. [Google Scholar] [CrossRef]
- Svanborg, C.; Godaly, G. Bacterial virulence in urinary tract infection. Infect. Dis. Clin. North Am. 1997, 11, 513–529. [Google Scholar] [CrossRef]
- Zalewska-Piątek, B.M.; Piątek, R.J. Alternative treatment approaches of urinary tract infections caused by uropathogenic Escherichia coli strains. Acta Biochim. Pol. 2019, 66, 129–138. [Google Scholar] [CrossRef]
- Day, C.J.; Lo, A.W.; Hartley-Tassell, L.E.; Argente, M.P.; Poole, J.; King, N.P.; Tiralongo, J.; Jennings, M.P.; Schembri, M.A. Discovery of Bacterial Fimbria–Glycan Interactions Using Whole-Cell Recombinant Escherichia coli Expression. mBio 2021, 12, e03664-20. [Google Scholar] [CrossRef]
- Samanta, P.; Doerksen, R.J. Identifying FmlH lectin-binding small molecules for the prevention of Escherichia coli-induced urinary tract infections using hybrid fragment-based design and molecular docking. Comput Biol Med 2023, 163, 107072. [Google Scholar] [CrossRef]
- Mulvey, M.A.; Lopez-Boado, Y.S.; Wilson, C.L.; Roth, R.; Parks, W.C.; Heuser, J.; Hultgren, S.J. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 1998, 282, 1494–1497. [Google Scholar] [CrossRef]
- Jones, C.H.; Pinkner, J.S.; Roth, R.; Heuser, J.; Nicholes, A.V.; Abraham, S.N.; Hultgren, S.J. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc. Natl. Acad. Sci. USA 1995, 92, 2081–2085. [Google Scholar] [CrossRef]
- Kalas, V.; Hibbing, M.E.; Maddirala, A.R.; Chugani, R.; Pinkner, J.S.; Mydock-McGrane, L.K.; Conover, M.S.; Janetka, J.W.; Hultgren, S.J. Structure-based discovery of glycomimetic FmlH ligands as inhibitors of bacterial adhesion during urinary tract infection. Proc. Natl. Acad. Sci. USA 2018, 115, E2819–E2828. [Google Scholar] [CrossRef]
- Maddirala, A.R.; Klein, R.; Pinkner, J.S.; Kalas, V.; Hultgren, S.J.; Janetka, J.W. Biphenyl Gal and GalNAc FmlH Lectin Antagonists of Uropathogenic E. coli (UPEC): Optimization through Iterative Rational Drug Design. J Med Chem 2019, 62, 467–479. [Google Scholar] [CrossRef]
- Wurpel, D.J.; Totsika, M.; Allsopp, L.P.; Hartley-Tassell, L.E.; Day, C.J.; Peters, K.M.; Sarkar, S.; Ulett, G.C.; Yang, J.; Tiralongo, J.; et al. F9 fimbriae of uropathogenic Escherichia coli are expressed at low temperature and recognise Galβ1-3GlcNAc-containing glycans. PLoS ONE 2014, 9, e93177. [Google Scholar] [CrossRef]
- Conover, M.S.; Ruer, S.; Taganna, J.; Kalas, V.; De Greve, H.; Pinkner, J.S.; Dodson, K.W.; Remaut, H.; Hultgren, S.J. Inflammation-induced adhesin-receptor interaction provides a fitness advantage to uropathogenic E. coli during chronic infection. Cell Host Microbe 2016, 20, 482–492. [Google Scholar] [CrossRef]
- Mydock-McGrane, L.K.; Hannan, T.J.; Janetka, J.W. Rational design strategies for FimH antagonists: New drugs on the horizon for urinary tract infection and Crohn’s disease. Expert Opin. Drug Discov. 2017, 12, 711–731. [Google Scholar] [CrossRef]
- Jarvis, C.; Han, Z.; Kalas, V.; Klein, R.; Pinkner, J.S.; Ford, B.; Binkley, J.; Cusumano, C.K.; Cusumano, Z.; Mydock-McGrane, L.; et al. Antivirulence Isoquinolone Mannosides: Optimization of the Biaryl Aglycone for FimH Lectin Binding Affinity and Efficacy in the Treatment of Chronic UTI. ChemMedChem 2016, 11, 367–373. [Google Scholar] [CrossRef]
- Mydock-McGrane, L.; Cusumano, Z.; Han, Z.; Binkley, J.; Kostakioti, M.; Hannan, T.; Pinkner, J.S.; Klein, R.; Kalas, V.; Crowley, J.; et al. Antivirulence C-Mannosides as Antibiotic-Sparing, Oral Therapeutics for Urinary Tract Infections. J. Med. Chem. 2016, 59, 9390–9408. [Google Scholar] [CrossRef]
