Cross-Cultural Adaptation and Psychometric Characteristics of the Greek Functional Gait Assessment Scale in Healthy Community-Dwelling Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. (I) Cross-Cultural Adaptation
2.2. (II) FGAGR Psychometric Testing
2.2.1. Sample
2.2.2. Measures
2.2.3. Assessors
2.2.4. Reliability Testing of the FGAGR
2.2.5. Validity Testing of the FGAGR
2.3. Experimental Settings and Procedure
2.4. Data Analysis
2.4.1. Sample Size Calculations
2.4.2. Statistical Analysis
3. Results
3.1. Translation and Adaptation Process
3.2. Psychometric Testing of Final FGAGR
3.2.1. Reliability
3.2.2. MDC95, SEM, Distribution of the Scores
3.2.3. Validity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blair, S.; Lake, M.J.; Ding, R.; Sterzing, T. Magnitude and variability of gait characteristics when walking on an irregular surface at different speeds. Hum. Mov. Sci. 2018, 59, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K. Neurophysiology of gait: From the spinal cord to the frontal lobe. Mov. Disord. 2013, 28, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Delval, A.; Bayot, M.; Defebvre, L.; Dujardin, K. Cortical Oscillations during Gait: Wouldn’t Walking be so Automatic? Brain Sci. 2020, 10, 90. [Google Scholar] [CrossRef]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pirker, W.; Katzenschlager, R. Gait disorders in adults and the elderly: A clinical guide. Wien. Klin. Wochenschr. 2017, 129, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Osoba, M.Y.; Rao, A.K.; Agrawal, S.K.; Lalwani, A.K. Balance and gait in the elderly: A contemporary review. Laryngoscope Investig. Otolaryngol. 2019, 4, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, P.; Kiechl, S.; Bloem, B.R.; Willeit, J.; Scherfler, C.; Gasperi, A.; Rungger, G.; Poewe, W.; Seppi, K. Prevalence and burden of gait disorders in elderly men and women aged 60-97 years: A population-based study. PLoS ONE 2013, 8, e69627. [Google Scholar] [CrossRef]
- Auvinet, B.; Touzard, C.; Montestruc, F.; Delafond, A.; Goeb, V. Gait disorders in the elderly and dual task gait analysis: A new approach for identifying motor phenotypes. J. Neuroeng. Rehabil. 2017, 14, 7. [Google Scholar] [CrossRef]
- Mancini, M.; Horak, F.B. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur. J. Phys. Rehabil. Med. 2010, 46, 239–248. [Google Scholar]
- Francis, C.A.; Franz, J.R.; O’Connor, S.M.; Thelen, D.G. Gait variability in healthy old adults is more affected by a visual perturbation than by a cognitive or narrow step placement demand. Gait Posture 2015, 42, 380–385. [Google Scholar] [CrossRef]
- Borowicz, A.; Zasadzka, E.; Gaczkowska, A.; Gawłowska, O.; Pawlaczyk, M. Assessing gait and balance impairment in elderly residents of nursing homes. J. Phys. Ther. Sci. 2016, 28, 2486–2490. [Google Scholar] [CrossRef]
- Klöpfer-Krämer, I.; Brand, A.; Wackerle, H.; Müßig, J.; Kröger, I.; Augat, P. Gait analysis–Available platforms for outcome assessment. Injury 2020, 51, S90–S96. [Google Scholar] [CrossRef] [PubMed]
