1. Introduction
The worldwide energy crisis, the rising costs of crude oil and natural gas, environmental pollution and climate change in recent years urgently require a transition to sustainable and cleaner energy forms. Among fossil fuels, coal covers about 29% of primary energy and 40% of electricity production globally [
1]. Its great reserves and lower costs indicate that coal will continue to be the main resource of energy in the future [
2] if clean technologies are used, which could concomitantly maintain the economic sustainability of power plants.
One such clean technology is coal gasification, converting coal into valuable fuel gas (CO, H
2, CH
4) through a reaction with non-stoichiometric air, steam or carbon dioxide, at high temperatures (above 700 °C). This process is not only environmentally superior to combustion, avoiding gaseous emissions that cause acid rain, but also offers greater flexibility for the generated gas to be used, besides as an energy source, for the production of biofuels and added-value chemicals [
3]. When steam is used as the gasifying agent, the yield of high-quality hydrogen gas is enhanced [
4,
5,
6], whereas catalysts [
7,
8], absorbent materials, or a pre-pyrolysis process can increase the reactivity of the fuels and reduce the amount of tar and carbon dioxide in the product mixture [
3], and thus, reduce consequent operational and environmental problems. On the other hand, the use of carbon dioxide from residual streams as the gasifying agent could provide a potential solution to the greenhouse gas effect [
9]. The reaction of carbon with carbon dioxide is also important in large-scale fixed-bed gasifiers, where the feedstock is subjected to different reaction zones such as drying, pyrolysis, air oxidation and carbon dioxide gasification [
10].
For coal gasification, however, in order to achieve high efficiency under less severe operating conditions and generate a gas free of by-products, the cost is drastically higher [
6]. Therefore, a sustainable solution to this problem could be achieved through the partial substitution of coal with alternative renewable energy sources. As such, biomass presenting great similarities to coal, in terms of chemical composition and methods of utilization [
11,
12], seems the most attractive candidate. Biomass, accounting for about 15% of the world’s energy supply [
12], offers not only low-cost availability, but also carbon neutrality [
3,
12], in line with global policies for de-carbonized energy and mitigation of the carbon footprint on the environment through the promotion of renewables and circular economy [
13]. Additionally, the co-gasification of coal and biomass overcomes the drawbacks of large investments in new biomass plants, which suffer due to the seasonal availability of materials, their low energy density, or their slagging/fouling tendency [
14,
15].
As agricultural wastes are generated in large quantities in most countries around the world, at low or no cost, with energy availability ranging between 5 EJ/y and 27 EJ/y [
11], the majority of past investigations have studied their co-gasification with high-rank coals, in order to promote their penetration of energy markets and fulfill the Agricultural and Renewables Policy [
11,
16], as well as promoting circular economy [
17,
18,
19]. Sorghum, fruit waste, spirit-based distillers, empty fruit bunches, sugar cane bagasse, rice straw and walnut shells [
20,
21,
22,
23] were among the residues tested. The reaction temperature, flow rate of the gasifying agent, blending ratio, chemical and structural characteristics of feedstocks and composition of ashes were addressed as the key factors affecting the performance of the co-gasification process. Synergetic effects were mostly reported between coal and biomass depending on these factors, increasing or decreasing the reactivity of the fuels and the yield of product gases [
17,
18,
19,
20,
21]. Alkali and alkaline earth metals in biomass ash were found to enhance the reaction rate of the blends more than expected [
17,
19,
20,
24], while Si, Al and P contents in coals were found to inhibit the process [
24]. External catalysts, such as Ca(OH)
2, dolomite, olivine and Na-Fe, were also used [
7,
8,
25] to increase the yield of obtained hydrogen and reduce tar.
The co-gasification of low-rank coals with agricultural wastes for the production of syngas under a steam or carbon dioxide atmosphere has not been sufficiently studied. The co-processing of lignite with wheat straw [
26] or sunflower seed cake [
6] via steam gasification showed that the biomass material exhibited a positive synergetic effect on the blends, attributed to the volatile content of biomass and the inherent alkali/alkaline earth metals. Similar results were obtained by the carbon dioxide gasification of lignite with soybean stalk or orange peel [
14]. On the other hand, the reaction rate of lignite/corn stalk mixtures [
27] during steam gasification was inhibited in comparison to that of component fuels, due to the evolution of the chemical structure during the process. Lignite is a principal fuel in many countries worldwide, including Germany, Poland, the Czech Republic, Bulgaria and Greece in Europe. Having higher proximity with biomass materials than high-rank coals, its co-processing with them will increase the operating flexibility of power plants and thereby their economic sustainability. A comparative assessment of the gasification performance of lignite/barley straw mixtures under different gasifying agents has not been reported so far, to the best of our knowledge. Additionally, the fusibility behavior of ashes at the high temperatures prevailing in the reactor and their environmental impact, as well as the evaluation of liquid or gaseous by-products through quantitative analyses, have not been addressed.
Based on the above discussion, the current study aimed to investigate and compare the gasification performance of lignite/barley straw mixtures for syngas production, through experiments under steam or carbon dioxide in a fixed-bed system and a thermogravimetric–mass spectrometry system. The thermal behavior, reactivity, conversion, product gas composition and interactions between fuels were determined and correlated with the structural characteristics and inherent minerals in ashes, which were analyzed via mineralogical, chemical and fusibility tests.
2. Materials and Methods
2.1. Raw Materials and Characterization Procedures
The materials selected for the present study were one lignite from the open-pit mine of Kardia (KL) located in the area of Western Macedonia in North Greece and barley straw residues (BS) collected from the fields near the power plant of Kardia, all provided by the Public Power Corporation of Greece. After air drying, the materials were ground to a particle size below 250 μm, in order to avoid heat and mass transfer effects during the tests. The lignite was ground in a jaw crusher and ball mill, whereas barley straw was ground in a cutting mill. Following riffling, mixtures of lignite and straw were prepared with blending ratios of 75:25, 50:50 and 25:75, respectively. Representative fuel samples were characterized on the basis of the ASTM standards (D5142, D5373, D4239, D5865) in the case of lignite and CEN/TC335 European standards in the case of biomass, in order to determine volatiles, fixed carbon and ash contents, elemental composition and calorific value.
Structural characteristics were derived through liquid nitrogen adsorption experiments, at relative pressures of 0.03–0.3, by applying the BET method. Prior to measurement, the samples were out-gassed under vacuum overnight at 150 °C. The equipment used was volumetric apparatus, namely, the Quantachrome Nova 2200 model (Quantachrome, FL, USA).
The fusibility behavior of ashes was studied through the use of a heating microscope, equipped with a high-definition video camera, in accordance with the DDCEN/TS, 15370-1:2006 European standards. Each sample was heated in air up to 1500 °C with a heating rate of 5 °C/min, and the initial deformation temperature (IDT), the softening temperature (ST), the hemispherical temperature (HT) and the fluid temperature (FT) were recorded. To support such results and obtain a deeper insight, the ashes were analyzed by a D8 Advance X-ray diffractometer (XRD) from Bruker AXS and their mineral phases were identified using the JCPDS database in conjunction with DIFFRAC plus software v.2004. Furthermore, trace elements in ashes were determined by an inductively coupled plasma mass spectrometer ICP-MS 7500cx (Agilent Technologies, Santa Clara, CA, USA) equipped with an Autosampler Series 3000 from Agilent Technologies and assisted by an Anton Paar Multiwave 3000 oven for sample digestion (detection limits of 0.4–34 ppb, depending on element measured).
