Abstract
Internal bleaching is a conservative treatment applied to a darkened endodontically treated tooth to restore its original colour, either as a single treatment or as a treatment prior to a subsequent prosthetic phase. The aim of this study was to objectively measure and compare using an experimental model the expansive capacity of four bleaching groups: carbamide peroxide 37% (CP 37%), hydrogen peroxide 30% (HP 30%), HP 30% mixed with sodium perborate (SP) and SP mixed with distilled water. A total of 150 lower incisors (n = 30 in each group) were prepared for the Walking Bleach technique and a glass tube with oil was introduced into the coronal access cavity to measure the expansive capacity through oil displacement, due to the reaction that occurs when bleaching agents interact with dental tissues. The results after 10 days were analysed with the Games–Howell post hoc test to compare the samples. Significant higher expansion was observed with HP 30% (p < 0.05) and lower expansion with SP (p < 0.05) compared to the other groups. No significant differences were observed between CP 37% (p > 0.05) and HP 30% + SP (p > 0.05). This provides significant and observable information on the behaviour of each bleaching group and its evolution after 10 days.
1. Introduction
Over the last few decades, there has been a growing concern about dental aesthetics and smile appearance among the population [1,2]. Since tooth colour is one of the main factors determining the appearance of a smile, the darkening or alteration of the colour can be of real concern for patients, and even more so when only one tooth in the anterior area undergoes colour change and no longer matches the rest [3,4,5,6,7]. Patients with this type of problem tend to be more worried about appearance when the darkening also becomes more pronounced over time. Therapeutic procedures aimed at treating and resolving this type of problem are thus relatively common in the dental clinic. Normally, this type of colour alteration is due to intrinsic staining, although it is necessary to study and individualise each case in order to prescribe the appropriate treatment [8]. Colour changes of intrinsic origin are more noticeable and more difficult to treat due to their colour distribution over the entire tooth structure. The most common causes of intrinsic tooth staining are diverse: pulp haemorrhage usually caused due to trauma in anterior teeth, pulp necrosis, necrotic tissue remaining in the pulp chamber after endodontic treatment, pulp calcification, infections and regenerative endodontic protocols using triple antibiotic pastes containing minocycline and mineral trioxide aggregate (MTA) as an intracanal barrier over a blood clot induced at the cementoenamel junction (CEJ) [9,10,11]. Such staining has also been associated with iatrogenic factors (application of various irrigants used for the chemical treatment of canals, drugs and endodontic sealants) [10,11]. In these cases, the simplest and most conservative treatment option for restoring the original colour of the darkened tooth is internal bleaching [12].
Endodontically treated teeth can be bleached externally, internally or through a combination of both [13]. There are several internal bleaching techniques: intracoronal bleaching using the thermocatalytic technique, the Walking Bleach technique or a combination of both [9,14,15]. Walking Bleach is currently the intracoronal bleaching method of choice for whitening darkened teeth, because it is considered safer for the patient and has a high success rate [6]. Different concentrations of hydrogen peroxide (HP) are used in tooth whitening, which can be applied directly or can also be generated from sodium perborate (SP) or carbamide peroxide (CP) [10,12]. Generally, HP is used at higher concentrations for nonvital teeth whitening than for vital teeth whitening. However, it also depends on whether the whitening is performed in the dentist’s office or at home. Similar concentrations are applied for both vital and nonvital teeth in the dental office under professional supervision, but in lower concentrations in home bleaching for safety reasons [11]. Hydrogen peroxide acts as an active agent in an oxidative process that degrades tooth-darkening compounds and larger stains. These bleaching agents are introduced into the pulp chamber of the endodontically treated tooth between 5 and 14 days, always after checking that the endodontic filling is perfect and without problems in the apex with X-rays, sealing the cavity with temporary restorative material [16,17]. If the desired shade has not been achieved after this time, the procedure can be repeated. The HP present in all whitening products penetrates through tooth structures and produces reactive oxygen species, free radicals and HP anions, which oxidatively rupture the double bonds of the chromogens deposited on the tooth and which are responsible for tooth darkening, thereby resulting in whiter teeth [15,18]. Internal bleaching is also sometimes performed with the aim of lightening the tooth substrate as a step prior to the placement of a subsequent aesthetic prosthetic restoration [19].
However, some aspects of the behaviour of bleaching agents remain to be elucidated. With the Walking Bleach intracoronal bleaching technique, it has been observed that the temporary seal that is placed once the bleaching agents have been introduced into the chamber becomes deficient. This probably occurs due to an increase in intracameral pressure caused by the interaction of the bleaching agents with the dental tissues [20,21,22,23]. Inadequate sealing may lead to certain problems, such as intracameral contamination due to the introduction of bacteria through the microscopic gap between the restoration and the tooth. Likewise, HP could escape into the oral environment and cause disruption of mucosal or gingival tissues [24,25]. In addition, the bleaching procedure would prove ineffective and treatment time would be prolonged.
Another less frequent but very harmful complication that places the survival of the tooth at risk is the occurrence of invasive external cervical resorption (ECR). As it is usually symptomless until it reaches an advanced stage, the diagnosis may be delayed and therefore negatively impact upon the treatment possibilities [19]. Furthermore, it has been observed that after internal bleaching, HP can remain active for some time within the dentinal tubules and continue to release oxygen by further decomposing two to four weeks after the bleaching treatment has been performed. This same oxygen can reduce the adhesion of restorative materials through the interaction of residual oxygen in the form of free radicals with composite resins [26,27].
Due to the inconveniences and complications that can be encountered when performing nonvital bleaching, and because the mechanism of bleaching is not yet fully understood, the aim of the present experimental study was to analyse and compare the expansive behaviour of different bleaching groups in vitro—CP 37%, HP 30% and HP 30% mixed with SP and SP with distilled water—and thus to contribute to a better understanding of the reactions that take place and try to prevent adverse effects in order to whiten more effectively and safely.
2. Materials and Methods
The present study focused on the bleaching of nonvital teeth using the Walking Bleach intracoronal technique, and was approved by the University Ethics Committee (Ref.: UCV/2019-2020/037).