- Middleton, A.; Fritz, S.L. Assessment of Gait, Balance, and Mobility in Older Adults: Considerations for Clinicians. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 2013, 2, 205–214. [Google Scholar] [CrossRef]
- Wrisley, D.M.; Marchetti, G.F.; Kuharsky, D.K.; Whitney, S.L. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys. Ther. 2004, 84, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.L.; Austin, A.G.; Banke, G.M.; Foxx, S.R.; Gaetano, L.; Gardner, L.A.; McElhiney, J.; Morris, K.; Penn, L. Reference Group Data for the Functional Gait Assessment. Phys. Ther. 2007, 87, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Wrisley, D.M.; Kumar, N.A. Functional gait assessment: Concurrent, discriminative, and predictive validity in community-dwelling older adults. Phys. Ther. 2010, 90, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Y.; Zhou, Y.; Chen, C.; Xing, D.; Wang, C. Validity of the Functional Gait Assessment in Patients With Parkinson Disease: Construct, Concurrent, and Predictive Validity. Phys. Ther. 2014, 94, 392–400. [Google Scholar] [CrossRef]
- Forsberg, A.; Andreasson, M.; Nilsagård, Y. The Functional Gait Assessment in People with Multiple Sclerosis: Validity and Sensitivity to Change. Int. J. MS Care 2017, 19, 66–72. [Google Scholar] [CrossRef]
- Price, R.; Choy, N.L. Investigating the Relationship of the Functional Gait Assessment to Spatiotemporal Parameters of Gait and Quality of Life in Individuals with Stroke. J. Geriatr. Phys. Ther. 2019, 42, 256–264. [Google Scholar] [CrossRef]
- Kahn, J.H.; Ohlendorf, A.; Olsen, A.; Gordon, K.E. Reliability and Validity of the Functional Gait Assessment in Incomplete Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2020, 26, 268–274. [Google Scholar] [CrossRef]
- Beninato, M.; Ludlow, L.H. The Functional Gait Assessment in Older Adults: Validation Through Rasch Modeling. Phys. Ther. 2016, 96, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Schwieterman, M.; Fier, K.; Berni, J.; Swartz, N.; Phillips, R.S.; Reneker, J.C. Reliability and Validity of the Functional Gait Assessment: A Systematic Review. Phys. Occup. Ther. Geriatr. 2016, 34, 88–103. [Google Scholar] [CrossRef]
- Thieme, H.; Ritschel, C.; Zange, C. Reliability and validity of the functional gait assessment (German version) in subacute stroke patients. Arch. Phys. Med. Rehabil. 2009, 90, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Kamrani, A.A.A. Concurrent Validity of Functional Gait Assessment, Timed Up and Go, and Gait Speed Tests in the Persian Community-Dwelling elderly. Iran. Rehabil. J. 2010, 8, 15–20. [Google Scholar]
- Kirkwood, R.N.; Batista, N.C.L.; Marques, L.B.F.; de Melo Ocarino, J.; Neves, L.L.A.; de Souza Moreira, B. Cross-cultural adaptation and reliability of the Functional Gait Assessment in older Brazilian adults. Braz. J. Phys. Ther. 2021, 25, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.B.F.; Moreira, B.S.; Ocarino, J.M.; Sampaio, R.F.; Bastone, A.C.; Kirkwood, R.N. Construct and criterion validity of the functional gait assessment-Brazil in community-dwelling older adults. Braz. J. Phys. Ther. 2021, 25, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.D.; Rojjanasrirat, W. Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: A clear and user-friendly guideline. J. Eval. Clin. Pract. 2011, 17, 268–274. [Google Scholar] [CrossRef]
