Activated Carbons as Effective Adsorbents of Non-Steroidal Anti-Inflammatory Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Methods
2.2.1. Adsorption Equilibrium
2.2.2. Adsorption Kinetics
- Hereafter, the kinetic data were analyzed using a 1,2-mixed-order kinetic equation (MOE), which is a generalization of the first- and second-order kinetics. This dependence (mathematically) corresponds to a linear combination of first- and second-order units. The above-mentioned relationship might also be expressed as the relative progress of adsorption over time F.orwhere f2 < 1—the normalized share of the second-order process in the kinetics. One should notice that in some cases, the MOE equation may be reduced to the first-order (f2 = 0) and the second-order (f2 = 1) kinetic equations [28,29].
- The kinetic data were also analyzed by applying a modified MOE equation that takes into account heterogeneity effects. The fractal-like MOE equation (f-MOE) can be presented as:where p—fractal coefficient. Under particular conditions, the dependence can be reduced to the MOE (p = 0), f-FOE (f2 = 0), and f-SOE (f2 = 1) equations [30,31].
- The multi-exponential equation (m-exp) was also used for the specification of kinetic data. This relationship is often used to describe adsorption on heterogeneous solids and may indicate the occurrence of a series of first-order or subsequent processes. This can be written as:orwhere “i”—the term of the m-exp equation. ki—the rate coefficient. ueq = 1 − ceq/c0—the relative loss of adsorbate from the solution [8,26].
2.2.3. Zeta Potential Measurements
2.2.4. Thermal Analysis
3. Results
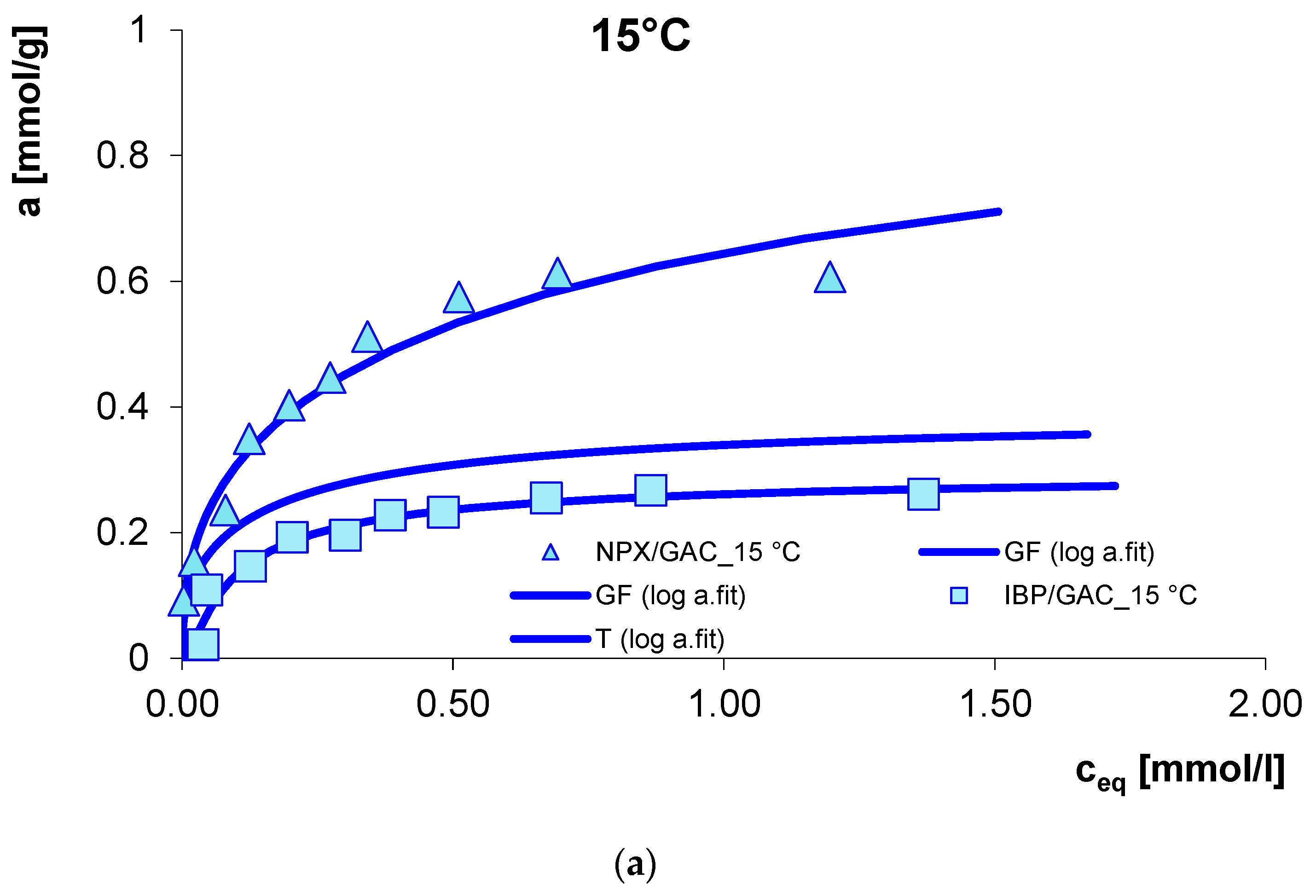
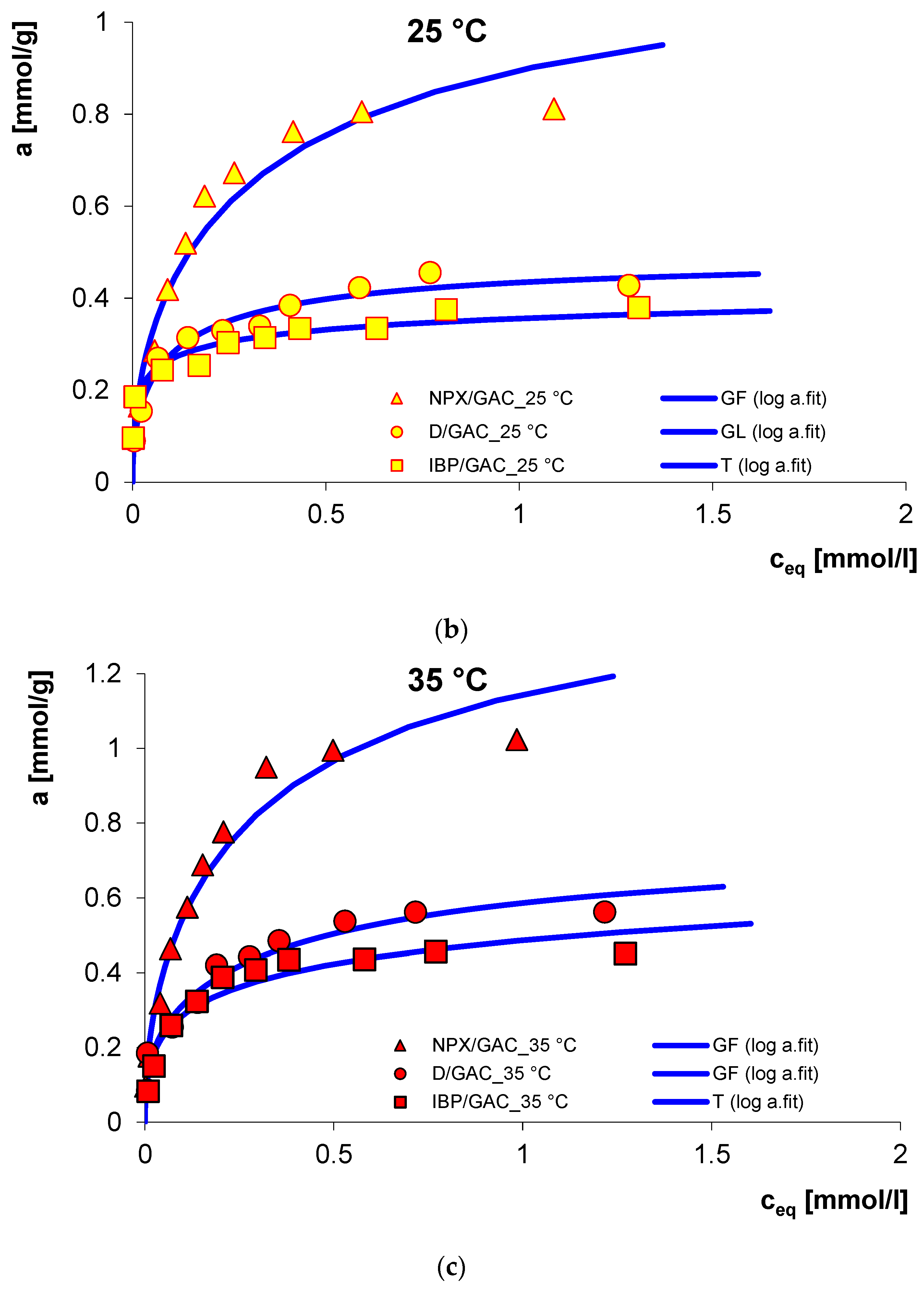
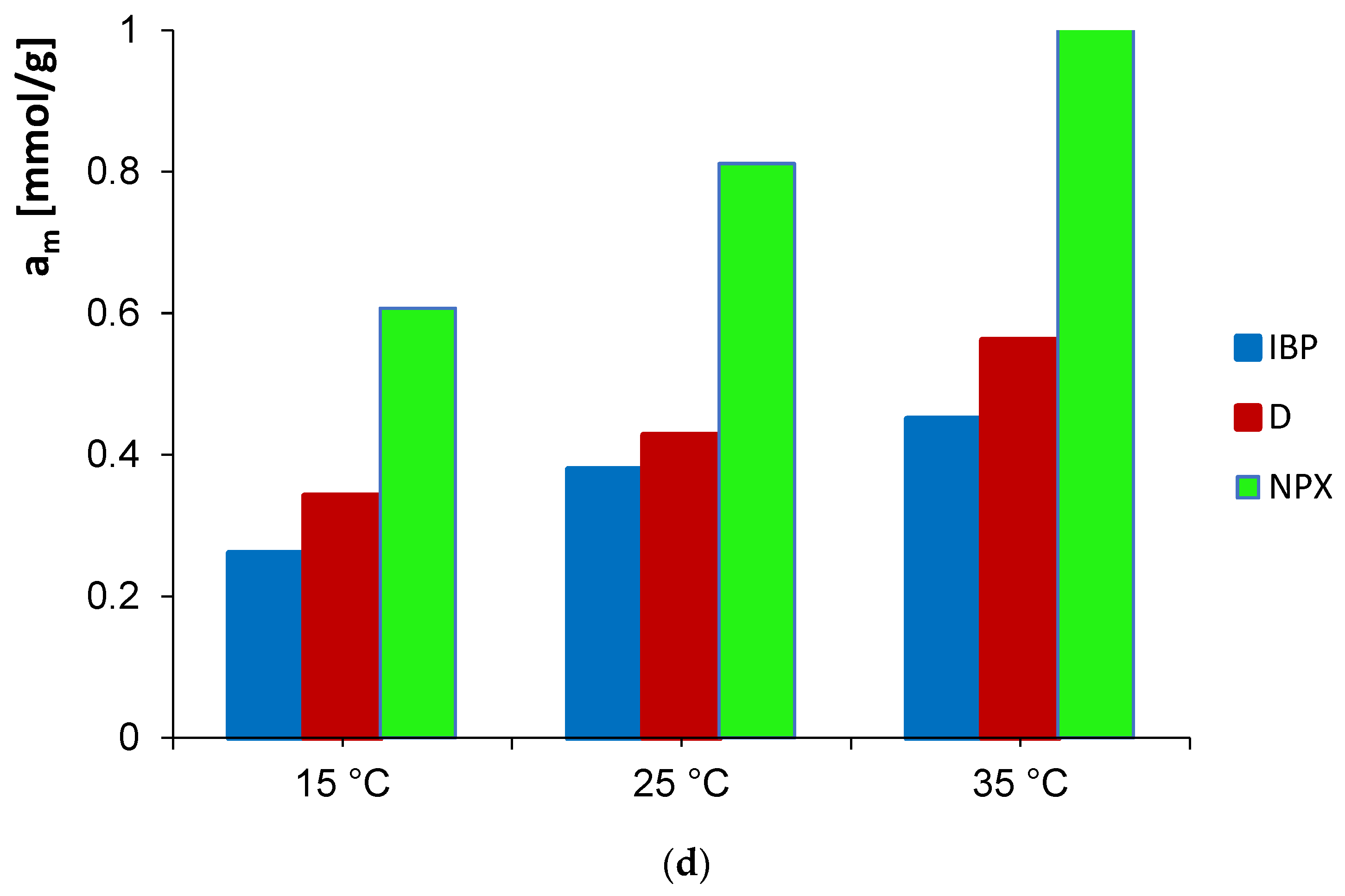
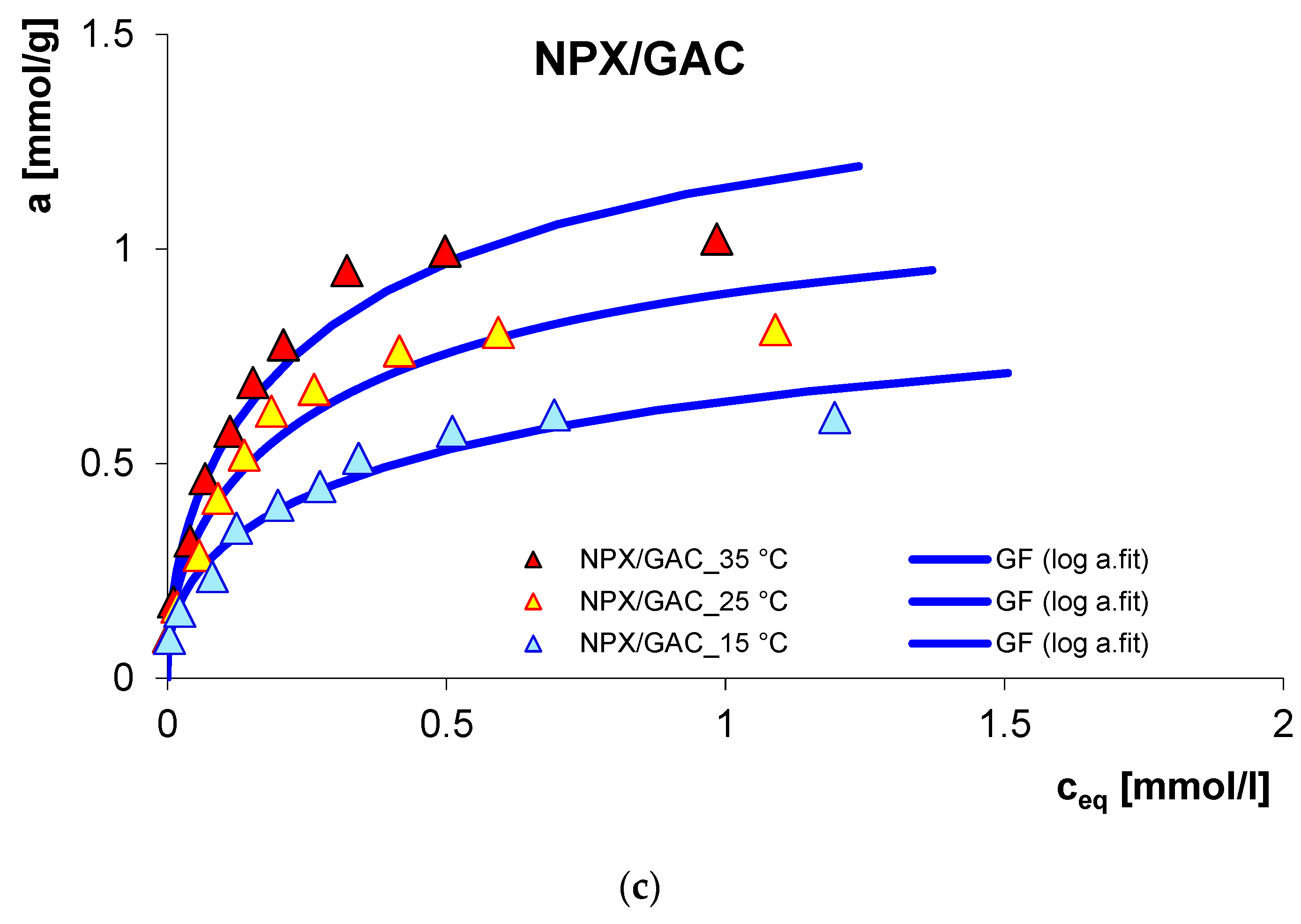
3.1. Adsorption Equilibrium
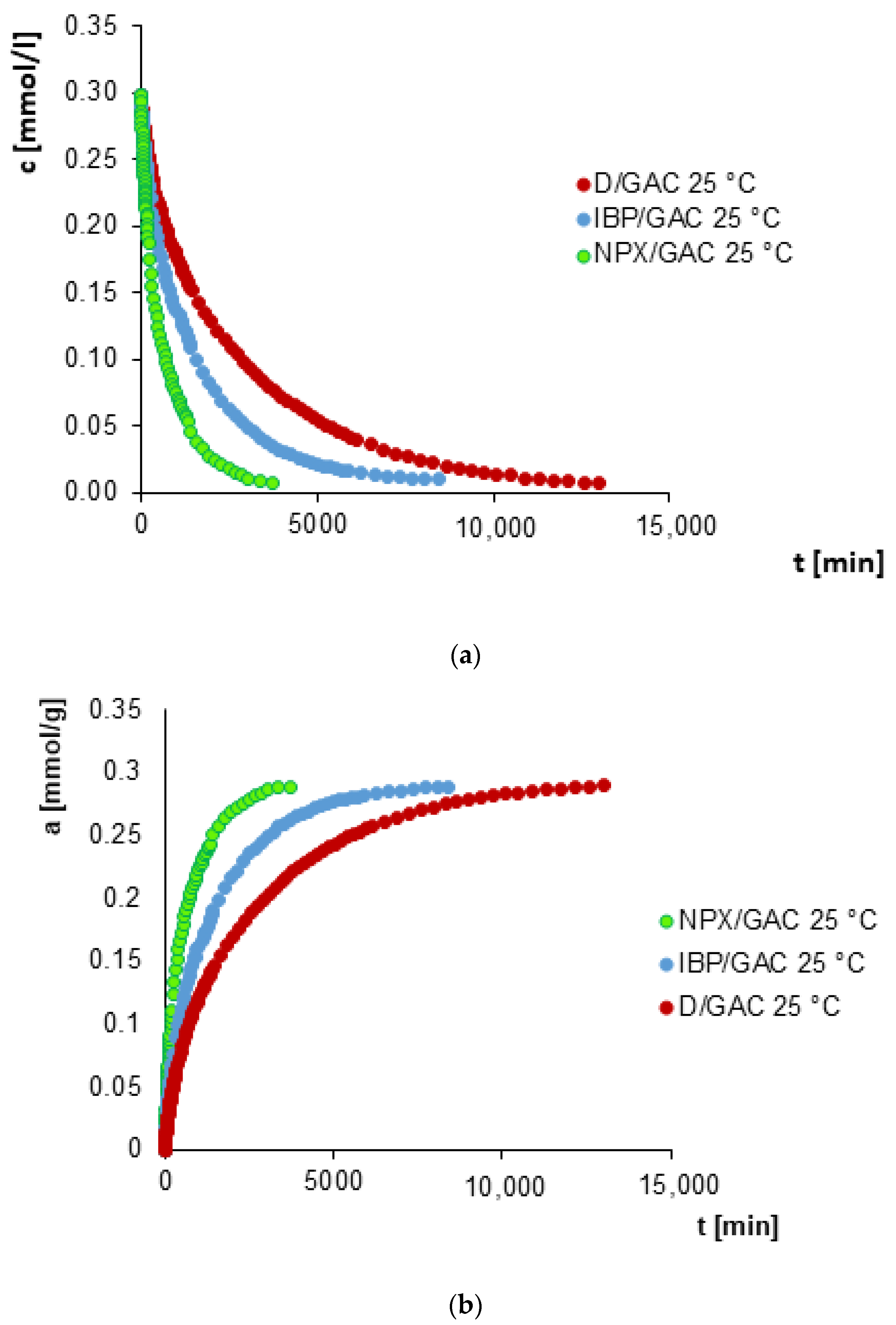
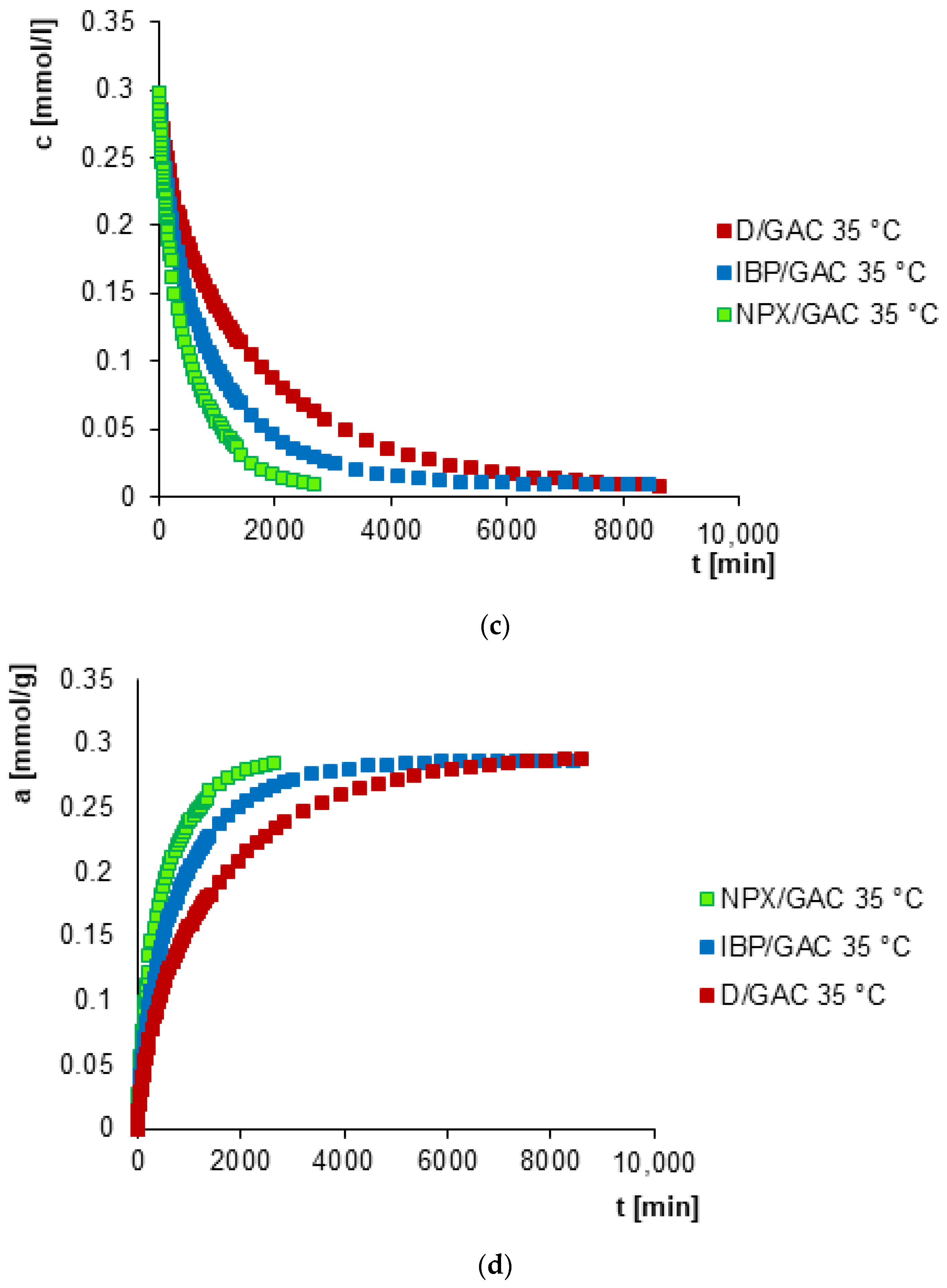
3.2. Adsorption Kinetics
3.3. Zeta Potential Measurements
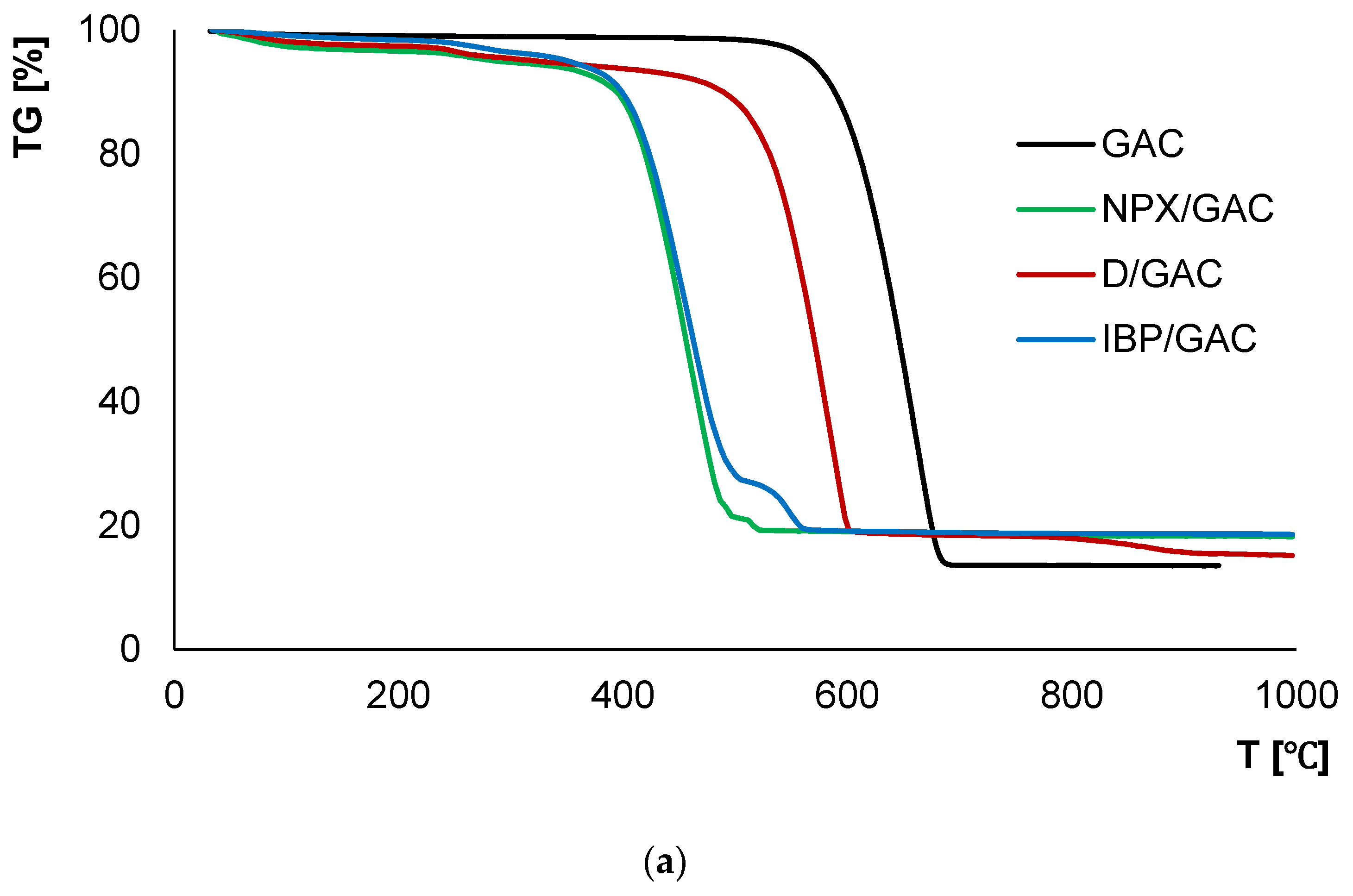