2.2. Preparation of Gasification Feedstocks
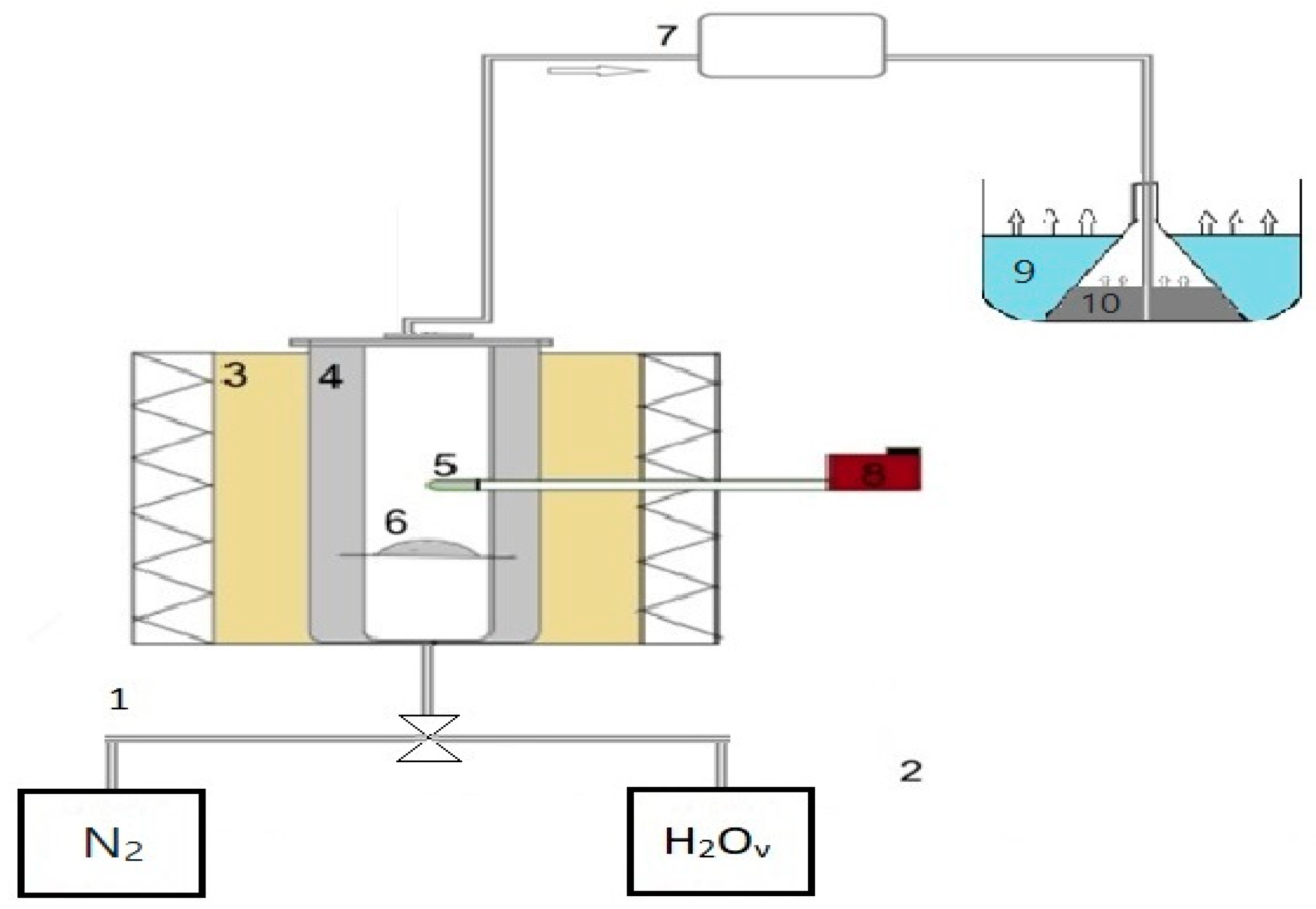
Gasification feedstocks were produced through pyrolysis experiments in a fixed-bed system (
Figure 1). This consisted of a stainless steel reactor, surrounded by a programmable high-temperature furnace (accuracy ± 3 °C), a Ni-Cr-Ni thermocouple just over the sample bed, inlet and outlet gas tubes and salt–ice baths. About 15–20 g of sample was charged into the reactor, which was sealed and introduced into the furnace and flushed with nitrogen at a flow rate og 150 mL/min for 30 min, to eliminate air. The furnace was set to 600 °C with a heating rate of 10 °C/min and held at this temperature for 30 min, followed by cooling. During the pyrolysis process, volatile products passed through the ice baths and condensable fractions were collected. Solid products were subjected to elemental and structural analyses, as described above for raw fuels, while liquid products were subjected to elemental analysis after centrifugation at 6000 rpm for 20 min in order to be separated by the water. The higher heating value of liquid oils was calculated according to their carbon (C), hydrogen (H) and oxygen (O) contents, using equation [
28]:
The higher heating value of gases was calculated after gas analysis in a TG-MS (thermogravimetric–mass spectrometry) system, using the TG/DTG-6 model from Perkin Elmer (Perkin Elmer, Hopkinton, MA, USA) and the MS QME-200 model from Balzers, respectively. The transfer line for transporting gases to the mass spectrometer was a fused silicon capillary (i.d. 0.32 mm) insulated and heated to 200 °C to prevent condensation. The tests were carried out up to 600 °C as before, under high-purity argon, at a 35 mL/min flow rate. Processing of data was performed using Pyris v3.5 and Quadstar422 v60 software. Tests with standard gases in argon provided the calibration factors required to determine the composition of each gaseous product, as explained elsewhere by the authors [
3].
2.3. Steam and Carbon Dioxide Gasification Experiments
The experiments under steam were conducted in the fixed-bed system as follows. Each char, or mixture of chars, was loaded into the reactor and heated up to 600 °C under nitrogen, as previously described. Distilled water was then injected by an automatic syringe pump through a 2 m pipe surrounding the reactor and transformed to steam of uniform flow (steam/char = 3), before passing through the char bed. The temperature was raised up to 1000 °C at a heating rate of 10 °C/min, with a retention time of 15 min. The exit gas stream was cooled down by the salt–ice baths and dried using silica gel within a quartz filter. Gas sampling was performed periodically during the experiments, using a PTFE Luer Lock gas syringe (Merck kGaA, Darmstadt, Germany). The TG-MS system, run off-line, enabled the quantitative analysis of product gas.
The experiments under carbon dioxide were conducted in the TG-MS system, which recorded continuously the weight loss and rate of weight loss of each sample as a function of temperature and time, as well as the gaseous products evolved. Each char, or mixture of chars, was heated up to 1000 °C with a heating rate of 10 °C/min, within a carbon dioxide flow of 35 mL/min. The purge gas had an argon of flow rate 45 mL/min. The reproducibility of the tests was high and is expressed as the relative standard deviation (RSD), reported below. For the evaluation of gas composition via MS, the procedure previously described was followed.
The efficiency of gasification was expressed by the equation:
where m
g and m
c are the masses of product gas and initial char, respectively.
The syngas (H
2 + CO) yield was expressed as:
where x
syn is the volume fraction of syngas in the product gas and V
g is the volume of total gas (m
3).
3. Results and Discussion
3.1. Characterization of Gasification Feedstocks
Table 1 represents the proximate analysis, the ultimate analysis, the higher heating value and the structural characteristics of the raw fuels and their chars. As observed, the lignite, having lower carbon and hydrogen contents than barley straw but a 3-times higher ash content, presented a reduced higher heating value. The concentrations of nitrogen and especially sulfur were very low, revealing insignificant toxic emissions during the thermal processing of both materials. Upon pyrolysis, volatile compounds consisting mostly of hydrogen and oxygen species were released, leading to the enrichment of chars in carbon. This, in turn, resulted in a higher heating value of chars in comparison to raw fuels, and the effect was more pronounced in the case of barley straw, the volatile matter of which was double that of the lignite. Furthermore, the evolution of volatile matter caused an increase in pore volume, as can be seen from the table, and accordingly, about a 30–90-fold increase in the specific surface area of chars with respect to the raw samples. The enhancement of carbon stability, aromaticity and surface area after thermal treatment is very important for the reactivity of fuels [
3,
27] and their gasification efficiency, as will be shown below.