2.1. Whitening Agents
The following bleaching agents were used: liquid HP 30% (H2O2) (Foret, Peroxfarma, Barcelona, Spain), CP 37% gel (CH4N2O·H2O2) (Whiteness Super 37 FGM, Joinville, SC, Brazil) and SP powder (NaBO3) (Acofarma Distribution, Madrid, Spain).
Whitening groups:
- Group 1: CP 37%;
- Group 2: HP 30%;
- Group 3: HP 30% + SP (ratio 2 g/mL);
- Group 4: SP with distilled water (ratio 2 g/mL);
- Group 5: Control.
The liquid (HP or distilled water) and powder (SP) in groups 3 and 4 were mixed in a dappen glass with a cement spatula. In the control group, only distilled water was used.
2.2. Sample Preparation
A total of 150 lower incisors extracted for orthodontic or periodontal reasons and without caries, cracks, fractures or any restorative or root canal treatment were used. All bacterial plaque debris was cleaned with a prophylaxis brush and sterile gauze, and the teeth were immediately immersed in Hank’s balanced salt solution (HBSS). The teeth were used within three months of extraction.
All teeth were subjected to endodontic treatment, performed using a single operator, with the aim of ensuring maximum homogeneity of internal bleaching in all teeth equally. First, all remaining pulp tissue was removed and the access cavities to the canal system were made on the lingual surface of the anterior teeth with a high-speed handpiece and diamond bur (FG ML 200442AA ISO198016, Diatech; Coltene/Whaledent, Altstätten, Switzerland). The teeth were prepared to working length with the ProTaper® system up to the F3 file (Dentsply Maillefer, Ballaigues, Switzerland) and an X-SmartTM endodontic micromotor (Dentsply, Weybridge, UK). The canals were obturated with AH Plus cement sealer and gutta-percha via lateral condensation, also using accessory gutta-percha and spacer (Dentsply Sirona, York, PA, USA).
Finally, a 3 mm endodontic filling was removed apical to the cementoenamel junction (CEJ) with a tungsten carbide bur (Tungsten bur, FG 330, Kerr, Bolzano, Italy); then, a 2 mm seal was applied using a resin-reinforced glass ionomer cement (Vitrebond, 3M ESPE, Maplewood, MN, USA).
2.3. Experimental Model
The experimental design of this study focuses on the measurement of oxygen expansion during Walking Bleach using different bleaching agents: CP 37%, HP 30%, HP 30% + SP and SP.
After endodontic treatment, the enamel around the access cavity was prepared and etched with 37% orthophosphoric acid (Scotchbond Etchant, 3M ESPE, Saint Paul, MN, USA). After rinsing with water and drying, adhesive (Excite DSC F, Ivoclar Vivadent, Schaan, Liechtenstein) was applied so that 1 mm diameter glass tubes (2940211, Paul Marienfeld Superior, Lauda-Konigshofen, Germany) could be assembled with adhesive and composite to the access cavity.
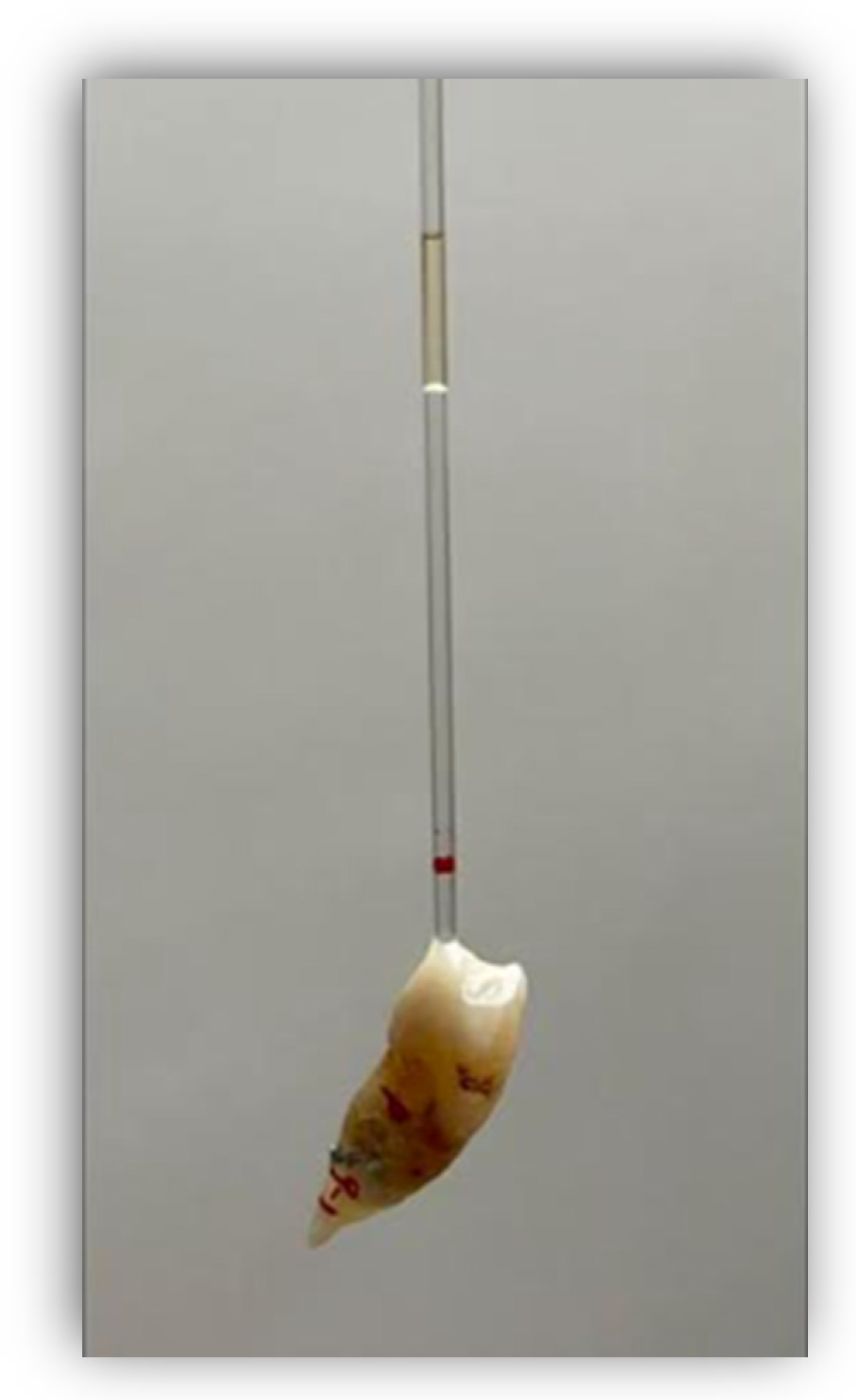
Before fixing the tubes, the corresponding bleaching agent was placed for each group of teeth and a volume of oil covering a length of 10 mm was introduced into the tubes in order to retain the fluids and to measure the expansion through their displacement along the glass tube, using an irrigation syringe with a 25G needle (25G, C80, Larident, Tribogna, Italy) (Figure 1). If necessary to control the continued displacement of the oil, the tubes were joined with adhesive and composite to allow the oil to continue to rise. Expansion was measured every 24 h for 10 days using a digital meter. Each time that the expansion was measured, a mark was made with a permanent marker pen so that the expansion could be measured from that mark on subsequent days. The preparation of the samples and bleaching products was carried out by one operator, while the measurements were performed by another operator who did not know what was in each sample.