3.4. Thermal Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pap, S.; Taggrat, M.A.; Shearer, L.; Li, Y.; Radovic, S.; Turk Sekulic, M. Removal behaviour of NSAIDs from wastewater using a P-functionalised microporous carbon. Chemosphere 2021, 264, 128439. [Google Scholar] [CrossRef] [PubMed]
- Mussa, Z.H.; Al-Qaim, F.F.; Jawad, A.H.; Scholz, M.; Yaseen, Z.M. A Comprehensive Review for Removal of Non-Steroidal Anti-Inflammatory Drugs Attained from Wastewater Observations Using Carbon-Based Anodic Oxidation Process. Toxics 2022, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Ayati, A.; Farghali, M.; Krivoshapkin, P.; Tanhaei, B.; Karimi-Maleh, H.; Krivoshapkina, E.; Taheri, P.; Tracey, C.; Al-Fatesh, A.; et al. Advanced adsorbents for ibuprofen removal from aquatic environments: A review. Environ. Chem. Lett. 2023. [Google Scholar] [CrossRef]
- Rastogi, A.; Tiwari, M.K.; Ghangrekar, M.M. A review on environmental occurrence, toxicity and microbial degradation of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). J. Environ. Manag. 2021, 300, 113694. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Maric, F. Mitigating the environmental impact of NSAIDs—Physiotherapy as a contribution to One Health and the SDGs. Eur. J. Physiother. 2023, 25, 51. [Google Scholar] [CrossRef]
- Arguello-Pérez, M.Á.; Ramírez-Ayala, E.; Mendoza-Pérez, J.A.; Monroy-Mendieta, M.M.; Vázquez-Guevara, M.; Lezama-Cervantes, C.; Godínez-Domínguez, E.; de Asís Silva-Bátiz, F.; Tintos-Gómez, A. Determination of the Bioaccumulative Potential Risk of Emerging Contaminants in Fish Muscle as an Environmental Quality Indicator in Coastal Lagoons of the Central Mexican Pacific. Water 2020, 12, 2721. [Google Scholar] [CrossRef]
- Chaves, M.J.S.; Kulzer, J.; Pujol de Lima, P.R.; Barbosa, S.C.; Primel, G.P. Updated knowledge, partitioning and ecological risk of pharmaceuticals and personal care products in global aquatic environments. Environ. Sci. Process. Impacts 2022, 24, 1982. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, M.; Marczewski, A.W.; Deryło-Marczewska, A.; Sternik, D. Nitrophenols removal from aqueous solutions by activated carbon—Temperature effect of adsorption kinetics and equilibrium. J. Environ. Chem. Eng. 2021, 9, 105459–105478. [Google Scholar] [CrossRef]