- Gluhm, S.; Goldstein, J.; Loc, K.; Colt, A.; Liew, C.V.; Corey-Bloom, J. Cognitive performance on the mini-mental state examination and the montreal cognitive assessment across the healthy adult lifespan. Cogn. Behav. Neurol. 2013, 26, 1–5. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphinee, S.; Williams, J.I.; Gayton, D. Measuring balance in the elderly: Preliminary development of an instrument. Physiother Can. 1989, 41, 304–311. [Google Scholar] [CrossRef]
- Lima, C.A.; Ricci, N.A.; Nogueira, E.C.; Perracini, M.R. The Berg Balance Scale as a clinical screening tool to predict fall risk in older adults: A systematic review. Physiotherapy 2018, 104, 383–394. [Google Scholar] [CrossRef]
- Franchignoni, F.; Horak, F.; Godi, M.; Nardone, A.; Giordano, A. Using psychometric techniques to improve the Balance Evaluation Systems Test: The mini-BESTest. J. Rehabil. Med. 2010, 42, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Godi, M.; Franchignoni, F.; Caligari, M.; Giordano, A.; Turcato, A.M.; Nardone, A. Comparison of reliability, validity, and responsiveness of the mini-BESTest and Berg Balance Scale in patients with balance disorders. Phys. Ther. 2013, 93, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulou, S.I.; Billis, E.; Gedikoglou, I.A.; Michailidou, C.; Nowicky, A.V.; Skrinou, D.; Michailidi, F.; Chandrinou, D.; Meligkoni, M. Reliability, validity and minimal detectable change of the Mini-BESTest in Greek participants with chronic stroke. Physiother. Theory Pract. 2019, 35, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Viccaro, L.J.; Perera, S.; Studenski, S.A. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J. Am. Geriatr. Soc. 2011, 59, 887–892. [Google Scholar] [CrossRef]
- Whitney, S.L.; Marchetti, G.F.; Schade, A.; Wrisley, D.M. The sensitivity and specificity of the Timed “Up & Go” and the Dynamic Gait Index for self-reported falls in persons with vestibular disorders. J. Vestib. Res. 2004, 14, 397–409. [Google Scholar] [PubMed]
- Yardley, L.; Beyer, N.; Hauer, K.; Kempen, G.; Piot-Ziegler, C.; Todd, C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005, 34, 614–619. [Google Scholar] [CrossRef] [PubMed]
- McGarrigle, L.; Yang, Y.; Lasrado, R.; Gittins, M.; Todd, C. A systematic review and meta-analysis of the measurement properties of concerns-about-falling instruments in older people and people at increased risk of falls. Age Ageing 2023, 52, afad055. [Google Scholar] [CrossRef]
- Billis, E.; Strimpakos, N.; Kapreli, E.; Sakellari, V.; Skelton, D.A.; Dontas, I.; Ioannou, F.; Filon, G.; Gioftsos, G. Cross-cultural validation of the Falls Efficacy Scale International (FES-I) in Greek community-dwelling older adults. Disabil. Rehabil. 2011, 33, 1776–1784. [Google Scholar] [CrossRef]
