Coniferous Honeydew Honey: Antibacterial Activity and Anti-Migration Properties against Breast Cancer Cell Line (MCF-7)
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Physicochemical Evaluation
2.2.1. Water Content and Water Activity
2.2.2. Conductivity
2.2.3. pH and Free Acidity
2.2.4. Color Analysis
2.2.5. 5-Hydroxymethylfurfural Analysis
2.2.6. Total Phenolic Content and Antioxidant Capacity
2.2.7. Protein Content
2.2.8. Enzyme Analysis
2.2.9. Jack Bean Urease Inhibition Assay
2.3. Bacterial Strains and Growth Conditions
2.4. Determination of Antibacterial Activity
2.5. Cell Lines
2.6. Determination of Cytotoxicity
2.7. Measurement of Cell Migration Using Scratch Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Evaluation
3.2. Total Phenolic Content and Antioxidant Capacity
3.3. Protein Content and Enzymatic Activity
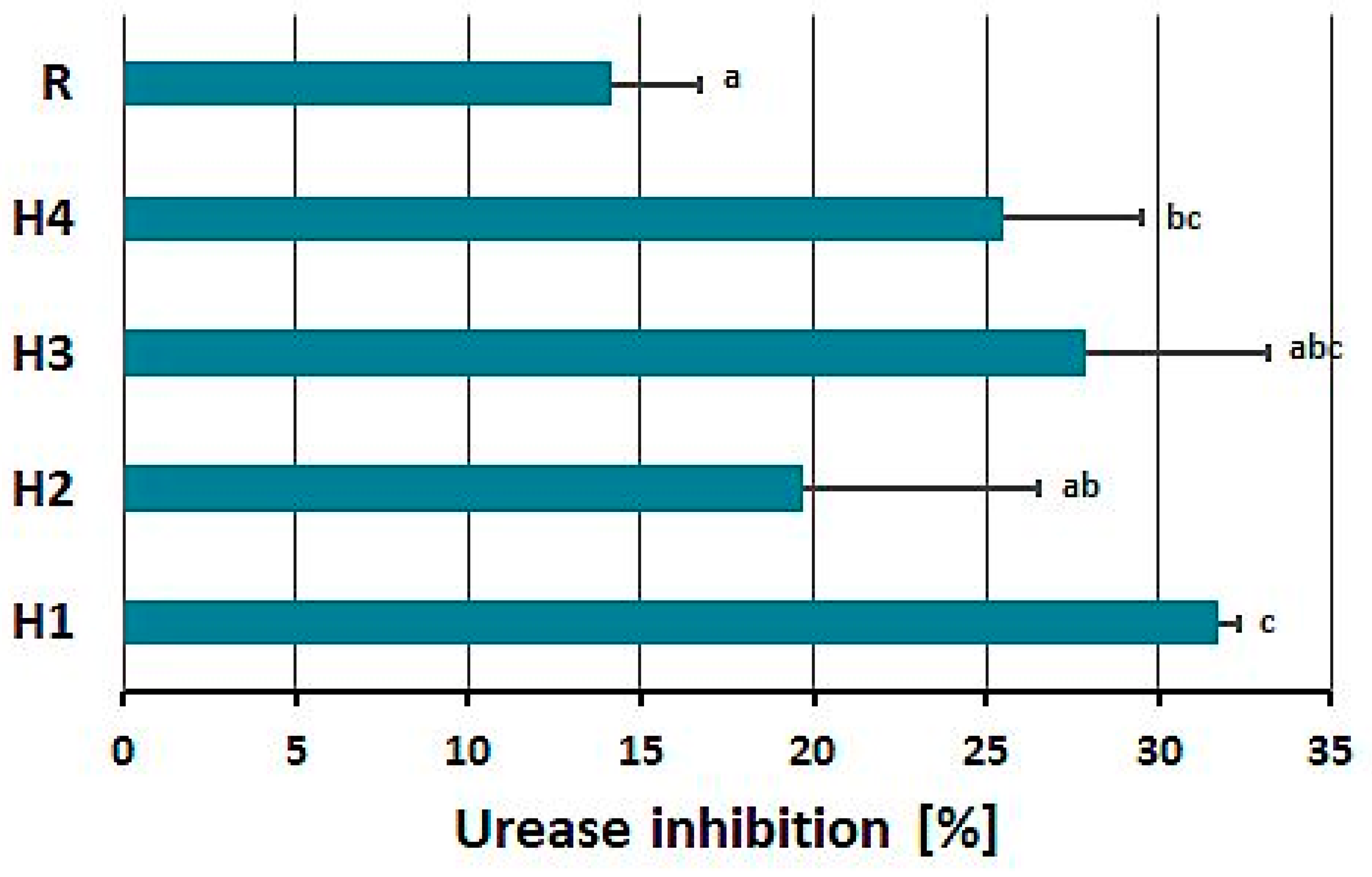
3.4. Urease Inhibition
3.5. Antibacterial Activity
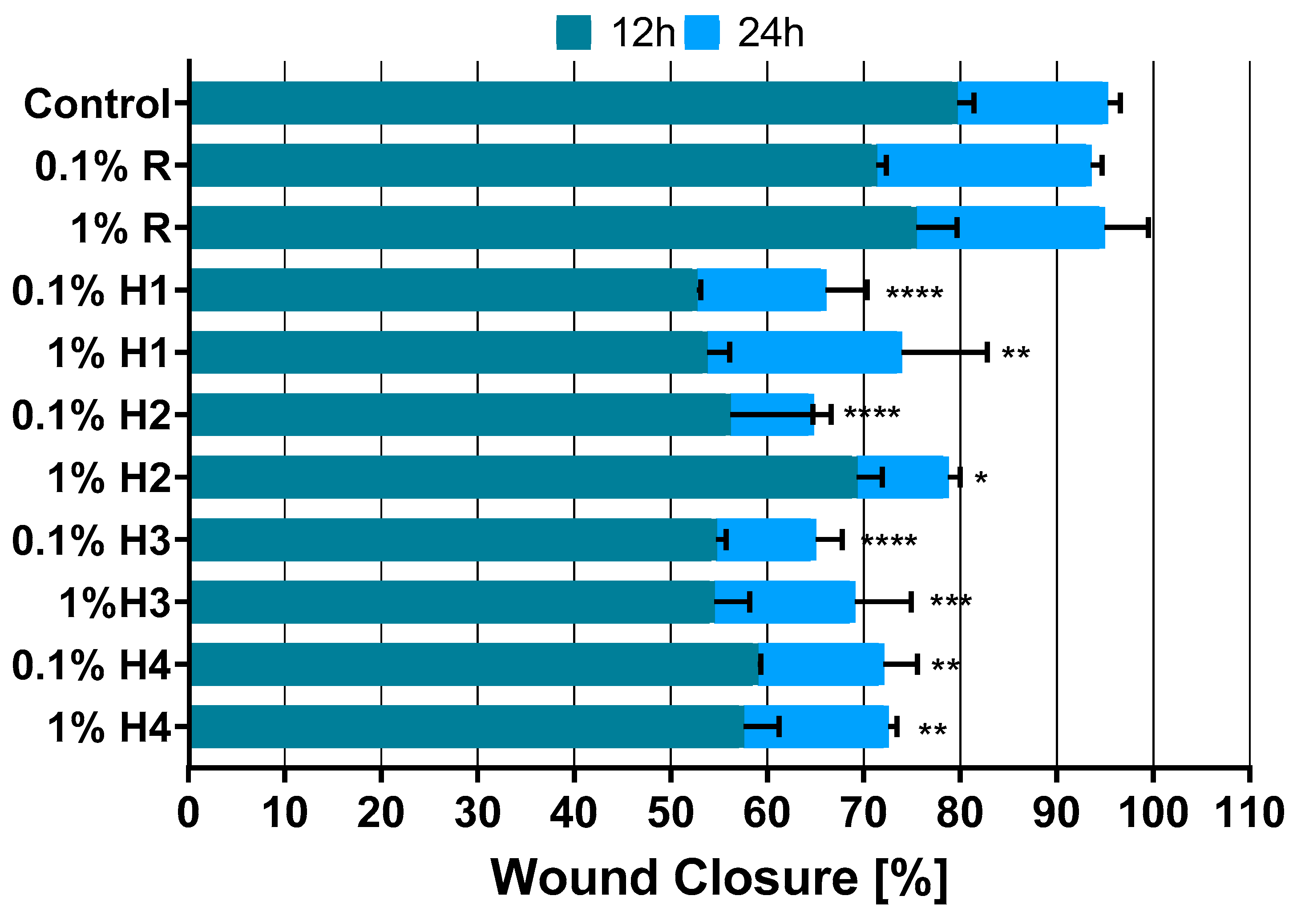