- Rehman, A.; Park, M.; Park, S.-J. Current Progress on the Surface Chemical Modification of Carbonaceous Materials. Coatings 2019, 9, 103. [Google Scholar] [CrossRef]
- Andreoli, S.; Eser, S. Composition of Surface Groups and Adsorption Properties of Activated Carbons Derived from Different Bio-Precursors. Chem. Eng. Trans. 2021, 86, 637. [Google Scholar] [CrossRef]
- Negara, D.N.K.P.; Nindhia, T.G.T.; Septiadi, W.N. Surface Properties and Adsorption Capacities of Rice Bran-Activated Carbon. J. Mech. Eng. Sci. Technol. 2020, 4, 115. [Google Scholar] [CrossRef]
- Wolak, E.; Orzechowska-Zięba, A. Change of the surface and structure of activated carbon as a result of HNO3 modification. Adsorption 2023. [Google Scholar] [CrossRef]
- Vojnović, B.; Cetina, M.; Franjković, P.; Sutlović, A. Influence of Initial pH Value on the Adsorption of Reactive Black 5 Dye on Powdered Activated Carbon: Kinetics, Mechanisms, and Thermodynamics. Molecules 2022, 27, 1349. [Google Scholar] [CrossRef]
- Bai, H.; Chen, J.; Zhou, X.; Hu, C. Single and binary adsorption of dyes from aqueous solutions using functionalized microcrystalline cellulose from cotton fiber. Korean J. Chem. Eng. 2020, 37, 1926. [Google Scholar] [CrossRef]
- Delaroza, R.; Wijayanti, A.; Kusumadewi, R.A.; Hadisoebroto, R. The effect of mixing speed to adsorption heavy metal Cu2+ and color using kepok banana peel waste. IOP Conf. Ser. Earth Environ. Sci. 2020, 426, 012024. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 12 December 2023).
- Available online: https://www.chemsrc.com/en/ (accessed on 12 December 2023).
- Safety Data Sheet for Activated Carbon GAC 1240W, Owned by Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/PL/en/sds/sigald/37771?userType=anonymous (accessed on 10 January 2024).