- Delbaere, K.; Close, J.C.; Mikolaizak, A.S.; Sachdev, P.S.; Brodaty, H.; Lord, S.R. The Falls Efficacy Scale International (FES-I). A comprehensive longitudinal validation study. Age Ageing 2010, 39, 210–216. [Google Scholar] [CrossRef]
- De Vet, H.C.W.; Terwee, C.B.; Knol, D.L.; Bouter, L.M. When to use agreement versus reliability measures. J. Clin. Epidemiol. 2006, 59, 1033–1039. [Google Scholar] [CrossRef]
- Bonett, D.G. Sample size requirements for estimating intraclass correlations with desired precision. Stat. Med. 2002, 21, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Buchner, A.; Erdfelder, E.; Lang, A.G. G*Power Version 3.1.9.2 [Computer Software]. Germany: Uiversität Kiel. 2014. Available online: http://www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3/download-and-register (accessed on 1 January 2018).
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Denham, E.B. Categorical Statistics for Communication Research, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 232–254. [Google Scholar] [CrossRef]
- Munro, B. Statistical Methods for Health Care Research, 5th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Field, A. Discovering Statistics Using IBM SPSS, 5th ed.; Sage Publications: London, UK, 2017. [Google Scholar]
- Dontje, M.L.; Dall, P.M.; Skelton, D.A.; Gill, J.M.R.; Chastin, S.F.M.; Seniors USP Team. Reliability, minimal detectable change and responsiveness to change: Indicators to select the best method to measure sedentary behaviour in older adults in different study designs. PLoS ONE 2018, 13, e0195424. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Almeida, S.; Carvalho, J.; Cruz, J.; Oliveira, A.; Jácome, C. Reliability, Validity, and Ability to Identify Fall Status of the Balance Evaluation Systems Test, Mini-Balance Evaluation Systems Test, and Brief-Balance Evaluation Systems Test in Older People Living in the Community. Arch. Phys. Med. Rehabil. 2016, 97, 2166–2173.e1. [Google Scholar] [CrossRef] [PubMed]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Greiner, M.; Pfeiffer, D.; Smith, R.D. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev. Vet. Med. 2000, 45, 23–41. [Google Scholar] [CrossRef]
- Ranganathan, P.; Aggarwal, R. Common pitfalls in statistical analysis: Understanding the properties of diagnostic tests–Part 1. Perspect. Clin. Res. 2018, 9, 40–43. [Google Scholar] [CrossRef]
- Ranganathan, P.; Aggarwal, R. Understanding the properties of diagnostic tests–Part 2: Likelihood ratios. Perspect. Clin. Res. 2018, 9, 99–102. [Google Scholar] [CrossRef]
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef]
- Leddy, A.L.; Crowner, B.E.; Earhart, G.M. Functional gait assessment and balance evaluation system test: Reliability, validity, sensitivity, and specificity for identifying individuals with Parkinson disease who fall. Phys. Ther. 2011, 91, 102–113. [Google Scholar] [CrossRef]