3.6. Viability and Cell Migration Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, A.; Ahmed Zia, S.; Rafi, M.; Mehmood, A.; Aslam, S.; Chaudhry, M.T. Quantification of honeydew production caused by dubas bug on three date palm cultivars wasps fauna of Pakistan. J. Entomol. Zool. Stud. 2016, 4, 278–284. [Google Scholar]
- Fischer, M.K.; Völkl, W.; Hoffmann, K.H. Honeydew production and honeydew sugar composition of polyphagous black bean aphid, Aphis fabae (Hemiptera: Aphididae) on various host plants and implications for ant-attendance. Eur. J. Entomol. 2005, 102, 155–160. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 710/2010 of 6 August 2010 on entering a name in the register of protected designations of origin and protected geographical indications („Podkarpacki Miód Spadziowy” (PDO)). Off. J. Eur. Communities 2010, 208, 1–2.
- Dżugan, M.; Zaguła, G.; Wesołowska, M.; Sowa, P.; Puchalski, C. Levels of toxic and essential metals in varietal honeys from podkarpacie. J. Elem. 2017, 22, 1039–1048. [Google Scholar] [CrossRef]
- Tomczyk, M.; Bocian, A.; Sidor, E.; Miłek, M.; Zaguła, G.; Dżugan, M. The Use of HPTLC and SDS-PAGE Methods for Coniferous Honeydew Honey Fingerprinting Compiled with Mineral Content and Antioxidant Activity. Molecules 2022, 27, 720. [Google Scholar] [CrossRef]
- Tomczyk, M.; Zaguła, G.; Puchalski, C.; Dżugan, M. Transfer of some toxic metals from soil to honey depending on bee habitat conditions. Acta Univ. Cibiniensis Ser. E Food Technol. 2020, 24, 49–59. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Muzolf-Panek, M.; Tomaszewska-Gras, J.; Konieczny, P. Predicting the botanical origin of honeys with chemometric analysis according to their antioxidant and physicochemical properties. Polish J. Food Nutr. Sci. 2019, 69, 191–201. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Silva, B.; Bergamo, G.; Brugnerotto, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. An overview of physicochemical characteristics and health-promoting properties of honeydew honey. Food Res. Int. 2019, 119, 44–66. [Google Scholar] [CrossRef]
- Directive 2014/63/EU of the European Parliament and of the Council amending Council Directive 2001/110/EC relating tohoney. Off. J. Eur. Communities 2014, 164, 1–5.