- Marton, M.; Ilavský, J.; Barloková, D. Adsorption of specific chloroacetanilidies on granular activated carbon. IOP Conf. Ser. Mater. Sci. Eng. 2020, 867, 012031. [Google Scholar] [CrossRef]
- Marczewski, A.W.; Sęczkowska, M.; Deryło-Marczewska, A.; Błachnio, M. Adsorption equilibrium and kinetics of selected phenoxyacid pesticides on activated carbon—Effect of temperature. Adsorption 2016, 22, 777–790. [Google Scholar] [CrossRef]
- Deryło-Marczewska, A.; Miroslaw, K.; Marczewski, A.W.; Sternik, D. Studies of adsorption equilibria and kinetics of o-. m-. p-nitro- and chlorophenols on microporous carbons from aqueous solutions. Adsorption 2010, 16, 359–375. [Google Scholar] [CrossRef]
- Marczewski, A.W.; Jaroniec, M. A new isotherm equation for single-solute adsorption from dilute solutions on energetically heterogeneous solids—Short communication. Monatshefte Chem. Chem. Mon. 1983, 114, 711–715. [Google Scholar] [CrossRef]
- Jaroniec, M.; Marczewski, A.W. Physical adsorption of gases on energetically heterogeneous solids I. Generalized Langmuir equation and its energy distribution. Monatshefte Chem. Chem. Mon. 1984, 115, 997–1012. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Geloster Stoffe. K. Sven. Vetenskapsakademiens. Handl. 1898, 24, 1–39. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef]
- Marczewski, A.W. Kinetics and equilibrium of adsorption of organic solutes on mesoporous carbons. Appl. Surf. Sci. 2007, 253, 5818–5826. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1988, 70, 115–124. [Google Scholar] [CrossRef]
- Marczewski, A.W. Application of mixed order rate equations to adsorption of methylene blue on mesoporous carbons. Appl. Surf. Sci. 2010, 256, 5145–5152. [Google Scholar] [CrossRef]
- Marczewski, A.W. Analysis of kinetic Langmuir model. Part I: Integrated kinetic Langmuir equation (IKL): A new complete analytical solution of the Langmuir rate equation. Langmuir 2010, 26, 15229–15238. [Google Scholar] [CrossRef] [PubMed]
- Marczewski, A.W.; Deryło-Marczwska, A.; Słota, A. Adsorption and desorption kinetics of benzene derivatives on mesoporous carbons. Adsorption 2013, 19, 391–406. [Google Scholar] [CrossRef]