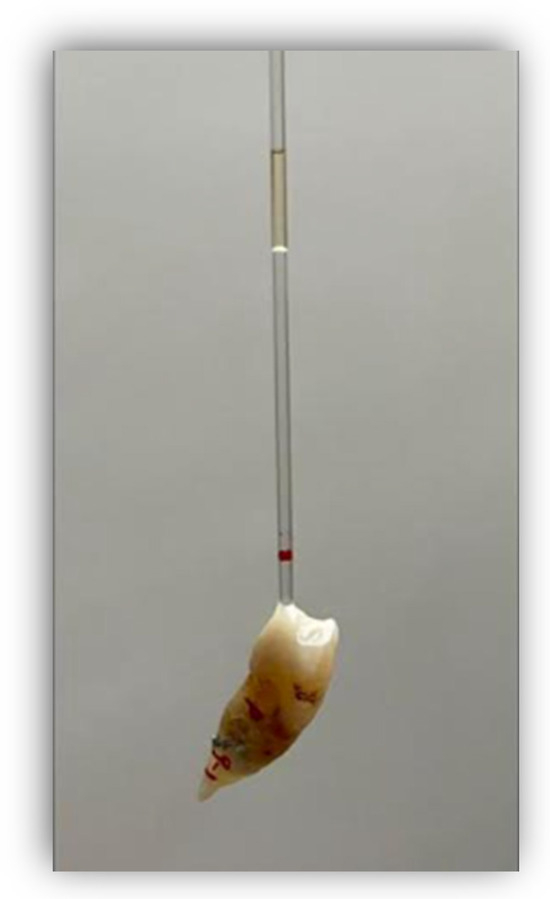
Figure 1.
The red line on the tube shows where the oil was originally, and the difference between the red line and the lower limit of the oil is the amount of displacement.
2.4. Statistical Analysis
It is hypothesised that an expansion reaction occurs within the pulp chamber when bleaching agents are introduced into the pulp chamber during the Walking Bleach nonvital whitening technique. Oxygen expansion was observed and measured for 10 days and all data were entered into an MS Excel spreadsheet for further statistical analysis using the SPSS version 23 statistical package (IBM Corp., Armonk, NY, USA) with a confidence level of 95% and considering statistically significance for p < 0.05.
First, Welch’s ANOVA test was performed, which showed that the mean final expansion of at least two of the four bleaching groups was significantly different (p-value < 0.001). In order to assess these differences, a Games–Howell post hoc test was used to assess the observed differences in expansion in the different bleaching groups.
3. Results
Only the study groups were compared, because there was no oil displacement in the control group. This also shows that the procedure was valid for measuring the oxygen expansion resulting from the interaction between the bleaching agents and the dental tissues.
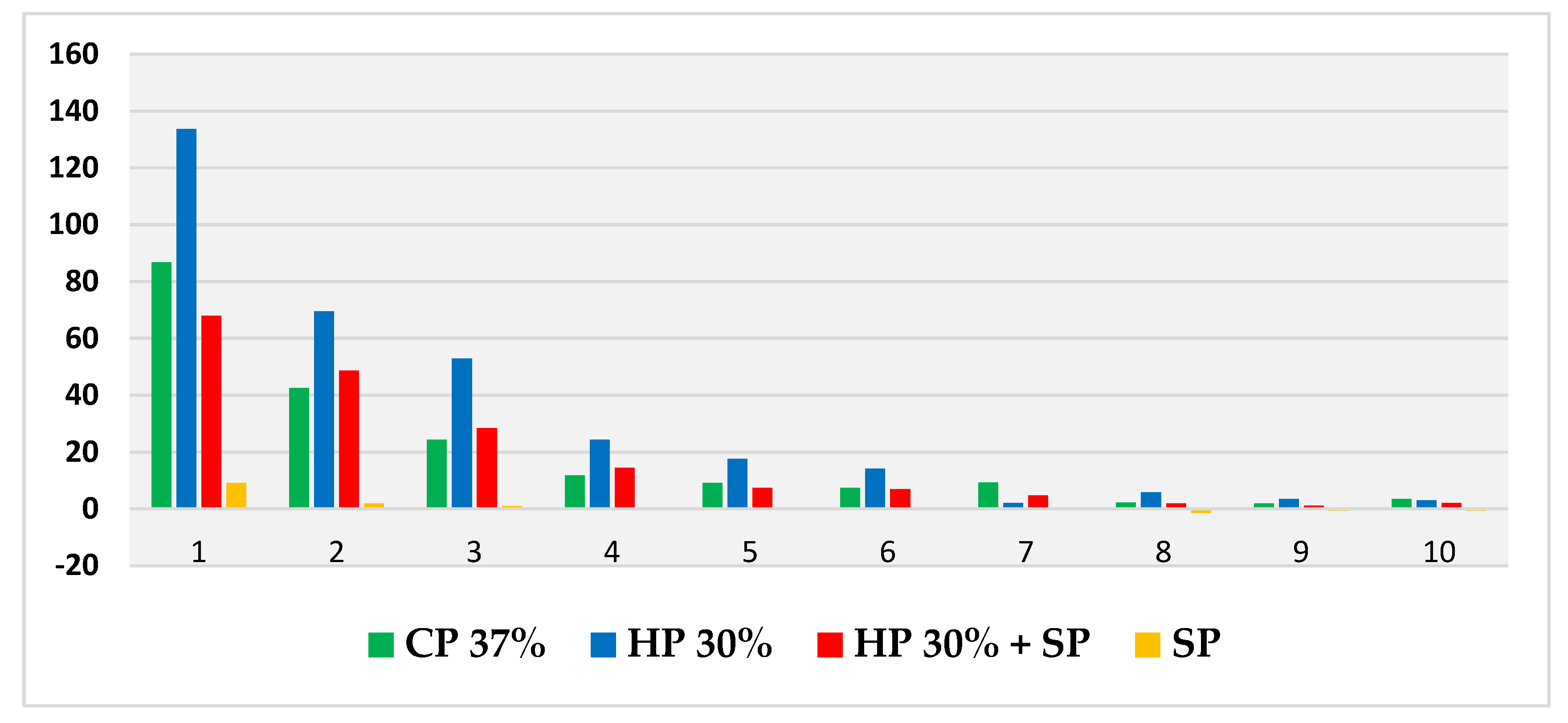
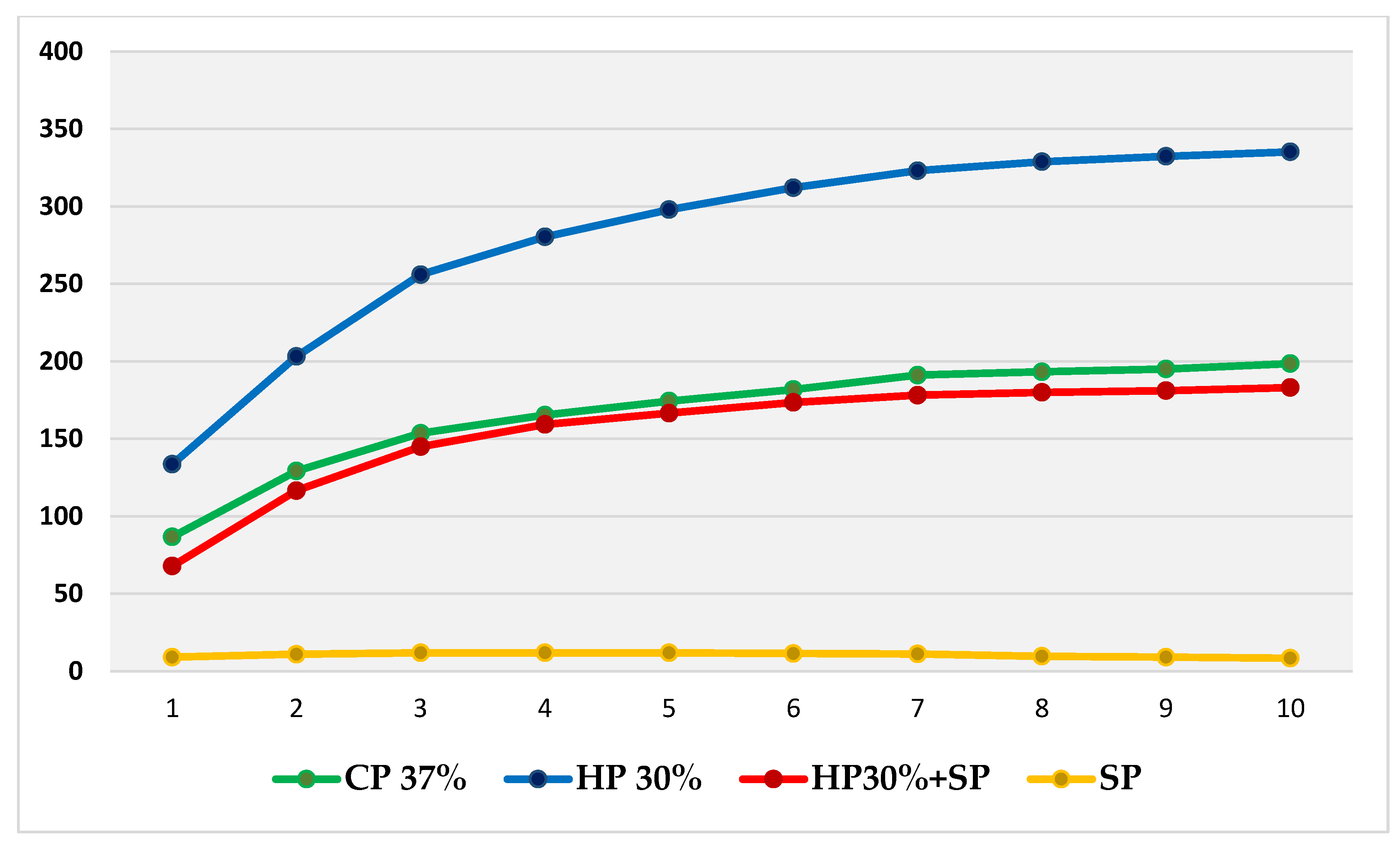