- Zielińska, S.; Wesołowska, M.; Bilek, M.; Kaniuczak, J.; Dżugan, M. The saccharide profile of Polish honeys depending on their botanical origin. J. Microbiol. Biotechnol. Food Sci. 2014, 3, 387–390. [Google Scholar]
- Kuś, P.M.; Jerković, I.; Marijanović, Z.; Tuberoso, C.I.G. Screening of Polish Fir Honeydew Honey Using GC/MS, HPLC-DAD, and Physical-Chemical Parameters: Benzene Derivatives and Terpenes as Chemical Markers. Chem. Biodivers. 2017, 14, e1700179. [Google Scholar] [CrossRef]
- Recklies, K.; Peukert, C.; Kölling-Speer, I.; Speer, K. Differentiation of Honeydew Honeys from Blossom Honeys and According to Their Botanical Origin by Electrical Conductivity and Phenolic and Sugar Spectra. J. Agric. Food Chem. 2021, 69, 1329–1347. [Google Scholar] [CrossRef]
- Porcza, L.; Simms, C.; Chopra, M. Honey and Cancer: Current Status and Future Directions. Diseases 2016, 4, 30. [Google Scholar] [CrossRef]
- Puścion-Jakubik, A.; Karpińska, E.; Moskwa, J.; Socha, K. Content of Phenolic Acids as a Marker of Polish Honey Varieties and Relationship with Selected Honey-Quality-Influencing Variables. Antioxidants 2022, 11, 1312. [Google Scholar] [CrossRef]
- Pita-Calvo, C.; Vázquez, M. Differences between honeydew and blossom honeys: A review. Trends Food Sci. Technol. 2017, 59, 79–87. [Google Scholar] [CrossRef]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Stryjecka, M.; Teter, A.; Skałecki, P.; Domaradzki, P.; Florek, M. Relationships between the content of phenolic compounds and the antioxidant activity of polish honey varieties as a tool for botanical discrimination. Molecules 2021, 26, 1810. [Google Scholar] [CrossRef]
- Fernández-Estellé, M.; Hernández-González, V.; Saurina, J.; Núñez, O.; Sentellas, S. Characterization and Classification of Spanish Honeydew and Blossom Honeys Based on Their Antioxidant Capacity. Antioxidants 2023, 12, 495. [Google Scholar] [CrossRef]
- Tsiapara, A.V.; Jaakkola, M.; Chinou, I.; Graikou, K.; Tolonen, T.; Virtanen, V.; Moutsatsou, P. Bioactivity of Greek honey extracts on breast cancer (MCF-7), prostate cancer (PC-3) and endometrial cancer (Ishikawa) cells: Profile analysis of extracts. Food Chem. 2009, 116, 702–708. [Google Scholar] [CrossRef]
- Martiniakova, M.; Kovacova, V.; Mondockova, V.; Zemanova, N.; Babikova, M.; Biro, R.; Ciernikova, S.; Omelka, R. Honey: A Promising Therapeutic Supplement for the Prevention and Management of Osteoporosis and Breast Cancer. Antioxidants 2023, 12, 567. [Google Scholar] [CrossRef]
- Erejuwa, O.O. Effect of honey in diabetes mellitus: Matters arising. J. Diabetes Metab. Disord. 2014, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Mandal, M. Honey Constituents and their apoptotic effect in colon cancer cells. J. Apiprod. Apimed. Sci. 2009, 1, 29–36. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Topal, E.; Balkanska, R.; Yücel, B.; Oravecz, T.; Cornea-Cipcigan, M.; Vodnar, D.C. Monofloral honeys as a potential source of natural antioxidants, minerals and medicine. Antioxidants 2021, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Eteraf-Oskouei, T.; Najafi, M. Uses of Natural Honey in Cancer: An Updated Review. Adv. Pharm. Bull. 2022, 12, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Sowa, P.; Grabek-Lejko, D.; Wesołowska, M.; Swacha, S.; Dżugan, M. Hydrogen peroxide-dependent antibacterial action of Melilotus albus honey. Lett. Appl. Microbiol. 2017, 65, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Alygizou, A.; Grigorakis, S.; Gotsiou, P.; Loupassaki, S.; Calokerinos, A.C. Quantification of Hydrogen Peroxide in Cretan Honey and Correlation with Physicochemical Parameters. J. Anal. Methods Chem. 2021, 2021, 5554305. [Google Scholar] [CrossRef]