- Haerifar, M.; Azizian, S. Fractal-like adsorption kinetics at the solid/solution interface. J. Phys. Chem. C 2012, 116, 13111–13119. [Google Scholar] [CrossRef]
- Terzyk, A.P. Molecular properties and intermolecular forces—Factors balancing the effect of carbon surface chemistry in adsorption of organics from dilute aqueous solutions. J. Colloid Interface Sci. 2004, 275, 9–29. [Google Scholar] [CrossRef]
- Fontecha-Cámara, M.A.; López-Ramón, M.V.; Álvarez-Merino, M.A.; Moreno-Castilla, C. About the endothermic nature of the adsorption of the herbicide diuron from aqueous solutions on activated carbon fiber. Carbon 2006, 44, 2335–2338. [Google Scholar] [CrossRef]
- Godek, E.; Wasilewska, M.; Maciołek, U.; Grządka, E. New insight into the temperature effect on adsorption of cationic guar gum on halloysite—Properties of the obtain systems. Chem. Eng. Res. Des. 2024, 201, 399–408. [Google Scholar] [CrossRef]
- Kara, M.; Yuzer, H.; Sabah, E.; Celik, M.S. Adsorption of Cobalt from Aqueous Solutions onto Sepiolite. Water Res. 2003, 37, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Badot, P.-M. Sorption Processes and Pollution. Conventional and Nonconventional Sorbents for Pollutant Removal from Wastewaters. Presses Universitaires de Franche-Comté: Basancon, France, 2010. [Google Scholar]
- Aharoni, C.; Sideman, S.; Hoffer, E. Adsorption of phosphate ions by collodioncoated alumina. J. Chem. Technol. Biotechnol. 1979, 29, 404–412. [Google Scholar] [CrossRef]
- Costa, R.L.T.; do Nascimento, R.A.; de Araújo, R.C.S.; Vieira, M.G.A.; da Silva, M.G.C.; de Carvalho, S.M.L.; de Faria, L.J.G. Removal of non-steroidal anti-inflammatory drugs (NSAIDs) from water with activated carbons synthetized from waste murumuru (Astrocaryum murumuru Mart.): Characterization and adsorption studies. J. Mol. Liq. 2021, 343, 116980. [Google Scholar] [CrossRef]