- Han, Z.; Pinkner, J.S.; Ford, B.; Obermann, R.; Nolan, W.; Wildman, S.A.; Hobbs, D.; Ellenberger, T.; Cusumano, C.K.; Hultgren, S.J.; et al. Structure-based drug design and optimization of mannoside bacterial FimH antagonists. J. Med. Chem. 2010, 53, 4779–4792. [Google Scholar] [CrossRef]
- Cusumano, C.K.; Pinkner, J.S.; Han, Z.; Greene, S.E.; Ford, B.A.; Crowley, J.R.; Henderson, J.P.; Janetka, J.W.; Hultgren, S.J. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci. Transl. Med. 2011, 3, 109ra115. [Google Scholar] [CrossRef]
- Grabosch, C.; Hartmann, M.; Schmidt-Lassen, J.; Lindhorst, T.K. Squaric acid monoamide mannosides as ligands for the bacterial lectin FimH: Covalent inhibition or not? ChemBioChem 2011, 12, 1066–1074. [Google Scholar] [CrossRef]
- Kleeb, S.; Pang, L.; Mayer, K.; Eris, D.; Sigl, A.; Preston, R.C.; Zihlmann, P.; Sharpe, T.; Jakob, R.P.; Abgottspon, D.; et al. FimH antagonists: Bioisosteres to improve the in vitro and in vivo PK/PD profile. J. Med. Chem. 2015, 58, 2221–2239. [Google Scholar] [CrossRef]
- Chalopin, T.; Alvarez Dorta, D.; Sivignon, A.; Caudan, M.; Dumych, T.I.; Bilyy, R.O.; Deniaud, D.; Barnich, N.; Bouckaert, J.; Gouin, S.G. Second generation of thiazolylmannosides, FimH antagonists for E. coli-induced Crohn’s disease. Org. Biomol. Chem. 2016, 14, 3913–3925. [Google Scholar] [CrossRef]
- Mydock-McGrane, L.K.; Cusumano, Z.T.; Janetka, J.W. Mannose-derived FimH antagonists: A promising anti-virulence therapeutic strategy for urinary tract infections and Crohn’s disease. Expert Opin. Ther. Pat. 2016, 26, 175–197. [Google Scholar] [CrossRef]
- Ofek, I.; Hasty, D.L.; Sharon, N. Anti-adhesion therapy of bacterial diseases: Prospects and problems. FEMS Immunol. Med. Microbiol. 2003, 38, 181–191. [Google Scholar] [CrossRef]
- Hu, Y.; Stumpfe, D.; Bajorath, J. Recent Advances in Scaffold Hopping. J. Med. Chem. 2017, 60, 1238–1246. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, S.Q.; Xu, W.R.; Wang, R.L.; Chou, K.C. Design novel dual agonists for treating type-2 diabetes by targeting peroxisome proliferator-activated receptors with core hopping approach. PLoS ONE 2012, 7, e38546. [Google Scholar] [CrossRef]
- Schrödinger Release 2021-2: Core Hopping; Schrödinger, LLC: New York, NY, USA, 2021.
- Pasala, C.; Katari, S.K.; Nalamolu, R.M.; Aparna, R.B.; Amineni, U. Integration of core hopping, quantum-mechanics, molecular mechanics coupled binding-energy estimations and dynamic simulations for fragment-based novel therapeutic scaffolds against Helicobacter pylori strains. Comput. Biol. Chem. 2019, 83, 107126. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Schrödinger Release 2021-2: Glide; Schrödinger, LLC: New York, NY, USA, 2021.
- Samanta, P.; Mishra, S.K.; Pomin, V.H.; Doerksen, R.J. Docking and Molecular Dynamics Simulations Clarify Binding Sites for Interactions of Novel Marine Sulfated Glycans with SARS-CoV-2 Spike Glycoprotein. Molecules 2023, 28, 6413. [Google Scholar] [CrossRef]
- Maurya, A.K.; Sharma, P.; Samanta, P.; Shami, A.A.; Misra, S.K.; Zhang, F.; Thara, R.; Kumar, D.; Shi, D.; Linhardt, R.J.; et al. Structure, anti-SARS-CoV-2, and anticoagulant effects of two sulfated galactans from the red alga Botryocladia occidentalis. Int. J. Biol. Macromol. 2023, 238, 124168. [Google Scholar] [CrossRef]
- Samanta, P.; Doerksen, R.J. Identifying p56lck SH2 Domain Inhibitors Using Molecular Docking and In Silico Scaffold Hopping. Appl. Sci. 2024, 14, 4277. [Google Scholar] [CrossRef]
- Simulations Plus, Inc. Available online: http://www.simulations-plus.com (accessed on 3 October 2024).