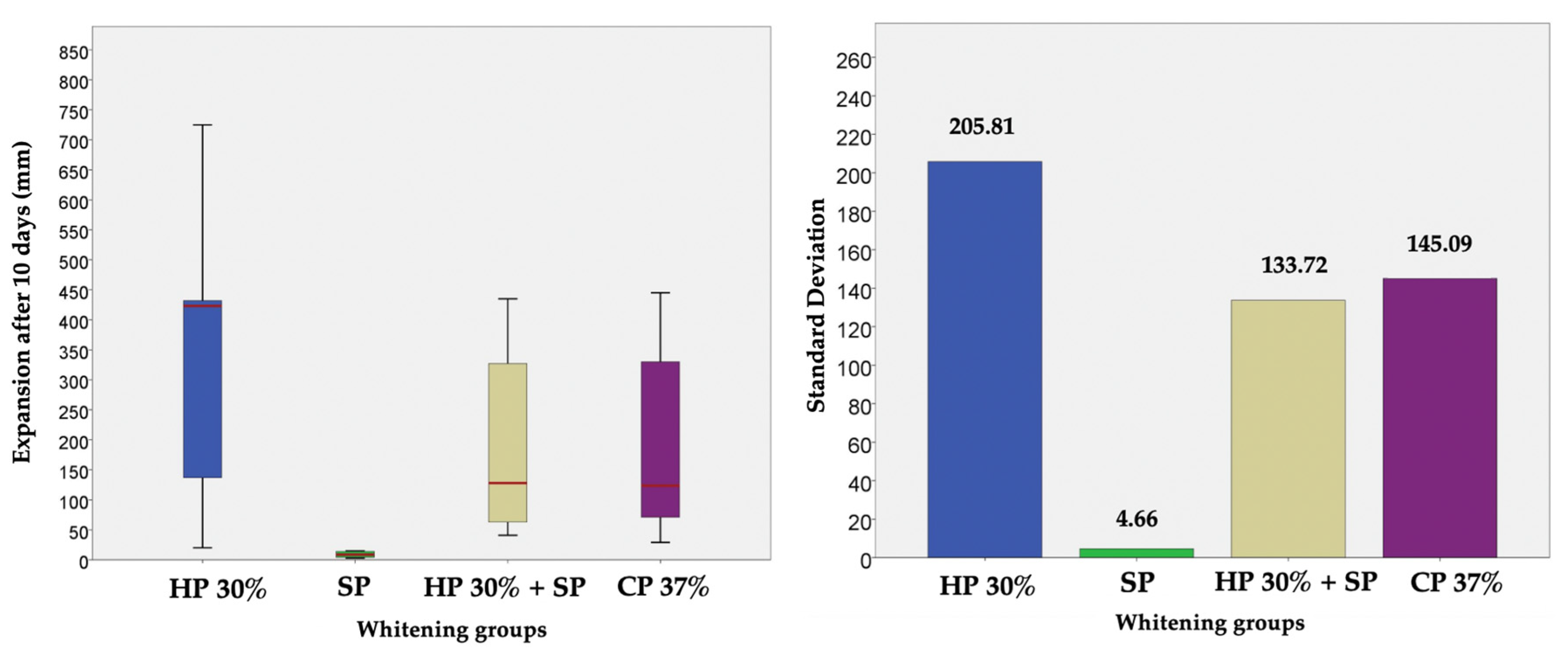
Thus, based on this method, the expansion (in mm) produced by CP 37%, HP 30%, HP30% + SP and SP in internal bleaching for 10 days in a situation similar to the usual conditions was measured and compared through a displacement of the oil introduced into the tubes. Figure 2 shows the evolution per day of the expansion in each bleaching group during the 10 days of recording. Figure 3, in turn, shows the cumulative sum for each group. Figure 4 shows a bar chart with the standard deviations and a box plot with the expansion values after 10 days.
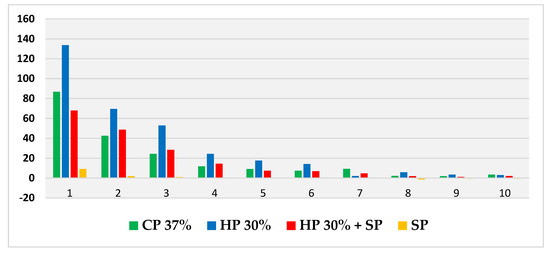
Figure 2.
Evolution of mean daily expansion (measured in mm) for each bleaching agent over the 10 days.
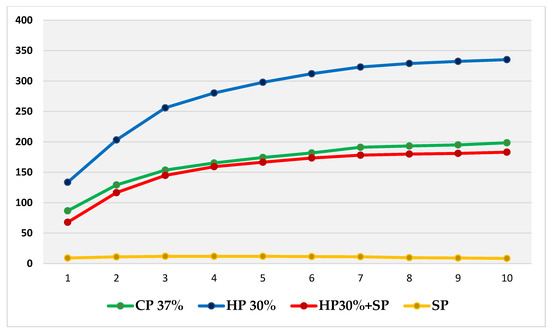
Figure 3.
Cumulative sum of mean daily expansions of each bleaching group during the 10 days, with plotting of the evolution shown over time.
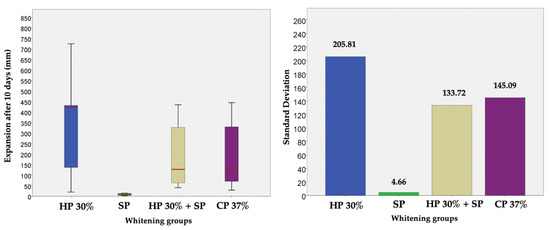
Figure 4.
Bar chart with the standard deviations and a box plot with the expansion values after 10 days, in which no outliers are observed.
In all four groups, the greatest variation in expansion occurred within the first 24 h, with HP 30% showing the highest expansion rate and SP with distilled water showing the lowest. The CP 37% and HP 30% + SP groups performed very similarly over the 10 days, while SP showed a more stable behaviour very different from that of the rest of the groups.
The Games–Howell post hoc test was used to assess the differences between the four groups (Table 1).

Table 1.
Multiple comparison of the expansion results obtained in each bleaching group to assess the significant differences in the expansion behaviour between them.
4. Discussion
The present in vitro study shows in an objective and quantifiable way the expansion behaviour that takes place after the interaction of the currently most commonly used internal bleaching agents with the dental tissues inside the pulp chamber. Specifically, measurement and comparison were made of the expansion between four bleaching groups.
The experimental model was designed to produce a situation as similar as possible to the conditions of the tooth when internal bleaching is carried out, and consisted of an extracted tooth to which a glass tube was affixed in the opening orifice in order to measure the expansion after endodontic treatment for the study. This is the same technique as that used in the study by Pallarés-Serrano et al. [23], but with the novelty that, in our study, we also included and compared CP 37%.
The bleaching agent with a significantly greater expansion versus the rest was HP 30%, followed by CP 37% and HP 30% + SP, with no significant differences between the latter. In turn, SP showed significantly less expansion with respect to the other groups.