- Baccar, R.; Sarrà, M.; Bouzid, J.; Feki, M.; Blànquez, P. Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. J. Chem. Eng. 2012, 211–212, 310–317. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Li, X.; Li, W.; Mao, S.; He, S.; Wu, X.; Tang, C.; Yu, J.; Pan, L.; et al. High efficiency removal of ibuprofen in water using activatedcarbon derived fromRadix Angelica Dahuricaresidue. Environ. Prog. Sustain. Energy 2023. [Google Scholar] [CrossRef]
- Jedynak, K.; Szczepanik, B.; Rędzia, N.; Słomkiewicz, P.; Kolbus, A.; Rogala, P. Ordered Mesoporous Carbons for Adsorption of Paracetamol and Non-Steroidal Anti-Inflammatory Drugs: Ibuprofen and Naproxen from Aqueous Solutions. Water 2019, 11, 1099. [Google Scholar] [CrossRef]
- Lach, J.; Szymonik, A. Adsorption of naproxen sodium from aqueous solutions on commercial activated carbons. J. Ecol. Eng. 2019, 20, 241–251. [Google Scholar] [CrossRef]
- Lach, J.; Szymonik, A. Adsorption of diclofenac sodium from aqueous solutions on commercial activated carbons. Desalin. Water. Treat. 2020, 186, 418–429. [Google Scholar] [CrossRef]
- Yaneva, Z.L. Nonsteroidal Anti-Inflammatory Drug Solid-State Microencapsulation on Green Activated Carbon—Mass Transfer and Host-Guest Interactions. Chem. Biochem. Eng. Q. 2019, 33, 249–269. [Google Scholar] [CrossRef]



| Adsorbate | Chemical Formula | Molar Mass [g/mol] | Solubility in H2O [mg/L at 25 °C] | Ionization Constant pKa | Melting Point [°C] | Boiling Point [°C] | Chemical Safety |
|---|---|---|---|---|---|---|---|
| Ibuprofen Sodium | 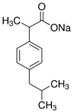 | 228.26 | 100 | 4.91 | 75–77 | 319.6 | Corrosive Irritant Health Hazard |
| Naproxen Sodium | 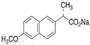 | 252.24 | 76 | 4.15 | 250–251 | 403.9 | Irritant Health Hazard |
| Diclofenac Sodium | 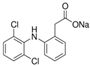 | 318.13 | 50 | 4.15 | 288–290 | 412 | Acute Toxic Irritant Health Hazard Environmental Hazard |
| System | Isotherm Type | am a | m b | n b | logK c | R2 d | SD(a) e |
|---|---|---|---|---|---|---|---|
| IBP/GAC_15 °C | T | 0.50 | 1 | 0.77 | 2.57 | 0.939 | 0.042 |
| IBP/GAC_25 °C | T | 0.41 | 1 | 0.61 | 3.93 | 0.954 | 0.045 |