- Lin, J.H.; Hsu, M.J.; Hsu, H.W.; Wu, H.C.; Hsieh, C.L. Psychometric comparisons of 3 functional ambulation measures for patients with stroke. Stroke 2010, 41, 2021–2025. [Google Scholar] [CrossRef] [PubMed]
- Dommershuijsen, L.J.; RagunathanJ, J.; Ruiter, R.; Groothof, D.; Mattace-Raso, F.U.S.; Ikram, M.A.; Polinder-Bos, H.A. Gait speed reference values in community-dwelling older adults–Cross-sectional analysis from the Rotterdam Study. Exp. Gerontol. 2022, 158, 111646. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, R.N.; Moreira, B.S.; Mingoti, S.A.; Faria, B.F.; Sampaio, R.F.; Resende, R.A. The slowing down phenomenon: What is the age of major gait velocity decline? Maturitas 2018, 115, 31–36. [Google Scholar] [CrossRef]
- Xie, Y.J.; Liu, E.Y.; Anson, E.R.; Agrawal, Y. Age-Related Imbalance Is Associated with Slower Walking Speed: An Analysis From the National Health and Nutrition Examination Survey. J. Geriatr. Phys. Ther. 2017, 40, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.K.R.; Scianni, A.A.; Lima, L.O.; de Carvalho Lana, R.; Rodrigues-De-Paula, F. The Mini-BESTest is an independent predictor of falls in Parkinson Disease. Braz. J. Phys. Ther. 2020, 24, 433–440. [Google Scholar] [CrossRef]
- Tsang, C.S.L.; Lin-Rong, L.; Chung, R.C.K.; Pang, M.Y.C. Psychometric Properties of the Mini- Balance Evaluation Systems Test (Mini-BESTest) in Community- Dwelling Individuals with Chronic Stroke. Phys. Ther. 2013, 93, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Wirz, M.; Müller, R.; Bastiaenen, C. Falls in persons with spinal cord injury: Validity and reliability of the Berg Balance Scale. Neurorehabil. Neural Repair 2010, 24, 70–77. [Google Scholar] [CrossRef]
- Ulus, Y.; Durmus, D.; Akyol, Y.; Terzi, Y.; Bilgici, A.; Kuru, O. Reliability and validity of the Turkish version of the Falls Efficacy Scale International (FES-I) in community-dwelling older persons. Arch. Gerontol. Geriatr. 2012, 54, 429–433. [Google Scholar] [CrossRef]
- Montero-Odasso, M.M.; Kamkar, N.; Pieruccini-Faria, F.; Osman, A.; Sarquis-Adamson, Y.; Close, J.; Hogan, D.B.; Hunter, S.W.; Kenny, R.A.; Lipsitz, L.A.; et al. Task Force on Global Guidelines for Falls in Older Adults. Evaluation of Clinical Practice Guidelines on Fall Prevention and Management for Older Adults: A Systematic Review. JAMA Netw. Open 2021, 4, e2138911. [Google Scholar] [CrossRef]
- Lee, D.; Tak, S.H. A concept analysis of fear of falling in older adults: Insights from qualitative research studies. BMC Geriatr. 2023, 23, 651. [Google Scholar] [CrossRef]
- MacKay, S.; Ebert, P.; Harbidge, C.; Hogan, D.B. Fear of Falling in Older Adults: A Scoping Review of Recent Literature. Can. Geriatr. J. 2021, 24, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Lusardi, M.M.; Pellecchia, G.L.; Schulman, M. Functional performance in community living older adults. J. Geriatr. Phys. Ther. 2003, 26, 14–22. [Google Scholar] [CrossRef]
- Nilsagård, Y.; Kollén, L.; Axelsson, H.; Forsberg, A. Functional gait assessment: Reliability and validity in people with peripheral vestibular disorders. Int. J. Ther. Rehabil. 2014, 21, 367–373. [Google Scholar] [CrossRef]