All bleaching groups showed greater oil displacement within the first 24 h, with stabilisation over the following days.
In the CP 37%, HP 30% and HP 30% + SP groups, oil displacement persisted after 10 days, showing that the reaction that produced expansion continued, although to a much lesser degree than in the first few days. However, in the case of SP, from the fourth day onwards, the oil ceased to displace and even began to decrease from the sixth day onwards. A very different behaviour was therefore observed in the SP group. These results are very similar to those reported in an in vitro study carried out by Pallarés-Serrano et al. [28], in which the authors separated the enamel from the dentine to observe the expansive behaviour of the bleaching agents upon both structures separately.
The same behaviour was observed as that obtained from mixing the bleaching agents with dentine. This could be due to the fact that in this study, the bleaching agents placed inside the pulp chamber were in direct contact with the dentine and not with the enamel.
While considered to be a safe treatment, the mechanism by which a tooth is bleached is not fully understood [10] and although it has many advantages, the procedure is not free from possible drawbacks or complications described as resulting from the oxidising behaviour of HP—such as the appearance of an ECR or adverse effects upon the cavity seal [11].
The reaction observed in this experimental model creates an increase in intracameral pressure and would be responsible for the displacement of the temporary restoration that has been observed in other studies over the years, which can leave a microscopic gap between the restoration and the tooth [21,23,28,29].
Displacement of the temporary restoration could result in prolonged treatment with more sessions due to failure to obtain the desired results, bacterial infiltration and even failure of the endodontic treatment, as well as irritation of the tissues with which the bleaching agent comes into contact as it seeps into the oral cavity through the open space [11,24,25,30,31].
Based on the results obtained in this study, HP 30% would be by far the bleaching agent with the greatest capacity to produce such a displacement.
In turn, the expansion observed in this study also agrees with the whitening capacity reported in other publications, with better results using HP 30% after a few days, coinciding with the days of greatest expansion observed, and whitening the teeth more quickly and effectively than with other agents [31,32,33,34].
These results also apply to SP, as many studies suggest that it has a more stable behaviour but with a lower bleaching capacity, as our findings also suggest [34].
On the other hand, it has been postulated that oxidising agents from internal bleaching can cause cement necrosis, an inflammation of the periodontal ligament and may contribute to ECR from the pulp chamber through the dentinal tubules [35].
In a study on the ultrastructural changes in dentin induced via intracoronal bleaching agents, Maleknejad et al. (2012) found that HP 35%, CP 45% and HP 30% + SP produced an increase in the diameter of dentinal tubules, with the exception of SP with distilled water, probably as a result of protein oxidation [36]. Similarly, Martin-Biedma et al. also studied in vitro the possible changes in enamel and dentine produced by SP for 7 days, and the results showed that it did not produce microstructural changes in enamel and dentine [37]. In addition, Serin et al. [38] and Keskin et al. [39] also observed in their in vitro studies changes in MTA produced by intracoronal bleaching agents, in surface morphology and chemical composition that could negatively affect the subsequent restoration.
These results are consistent with those of our study, which shows a greater reaction with these agents, while the reaction with SP would not have sufficient capacity to alter dentine in the same way. Furthermore, they also agree with the findings of Heling et al. (1995), who observed the ability of Streptococcus faecalis to penetrate through the dentinal tubules of teeth bleached with HP 30% and HP 30% + SP, but not of teeth bleached with SP [40].
This finding was relevant because it has been observed that bacteria in the gingival or pulp chamber sulci can subsequently colonise the empty dentinal tubules, cause the persistent inflammation of the surrounding tissues and may contribute to the development of ECR [19,36,41,42].
There are published studies on the relationship between the reduction in the organic content of enamel and dentine with changes in biomechanical properties during bleaching procedures. Since the dentine structure constitutes an important part of endodontically treated teeth, any alteration in the biomechanical properties of dentine may have an impact on the overall strength of the tooth [22,42,43].
Using extracted human teeth, Lewinstein et al. examined the effect of HP 30% and SP on dentine and enamel microhardness [43]. They observed that HP 30% reduced the microhardness of both, but not the treatment with HP 30% + SP, and therefore considered this combination as the treatment of choice for the Walking Bleach technique.
In a study by Berger et al., all the bleaching agents that they used reduced the elastic modulus of dentin up to 7 days after bleaching compared to an unbleached control group; however, at 14 days postbleaching, there was no significant difference between the study groups and the controls, even though the group with higher HP levels had not yet reached normal values [44].
These authors speculate that this may be due to the fact that after 14 days, the oxygen concentration was reduced, favouring the reversion of the values of the modulus of elasticity. In this study, we observed that after 10 days, the expansion had been greatly reduced with respect to the initial values, and we would expect that after 14 days, it would be even less or nonexistent.
According to the results of our study, after 10 days and in all the internal bleaching groups except for SP, the oil in the tubes was still displaced, i.e., the expansion reaction was still present after 10 days. Therefore, we estimate that the delay of the definitive restorative treatment should be at least two weeks, since one week would be insufficient time. Our results agree with those reported by Yoon et al. (2014), who bleached dentin with SP and subsequently used sodium ascorbate for different periods of time, and found no differences versus the control groups [45]. This may be due to the low expansion observed in this study with SP. Cavalli et al. (2018) indicated that the time the restoration or prosthesis should be delayed depends on the bleaching agent used and its concentration, and advised SP for this type of treatment in which the subsequent restoration phase is relatively urgent [46].
The present study has the limitations of in vitro research. Although the tooth has been treated in the same way as we would treat it in a patient, the results could be influenced by the wet conditions involved. Further, both in vitro and in vivo studies are needed to contrast the results obtained.
5. Conclusions
Within the limitations of this in vitro study, it could be observed that each bleaching group has a different expansion behaviour, with the exception of the HP 30% + SP and CP 37% groups that show a very similar behaviour. However, HP 30% shows a significantly higher oxygen expansion, and SP shows a significantly lower expansion than the rest and a very different and more stable behaviour.