| IBP/GAC_35 °C | T | 0.31 | 1 | 0.17 | 8.45 | 0.909 | 0.085 |
| D/GAC_15 °C | GF | 0.59 | 0.36 | 1 | 0.36 | 0.955 | 0.048 |
| D/GAC_25 °C | GL | 0.48 | 0.54 | 0.61 | 0.64 | 0.985 | 0.033 |
| D/GAC_35 °C | GF | 0.39 | 0.37 | 1 | 0.16 | 0.902 | 0.088 |
| NPX/GAC_15 °C | GF | 0.64 | 0.39 | 1 | −0.28 | 0.978 | 0.047 |
| NPX/GAC_25 °C | GF | 0.87 | 0.45 | 1 | 0.07 | 0.972 | 0.061 |
| NPX/GAC_35 °C | GF | 1.09 | 0.47 | 1 | 0.12 | 0.985 | 0.048 |
| System | ΔG° a [kJ/mol] | ΔH° b [kJ/mol] | ΔS° c [kJ/mol] |
|---|---|---|---|
| IBP/GAC_15 °C | −2.66 | ||
| IBP/GAC_25 °C | −3.07 | 22.73 | 0.07 |
| IBP/GAC_35 °C | −3.97 | ||
| D/GAC_15 °C | −1.98 | ||
| D/GAC_25 °C | −2.72 | 21.61 | 0.06 |
| D/GAC_35 °C | −3.25 | ||
| NPX/GAC_15 °C | −0.10 | ||
| NPX/GAC_25 °C | −0.73 | 26.41 | 0.087 |
| NPX/GAC_35 °C | −1.62 |
| System | m-exp [%] | FOE [%] | SOE [%] | MOE [%] | f-FOE [%] | f-SOE [%] | f-MOE [%] |
|---|---|---|---|---|---|---|---|
| IBP/GAC 25 °C | 0.526 | 2.568 | 3.191 | 1.145 | 0.683 | 2.584 | 0.664 |
| IBP/GAC 35 °C | 0.443 | 2.710 | 2.187 | 1.107 | 0.683 | 5.805 | 0.879 |
| D/GAC 25 °C | 0.335 | 2.771 | 3.276 | 1.499 | 0.620 | 2.615 | 0.520 |
| D/GAC 35 °C | 0.500 | 2.750 | 2.969 | 1.541 | 0.531 | 2.589 | 0.485 |
| NPX/GAC 25 °C | 0.695 | 3.242 | 2.407 | 1.360 | 0.829 | 2.336 | 0.778 |
| NPX/GAC 35 °C | 0.720 | 2.778 | 2.603 | 1.014 | 0.771 | 1.860 | 0.673 |
| System | f1 a, log k1 b | f2 a, log k2 b | f3 a, log k3 b | ueq c | t1/2 d [min] | SD(c)/co e [%] | 1 − R2 f |
|---|---|---|---|---|---|---|---|
| IBP/GAC 25 °C | 0.071, −3.931 | 0.746, −3.206 | 0.183, −2.211 | 1 | 866.4 | 0.526 | 2.3 × 10−4 |
| IBP/GAC 35 °C | 0.051, −4.259 | 0.719, −3.010 | 0.230, −2.148 | 1 | 495.4 | 0.443 | 1.5 × 10−4 |
| D/GAC 25 °C | 0.568, −3.606 | 0.275, −3.249 | 0.157, −2.372 | 1 | 1562.4 | 0.335 | 9.4 × 10−5 |
| D/GAC 35 °C | 0.486, −3.445 | 0.368, −2.996 | 0.146, −2.112 | 1 | 902.9 | 0.500 | 2.2 × 10−4 |
| NPX/GAC 25 °C | 0.667, −3.006 | 0.333, −2.174 | - | 1 | 355.9 | 0.695 | 4.6 × 10−4 |