- Vlachakis, D.; Bencurova, E.; Papangelopoulos, N.; Kossida, S. Chapter Seven–Current State-of-the-Art Molecular Dynamics Methods and Applications. Adv. Protein Chem. Struct. Biol. 2014, 94, 269–313. [Google Scholar]
- Tomasulo, P. ChemIDplus-super source for chemical and drug information. Med. Ref. Serv. Q 2002, 21, 53–59. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, D.E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the SC’06: Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, Tampa, FL, USA, 11–17 November 2006; p. 84-es. [Google Scholar]
- Schrödinger Release 2021-2: Desmond Molecular Dynamics System; D. E. Shaw Research: New York, NY, USA, 2021; Maestro-Desmond Interoperability Tools; Schrödinger: New York, NY, USA, 2021. Cf. Available online: https://www.schrodinger.com/citations/ (accessed on 9 October 2024).
- Li, J.; Abel, R.; Zhu, K.; Cao, Y.; Zhao, S.; Friesner, R.A. The VSGB 2.0 model: A next generation energy model for high resolution protein structure modeling. Proteins 2011, 79, 2794–2812. [Google Scholar] [CrossRef]
- Ludwig, S.G.; Kiyohara, C.L.; Carlucci, L.A.; Kisiela, D.; Sokurenko, E.V.; Thomas, W.E. FimH as a scaffold for regulated molecular recognition. J Biol Eng 2021, 15, 3. [Google Scholar] [CrossRef]
- Nilsson, L.M.; Thomas, W.E.; Sokurenko, E.V.; Vogel, V. Beyond induced-fit receptor-ligand interactions: Structural changes that can significantly extend bond lifetimes. Structure 2008, 16, 1047–1058. [Google Scholar] [CrossRef]
- Nilsson, L.M.; Thomas, W.E.; Trintchina, E.; Vogel, V.; Sokurenko, E.V. Catch bond-mediated adhesion without a shear threshold: Trimannose versus monomannose interactions with the FimH adhesin of Escherichia coli. J. Biol. Chem. 2006, 281, 16656–16663. [Google Scholar] [CrossRef]
- Schönemann, W.; Cramer, J.; Mühlethaler, T.; Fiege, B.; Silbermann, M.; Rabbani, S.; Dätwyler, P.; Zihlmann, P.; Jakob, R.P.; Sager, C.P.; et al. Improvement of Aglycone π-Stacking Yields Nanomolar to Sub-nanomolar FimH Antagonists. ChemMedChem 2019, 14, 749–757. [Google Scholar] [CrossRef]
- Roos, G.; Wellens, A.; Touaibia, M.; Yamakawa, N.; Geerlings, P.; Roy, R.; Wyns, L.; Bouckaert, J. Validation of Reactivity Descriptors to Assess the Aromatic Stacking within the Tyrosine Gate of FimH. ACS Med. Chem. Lett. 2013, 4, 1085–1090. [Google Scholar] [CrossRef]
- Fiege, B.; Rabbani, S.; Preston, R.C.; Jakob, R.P.; Zihlmann, P.; Schwardt, O.; Jiang, X.; Maier, T.; Ernst, B. The tyrosine gate of the bacterial lectin FimH: A conformational analysis by NMR spectroscopy and X-ray crystallography. ChemBioChem 2015, 16, 1235–1246. [Google Scholar] [CrossRef]
- Vanwetswinkel, S.; Volkov, A.N.; Sterckx, Y.G.; Garcia-Pino, A.; Buts, L.; Vranken, W.F.; Bouckaert, J.; Roy, R.; Wyns, L.; van Nuland, N.A. Study of the structural and dynamic effects in the FimH adhesin upon α-d-heptyl mannose binding. J. Med. Chem. 2014, 57, 1416–1427. [Google Scholar] [CrossRef]
- Singh, K.D.; Kirubakaran, P.; Nagarajan, S.; Sakkiah, S.; Muthusamy, K.; Velmurgan, D.; Jeyakanthan, J. Homology modeling, molecular dynamics, e-pharmacophore mapping and docking study of Chikungunya virus nsP2 protease. J. Mol. Model. 2012, 18, 39–51. [Google Scholar] [CrossRef]
- Halgren, T.A. Identifying and characterizing binding sites and assessing druggability. J. Chem. Inf. Model. 2009, 49, 377–389. [Google Scholar] [CrossRef]
- Halgren, T. New method for fast and accurate binding-site identification and analysis. Chem. Biol. Drug Des. 2007, 69, 146–148. [Google Scholar] [CrossRef]
- Schrödinger Release 2021-2: SiteMap; Schrödinger, LLC: New York, NY, USA, 2021.