- Feknous, N.; Boumendjel, M. Natural bioactive compounds of honey and their antimicrobial activity. Czech J. Food Sci. 2022, 40, 163–178. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Farida, F.; Miftakhul Nizar, A.; Ode Nurlina, W.; Agus, R. Effectiveness of Honey Dressing on Healing of Diabetic Foot Ulcer: Systematic Review. Int. J. Psychosoc. Rehabil. 2020, 24, 2020. [Google Scholar]
- Dżugan, M.; Miłek, M.; Kielar, P.; Stępień, K.; Sidor, E.; Bocian, A. SDS-PAGE Protein and HPTLC Polyphenols Profiling as a Promising Tool for Authentication of Goldenrod Honey. Foods 2022, 11, 2390. [Google Scholar] [CrossRef]
- Ben Mansour, R.; Ksouri, W.M.; Cluzet, S.; Krisa, S.; Richard, T.; Ksouri, R. Assessment of Antioxidant Activity and Neuroprotective Capacity on PC12 Cell Line of Frankenia thymifolia and Related Phenolic LC-MS/MS Identification. Evid.-Based Complement. Altern. Med. 2016, 2016, 2843463. [Google Scholar] [CrossRef]
- Santos, J.S.; Alvarenga Brizola, V.R.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Dżugan, M.; Miłek, M.; Sidor, E.; Buczkowicz, J.; Hęclik, J.; Bocian, A. The Application of SDS-PAGE Protein and HPTLC Amino Acid Profiling for Verification of Declared Variety and Geographical Origin of Honey. Food Anal. Methods 2023, 16, 1157–1171. [Google Scholar] [CrossRef]
- Tanaka, T.; Kawase, M.; Tani, S. Urease inhibitory activity of simple α,β-unsaturated ketones. Life Sci. 2003, 73, 2985–2990. [Google Scholar] [CrossRef]
- Bocian, A.; Ciszkowicz, E.; Hus, K.K.; Buczkowicz, J.; Lecka-Szlachta, K.; Pietrowska, M.; Petrilla, V.; Petrillova, M.; Legáth, Ľ.; Legáth, J. Antimicrobial activity of protein fraction from Naja ashei venom against Staphylococcus epidermidis. Molecules 2020, 25, 293. [Google Scholar] [CrossRef]
- Miłek, M.; Ciszkowicz, E.; Sidor, E.; Hęclik, J.; Lecka-Szlachta, K.; Dżugan, M. The Antioxidant, Antibacterial and Anti-Biofilm Properties of Rapeseed Creamed Honey Enriched with Selected Plant Superfoods. Antibiotics 2023, 12, 235. [Google Scholar] [CrossRef]
- Zapała, L.; Kosińska, M.; Woźnicka, E.; Byczyński, Ł.; Ciszkowicz, E.; Lecka-Szlachta, K.; Zapała, W.; Chutkowski, M. Comparison of spectral and thermal properties and antibacterial activity of new binary and ternary complexes of Sm(III), Eu(III) and Gd(III) ions with N-phenylanthranilic acid and 1,10-phenanthroline. Thermochim. Acta 2019, 671, 134–148. [Google Scholar] [CrossRef]
- Miłek, M.; Ciszkowicz, E.; Tomczyk, M.; Sidor, E.; Zaguła, G.; Lecka-Szlachta, K.; Pasternakiewicz, A.; Dżugan, M. Poplar-Type Polish Propolis Considering Local Flora Diversity Breast Cancer Cells. Molecules 2022, 27, 725. [Google Scholar] [CrossRef]
- Balkanska, R.; Ignatova, M. Determination of Selected Physicochemical Parameters of Bulgarian Honeydew Honey. J. Mt. Agric. Balk. 2014, 17, 558–571. [Google Scholar]