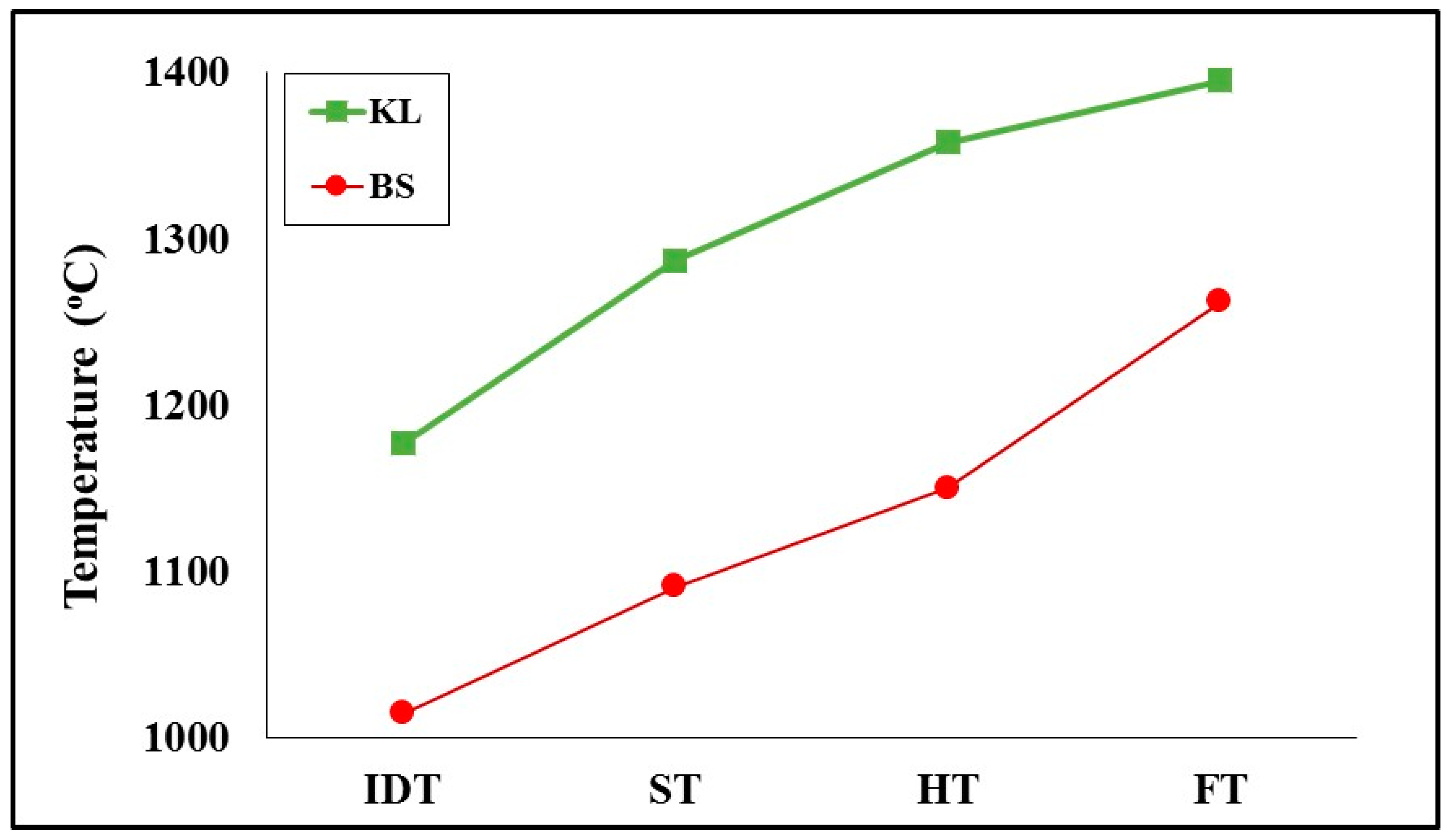
As gasification experiments were carried out up to 1000 °C, the fusibility behavior of ashes was investigated, and the data recorded by the heating microscope are compared in
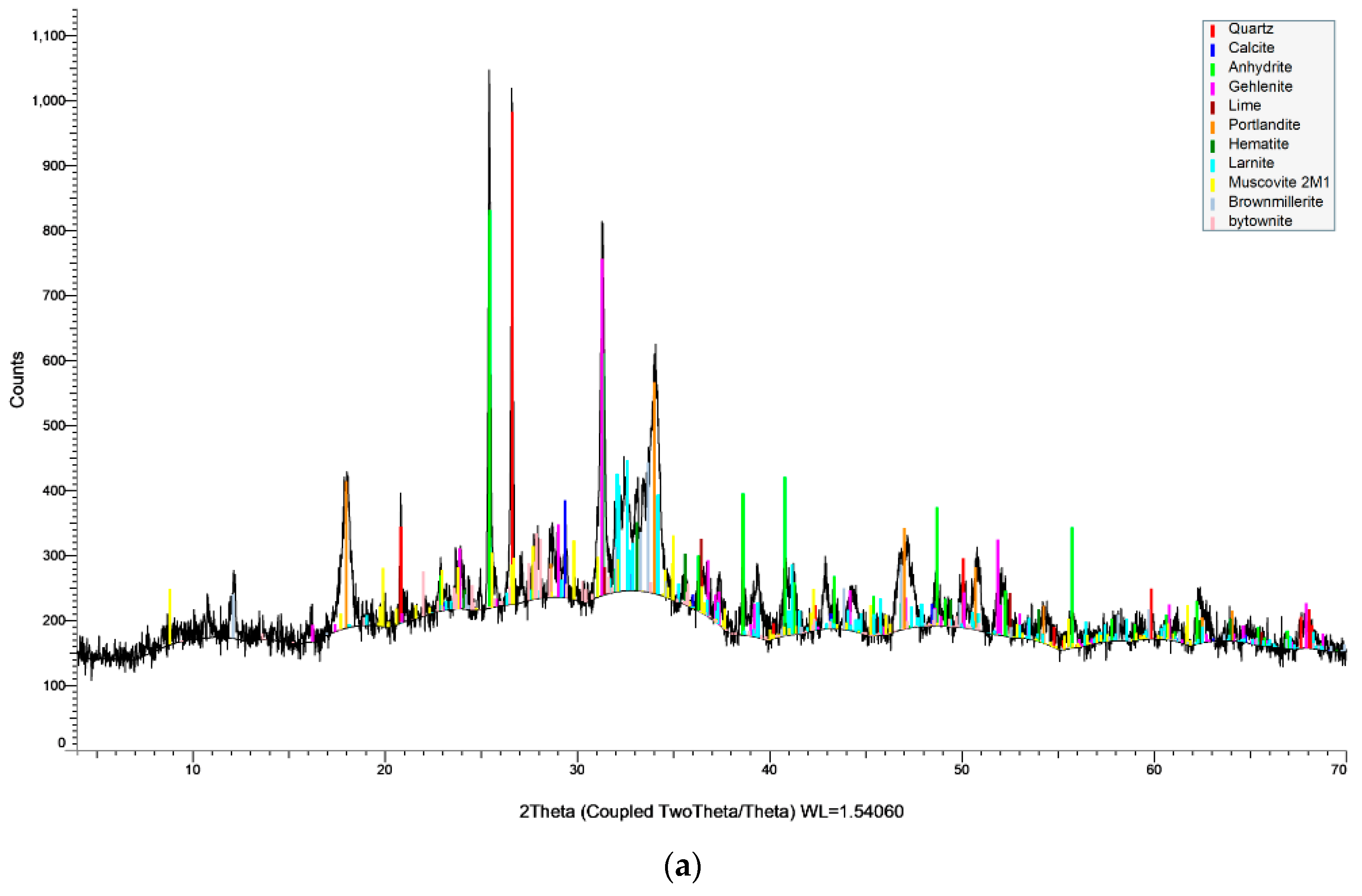
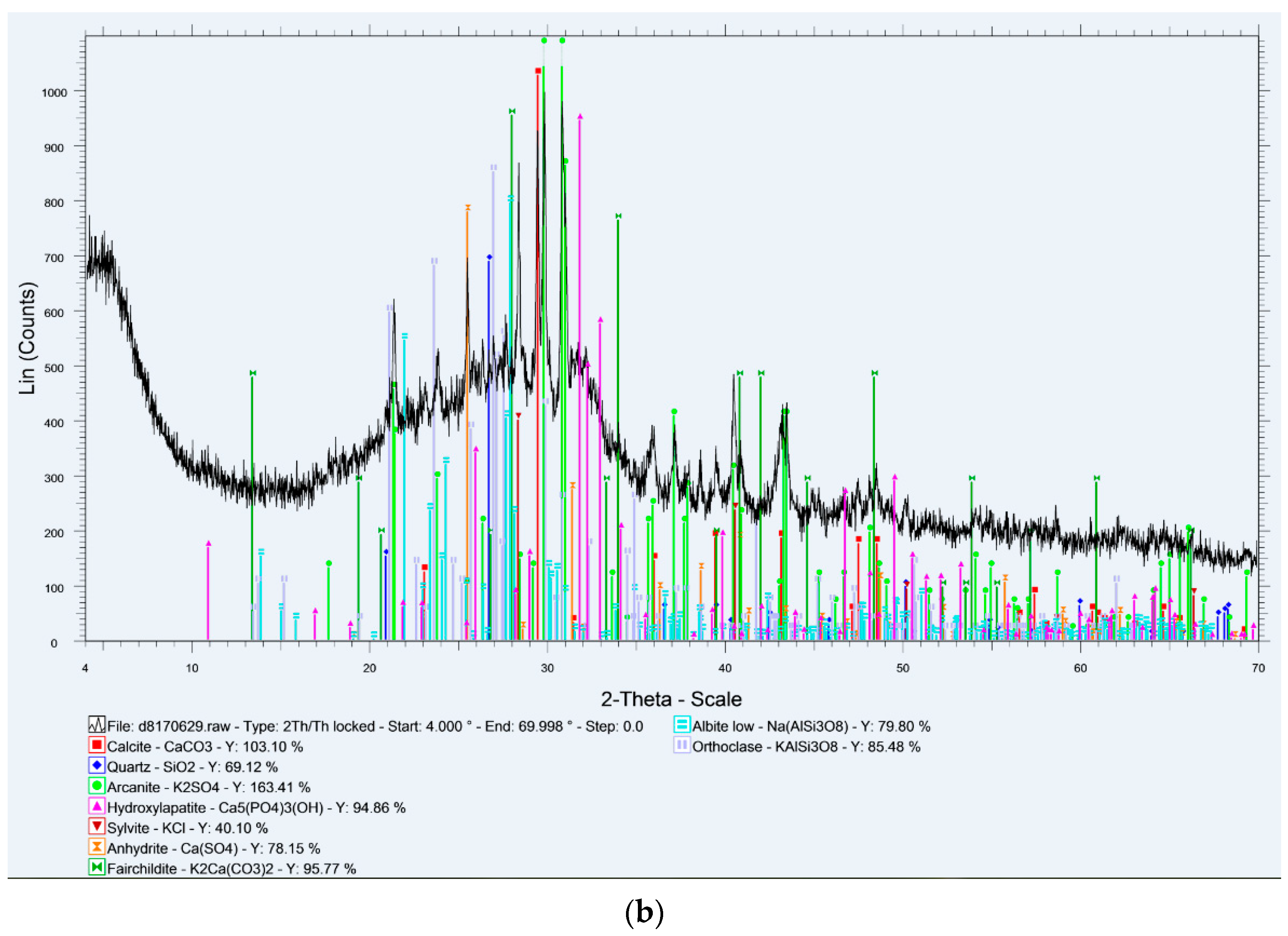
Figure 2. The initial deformation and softening temperatures of barley straw (1015 °C and 1091 °C) occurred about 160 °C and 190 °C earlier than those corresponding to lignite; however, both values imply deposition problems in boilers operating above 1000 °C. The fluid temperature of barley straw (1262 °C) was also lower than that of lignite (1395 °C). This behavior is attributed to the different mineral phases present in the two materials, melting at different temperatures. The XRD spectra of the ashes are illustrated in
Figure 3.