Author Contributions
Conceptualisation, A.P.-S. (Alba Pallarés-Serrano).; methodology, A.P.-S. (Alba Pallarés-Serrano) and A.P.-S. (Antonio Pallarés-Sabater); software, A.P.-S. (Antonio Pallarés-Serrano) and G.M.-M.; validation, A.P.-S. (Antonio Pallarés-Sabater) and A.P.-S. (Antonio Pallarés-Serrano); formal analysis, S.P.-S.; investigation, A.P.-S. (Alba Pallarés-Serrano); resources, G.M.-M.; data curation, S.P.-S. and G.M.-M.; writing—original draft preparation, A.P.-S. (Alba Pallarés-Serrano); writing—review and editing, A.P.-S. (Alba Pallarés-Serrano); visualisation, G.M.-M. and S.P.-S.; supervision, A.P.-S. (Antonio Pallarés-Sabater); project administration, S.P.-S. and G.M.-M.; funding acquisition, A.P.-S. (Antonio Pallarés-Sabater) and A.P.-S. (Antonoi Pallarés-Serrano). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the Catholic University of Valencia (Valencia, Spain) (protocol code UCV/2019-2020/037) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are reported within this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Horn, S.; Matuszewska, N.; Gkandtidis, N.; Verna, C.; Kanavakis, G. Smile dimensions affect self-perceived smile attractiveness. Sci. Rep. 2021, 2, 2779. [Google Scholar] [CrossRef]
- Batwa, W. The Influence of the Smile on the Perceived Facial Type Esthetics. Biomed. Res. Int. 2018, 2018, 3562916. [Google Scholar] [CrossRef] [PubMed]
- Tin-Oo, M.M.; Saddki, N.; Nurhidayati, H. Factors influencing patient satisfaction with dental appearance and treatments they desire to improve aesthetics. BMC. Oral. Health 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Samorodnitzky-Naveh, G.R.; Geiger, S.B.; Levin, L. Patients’ satisfaction with dental esthetics. JADA 2007, 138, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Van Der Geld, P.; Oosterveld, P.; Van Heck, G.; Kuijpers-Jagtman, A.M. Smile attractiveness: Self-perception and influence on personality. Angle Orthod. 2007, 77, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Bersezio, C.; Martin, J.; Peña, F.; Rubio, M.; Estay, J.; Vernal, R.; Fernández, E. Effectiveness and Impact of the Walking Bleach Technique on Esthetic Self-perception and Psychosocial Factors: A Randomized Double-blind Clinical Trial. Oper. Dent. 2017, 42, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Greta, D.C.; Colosi, H.A.; Gasparik, C.; Dudea, D. Color comparison between non-vital and vital teeth. J. Adv. Prosthodont. 2018, 10, 218–226. [Google Scholar] [CrossRef]
- Pandey, S.H.; Patni, P.M.; Jain, P.; Chaturvedi, A. Management of intrinsic discoloration using walking bleach technique in maxillary central incisors. Clujul Med. 2018, 91, 229–233. [Google Scholar] [CrossRef]
- Plotino, G.; Buono, L.; Grande, N.M.; Parameijer, C.H.; Somma, F. Nonvital Tooth Bleaching: A Review of the Literature and Clinical Procedures. J. Endod. 2008, 34, 394–407. [Google Scholar] [CrossRef]
- Kwon, S.R.; Wertz, P.W. Review of the Mechanism of Tooth Whitening. J. Esthet. Restor. Dent. 2015, 27, 240–257. [Google Scholar] [CrossRef]
- Kahler, B. Present status and future directions—Managing discoloured teeth. Int. Endod. J. 2022, 55, 922–950. [Google Scholar] [CrossRef]
- Sakalli, B.; Basmaci, F.; Dalmizrak, O. Evaluation of the penetration of intracoronal bleaching agents into the cervical region using different intraorifice barriers. BMC Oral Health 2022, 22, 266. [Google Scholar] [CrossRef] [PubMed]
- Greenwall-Cohen, J.; Greenwall, L.H. The single discoloured tooth: Vital and non-vital bleaching techniques. Br. Dent. J. 2019, 226, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Scaravilli, M.S.; Farella, M.; Riccitiello, F. Bleaching teeth treated endodontically: Long-term evaluation of a case series. J. Endod. 2006, 32, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, M.A. An overview of tooth-bleaching techniques: Chemistry, safety and efficacy. Periodontology 2000 2008, 48, 148–169. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.Q. Tooth-bleaching procedures and their controversial effects: A literature review. Saudi Dent. J. 2014, 26, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, K.; Wrbas, K.T. Management of calcified root canal during root canal therapy. J. Dent. Sci. 2023, 18, 1931–1932. [Google Scholar] [CrossRef] [PubMed]
- Karadas, M.; Demiburga, S. Influence of a short-time antioxidant application on the dentin bond strength after intracoronal bleaching. Microsc. Res. Tech. 2019, 82, 1720–1727. [Google Scholar] [CrossRef]
- Newton, R.; Hayes, J. The association of external cervical resorption with modern internal bleaching protocols: What is the current evidence? Br. Dent. J. 2020, 228, 333–337. [Google Scholar] [CrossRef]
- Waite, R.M.; Carnes, D.L.; Walker, W.A. Microleakage of TERM used with sodium perborate/water and sodium perborate/superoxol in the “walking bleach” technique. J. Endod. 1998, 24, 648–650. [Google Scholar] [CrossRef]
- Hosoya, N.; Cox, C.F.; Arai, T.; Nakamura, J. The walking bleach procedure: An in vitro study to measure microleakage of five temporary sealing agents. J. Endod. 2000, 26, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Chng, H.K.; Palamara, J.E.; Messer, H.H. Effect of hydrogen peroxide and sodium perborate on biomechanical properties of human dentin. J. Endod. 2002, 28, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Pallarés-Serrano, A.; Pallarés-Serrano, A.; Pallarés-Serrano, S.; Pallarés-Sabater, A. Study of the Intra-Coronal Pressure Generated by Internal Bleaching Agents and Its Influence on Temporary Restoration. Appl. Sci. 2022, 12, 2799. [Google Scholar] [CrossRef]