| Characteristics | Mean (Standard Deviation) |
|---|---|
| Total Sample | n = 24 |
| Age (y) | 74 (7) |
| Range (min-max) | 66–94 |
| Sex (m/f) | 6/18 |
| Height (cm) | 161 (7) |
| Weight (kg) | 74 (13) |
| Body Mass Index (kg/cm2) | 28 (4) |
| Mini-Mental State (/30) | 28 (1) |
| Characteristics | Men Mean (Standard Deviation) | Women Mean (Standard Deviation) | p Value |
|---|---|---|---|
| Age (years) | 72 (5) | 80 (9) | 0.093 |
| Body Mass Index (kg/cm2) | 27 (2) | 29 (5) | 0.560 |
| FGAGR total score (/30) | 25 (3) | 28 (3) | 0.064 |
| Mini-BESTest total score (/28) | 20 (3) | 18 (3) | 0.167 |
| TUG (seconds) | 13.3 (3.4) | 13.5 (2.8) | 0.891 |
| FES-I total score (/64) | 24 (5) | 26 (4) | 0.297 |
| Item of FGAGR | Single Items Agreement | Item/Total Correlation | |
|---|---|---|---|
| Inter-Rater | Test-Retest | ||
| 1 | 0.879 * | 0.818 * | 0.275 |
| 2 | 0.875 * | 0.871 * | 0.490 * |
| 3 | 0.903 * | 0.950 * | 0.485 * |
| 4 | 0.943 * | 1.000 * | 0.732 * |
| 5 | 0.833 * | 0.667 * | 0.575 * |
| 6 | 0.632 * | 0.514 * | 0.339 |
| 7 | 0.703 * | 0.652 * | 0.619 * |
| 8 | 0.936 * | 0.749 * | 0.524 * |
| 9 | 0.849 * | 1.000 * | 0.561 * |
| 10 | 1.000 * | 0.941 * | 0.375 |
| Variable | FGAGR |
|---|---|
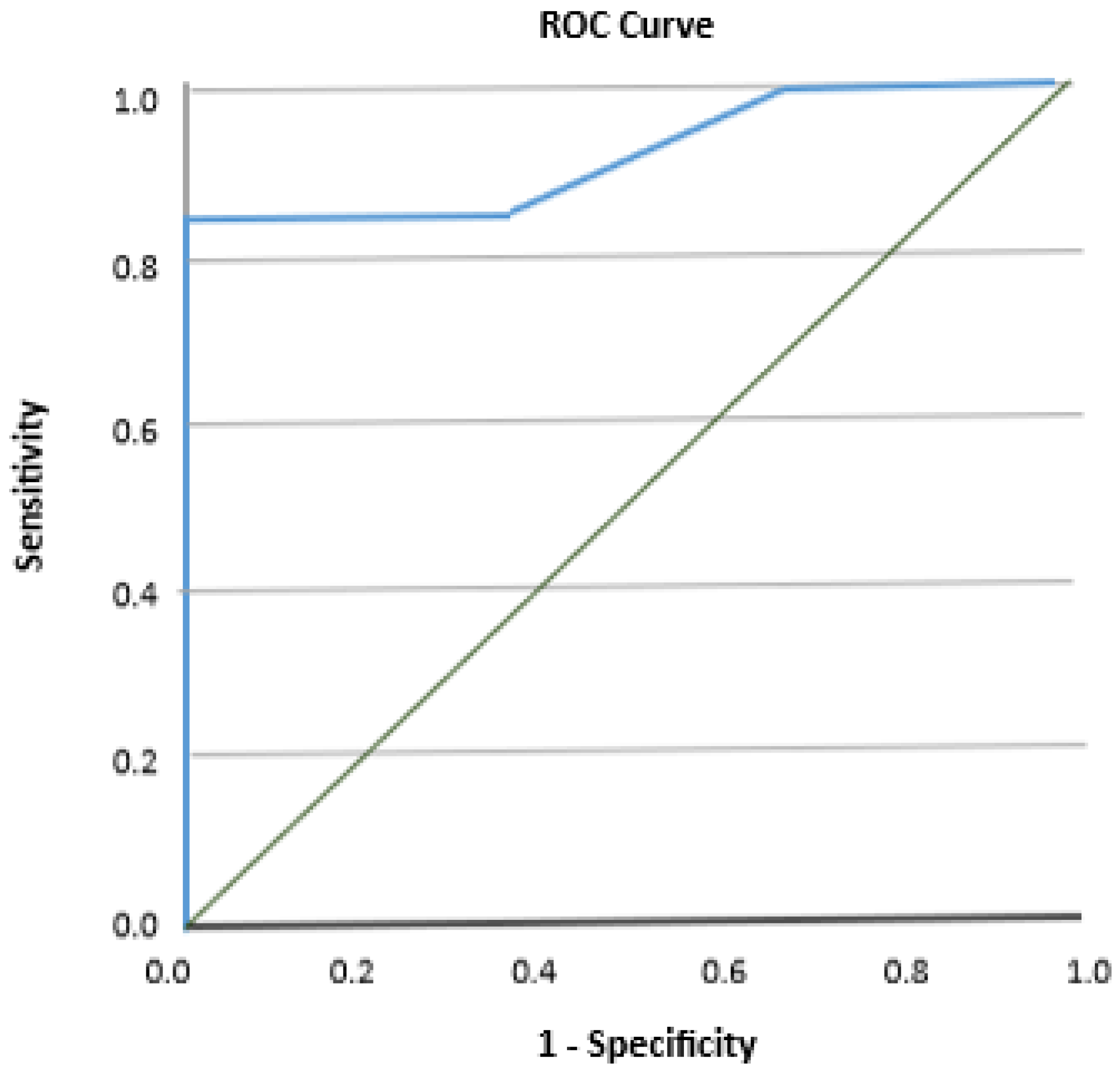
| AUC | 0.921 (0.80–1.00) |
| Cutoff point (/30) | 25 |
| Sensitivity (95% CI) | 0.84 (0.64–0.96) |
| Specificity (95% CI) | 0.50 (0.16–0.84) |
| True Positive | 16 |
| False Positive | 3 |
| True Negative | 3 |
| False Negative | 0 |
| Positive Likelihood Ratio (LR+) | 0 |
| Negative Likelihood Ratio (LR−) | 0.16 |
| Positive Predictive Value (%) | 100 |
| Negative Predictive Value (%) | 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampropoulou, S.; Kellari, A.; Gedikoglou, I.A.; Kozonaki, D.G.; Nika, P.; Sakellari, V. Cross-Cultural Adaptation and Psychometric Characteristics of the Greek Functional Gait Assessment Scale in Healthy Community-Dwelling Older Adults. Appl. Sci. 2024, 14, 520. https://doi.org/10.3390/app14020520
Lampropoulou S, Kellari A, Gedikoglou IA, Kozonaki DG, Nika P, Sakellari V. Cross-Cultural Adaptation and Psychometric Characteristics of the Greek Functional Gait Assessment Scale in Healthy Community-Dwelling Older Adults. Applied Sciences. 2024; 14(2):520. https://doi.org/10.3390/app14020520
Chicago/Turabian StyleLampropoulou, Sofia, Anthi Kellari, Ingrid A. Gedikoglou, Danai Gagara Kozonaki, Polymnia Nika, and Vasiliki Sakellari. 2024. "Cross-Cultural Adaptation and Psychometric Characteristics of the Greek Functional Gait Assessment Scale in Healthy Community-Dwelling Older Adults" Applied Sciences 14, no. 2: 520. https://doi.org/10.3390/app14020520
APA StyleLampropoulou, S., Kellari, A., Gedikoglou, I. A., Kozonaki, D. G., Nika, P., & Sakellari, V. (2024). Cross-Cultural Adaptation and Psychometric Characteristics of the Greek Functional Gait Assessment Scale in Healthy Community-Dwelling Older Adults. Applied Sciences, 14(2), 520. https://doi.org/10.3390/app14020520