These ashes were produced at 550 °C, in order to detect any mineral phases before being transformed, or volatilized at higher temperatures. Thus, the relatively low initial deformation and softening temperatures of barley straw sample were attributed to the fusible forms of arcanite (MP 1070 °C), sylvite and fairchildite (MP 815 °C) [
29] minerals, as
Figure 3b shows. On the other hand, Kardia lignite ash was rich in calcium and silicon phases, mainly in the forms of calcite (MP > 1300 °C), anhydrite (MP > 1300 °C) and quartz (MP 1700 °C) [
29], and contained considerable amounts of high-melting-point aluminum and iron minerals, while it had a lower content in alkali incorporated in clays and feldspars. The characteristic fusion temperatures of the lignite/barley straw 50:50 mixture, being intermediate to blend components, suggests the safe operation of gasification systems, in terms of fouling, at temperatures below 1000 °C.
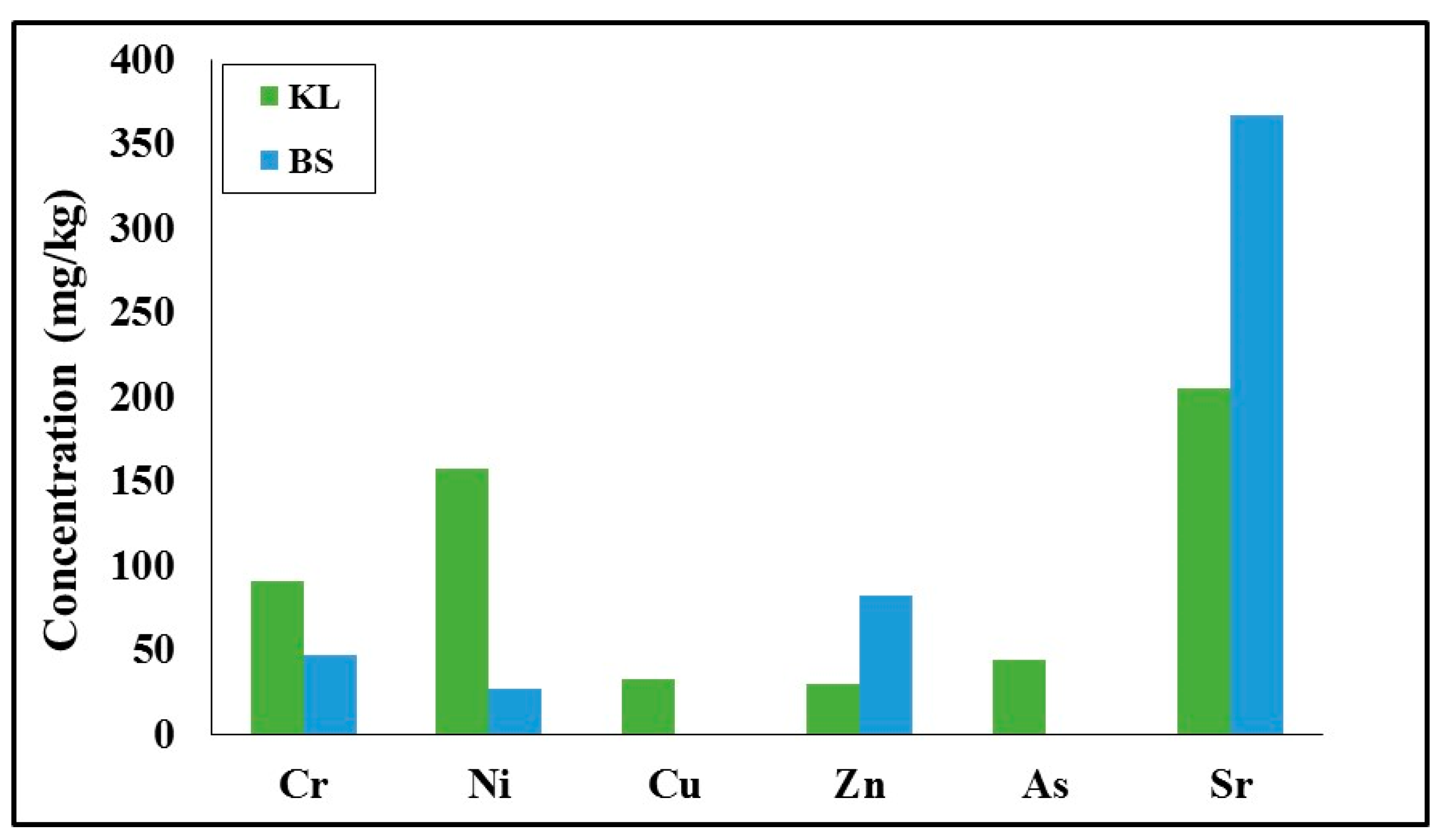
Concerning the impact of heavy metals in ashes on the environment upon disposal, it was found that none of the measured values exceeded the European Union limits [
30], and the toxic metals Hg, Pb, Cd and Co were below the detection limits of the ICP-MS analyzer (0.004–0.35 μg/L). From
Figure 4, it can be seen that biomass ash presented lower concentrations in Cr and Ni compared to lignite ash, whereas its concentrations were much higher in Sr and Zn.