- Atanassova, J.; Lazarova, M.; Yurukova, L. Significant parameters of Bulgarian honeydew honey. J. Cent. Eur. Agric. 2016, 17, 640–651. [Google Scholar] [CrossRef][Green Version]
- Damto, T. A Review on Status of Honey Adulteration and Their Detection Techniques in Ethiopia. J. Nutr. Food Sci. 2021, 11, 180. [Google Scholar]
- Serin, S.; Turhan, K.N.; Turhan, M. Correlation between water activity and moisture content of Turkish flower and pine honeys. Food Sci. Technol. 2018, 38, 238–243. [Google Scholar] [CrossRef]
- Cabrera, M.; Perez, M.; Gallez, L.; Andrada, A.; Balbarrey, G. Colour, antioxidant capacity, phenolic and flavonoid content of honey from the Humid Chaco Region, Argentina. Phyton 2017, 86, 124–130. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant activity, total phenolic content, individual phenolics and physicochemical parameters suitability for Romanian honey authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef]
- Chis, A.M.; Purcarea, C.; Dżugan, M.; Teusdea, A. Comparative antioxidant content and antioxidant activity of selected Romanian and Polish honeydew honey. Rev. Chim. 2016, 67, 214–218. [Google Scholar]
- Wilczyńska, A. Phenolic content and antioxidant activity of different types of polish honey—A short report. Polish J. Food Nutr. Sci. 2010, 60, 309–313. [Google Scholar]
- Dor, G.O.L.M.; Mahomoodally, M.F. Chemical profile and in vitro bioactivity of tropical honey from Mauritius. Asian Pac. J. Trop. Dis. 2014, 4, S1002–S1013. [Google Scholar] [CrossRef]
- Montaser, M.; Ali, A.T.; Sayed, A.M.; Abdelmohsen, U.R.; Zidan, E.W.; Orfali, R.; Rateb, M.E.; Zaki, M.A.; Hassan, H.M.; Mohammed, R.; et al. 1H-NMR Metabolic Profiling, Antioxidant Activity, and Docking Study of Common Medicinal Plant-Derived Honey. Antioxidants 2022, 11, 1880. [Google Scholar] [CrossRef]
- Ankley, L.M.; Monteiro, M.P.; Camp, K.M.; O’Quinn, R.; Castillo, A.R. Manuka honey chelates iron and impacts iron regulation in key bacterial pathogens. J. Appl. Microbiol. 2020, 128, 1015–1024. [Google Scholar] [CrossRef]
- Sánchez-Chino, X.M.; Jiménez-Martínez, C.; Ramírez-Arriaga, E.; Martínez-Herrera, J.; Corzo-Ríos, L.J.; Godínez García, L.M. Actividad antioxidante y quelante de metales de las mieles de Melipona beecheii y Frieseomelitta nigra originarias de Tabasco, México. TIP Rev. Espec. Ciencias Quím.-Biol. 2019, 22, 1–7. (In Spanish) [Google Scholar] [CrossRef]
- Wesołowska, M.; Dżugan, M. Aktywność i stabilność termiczna diastazy występującej w Podkarpackich miodach odmianowych. Zywn. Nauk. Technol. Jakosc/Food. Sci. Technol. Qual. 2017, 24, 103–112. (In Polish) [Google Scholar] [CrossRef]
- Sidor, E.; Tomczyk, M.; Dzugan, M. Application of Ultrasonic or Microwave Radiation to Delay Crystallization and Liquefy Solid Honey. J. Apic. Sci. 2021, 65, 243–253. [Google Scholar] [CrossRef]
- Dżugan, M.; Grabek-Lejko, D.; Sidor, E.; Tomczyk, M. The impact of ultrasound decrystallization on enzymatic, antioxidant and antibacterial properties of honey. Innov. Food Sci. Emerg. Technol. 2021, 71, 102709. [Google Scholar] [CrossRef]
- Karabournioti, S.; Zervalaki, P. The effect of heating on honey HMF and invertase. Apiacta 2001, 36, 177–181. [Google Scholar]
- Zhang, G.Z.; Tian, J.; Zhang, Y.Z.; Li, S.S.; Zheng, H.Q.; Hu, F.L. Investigation of the maturity evaluation indicator of honey in natural ripening process: The case of rape honey. Foods 2021, 10, 2882. [Google Scholar] [CrossRef]