- Paula, A.B.; Dias, M.I.; Ferreira, M.M.; Carrilho, T.; Marto, C.M.; Casalta, J.; Cabrita, A.S.; Carrilho, E. Effects on gastric mucosa induced by dental bleaching—An experimental study with 6% hydrogen peroxide in rats. J. Appl. Oral Sci. 2015, 23, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, M.; K-Kaneyama, J.R.; Yamada, M.; Senda, A.; Manabe, A.; Miyazaki, A. Cytotoxic Effects of Hydrogen Peroxide on Human Gingival Fibroblasts In Vitro. Oper. Dent. 2015, 40, 430–439. [Google Scholar] [CrossRef]
- Cannabrava, V.P.; Fernandes, S.L.; Calabria, M.P.; Magalhlaes, A.C.; Ishikiriama, S.K.; Atta, M.T.; Wang, L. Bleaching technique effect of dentin permeability. Am. J. Dent. 2014, 27, 145–148. [Google Scholar]
- Lima, A.F.; Fonseca, F.M.; Freitas, M.S.; Palialol, A.R.; Aguiar, F.H.; Marchi, G.M. Effect of bleaching treatment and reduced application time of an antioxidant on bond strength to bleached enamel and subjacent dentin. J. Adhes. Dent. 2011, 13, 537–542. [Google Scholar]
- Pallarés-Serrano, A.; Pallarés-Serrano, S.; Pallarés-Serrano, A.; Pallarés-Sabater, A. Assessment of Oxygen Expansion during Internal Bleaching with Enamel and Dentin: A Comparative In Vitro Study. Dent. J. 2021, 9, 98. [Google Scholar] [CrossRef]
- Traviglia, A.; Re, D.; De Micheli, L.; Bianchi, A.E.; Coraini, C. Speed bleaching: The importance of temporary filling with hermetic sealing. Int. J. Esthet. Dent. 2019, 14, 310–323. [Google Scholar]
- Lima, S.N.L.; Ribeiro, I.S.; Grisotto, M.A.; Fernandes, E.S.; Hass, V.; de Jesus Tavarez, R.R.; Pinto, S.C.S.; Lima, D.M.; Loguercio, A.D.; Bandeca, M.C. Evaluation of several clinical parameters after bleaching with hydrogen peroxide at different concentrations: A randomized clinical trial. J. Dent. 2018, 68, 91–97. [Google Scholar] [CrossRef]
- Bersezio, C.; Sánchez, F.; Estay, J.; Ledezma, P.; Vernal, R.; Garlet, G.; Oliveira, O.B.; Fernández, E. Inflammatory markers IL-1β and RANK-L assessment after non-vital bleaching: A 3-month follow-up. J. Esthet. Restor. Dent. 2020, 32, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.C.; Braga, S.R.M.; Ferreira, D.; Jacintho, F.F.; Scaramucci, T.; Sobral, M.A.P. Bleaching of severely darkened nonvital tooth case report-48 months clinical control. J. Esthet. Restor. Dent. 2021, 33, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, R.; Aruna, S.; Joyson, M.; Manikandan, D. Comparison of the bleaching efficacy of three different agents used for intracoronal bleaching of discolored primary teeth: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2013, 31, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.Y.; Lum, S.O.Y.; Poh, R.S.C.; Lee, G.P.; Lim, K.C. An in vitro comparison of the bleaching efficacy of 35% carbamide peroxide with established intracoronal bleaching agents. Int. Endod. J. 2004, 37, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M.; Lindvall, A.M. External root resorption following bleaching of pulpless teeth with oxygen peroxide. Endod. Dent. Traumatol. 1985, 1, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Maleknejad, F.; Ameri, H.; Kianfar, I. Effect of intracoronal bleaching agents on ultrastructure and mineral content of dentin. J. Conserv. Dent. 2012, 15, 174–177. [Google Scholar] [PubMed]
- Martin-Biedma, B.; Gonzalez-Gonzalez, T.; Lopes, M.; Vilar, R.; Bahillo, J.; Varela-Patiño, P. Colorimeter and scanning electron microscopy analysis of teeth submitted to internal bleaching. J. Endod. 2010, 36, 334–337. [Google Scholar] [CrossRef]
- Serin, K.T. Effects of intracoronal bleaching agents on the surface properties of mineral trioxide aggregate. Odontology 2019, 107, 465–472. [Google Scholar] [CrossRef]
- Keskin, C.; Sariyilmaz, E.; Keles, A. The effect of bleaching agents on the compressive strength of calcium silicate-based materials. Aust. Endod. J. 2019, 45, 311–316. [Google Scholar] [CrossRef]
- Heling, I.; Parson, A.; Rotstein, I. Effect of bleaching agents on dentin permeability to Streptococcus faecalis. J. Endod. 1995, 21, 540–542. [Google Scholar] [CrossRef]
- Heboyan, A.; Avetisyan, A.; Karobari, M.I.; Marya, A.; Khurshid, Z.; Rokaya, D.; Zafar, M.S.; Fernandes, G.V.O. Tooth root resorption: A review. Sci. Prog. 2022, 105, 368504221109217. [Google Scholar] [CrossRef] [PubMed]
- Kazemipoor, M.; Azad, S.; Farahat, F. Concurrent effect of bleaching materials and size of root canal preparation on cervical dentin microhardness. Iran. Endod. J. 2017, 12, 298–302. [Google Scholar] [PubMed]
- Lewistein, I.; Hirschfeld, Z.; Stabholz, A.; Rotstein, I. Effect of hydrogen peroxide and sodium perborate on the microhardness of human enamel and dentin. J. Endod. 1994, 20, 61–63. [Google Scholar] [CrossRef]
- Berger, S.B.; Pavan, S.; Vidal Cde, M.; Santos, P.H.; Giannini, M.; Bedran-Russo, A.K. Changes in the stiffness of demineralized dentin following application of tooth whitening agents. Acta Odontol. Scand. 2012, 70, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Burrow, M.F.; Wong, R.; Parashos, P. Effect of sodium ascorbate on resin bonding to sodium perborate-bleached dentin. Oper. Dent. 2014, 39, 98–106. [Google Scholar] [CrossRef]
- Cavalli, V.; Rosa, D.A.D.; Silva, D.P.D.; Kury, M.; Liporoni, P.C.S.; Soares, L.E.S.; Martins, A.A. Effects of experimental bleaching agents on the mineral content of sound and demineralized enamels. J. Appl. Oral Sci. 2018, 26, 20170589. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).