3.2. Characterization of Liquid and Gaseous By-Products
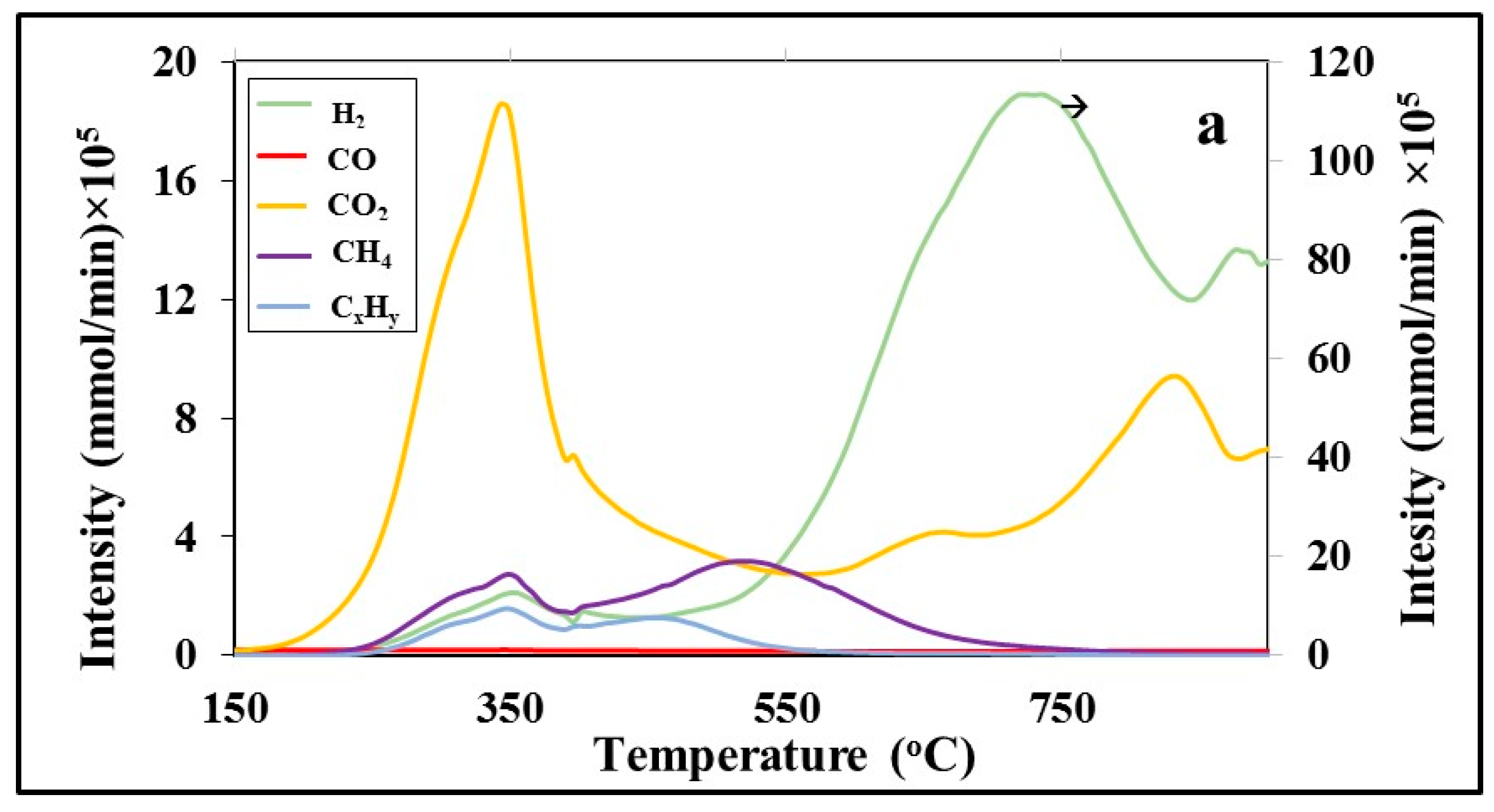
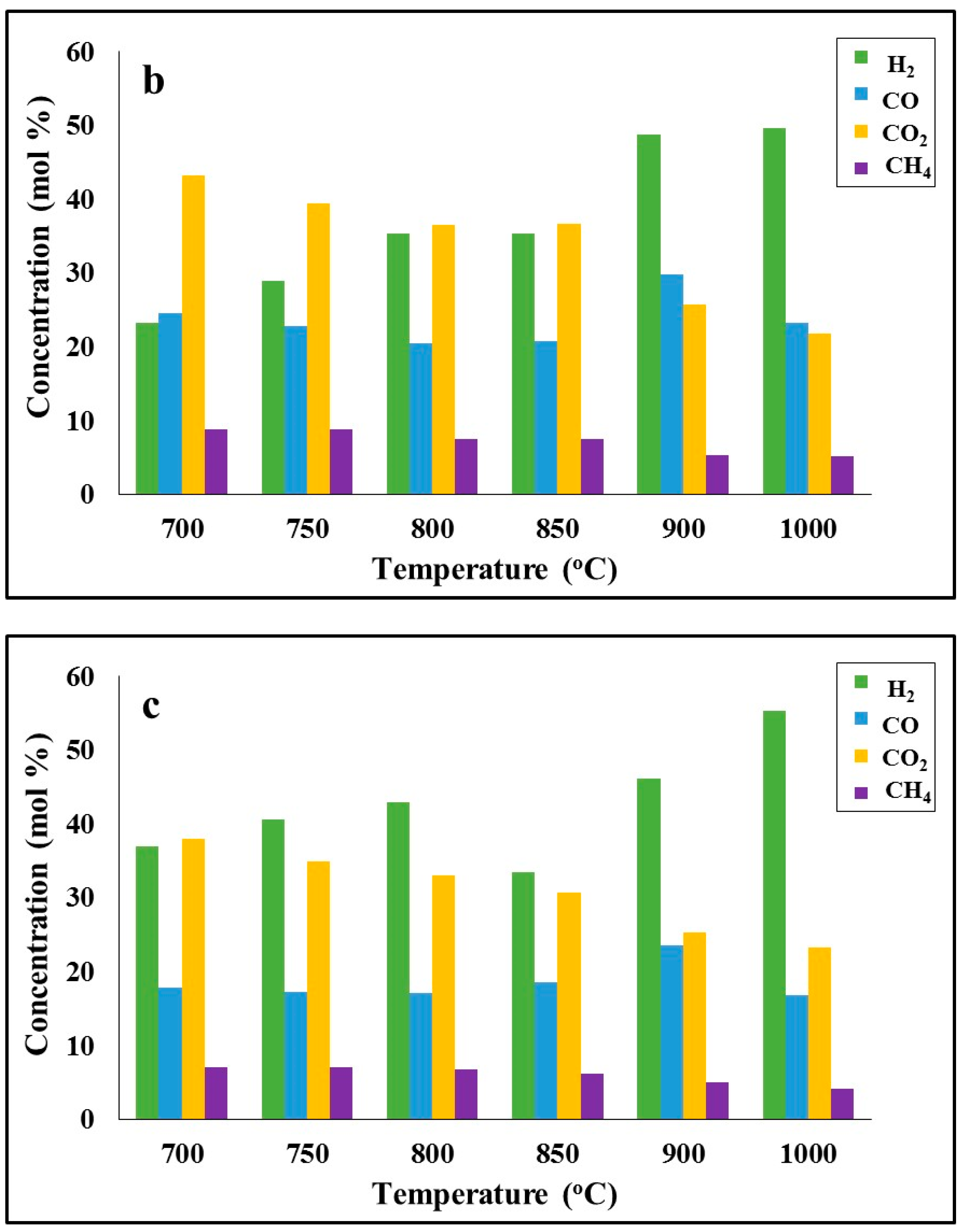
A representative graph of the evolution rate of light gas during the pyrolysis step of barley straw, as recorded by the TG-MS system, is indicated in
Figure 5a. The gas mixture consisted mainly of CO and CO
2, which were emitted between 300 °C and 750 °C. CH
4, light hydrocarbons and H
2 were evolved in lower amounts at higher temperatures, >450 °C, through the cracking of stronger bonds. In the case of barley straw, the emission of CH
4 and C
xH
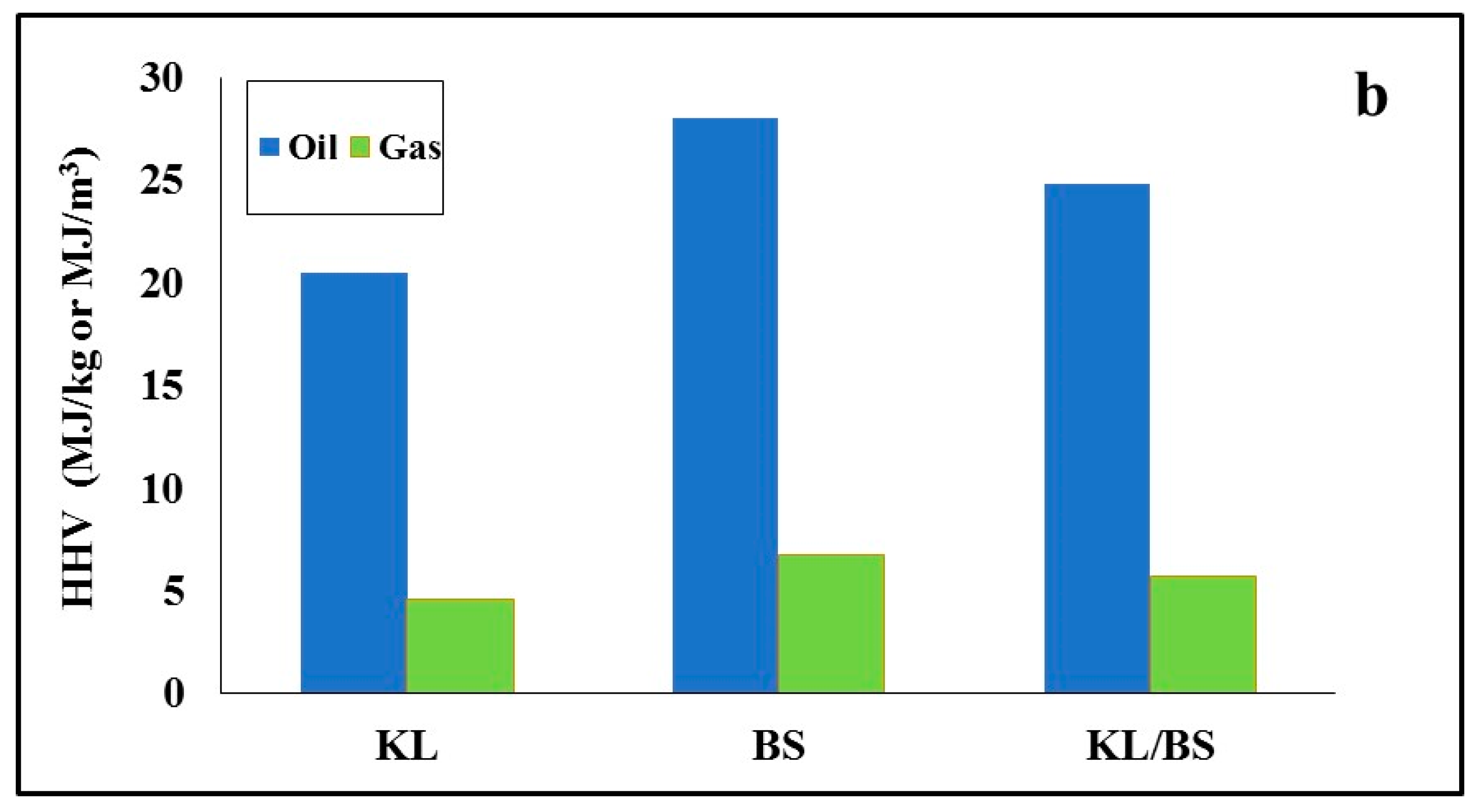
y started earlier, around 300 °C. The average heating values of gases produced from all fuels studied upon pyrolysis are compared in
Figure 5b, with the heating value of the oils collected after the centrifugation of the condensates. As can be seen, for barley straw fuel, these values were higher, at 28 MJ/kg and 6.8 MJ/m
3, respectively. Thus, liquid and gaseous by-products could offer valuable energy.
3.3. Gasification Performance of Fuels and Their Mixture with Steam Reagent
During steam gasification, several reactions occur simultaneously within the reactor, the most important of which are the following [
11]:
The water–gas shift reaction (8) is in equilibrium and the formation of H2 is favored at high temperatures, while reactions (7) and (9) are favored at high pressures.