- Sahin, H. Honey as an apitherapic product: Its inhibitory effect on urease and xanthine oxidase. J. Enzyme Inhib. Med. Chem. 2016, 31, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Rückriemen, J.; Klemm, O.; Henle, T. Manuka honey (Leptospermum scoparium) inhibits jack bean urease activity due to methylglyoxal and dihydroxyacetone. Food Chem. 2017, 230, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ni, M. Regulation of biofilm formation in Klebsiella pneumoniae. Front. Microbiol. 2023, 14, 1238482. [Google Scholar] [CrossRef]
- Muselius, B.; Sukumaran, A.; Yeung, J.; Geddes-McAlister, J. Iron Limitation in Klebsiella pneumoniae Defines New Roles for Lon Protease in Homeostasis and Degradation by Quantitative Proteomics. Front. Microbiol. 2020, 11, 546. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules 2014, 19, 2497–2522. [Google Scholar] [CrossRef]
- Dragan, S.R.; Nicola, T.; Ilina, R.Ş.; Ursoniu, S.; Kimar, A.; Nimade, S.; Nicola, T. Role of multi-component functional foods in the complex treatment of patients with advanced breast cancer. Rev. Med. Chir. Soc. Med. Nat. Iasi 2017, 111, 877–884. [Google Scholar]
- Hassan, M.I.; Mabrouk, G.M.; Shehata, H.H.; Aboelhussein, M.M. Antineoplastic effects of bee honey and nigella sativa on hepatocellular carcinoma cells. Integr. Cancer Ther. 2012, 11, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Bag, S.; Barui, A.; Banerjee, P.; Chatterjee, J. Honey dilution impact on in vitro wound healing: Normoxic and hypoxic condition. Wound Repair Regen. 2015, 23, 412–422. [Google Scholar] [CrossRef] [PubMed]


| Sample Code | Honey Type /Variety | Apiary Location | Organoleptic Evaluation | |||
|---|---|---|---|---|---|---|
| Texture | Color | Smell | Taste | |||
| H1 | fir honeydew | 49.68 N, 21.81 E | sticky, gummy | light brown | pleasant, specific, resinous | slightly resinous, spicy |
| H2 | 49.48 N, 22.43 E | viscous liquid | dark brown with black tint | resinous, slightly acidic | slightly resinous, caramel, spicy | |
| H3 | 49.47 N, 22.07 E | viscous liquid | dark brown with black tint | resinous, slightly acidic | slightly resinous, caramel, spicy | |
| H4 | 49.34 N, 22.19 E | viscous liquid | dark brown with black tint | woody, smoky | slightly resinous, spicy | |
| R | rapeseed | 49.81 N, 21.54 E | fully crystallized | pale yellow | specific for rape flower | very sweet, slightly, bland |
| Honey Sample | Water Content [%] | Water Activity | pH | Free Acidity [mval/kg] | Conductivity [mS/cm] | HMF [mg/kg] | Color [mm Pfund] | Color A450 nm |
|---|---|---|---|---|---|---|---|---|
| H1 | 17.10 ± 0.10 b | 0.522 ± 0.006 a | 4.69 ± 0.01 d | 27.35 ± 0.07 b | 1.21 ± 0.00 d | 3.42 ± 0.71 a | >150 b | 0.466 ± 0.011 b |
| H2 | 18.90 ± 0.10 d | 0.570 ± 0.003 c | 4.20 ± 0.02 b | 35.55 ± 0.07 c | 0.99 ± 0.01 b | 3.22 ± 0.51 a | >150 b | 0.831 ± 0.025 c |
| H3 | 15.60 ± 0.10 a | 0.567 ± 0.001 c | 4.38 ± 0.00 c | 39.80 ± 0.14 d | 1.13 ± 0.00 c | 6.58 ± 0.67 b | >150 b | 0.901 ± 0.009 d |
| H4 | 19.00 ± 0.30 d | 0.602 ± 0.002 d | 4.16 ± 0.02 b | 41.10 ± 0.14 e | 1.01 ± 0.00 b | 7.20 ± 0.53 b | >150 b | 0.859 ± 0.022 cd |
| R | 17.60 ± 0.10 c | 0.533 ± 0.003 b | 4.10 ± 0.02 a | 8.55 ± 0.07 a | 0.14 ± 0.00 a | <LOQ | 40 ± 0.3 a | 0.071 ± 0.010 a |