- Alonso-Caballero, A.; Schönfelder, J.; Poly, S.; Corsetti, F.; De Sancho, D.; Artacho, E.; Perez-Jimenez, R. Mechanical architecture and folding of E. coli type 1 pilus domains. Nat. Commun. 2018, 9, 2758. [Google Scholar] [CrossRef]
- Sivignon, A.; Bouckaert, J.; Bernard, J.; Gouin, S.G.; Barnich, N. The potential of FimH as a novel therapeutic target for the treatment of Crohn’s disease. Expert Opin. Ther. Targets 2017, 21, 837–847. [Google Scholar] [CrossRef]
- Segall, M.D. Multi-parameter optimization: Identifying high quality compounds with a balance of properties. Curr Pharm Des 2012, 18, 1292–1310. [Google Scholar] [CrossRef]
- Schwardt, O.; Rabbani, S.; Hartmann, M.; Abgottspon, D.; Wittwer, M.; Kleeb, S.; Zalewski, A.; Smieško, M.; Cutting, B.; Ernst, B. Design, synthesis and biological evaluation of mannosyl triazoles as FimH antagonists. Bioorg. Med. Chem. 2011, 19, 6454–6473. [Google Scholar] [CrossRef]
- Klein, T.; Abgottspon, D.; Wittwer, M.; Rabbani, S.; Herold, J.; Jiang, X.; Kleeb, S.; Lüthi, C.; Scharenberg, M.; Bezençon, J.; et al. FimH antagonists for the oral treatment of urinary tract infections: From design and synthesis to in vitro and in vivo evaluation. J. Med. Chem. 2010, 53, 8627–8641. [Google Scholar] [CrossRef]
- Renner, S.; Schneider, G. Scaffold-hopping potential of ligand-based similarity concepts. ChemMedChem 2006, 1, 181–185. [Google Scholar] [CrossRef]
- Franke, L.; Schwarz, O.; Müller-Kuhrt, L.; Hoernig, C.; Fischer, L.; George, S.; Tanrikulu, Y.; Schneider, P.; Werz, O.; Steinhilber, D.; et al. Identification of natural-product-derived inhibitors of 5-lipoxygenase activity by ligand-based virtual screening. J. Med. Chem. 2007, 50, 2640–2646. [Google Scholar] [CrossRef]
- Maeda, I.; Tamura, S.; Ogura, Y.; Serizawa, T.; Shimada, T.; Kunimoto, R.; Miyao, T. Scaffold-Hopped Compound Identification by Ligand-Based Approaches with a Prospective Affinity Test. J. Chem. Inf. Model. 2024, 64, 5557–5569. [Google Scholar] [CrossRef]
- Aparoy, P.; Reddy, K.K.; Reddanna, P. Structure and ligand based drug design strategies in the development of novel 5- LOX inhibitors. Curr. Med. Chem. 2012, 19, 3763–3778. [Google Scholar] [CrossRef]
- Fontaine, F.; Cross, S.; Plasencia, G.; Pastor, M.; Zamora, I. SHOP: A method for structure-based fragment and scaffold hopping. ChemMedChem 2009, 4, 427–439. [Google Scholar] [CrossRef]
- Johansson, E.; Caraballo, R.; Hurdiss, D.L.; Mistry, N.; Andersson, C.D.; Thompson, R.F.; Ranson, N.A.; Zocher, G.; Stehle, T.; Arnberg, N.; et al. Exploring the Effect of Structure-Based Scaffold Hopping on the Inhibition of Coxsackievirus A24v Transduction by Pentavalent N-Acetylneuraminic Acid Conjugates. Int. J. Mol. Sci. 2021, 22, 8418. [Google Scholar] [CrossRef]
- Bajorath, J. Computational Scaffold Hopping: Cornerstone for the Future of Drug Design? Future Med. Chem. 2017, 629–631. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).