| NPX/GAC 35 °C | 0.647, −2.908 | 0.353, −2.150 | - | 1 | 286.7 | 0.720 | 4.9 × 10−4 |
| System | f2 a | p b | log k1 c | ueq d | t1/2 e [min] | SD(c)/co f [%] | 1 − R2 g |
|---|---|---|---|---|---|---|---|
| IBP/GAC 25 °C | 0.332 | 0.685 | −3.03 | 1 | 830.7 | 0.664 | 3.7 × 10−4 |
| IBP/GAC 35 °C | 0.460 | 0.816 | −3.12 | 1 | 471.8 | 0.879 | 6.0 × 10−4 |
| D/GAC 25 °C | 0.435 | 0.654 | −3.19 | 1 | 1486.9 | 0.520 | 2.3 × 10−4 |
| D/GAC 35 °C | 0.388 | 0.666 | −3.03 | 1 | 872.7 | 0.485 | 2.2 × 10−4 |
| NPX/GAC 25 °C | 0.336 | 0.785 | −2.93 | 1 | 358.9 | 0.778 | 5.8 × 10−4 |
| NPX/GAC 35 °C | 0.440 | 0.839 | −2.88 | 1 | 288.9 | 0.673 | 4.3 × 10−4 |
| Sample | TG | DTG | DSC | ||
|---|---|---|---|---|---|
| ΔT [°C] | Mass Loss [%] | Total Mass Loss [%] | Tmin [°C] | endo/exo | |
| GAC | 30–480 | 1.34 | 86.50 | - | - |
| 480–930 | 85.16 | 653 | exo | ||
| IBP/GAC | 30–200 | 1.64 | 81.46 | 79.7 | endo |
| 200–600 | 79.24 | 274.1 454.7 | exo exo | ||
| 550.2 | exo | ||||
| 600–930 | 0.58 | - | - | ||
| D/GAC | 30–200 | 2.62 | 84.85 | 78.7 | endo |
| 200–600 | 78.24 | 252.7 583.6 | exo exo | ||
| 600–930 | 3.99 | 874.3 | endo | ||
| NPX/GAC | 30–200 | 3.49 | 81.79 | 81.6 | endo |
| 200–600 | 77.44 | 266.6 516.6 | exo exo | ||
| 600–930 | 0.86 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilewska, M.; Grządka, E. Activated Carbons as Effective Adsorbents of Non-Steroidal Anti-Inflammatory Drugs. Appl. Sci. 2024, 14, 743. https://doi.org/10.3390/app14020743
Wasilewska M, Grządka E. Activated Carbons as Effective Adsorbents of Non-Steroidal Anti-Inflammatory Drugs. Applied Sciences. 2024; 14(2):743. https://doi.org/10.3390/app14020743
Chicago/Turabian StyleWasilewska, Małgorzata, and Elżbieta Grządka. 2024. "Activated Carbons as Effective Adsorbents of Non-Steroidal Anti-Inflammatory Drugs" Applied Sciences 14, no. 2: 743. https://doi.org/10.3390/app14020743
APA StyleWasilewska, M., & Grządka, E. (2024). Activated Carbons as Effective Adsorbents of Non-Steroidal Anti-Inflammatory Drugs. Applied Sciences, 14(2), 743. https://doi.org/10.3390/app14020743