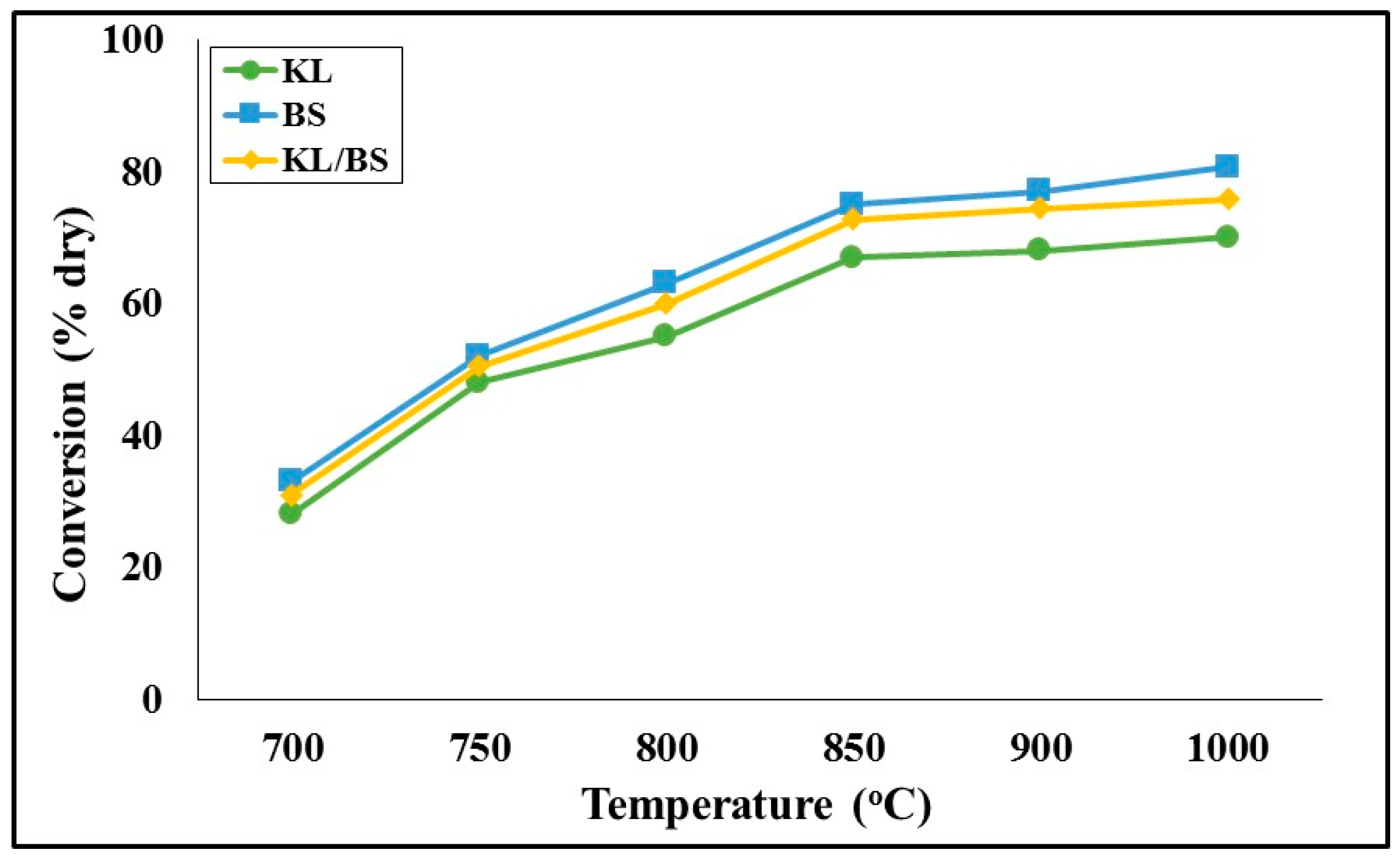
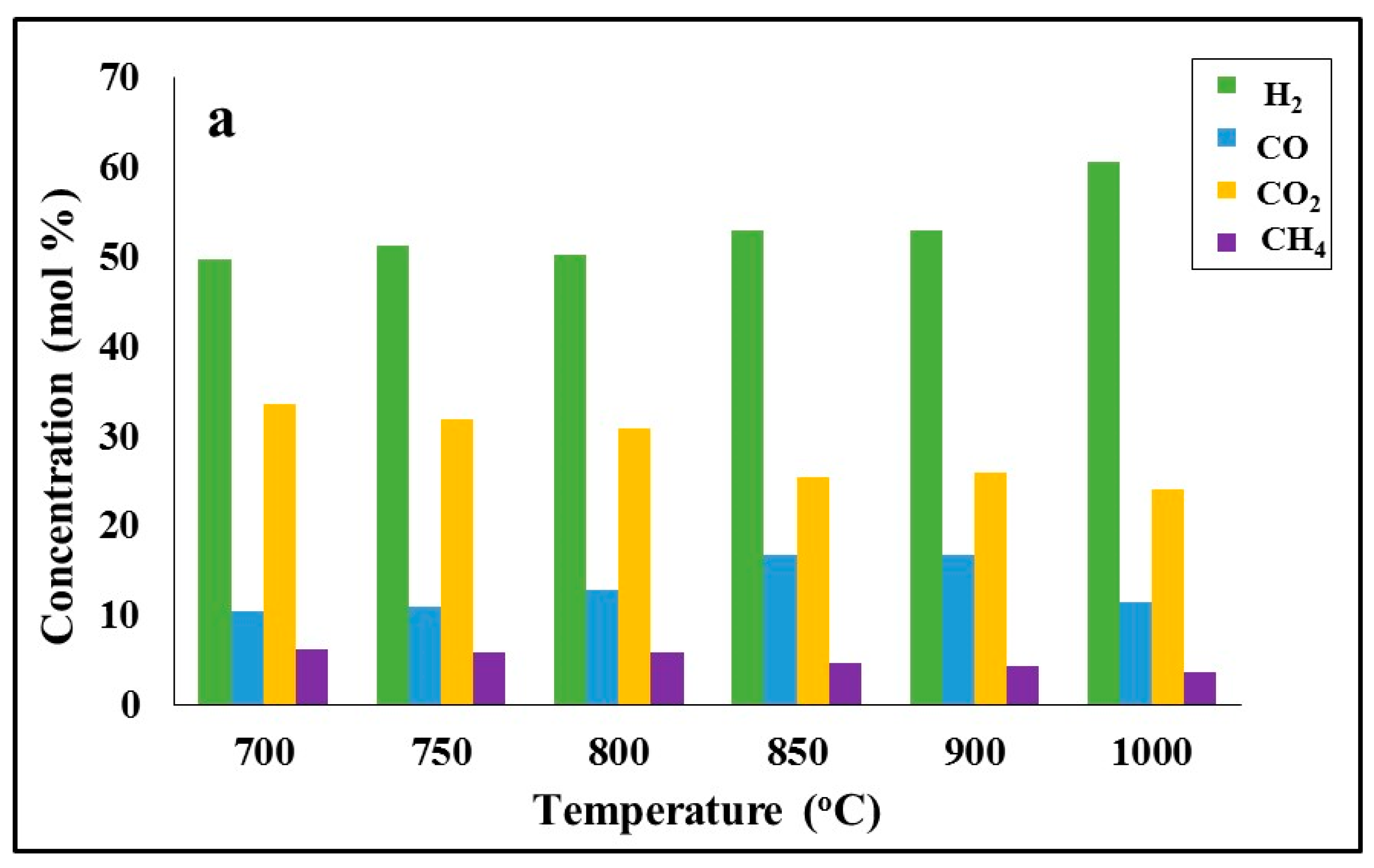
Figure 6 illustrates the effect of gasification temperature on fuel conversion, and
Figure 7 illustrates the composition of the product gas as a function of temperature during the process for the lignite, the biomass and their mixture. As the reaction temperature was increased from 600 °C to 1000 °C, gasification reactions were promoted and conversion increased. At the final temperature, the lignite was converted to gas by 70%, barley straw by 80.7% and the mixture by 73.5%. The higher conversion of barley straw biochar is attributed to its enhanced specific surface area (
Table 1) in comparison to the lignite, as well as its enrichment in potassium minerals such as arcanite, sylvite and fairchildite, previously presented in
Figure 3, which are known to act as catalysts during the gasification process, increasing the reaction rate and thereby the conversion [
4,
6,
17,
19]. From
Figure 7, it can be seen that the product gas mixture of both fuels consisted mainly of H
2, CO
2 and CO and lower amounts of CH
4. Hydrocarbons were produced in minor quantities (0.03–0.1% mol), and thus, were excluded from the graph. As the temperature was raised from 700 °C to 900 °C, the concentrations of H
2 and CO in the product gas increased, whereas that of CO
2 decreased. Thus, it can be speculated that reactions (4) and (6) and the reverse water–gas shift reaction (8) prevailed during the process. Above 900 °C, reactions (4), (5) and (8) were promoted, further increasing the percentage of H
2 in the product gas at the expense of CO. Also, the drop in the concentration of CH
4 with increasing temperature shows that CH
4 produced by reaction (7) was further consumed by reaction (9).
Figure 7 shows that the amount of syngas (CO + H
2) produced from these materials varied between 70% and 72% mol. A comparison of the present results with those of previous investigations is difficult, because of the variety of fuels and experimental conditions used.