| Honey Sample | TPC [mg GAE/kg] | DPPH [μmol TE/kg] | FRAP [μmol TE/kg] | CUPRAC [μmol TE/kg] | Fe2+ Chelating Ability [mg EDTA/kg] | Cu2+ Chelating Ability [mg EDTA/kg] |
|---|---|---|---|---|---|---|
| H1 | 657.24 ± 80.91 c | 773.98 ± 48.26 bc | 1443.53 ± 33.72 b | 8243.59 ± 683.80 b | 2498.31 ± 224.56 c | 1123.91 ± 20.46 bc |
| H2 | 823.91 ± 43.67 b | 710.36 ± 43.17 b | 1628.29 ± 18.32 c | 10666.67 ± 385.26 c | 969.39 ± 44.06 b | 1075.50 ± 31.44 b |
| H3 | 1001.98 ± 23.07 d | 941.73 ± 141.93 c | 2213.82 ± 47.44 d | 11160.26 ± 664.04 c | 2081.72 ± 297.54 c | 1164.75 ± 11.42 bc |
| H4 | 980.65 ± 7.10 d | 865.03 ± 90.22 bc | 2126.10 ± 83.77 d | 11166.67 ± 122.13 c | 2038.96 ± 93.61 c | 1191.98 ± 14.59 c |
| R | 195.44 ± 2.27 a | 125.98 ± 13.27 a | 272.48 ± 2.51 a | 2237.18 ± 154.25 a | 441.63 ± 95.88 a | 582.37 ± 87.21 a |
| Honey Sample | Protein [mg/100 g] | Diastase Number [DN] | NAG [mU/g] | β-GAL [mU/g] | α-GLU [mU/g] |
|---|---|---|---|---|---|
| H1 | 110.71 ± 15.40 b | 14.64 ± 0.18 e | 3.61 ± 0.51 a | 12.51 ± 0.73 b | 13.42 ± 0.11 b |
| H2 | 199.64 ± 12.83 c | 10.04 ± 0.10 a | 12.74 ± 0.17 c | 12.62 ± 1.57 b | 12.86 ± 1.12 b |
| H3 | 276.77 ± 32.08 d | 11.92 ± 0.05 b | 15.64 ± 0.79 d | 17.79 ± 0.45 c | 18.54 ± 1.18 c |
| H4 | 335.75 ± 10.27 e | 13.51 ± 0.213 c | 24.22 ± 0.67 e | 29.74 ± 0.73 d | 30.45 ± 0.62 d |
| R | 69.87 ± 3.85 a | 14.20 ± 0.13 d | 5.92 ± 0.28 b | 6.11 ± 0.34 a | 6.99 ± 0.22 a |
| Bacterial Strain | Escherichia coli ATCC 10536 | Klebsiella pneumoniae ATCC 13883 | Listeria monocytogenes SLR 2249 | Staphylococcus aureus ATCC 6538 | Staphylococcus epidermidis ATCC 35984 | Streptococcus agalactiae DSM 2134 | Streptococcus pyogenes DSM 20565 |
|---|---|---|---|---|---|---|---|
| MIC/MBC [% w/v] | |||||||
| H1 | ND/ND | 12.5/25.0 | ND/ND | ND/ND | ND/ND | 50.0/ND | 50.0/ND |
| H2 | ND/ND | 25.0/ND | ND/ND | ND/ND | ND/ND | ND/ND | 25.0/ND |
| H3 | ND/ND | 12.5/25.0 | ND/ND | ND/ND | ND/ND | 50.0/50.0 | 50.0/ND |
| H4 | ND/ND | 12.5/25.0 | ND/ND | ND/ND | ND/ND | 50.0/50.0 | 25.0/50.0 |
| R | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND | ND/ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dżugan, M.; Ciszkowicz, E.; Tomczyk, M.; Miłek, M.; Lecka-Szlachta, K. Coniferous Honeydew Honey: Antibacterial Activity and Anti-Migration Properties against Breast Cancer Cell Line (MCF-7). Appl. Sci. 2024, 14, 710. https://doi.org/10.3390/app14020710
Dżugan M, Ciszkowicz E, Tomczyk M, Miłek M, Lecka-Szlachta K. Coniferous Honeydew Honey: Antibacterial Activity and Anti-Migration Properties against Breast Cancer Cell Line (MCF-7). Applied Sciences. 2024; 14(2):710. https://doi.org/10.3390/app14020710
Chicago/Turabian StyleDżugan, Małgorzata, Ewa Ciszkowicz, Monika Tomczyk, Michał Miłek, and Katarzyna Lecka-Szlachta. 2024. "Coniferous Honeydew Honey: Antibacterial Activity and Anti-Migration Properties against Breast Cancer Cell Line (MCF-7)" Applied Sciences 14, no. 2: 710. https://doi.org/10.3390/app14020710
APA StyleDżugan, M., Ciszkowicz, E., Tomczyk, M., Miłek, M., & Lecka-Szlachta, K. (2024). Coniferous Honeydew Honey: Antibacterial Activity and Anti-Migration Properties against Breast Cancer Cell Line (MCF-7). Applied Sciences, 14(2), 710. https://doi.org/10.3390/app14020710