For coals of various rank, the concentration of syngas has been reported to range between 58% and 83% [
6,
17,
26], while for some agricultural residues, such as cotton straw, corn stalk and rice husk, it is reported to vary between 61% and 74% at temperatures of 800–900 °C [
4,
5,
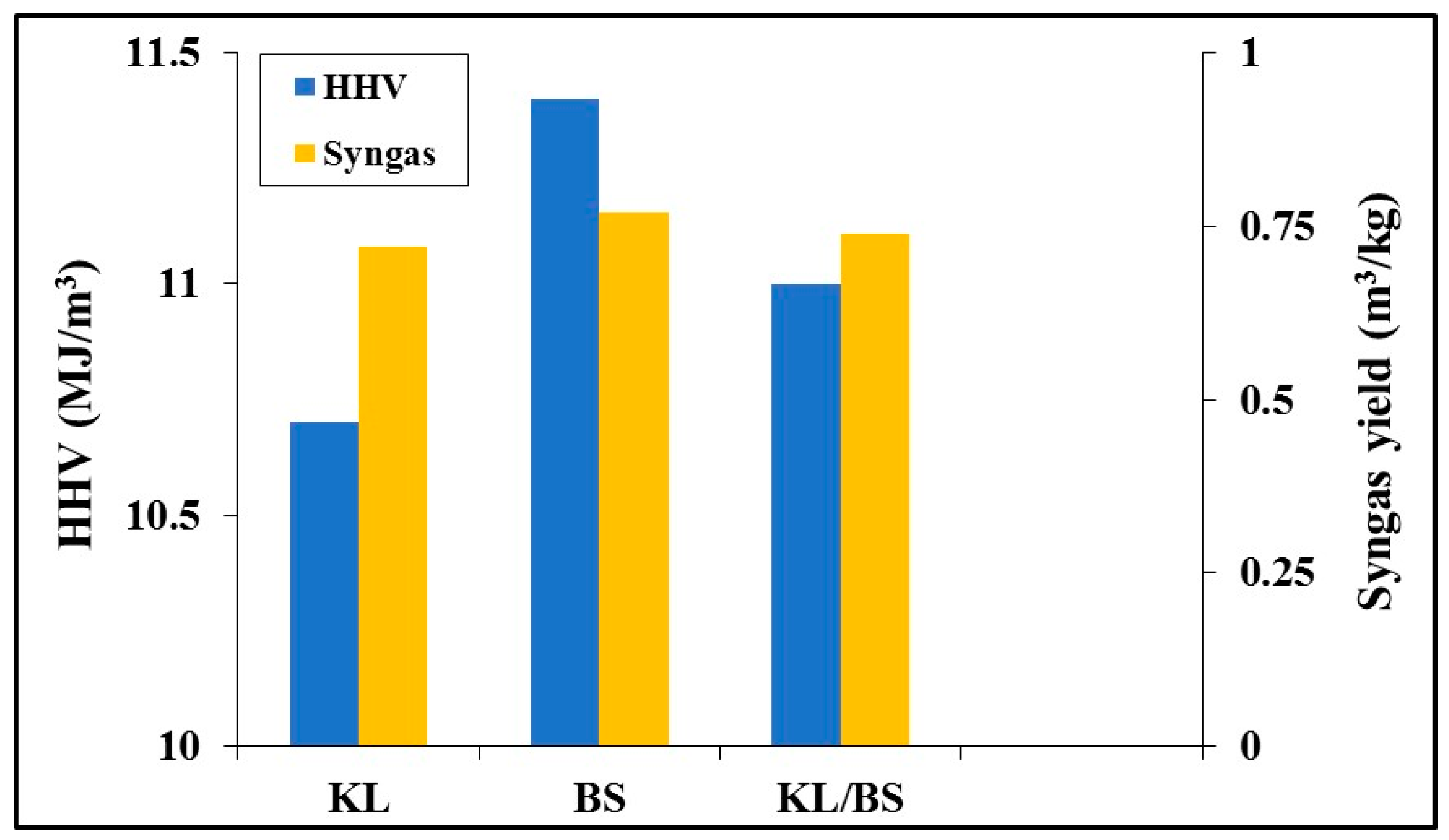
31]. The distribution of gases within the generated product at 1000 °C was reflected in the higher heating value of the gas mixture from the gasification of the fuels studied. The results are indicated in
Figure 8, together with the yield of syngas. The heating value of barley straw was higher than that of lignite, due to its increased content in combustible gases (H
2, CO, CH
4). Furthermore, the yield of syngas was also higher in this case, due to the increase in the conversion of this biomass material. When the lignite was blended with barley straw, the syngas yield and the higher heating value of the product gas from the blend were enhanced, in comparison to the values corresponding to lignite.
3.4. Gasification Performance of Fuels and Their Mixture with Carbon Dioxide Reagent
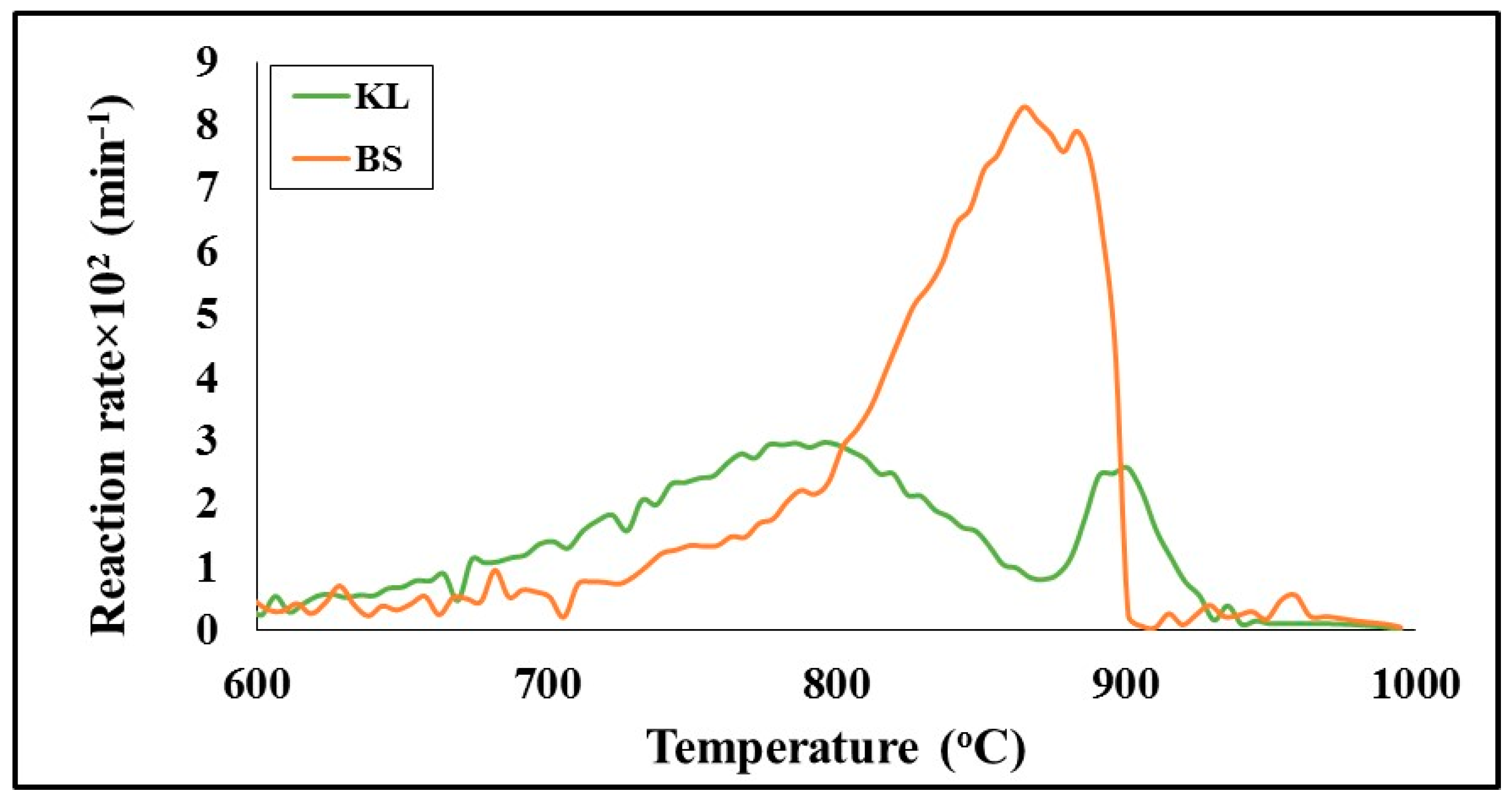
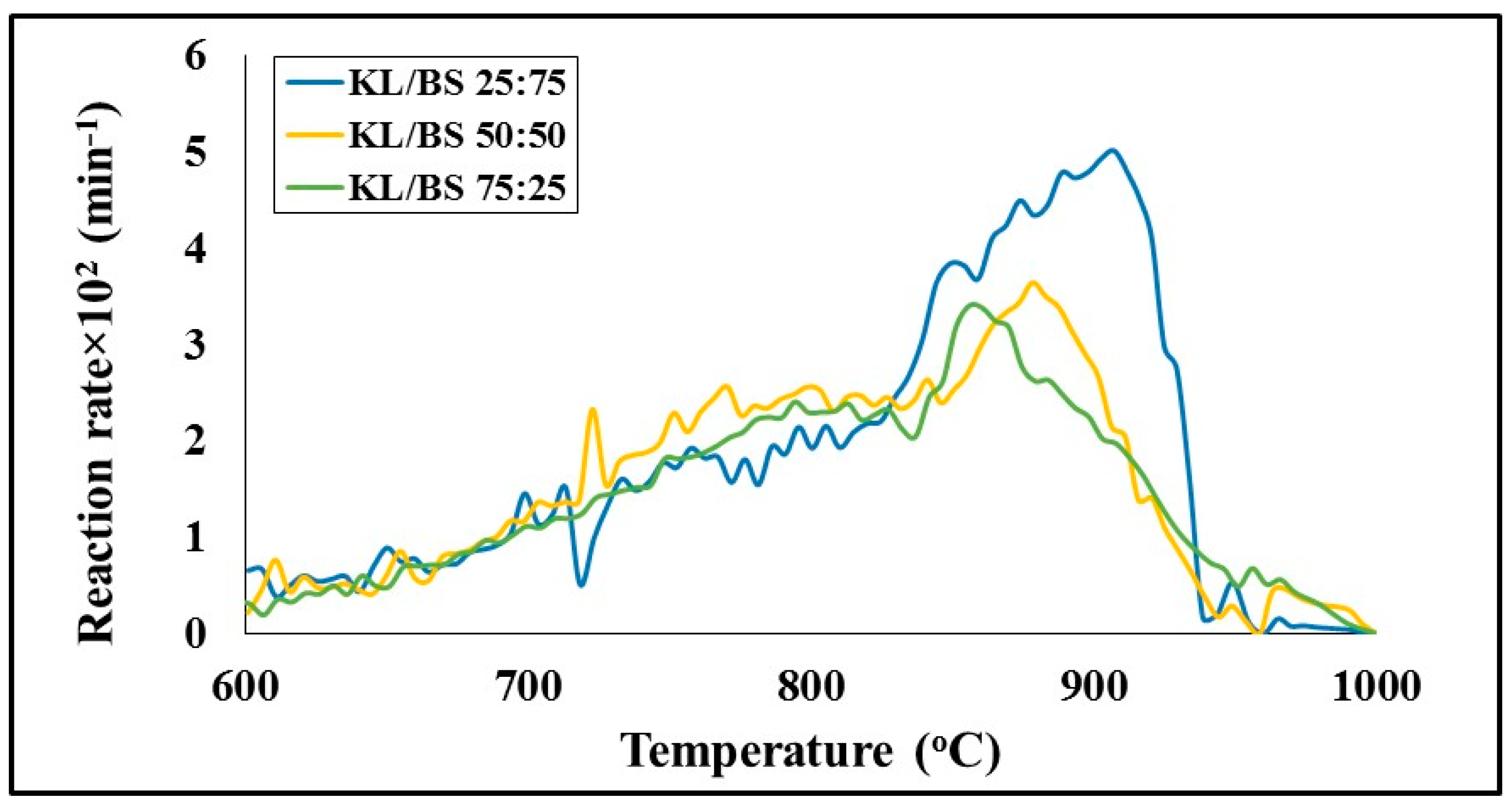
A comparison of the gasification profiles of the lignite and barley straw chars as a function of temperature, using carbon dioxide as the gasifying agent by conducting experiments in the TG-MS system, is made in
Figure 9.
Table 2 summarizes the characteristic parameters of the process, such as peak temperature and rate, reactivity, conversion and product gas composition. As can be seen, the lignite started to decompose earlier and its maximum (T
max) was centered at 789 °C. On the other hand, barley straw char, although it presented a much higher peak temperature 865 °C, displayed a 3-times higher rate (R
max) than the lignite, which resulted in enhanced reactivity, as expressed by the R
max/T
max ratio. Furthermore, the conversion of barley straw to carbon monoxide gas through the Boudouard reaction (6) was about 95%, in contrast to that corresponding to lignite char, which was only 62.9%. This low reactivity of lignite fuel is principally attributed to its elevated amount of ash (
Table 1), serving as a physical barrier to the carbon dioxide reagent, as well as its reduced specific surface area (
Table 1) available for reaction. Barley straw fuel, however, with a 4-times higher specific surface area and a significant amount of potassium, as previously presented (18% K
2O in ash), known to catalyze the gasification process [
32], exhibited a higher rate and final conversion.
Table 2 also shows that the product gas consisted mainly of carbon monoxide and small amounts of hydrogen, methane and hydrocarbons. The increased higher heating value of lignite gas is explained by its higher content in methane, which was most probably formed by the reaction of hydrogen, from the splitting of aromatic rings at high temperatures [
22], with carbon in char. On the other hand, the yield of syngas produced, which was directly related to the conversion and gaseous composition, was greater in the case of barley straw (0.84 m
3/kg versus 0.54 m
3/kg of lignite).
The TG/DTG gasification profiles of the lignite/barley straw char blends are illustrated in
Figure 10. Comparing
Figure 9 and
Figure 10, it can be observed that the blend curves lie between those of the component fuels. However, as also confirmed by
Table 2, the inflection temperatures were shifted to higher values than those theoretically expected from the contribution of each component material, and the reaction rates were lower that the weighted average values. This, in turn, resulted in lower reactivity and final conversion, which did not present a linear relationship with the amount of blending, agreeing with earlier studies reporting a lower reaction rate of blends in comparison to component fuels [
27]. Therefore, interactions occurred between the two fuels upon mixing, leading to synergistic effects. Considering that the experiments were carried out under exactly the same conditions, the reasons behind this behavior could be attributed to the physical structure and chemical composition of the two fuels. The high amount of lignite ash could have had a negative effect on the potassium catalytic effect of barley straw char. Additionally, the much lower O/C molar ratio of the blends (0.03–0.07) compared to lignite (0.11), implying a higher order and more stable aromatic structure [
3], could have caused a drop in gasification reactivity. Nevertheless, by blending the lignite with the barley straw biomass material, upgraded chars were produced with higher organic matter, exhibiting higher gasification reactivity and conversion than the lignite.
As to the effect of the gasifying agent on the gasification efficiency of the fuels studied, the above results show that in the case of lignite, although the heating value of the product gas was higher under a carbon dioxide atmosphere, the reaction under a steam atmosphere increased conversion and syngas yield. On the other hand, for barley straw fuel, upon carbon dioxide gasification, conversion and syngas yield were enhanced, whereas the heating value of the product gas was also higher, compared to steam gasification.
3.5. Industrial Application Prospects
Lignite is an important fuel for many national economies around the world and has higher proximity to biomass materials than high-rank coals. On the other hand, agricultural wastes are generated in large quantities in most countries at low or no cost and possess high energy availability. Therefore, the co-processing of these fuels will increase the operating flexibility of power plants, and thereby, their economic sustainability, reducing at the same time the carbon footprint on the environment. The development of transportation and storage methods of biomass materials is necessary for their use together with lignite in power plants.
The results of this study are promising, suggesting that agricultural wastes such as barley straw could be used as feedstocks in co-gasification processes with lignite for syngas production. However, further tests on a larger scale and techno-economic analyses are required prior to industrial applications. The expansion and optimization of operating parameters, the use of catalysts to increase the rate of gasification reactions and yield of syngas, the use of additives to capture carbon dioxide during the steam gasification process, as well as computational modeling studies are among the important issues to be investigated. The energy source for heating up the gasifier could be provided by coupling the process with a power plant, or could have a renewable origin, such as the by-products of the biomass pyrolysis step.
Unlike steam gasification, which is quite an established process, for the deployment of carbon dioxide gasification technology on an industrial scale, additional aspects must be investigated and implemented. A principal challenge is to examine the optimum concentration of carbon dioxide in the feed gas, in terms of gasification efficiency and syngas quality, by mixing it with other gasifying agents, as the use of pure carbon dioxide in commercial gasifiers is not feasible.
4. Conclusions
Kardia lignite contained a great amount of ash (33.3%) rich in calcium and silicon phases, leading to a low calorific value (12 MJ/kg). Barley straw biomass material had a lower content of ash (11.5%) enriched in potassium and a high calorific value (21.3 MJ/kg). Devolatilization of the materials up to 600 °C resulted in the carbon enrichment of chars and a 30–90-fold increase in the specific surface area. Gaseous and liquid by-products with higher heating values of 5–7 MJ/m3 and 20–28 MJ/kg could offer valuable energy.
Upon steam gasification up to 1000 °C, avoiding deposition phenomena from ashes, conversion increased with temperature, and the product gas was enriched in hydrogen and carbon monoxide. The syngas yield and heating value of the gas mixture were higher for barley straw fuel (0.77 m3/kg, 11.4 MJ/m3), which, when blended with the lignite, produced upgraded products.
Upon carbon dioxide gasification up to 1000 °C, barley straw char exhibited a 3-times higher rate than lignite, as well as higher conversion (94.5% vs. 62.9%) and syngas yield (0.84 m3/kg). Lignite/barley straw blends showed synergistic effects and presented higher gasification reactivity and conversion in comparison to lignite.
The overall performance of lignite was improved with the steam reagent, while that of barley straw was improved with the carbon dioxide reagent.